Abstract
Heart failure (HF) is a threat to public health. Heterogeneities in aetiology and phenotype complicate the diagnosis and management of HF. This is especially true when considering HF with preserved ejection fraction (HFpEF), which makes up 50% of HF cases. Natriuretic peptides may aid in establishing a working diagnosis in patients suspected of HF, but echocardiography remains the optimal choice for diagnosing HF. Echocardiography provides important prognostic information in both HF with reduced ejection fraction (HFrEF) and HFpEF. Traditionally, emphasis has been put on the left ventricular ejection fraction (LVEF). LVEF is useful for both diagnosis and prognosis in HFrEF. However, echocardiography offers more than this single parameter of systolic function, and for optimal risk assessment in HFrEF, an echocardiogram evaluating systolic, diastolic, left atrial and right ventricular function is beneficial. In this assessment echocardiographic modalities such as global longitudinal strain (GLS) by 2D speckle-tracking may be useful. LVEF offers little value in HFpEF and is neither helpful for diagnosis nor prognosis. Diastolic function quantified by E/e′ and systolic function determined by GLS offer prognostic insight in HFpEF. In HFpEF, other parameters of cardiac performance such as left atrial and right ventricular function evaluated by echocardiography also contribute with prognostic information. Hence, it is important to consider the entire echocardiogram and not focus solely on systolic function. Future research should focus on combining echocardiographic parameters into risk prediction models to adopt a more personalized approach to prognosis instead of identifying yet another echocardiographic biomarker.
Keywords: 2D echocardiography, 2D speckle-tracking echocardiography, heart failure, mechanics
Introduction
Chronic heart failure (HF) represents a large societal burden of disease and has recently been characterized as an emerging epidemic (1). HF is associated with significant mortality and morbidity (1). Furthermore, healthcare expenditures are only expected to increase due to ageing of the population (2). As a result, strategies to prevent HF and improve the efficiency and quality of care are needed. HF is a clinical syndrome characterized by heterogeneities in both aetiology and phenotype, making management and intervention difficult. For example, it has become apparent that almost 50% of HF patients may have HF with preserved left ventricular (LV) ejection fraction (HFpEF) (3), a disease that represents a diagnostic, prognostic and therapeutic challenge. Echocardiography provides a large amount of detailed information regarding cardiac structure and function in an easily accessible and cost-effective manner and is currently recommended in the diagnostic workup of patients in whom HF cannot be ruled out clinically (4). Additionally, biomarkers such as type B natriuretic peptides (BNP) and N-terminal prohormone BNP (NT-proBNP) may aid in the diagnosis of HF (5). This review summarizes the important features, strengths and limitations of echocardiography and BNP HF with respect to diagnosis, prognosis and risk prediction.
Diagnosis of HF
The diagnosis of non-acute HF relies on the presence of HF-related symptoms and the subsequent quantification of cardiac dysfunction. Cardinal symptoms include but are not limited to dyspnoea, reduced exercise capacity and peripheral oedema. Comorbidities such as previous myocardial infarction increase the likelihood of a HF diagnosis (6). Many of these symptoms are non-specific for HF (7), especially in the setting of chronic lung disease (7). Therefore, in general, patients presenting with signs and/or symptoms of HF should undergo an echocardiogram to confirm HF diagnosis and to determine the underlying aetiology in order to guide treatment and management (4). In current guidelines, natriuretic peptides are recommended as an alternative initial screening protocol potentially capable of ruling out the presence of HF (4). BNP and NT-proBNP both display a questionable positive predictive value, but a very high negative predictive value with respect to ruling out the presence of HF with reduced ejection fraction (HFrEF) (8, 9). The high negative predictive value but low positive predictive value is likely due to contemporary cut-offs being very low. Current guidelines emphasize that patients suspected of HF with a BNP >35 pg/mL or a NT-proBNP >125 pg/mL must undergo echocardiography to confirm HF diagnosis (4) and that patients with values below the cut-offs are very unlikely to have HF. However, natriuretic peptide levels have been shown to increase significantly with age and female sex (10), and age-adjusted cut-offs may offer better discriminatory value in the elderly and avoid unnecessary echocardiograms (11). Also, in a recent study of patients with valvular disease and adverse cardiac remodelling but with normal LV systolic function, the majority of patients had normal BNP levels (12). More research is required to determine whether valvular disease may affect the diagnostic value of BNP. Still, echocardiography to confirm HF diagnosis is not recommended in contemporary guidelines if values of natriuretic peptides are below reported cut-offs (4). The rationale for this approach is sound, since a blood-based biomarker capable of ruling out HF allows for the prevention of unnecessary echocardiograms. Additionally, it allows the clinician to search for the true cause of the patient’s symptoms. However, it is known that values of NT-proBNP and BNP are lower in HFpEF than in HFrEF (13).
Natriuretic peptides are secreted in response to myocardial wall stress. HFpEF is characterized by a small LV cavity and thickened LV walls (14). Since the law of Laplace (Fig. 1) dictates that LV wall stress is inversely proportional with LV wall thickness and directly proportional to LV radius, HFpEF does not elevate LV wall stress in the same way as seen in HFrEF (14, 15). Furthermore, it is known that values of natriuretic peptides are consistently lower in obese patients (16, 17, 18). Accordingly, it has been shown that obese HFpEF patients have lower levels of natriuretic peptides when compared to non-obese HFpEF patients (19). The mechanisms responsible for the lower levels of natriuretic peptides seen in obese HFpEF patients are currently unclear; however, it has been hypothesized that increased epicardial fat mass in obesity may subject the heart to an increased external pressure (19, 20). This increased external pressure then attenuates some of the intraventricular pressure that is believed to stimulate natriuretic peptide release, leading to reduced natriuretic peptide release (19, 20). When considering that almost 50% of all HF patients display a preserved EF phenotype (3) and that obesity is closely associated with HFpEF (21, 22), caution must be taken when excluding a HF diagnosis on the basis of a BNP measurement of <35 pg/mL or a NT-proBNP <125 pg/mL as recommended in current guidelines (4). The high prevalence of morbid obesity in HFpEF decreases the diagnostic value of natriuretic peptides, and it also complicates the estimation of jugular venous pressure and other diagnostic signs such as oedema. It should be noted that common cardiovascular medications such as angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and diuretics may reduce circulating levels of BNP (23, 24, 25, 26). Therefore, low BNP values must be interpreted with care in patients already taking these medications. Hence, diagnosing HFpEF remains challenging, and the clinician must remain vigilant. The possibility of HFpEF despite near-normal levels of natriuretic peptides especially in the setting of morbidly obese patients must still be considered and, if suspected, followed up by echocardiography.
Figure 1.
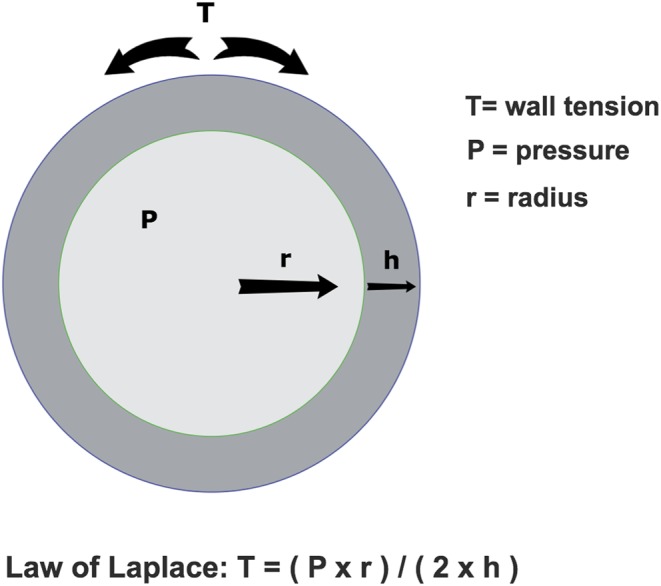
This figure shows the law of Laplace applied to a cross-sectional diagram of LV. The law of Laplace dictates that the LV wall tension is directly proportional to the product of the LV pressure and the LV radius. The LV wall tension is also inversely proportional to the LV wall thickness. LV, left ventricle.
HFrEF is easily diagnosed by echocardiography. The diagnosis of HFrEF, is by definition HF symptoms and left ventricular ejection fraction (LVEF) <40%, usually quantified by the Simpson biplane method (4).
HFpEF is more difficult to diagnose and the diagnosis includes, in addition to HF symptoms and a LVEF ≥50%, structural or functional signs of diastolic dysfunction or LV hypertrophy. These include either left atrial dilation (left atrial volume index ≥34 m/m2), LV hypertrophy (left ventricular mass index ≥115 g/m2 for men and ≥95 g/m2 for women) or an E/e′ ≥13 (4). As has been shown for the NT-proBNP and BNP cut-offs values, these criteria perform mediocre at best in diagnosing HFpEF (27). However, a recent study by Obokata et al. suggests that adding E/e′ measured during exercise to current guidelines may increase the sensitivity and negative predictive value for ruling out HFpEF (27). Importantly, it must be noted that Obokata et al. used E/e′ cut-offs from the American Society of Echocardiography/European Associaton of Cardiovascular Imaging (ASE/EACV) guidelines for assessment of diastolic dysfunction (27, 28). Therefore, the E/e′ cut-off for diastolic dysfunction measured during exercise differs with the measurement position of e′. When using only e′ measured in the lateral mitral annulus, a cut-off of E/e′ >15 is employed, while an E/e′ cut-off value of E/e′ >14 is used when e′ is averaged from both the septal and lateral mitral annular position (27, 28). Hence, when using exercise E/e′ in HFpEF diagnostics the e′ measurement position must be accounted for. New techniques such as myocardial strain deformation imaging by 2D speckle-tracking (2DS) have been shown to detect impaired systolic function in HFpEF despite normal LVEF (29). Furthermore, 2DS at rest has been demonstrated to identify patients with an increasing filling pressure during exercise among patients with unexplained dyspnoea and a normal LVEF (30). Hence, in the time to come, deformation imaging by 2DS may ease the diagnosis of HFpEF.
Finally, a new group of patients has been introduced in the latest 2016 European Society of Cardiology (ESC) HF guidelines. This patient group has been termed ‘heart failure with mid-range ejection fraction’ (HFmrEF), and comprises patients with heart failure symptoms, a LVEF of 40–49%, elevated levels of natriuretic peptides and either relevant structural heart disease (LV hypertrophy or left atrial (LA) enlargement) or diastolic dysfunction (4). According to the ESC, introducing this patient group as an entity independent of HFpEF and HFrEF was done to ‘stimulate research into the underlying characteristics, pathophysiology and treatment of this group of patients’ (4). Patients with HFmrEF are estimated to comprise 10–20% of all HF patients and currently occupies a ‘grey zone’ in the HF literature (31). In the cardiovascular health study, the mortality of HFmrEF patients was intermediate between HFrEF and HFpEF (32). It is interesting that some of the diagnostic criteria for HFmrEF are identical to those of HFpEF (signs of relevant structural heart disease or diastolic dysfunction) (4). This in accordance with recent evidence suggesting that HFmrEF may constitute a subset of HFpEF patients who are more affected by coronary artery disease (31). Coronary artery disease in HFpEF is associated with worse outcome and greater deterioration in LVEF, and some HFmrEF patients may therefore be HFpEF patients who may be progressing to HFrEF (33). However, large gaps in evidence regarding HFmrEF exist, and the introduction of HFmrEF as a diagnostic entity independent of HFrEF and HFpEF in current HF guidelines is likely to spur much-needed future research into this conundrum.
Prognosis and risk prediction in HF
In the current guidelines, echocardiography is recommended in the diagnostic workup of suspected HF patients in order to establish a diagnosis of either HFrEF or HFpEF (4). Echocardiography is also recommended in HFrEF patients to assess LVEF in order to guide evidence-based pharmacological treatment and device therapy (implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT)) and to quantify valvular disease (4). In addition, echocardiographic assessment of HF patients provides very important prognostic information. This is essential in helping patients, families and clinicians decide on appropriate type and timing of therapy.
HF patients who have undergone echocardiographic examination show better survival rates due to intensified medical treatment and intervention (34). Many echocardiographic markers have displayed prognostic value in HF (Table 1), and echocardiography is vital in the risk stratification of HF patients (35). LVEF determined by echocardiography is widely used in clinical practice and currently guides both diagnosis and therapy in HF (4). However, new and promising methods such as strain imaging by 2DS and tissue Doppler imaging (TDI) have emerged. Particularly, strain imaging has proven beneficial in detecting impaired systolic function in HFpEF despite normal LVEF values. The following sections will discuss risk prediction in HF including new methods such as 2DS and TDI.
Table 1.
The results of selected studies that have identified echocardiographic prognostic markers in both HFrEF and HFpEF.
| Study (year) | Echo parameter | Outcome | N | Follow-up | Comment |
|---|---|---|---|---|---|
| HFrEF | |||||
| Systolic function | |||||
| Curtis et al. 2003 (37) | LVEF | All-cause mortality | 7788 | 37 months (mean) | |
| Pocock et al. 2006 (38) | LVEF | All-cause mortality, cardiac death and HF hospitalization (composite) | 7599 | 38 months (median) | |
| Sengeløv et al. 2015 (39) | GLS | All-cause mortality | 1065 | 40 months (median) | Superior to LVEF |
| Hasselberg et al. 2015 (40) | GLS | Exercise capacity | 63 | N/A | Superior to LVEF |
| Risum et al. 2013 (44) | LV dyssynchrony by TDI | CRT response (all-cause mortality, cardiac transplantation or LVAD) (composite) | 131 | 47 months (truncated) | |
| Haugaa et al. 2012 (114) | LV mechanical dispersion* | Ventricular fibrillation or tachycardia (composite) | 569 | 30 months (median) | Following myocardial infarction |
| Biering-Sørensen et al. 2017 (48) | LV strain the inferior wall | Ventricular fibrillation or tachycardia (composite) | 1064 | 35 months (median) | MADIT-CRT sub-study |
| Biering-Sørensen et al. 2016 (49) | Inferior wall late diastolic velocity (a′) by TDI | Ventricular fibrillation or tachycardia or cardiac death(composite) | 151 | 28 months (median) | |
| Modin et al. 2017 (53) | GLS corrected by RR-interval | All-cause mortality | 151 | 32 months (median) | HFrEF with atrial fibrillation during examination |
| Diastolic and RV function | |||||
| Pinamonti et al. 1993 (54) | Restrictive filling pattern by E/A and DT | All-cause mortality or cardiac transplantation (composite) | 79 | 22 months | |
| Xie et al. 1994 (55) | Restrictive filling pattern by E/A and DT | Cardiac death | 100 | 16 months (mean) | |
| Acil et al. 2005 (57) | E/e′ | Cardiac death, cardiac transplantation or HF hospitalization (composite) | 132 | 7.5 months (mean) | |
| Rossi et al. 2009 (58) | LA area | All-cause mortality or HF hospitalization (composite) | 1157 | N/A | Meta-analysis of 18 prospective studies |
| Hsiao & Chiou 2013 (59) | LA expansion index | All-cause mortality and HF admission (composite) | 1735 | 31 months (median) | Dyspnoea patients |
| Ghio et al. 2001 (60) | RV ejection fraction | All-cause mortality or Cardiac transplant (composite) | 377 | 17 months (median) | |
| HFpEF | |||||
| Systolic function | |||||
| Shah et al. 2015 (89) | GLS | Cardiovascular death, HF hospitalization or aborted cardiac arrest (composite) | 447 | 31 months (median) | TOPCAT sub-study |
| Huang et al. 2017 (90) | GLS | All-cause mortality or HF hospitalization (composite) | 54 | At least 3 years | |
| Biering-Sørensen et al. 2017 (30) | GLS | Exercise-induced rise in pulmonary arterial wedge pressure | 85 | N/A | Unexplained dyspnoea patients |
| Hasselberg et al. 2015 (40) | GLS | Exercise capacity | 37 | N/A | |
| Wang et al. 2015 (92) | GLS during exercise | All-cause mortality or HF hospitalization (composite) | 80 | 36 months | |
| Other parameters | |||||
| Okura et al. 2009 (96) | E/e′ | All-cause mortality or HF hospitalization (composite) | 50 | 19 months (mean) | |
| Santos et al. 2016 (105) | LA strain | Cardiovascular death, HF hospitalization or aborted cardiac arrest (composite) | 357 | 31 months (mean) | TOPCAT sub-study |
| Melenovsky et al. 2015 (115) | LA emptying fraction | All-cause mortality | 101 | 12 months (median) | |
| Lam et al. 2009 (103) | Tricuspid regurtitant velocity | All-cause mortality | 244 | 36 months (median) | |
| Melenovsky et al. 2014 (116) | RV fractional area change | All-cause mortality | 96 | 18 months (median) | |
| Mohammed et al. 2014 (105) | TAPSE | All-cause mortality, cardiovascular mortality and HF hospitalization (not composite) | 562 | 55 months | |
CRT, cardiac resynchronization therapy; DT, deceleration time of the E-wave; GLS, global longitudinal strain; HF, heart failure; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging.
Risk prediction in HFrEF
Echocardiography is very valuable in the risk stratification of HFrEF patients. In 1962, Folse and Braunwald published results describing how to measure the ‘fraction of LV volume ejected per beat’ (36). This study marked an era spanning decades in which LVEF was the single most important metric in echocardiography, and particularly so in HFrEF. We now know that both anatomical structure and cardiac function offer prognostic insight in HFrEF and for that reason, it is important to do a comprehensive echocardiogram. This includes evaluating both LV systolic and diastolic function in addition to right ventricular and LA function. During the past 10–15 years, it has become increasingly apparent that advanced methods such as 2DS and TDI provide valuable insight into the prognosis and natural history of HF. This is acknowledged in current HF guidelines, since advanced methods should be considered for the detection of subclinical cardiac dysfunction in individuals at high risk of developing HF (4). LVEF still remains an important measurement in HFrEF (4), but as we now know, echocardiography has more information to offer.
Systolic function and prognosis in HFrEF
Reduced systolic function confers an adverse prognosis in HFrEF. LVEF remains the most widely used echocardiographic parameter for quantification of systolic function and is an established predictor of mortality in HFrEF (37, 38). However, LVEF relies on geometric assumptions and may therefore not reflect actual LV deformation. Recently, global longitudinal strain (GLS) has been demonstrated as a superior predictor of mortality in HFrEF when compared to LVEF (39). GLS is also superior to LVEF in predicting reduced exercise capacity in HFrEF (40). This suggests that GLS may be able to quantify the extent of systolic dysfunction in HFrEF more accurately and that it may be a superior prognostic factor to LVEF.
Two major causes of death in HFrEF are cardiac pump failure and sudden death from malignant ventricular arrhythmias. Device-based therapy such as CRT and CRT-ICD has been shown to reduce mortality and rehospitalization rates and improve prognosis in selected subsets of HFrEF patients (41). However, this therapy is very costly, and in several trials, it has been noted that approximately 1/3 of patients under current HFrEF selection criteria do not benefit clinically or hemodynamically (i.e. with an increased LVEF) from this treatment (42). Furthermore, the heterogeneous pathophysiology underlying HFrEF complicates the selection of patients. Current selection criteria are LVEF ≤35%, a wide QRS complex (≥150 ms) and symptomatic HF (43). Previous attempts to use parameters derived from echocardiography for the selection of CRT candidates have failed. However, mechanical dyssynchrony assessed by TDI has been associated with long-term survival in CRT patients (44), and measures of LV dyssynchrony based on longitudinal strain imaging appear to be strong prognostic factors of malignant arrhythmias in HFrEF (45, 46). Thus, these methods are promising for improving the selection of HFrEF patients for CRT and CRT-ICD.
Beside the geometric assumptions, another significant disadvantage of the LVEF is the lack of ability to quantify regional myocardial function. In mammalian hearts, reentry circuits arise when a wave of electrical activation abnormally reenters the myocardium instead of propagating normally throughout the cardiac tissue to die out in its periphery. Reentry plays a significant role in the pathophysiology underlying life-threatening ventricular arrhythmias such as ventricular tachycardia (VT) or ventricular fibrillation (VF) (47). Arrhythmias sustained by reentry mechanisms rely primarily on heterogeneities in cardiac structure and function. Localized areas of abnormal cardiac anatomical structure (such as scarring/fibrosis) or electrophysiological properties (such as subclinical ischaemia) may contribute to arrhythmogenesis. These localized areas can be missed by dilution with global measures of cardiac function such as the LVEF. In HFrEF patients receiving ICD-CRT therapy from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial, only reduced peak longitudinal strain in the inferior wall predicted VT/VF (48). This prognostic value was incremental to clinical and echocardiographic parameters (including GLS). Furthermore, in HFrEF patients with ischaemic aetiology receiving ICD therapy, only the late diastolic velocity (a′) measured by TDI in the inferior wall predicted a combined outcome of VT/VF and cardiovascular death (49). These results show that regional function is important in the diagnosis, treatment and prognosis of HFrEF.
It is now apparent that quantification of systolic function offers much prognostic value in HFrEF. However, the high prevalence of atrial fibrillation (AF) in HFrEF represents a challenge to current echocardiographic methods. In AF rhythm, the varying RR-interval and changing loading conditions impairs systolic measurements and thus the usefulness of these to predict outcome (50). Hence, AF patients are often excluded from echocardiographic studies. This is an issue when considering the very high prevalence of AF in HFrEF (51). A novel method of correcting GLS values by the RR-interval has been suggested (52) and has recently been demonstrated to be a superior prognostic marker to LVEF in HFrEF patients with AF during examination (53). This method may allow risk stratification of HFrEF patients despite AF rhythm.
Comprehensive cardiac assessment and prognosis in HFrEF
In HFrEF, much emphasis is put on the quantification of systolic function. Other aspects of cardiac structure and function also contribute with prognostic value. The quantification of LV filling pressure holds prognostic value in HFrEF: A restricted filling pattern by Doppler echocardiography as determined by E/A ratio and deceleration time of the E-wave is highly prognostic in HFrEF (54, 55). The ratio of transmitral early LV filling velocity to early diastolic TDI velocity of the mitral annulus (E/e′) is a measure of LV filling pressure and diastolic function. E/e′ is an independent predictor of mortality and hospitalization in HFrEF (56, 57). LA volume and function, important measures of diastolic function and markers of LV filling pressure, also contribute with independent prognostic value in HFrEF. LA size has been demonstrated as a powerful predictor of mortality and hospitalization in a meta-analysis of 18 studies of HFrEF patients (58). Particularly, the quantification of LA function through the LA emptying fraction and the LA expansion index seems promising. In a study of 1735 dyspnoea patients, LA expansion index was superior to LA volume in predicting mortality and hospitalization for HF (59). Thus, information about LV diastolic function provides much prognostic information in HFrEF.
The left side of the heart is not the sole contributor to risk stratification in HFrEF. The right ventricle (RV) holds significant prognostic value in HFrEF. A common misconception – it is thought that RV systolic function is exclusively determined by the afterload posed by decreasing LV function. However, RV ejection fraction and pulmonary artery systolic pressure both independently predict outcome in HFrEF (60). Thus, the prognostic value of RV systolic function is independent of RV afterload secondary to LV dysfunction and decreased RV systolic function likely marks a stage of advanced disease in which RV compensation is no longer possible.
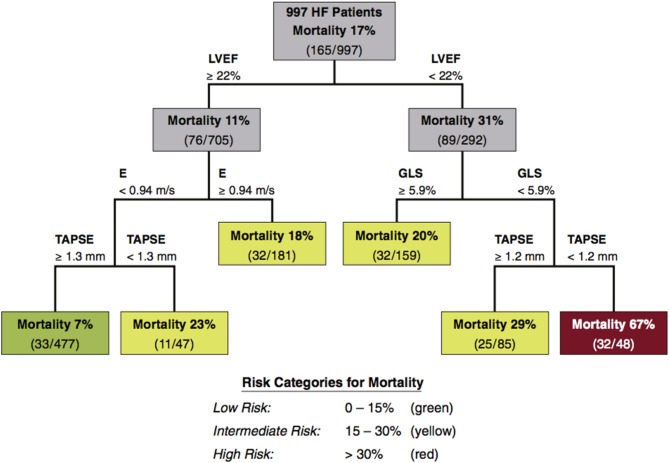
The aforementioned are in no way an exhaustive list of every echocardiographic marker or parameter that holds prognostic value in HFrEF. There are many more, such as the quantification of chamber geometry and valvular disease and indices of LV mass and hypertrophy. It serves to illustrate that the prognostic value of echocardiography in HFrEF goes far beyond the LVEF. Even though GLS shows promise as a universal marker of cardiac function, no single prognostic factor is sufficient for risk assessment in HFrEF. This was elegantly demonstrated by Sengeløv et al. in a study of 1065 HFrEF patients (39). In this study, GLS was the best prognostic factor out of all echocardiographic parameters determined by multivariable Cox regression and univariable C-statistics. Authors also performed a classification and regression tree (CART) analysis of echocardiographic parameters included in the study. CART is a statistical technique used to determine the best binary risk assessment scheme with respect to prediction of an outcome (61). Through the CART analysis, when considering all echocardiographic parameters included in their study, Sengeløv et al. found that LVEF, GLS, E and tricuspid annular plane systolic excursion (TAPSE) were important in the risk stratification of their HFrEF cohort (39) (Fig. 2). These results emphasize the need to evaluate both systolic, diastolic and RV function when predicting risk in HFrEF (Table 1). They also serve to emphasize that no single prognostic marker is sufficient to predict prognosis in HFrEF and that the results of different echocardiographic parameters must be interpreted together and not as a collection of single markers of risk, independent of each other Table 1 provides a selection of the many echocardiographic predictors of outcome in HFrEF (Table 1).
Figure 2.
A risk stratification tree obtained by CART analysis. A CART analysis includes many echocardiographic parameters to determine the most important predictors of mortality in HFrEF patients. The analysis selected LVEF, GLS, peak early diastolic filling velocity (E) and TAPSE as the most important predictors of mortality in HFrEF and combined them into a binary risk assessment scheme. CART, classification and regression tree analysis; GLS, global longitudinal strain; HF, heart failure; HFrEF, HF with reduced ejection fraction; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion. Reprinted from JACC: Cardiovascular Imaging, Vol 8, Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K & Biering-Sørensen T, Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction, Pages 1351–1359, Copyright (2015), with permission from Elsevier .
B-type natriuretic peptides and prognosis in HFrEF
The measurement of BNP to aid in risk stratification of chronic HF patients is recommended in current guidelines (recommendation Class 1A). Hence, BNP assessment is useful for determining risk of adverse outcome in chronic HF (62). BNP predicts all-cause mortality (63, 64) and sudden death in HFrEF (65). Furthermore, changes in BNP over a 6-month period have been shown to predict adverse outcome independently of baseline BNP levels (66). Thus, BNP levels offer easily accessible prognostic value in HFrEF and may be helpful in management and monitoring of HFrEF.
Risk prediction in HF with preserved ejection fraction
HFpEF currently represents a substantial clinical conundrum. Although spironolactone has been shown to reduce heart failure hospitalization rates in HFpEF (67), no therapeutic treatment has been shown to consistently improve survival (4). When considering that up to around 50% of HF patients may have HFpEF (3), this lack of effective therapeutic treatment represents a large unmet need in current cardiology practice. In order to properly orchestrate trials and to guide clinical decision making, thorough and accurate risk prediction is vital.
Impaired systolic function in HFpEF despite preserved LVEF
HFpEF was originally thought to result from diastolic dysfunction. This was based on invasive hemodynamic studies displaying increased LV stiffness, impaired diastolic relaxation and increased filling pressure in HFpEF (68, 69). However, even though LVEF may be preserved in HFpEF, systolic function is still abnormal. The LV contraction comprises longitudinal shortening, circumferential shortening and radial thickening. Both mitral annular plane longitudinal descent and velocity are impaired in HFpEF indicating decreased longitudinal function (70). GLS quantifies LV wall shortening during the cardiac cycle and particularly reflects longitudinal function (71). Accordingly, GLS has been shown to be impaired in HFpEF (29). Thus, despite a normal LVEF, systolic function is indeed abnormal in HFpEF.
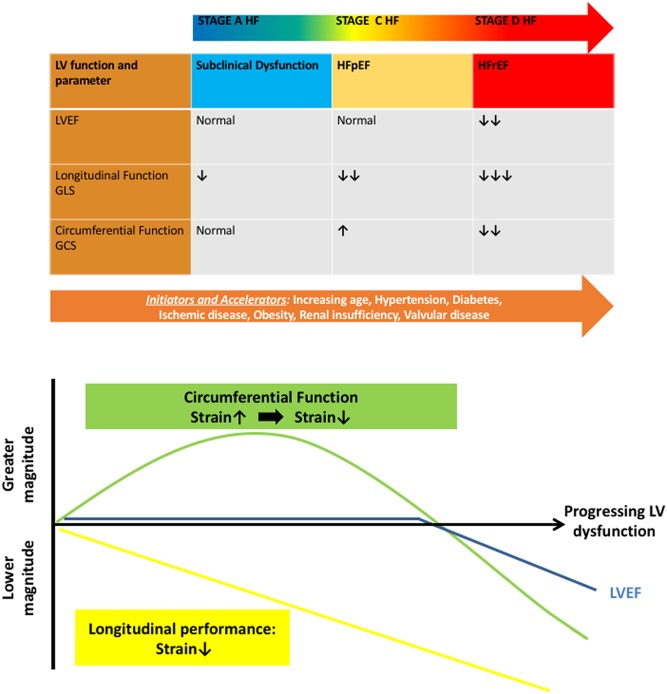
How LVEF can be preserved, despite the presence of systolic impairment in HFpEF, is not entirely clear. An analysis of the LV fibre and contraction pattern may offer some insight into the conundrum that is HFpEF. The LV muscular wall comprises three overall compartments: the subendocardium, the midmyocardium and the subepicardium (72). Circumferential fibres occupy the midmyocardium and produce primarily circumferential shortening, while longitudinal fibres in the subendocardium and subepicardium form a right-handed and left-handed helix, respectively (72). Thus, the subendocardial and subepicardial fibres form two oppositely directed spirals, with a net difference in angulation between these two spirals ranging from +60° to −60° (Fig. 3) (73). As a result, the circumferential components of subendocardial and subepicardial fibre contraction balance each other out and produce little net circumferential shortening in the normal heart (Fig. 3). The subendocardial fibres appear to be the most susceptible to injury (74, 75). Impairments in subendocardial fibre function lead to decreased right-handed helix shortening and thus reduced longitudinal function. Additionally, impairments in subendocardial fibre function may leave the left-handed helix shortening by subepicardial fibres unbalanced, potentially resulting in increased circumferential shortening (Fig. 3) (29, 76, 77, 78). This mechanism of exaggerated circumferential shortening by subendocardial fibre dysfunction may explain a distinct pattern of contraction observed in many conditions of subclinical LV dysfunction predisposing to HFpEF (Fig. 4). In increasing age (79), hypertension (80), diabetes mellitus (81) and obesity (82), GLS is reduced, reflecting subendocardial fibre dysfunction; yet, LVEF is preserved. Accordingly, in many of these conditions, circumferential shortening appears to be preserved or increased (83, 84, 85, 86). This may be extended to explain the decreased longitudinal yet preserved or exaggerated circumferential function seen in HFpEF (85, 87) and may also explain how LVEF can be preserved yet systolic function impaired in HFpEF (Fig. 4). These considerations serve to emphasize the limitations of LVEF as the sole marker of LV systolic function.
Figure 3.
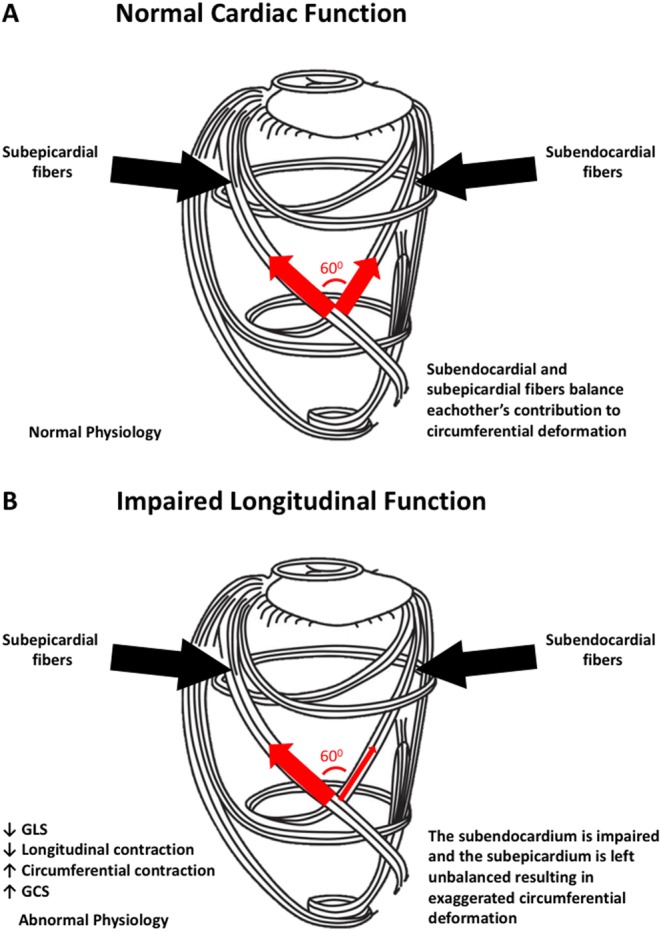
This figure depicts the myocardial fibre orientation of the left ventricular wall and their directions of contraction. In the subepicardium, myocardial fibres are oriented in a left-handed helix, while they run in a right-handed helix in the subendocardium. The cardiac midwall comprises circumferentially oriented fibres. (A) In the normal heart, the subepicardial left-handed helical fibres are balanced by the subendocardial right-handed helical fibres and longitudinal function is normal. (B) The subendocardial fibres are most susceptible to dysfunction from hypertension, increasing age, diabetes and other cardiovascular risk factors. When subendocardial function is lost, longitudinal contraction is impaired and the subepicardial fibres are left unbalanced. This results in decreased GLS and exaggerated circumferential contraction and GCS. This pattern of contraction is common in the presence of cardiovascular risk factors such as hypertension, increasing age and diabetes. GLS, global longitudinal strain; GCS, global circumferential strain. Adapted, under the terms of the original Creative Commons Attribution licence, from Nakatani 2011 (113).
Figure 4.
A model of progressive abnormalities in left ventricular function in heart failure across LVEF spectrum. Subclinical myocardial dysfunction triggered by cardiovascular risk factors such as age, hypertension and diabetes may present as depressed longitudinal deformation and decreased GLS but increased circumferential deformation and GCS. Progression is characterized by continuous impairment in longitudinal deformation. LVEF decreases at a point when circumferential function also starts to decline. GLS, global longitudinal strain; GCS, global circumferential strain; LVEF, LV ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Systolic function and prognosis in HFpEF
In the Candesartan in heart failure - assessment of mortality and morbidity (CHARM) trials, which studied 7599 HF patients with a broad spectrum of LVEFs, LVEF did not accurately discriminate risk of cardiovascular outcome in patients with an LVEF >45% (88). A similar relationship between LVEF and mortality was found in the Digitalis investigation group (DIG) trial of 7788 HF patients (37). This suggests that LVEF does not accurately quantify risk of adverse outcome in HFpEF. However, as discussed previously, this does not mean that systolic function is normal in HFpEF, since longitudinal function determined by GLS has been shown to be impaired (29). In the recent years, GLS has emerged as a powerful prognostic factor of cardiovascular death and hospitalization in HFpEF (89). GLS was also a strong prognostic marker of mortality in index hospitalized HFpEF patients (90). Furthermore, GLS predicts reduced exercise capacity in HFpEF (40). Exercise capacity is a strong prognostic parameter in HFpEF (91). GLS measured during bicycle ergometer testing has also been demonstrated as a strong prognostic marker in HFpEF (92). Thus, GLS shows great promise in risk stratification of HFpEF patients (Table 1). In the future, GLS may become valuable in guiding patient selection for HFpEF trials and for directing therapeutic treatment.
Other echocardiographic parameters with prognostic value in HFpEF
HFpEF is usually characterized by a small LV cavity, hypertrophied LV walls and severe diastolic dysfunction (93). Naturally, LV filling pressures are elevated in HFpEF (94) and LV compliance and relaxation is impaired, particular so during exercise (95). E/e′ is an estimate of LV filling pressure and has been shown to predict cardiac events in HFpEF (96). However, in the most recent guidelines for the quantification of diastolic function, it is stated that optimal assessment of diastolic function cannot be made by any one measure and is best assessed by several echocardiographic parameters (97). Accordingly, it was recently shown that E/e′ did not accurately estimate LV filling pressure and neither did it identify increased LV filling pressure in patients with dyspnoea (98). As such, it appears that a multi-parameter approach to the assessment of diastolic function in HFpEF is needed (97). The parameters recommended for this purpose are mitral E/A ratio, E/e′, LA volume indexed to body surface area and tricuspid regurgitant velocity (97).
As previously discussed, LA structure and function is a sensitive barometer of LV filling pressure. Chronic exposure of the LA to elevated LV filling pressure causes LA dilatation (99). LA dilation is found in about half of all HFpEF patients (93). HFpEF patients rely more than HFrEF patients on atrial pump function to adequately fill their stiff and non-compliant LV. This puts great strain on the LA and, as a result, the prevalence of AF is high in HFpEF (approx. 40%) (100). When LA function ceases, LV filling in HFpEF becomes severely impaired and thus marks a stage of advanced disease. As such, AF in HFpEF is an independent predictor of mortality and hospitalization (100). Recent application of 2DS of the LA has resulted in the measurement of LA peak reservoir strain. This new parameter shows promise in categorizing diastolic dysfunction (101) and may have prognostic value in HFpEF (102). Nevertheless, the dependence of LA peak reservoir strain on LA size and LV longitudinal function should always be taken into account when assessing LA peak reservoir strain.
RV function is closely related to LV diastolic function, since high LV filling pressure will increase pulmonary pressures and cause greater RV afterload. Thus, pulmonary hypertension and RV dysfunction are highly prevalent in HFpEF (103). Pulmonary hypertension quantified by tricuspid regurgitation velocity is an independent predictor of mortality in HFpEF (103, 104). Furthermore, the presence of RV systolic dysfunction has incremental value in addition to the presence of pulmonary hypertension. RV systolic dysfunction may mark a stage in which the RV is no longer able to compensate for the increased afterload in HFpEF or it may be a marker of generalized cardiomyopathy affecting both the LV and the RV. Nevertheless, RV systolic dysfunction assessed by TAPSE is associated with AF and the comorbidity burden in HFpEF and is predictive of poor outcomes (105). The complicated geometry of the RV makes imaging challenging, but 2DS has recently been applied to the RV free wall and was shown to predict outcome in pulmonary hypertension (106). RV free wall strain may offer intriguing prognostic value in HFpEF.
Once more, it becomes apparent that not one echocardiographic marker of cardiac structure or function is sufficient in HFpEF. A comprehensive examination is needed and results must be interpreted by the clinician on a personalized basis. We see that LV systolic and diastolic function, LA function and RV function offer prognostic value in HFpEF. As is stated in the current guidelines, the assessment of diastolic function is multifaceted and requires the assessment and interpretation of multiple echocardiographic indices (97). This is particularly true in HFpEF. Table 1 provides a list of studies that have identified echocardiographic prognostic parameters in HFpEF (Table 1).
B-type natriuretic peptides and prognosis in HFpEF
BNP levels are lower in HFpEF than in HFrEF (64). This is likely due to lower LV wall stress in HFpEF compared to HFrEF, since the increased wall thickness and the reduced LV radius both decrease LV wall stress in HFpEF (Fig. 1). Despite the lower levels of BNP observed in HFpEF, the usability to predict all-cause mortality appears to be similar to HFrEF (64). BNP levels predict death due to worsening of HF, HF hospitalization and sudden death in HFpEF (107). Changes in BNP levels have also displayed prognostic value in HFpEF: In a study of 2612 HFpEF patients (the I-Preserve study), an increase in BNP levels over 6 months was associated with an increased risk of cardiovascular death and HF hospitalization, while a decrease in BNP levels at 6 months was associated with a trend towards a decreased risk of cardiovascular death and HF hospitalization. BNP levels may therefore become a valuable tool for guiding management and treatment in HFpEF patients.
Echocardiographic risk prediction models in HF
It is now apparent that many echocardiographic markers hold prognostic value in HF. However, single measures of risk are rarely sufficient for the accurate estimation of prognosis in complex diseases such as HF (108). The topic of prognosis is essential to medicine and lays the foundation for clinical decision making. Accurate risk stratification allows clear communication of realistic expectations to patients and families and is instrumental in guiding evidence- and device-based therapies (22). Therefore, risk prediction in HF might benefit from the development of simple risk prediction schemes similar to the Systematic Coronary Risk Evaluation (SCORE) risk chart (109), and other prediction models currently used to estimate the risk of future cardiovascular disease in the general population. The estimation of risk from multivariable prediction models built upon the numerous established single-marker studies may offer more clinical value than simply identifying new single markers of risk (110, 111, 112). Many prediction models, with great heterogeneity in the number and types of predictors utilized, exist for HF; however, none have been deemed satisfactory (4, 110). Building a risk prediction model based on the accumulated data regarding echocardiographic predictors of risk in HF is cost-effective and feasible and may therefore represent a high-gain field of study in comparison to identifying, yet another prognostic marker. Echocardiography may be an optimal tool for personalizing risk stratification of HF patients and such efforts may help to maximize clinical applicability of the echocardiographic prognostic markers identified thus far.
Conclusion
B-type natriuretic peptides are useful in the exclusion of suspected HF; however, caution is warranted in the morbidly obese suspected of HFpEF. Echocardiography remains an essential procedure in HF. Echocardiography allows for accurate diagnosis and prognosis in both HFrEF and also in HFpEF. Future research should focus on combining echocardiographic prognostic markers into easily applicable prediction models in order to aid clinical decision making.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
Daniel Modin was supported by a scholarship from the Medical Society in Copenhagen during the preparation of this manuscript. Tor Biering-Sørensen was supported by the Fondsbørsvekselerer Henry Hansen og Hustrus Hovedlegat 2016. The sponsors had no role in the study design, data collection, data analysis, data interpretation or writing of the article.
References
- 1.Roger VL. Epidemiology of heart failure. Circulation Research 2013. 113 646–659. ( 10.1161/CIRCRESAHA.113.300268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation: Heart Failure 2013. 6 606–619. ( 10.1161/HHF.0b013e318291329a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. New England Journal of Medicine 2006. 355 260–269. ( 10.1056/NEJMoa051530) [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016. 37 2129–2200. ( 10.1093/eurheartj/ehw128) [DOI] [PubMed] [Google Scholar]
- 5.Tang WHW, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: clinical utilization of cardiac biomarker testing in heart failure. Circulation 2007. 116 e99–e109. ( 10.1161/CIRCULATIONAHA.107.185267) [DOI] [PubMed] [Google Scholar]
- 6.Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? Quarterly Journal of Medicine 1997. 90 335–339. ( 10.1093/qjmed/90.5.335) [DOI] [PubMed] [Google Scholar]
- 7.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. European Journal of Heart Failure 2009. 11 130–139. ( 10.1093/eurjhf/hfn013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto K, Burnett JC, Bermudez EA, Jougasaki M, Bailey KR, Redfield MM. Clinical criteria and biochemical markers for the detection of systolic dysfunction. Journal of Cardiac Failure 2000. 6 194–200. ( 10.1054/jcaf.2000.9676) [DOI] [PubMed] [Google Scholar]
- 9.Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, Hardman SM, Dargie HJ, Cowie MR. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. European Journal of Heart Failure 2005. 7 537–541. ( 10.1016/j.ejheart.2005.01.022) [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. Journal of the American College of Cardiology 2002. 40 976–982. ( 10.1016/S0735-1097(02)02059-4) [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt P, Collinson PO, Doughty RN, Fuat A, Gaze DC, Gustafsson F, Januzzi J, Rosenberg J, Senior R, Richards M. Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care. European Heart Journal 2010. 31 1881–1889. ( 10.1093/eurheartj/ehq163) [DOI] [PubMed] [Google Scholar]
- 12.Sharma V, Stewart RA, Lee M, Gabriel R, Van Pelt N, Newby DE, Kerr AJ. Plasma brain natriuretic peptide concentrations in patients with valvular heart disease. Open Heart 2016. 3 e000184 ( 10.1136/openhrt-2014-000184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002. 288 2144–2150. ( 10.1001/jama.288.17.2144) [DOI] [PubMed] [Google Scholar]
- 14.Maeder MT, Thompson BR, H-P Brunner-La Rocca, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. Journal of the American College of Cardiology 2010. 56 855–863. ( 10.1016/j.jacc.2010.04.040) [DOI] [PubMed] [Google Scholar]
- 15.Maeder MT, Mariani JA, Kaye DM. Hemodynamic determinants of myocardial B-type natriuretic peptide release: relative contributions of systolic and diastolic wall stress. Hypertension 2010. 56 682–689. ( 10.1161/HYPERTENSIONAHA.110.156547) [DOI] [PubMed] [Google Scholar]
- 16.Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. International Journal of Cardiology 2014. 176 611–617. ( 10.1016/j.ijcard.2014.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. Journal of the American College of Cardiology 2006. 47 85–90. ( 10.1016/j.jacc.2005.08.050) [DOI] [PubMed] [Google Scholar]
- 18.Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B-type natriuretic peptide levels in heart failure. Journal of the American College of Cardiology 2004. 43 1590–1595. ( 10.1016/j.jacc.2003.10.066) [DOI] [PubMed] [Google Scholar]
- 19.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017. 136 6–19. ( 10.1161/CIRCULATIONAHA.116.026807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clerico A, Zaninotto M, Passino C, Plebani M. Obese phenotype and natriuretic peptides in patients with heart failure with preserved ejection fraction. Clinical Chemistry and Laboratory Medicine 2018. [epub]. ( 10.1515/cclm-2017-0840) [DOI] [PubMed] [Google Scholar]
- 21.Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clinic Proceedings 2017. 92 266–279. ( 10.1016/j.mayocp.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 22.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circulation: Heart Failure 2016. 9 e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggioni AP, Anand I, Gottlieb SO, Latini R, Tognoni G, Cohn JN. & Val-HeFT Investigators (Valsartan Heart Failure Trial). Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. Journal of the American College of Cardiology 2002. 40 1414–1421. ( 10.1016/S0735-1097(02)02304-5) [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura M, Mizuno Y, Nakayama M, Sakamoto T, Sugiyama S, Kawano H, Soejima H, Hirai N, Saito Y, Nakao K, et al. B-type natriuretic peptide as a marker of the effects of enalapril in patients with heart failure. American Journal of Medicine 2002. 112 716–720. ( 10.1016/S0002-9343(02)01121-X) [DOI] [PubMed] [Google Scholar]
- 25.Paterna S, Di Pasquale P, Parrinello G, Fornaciari E, Di Gaudio F, Fasullo S, Giammanco M, Sarullo FM, Licata G. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure: a double-blind study. Journal of the American College of Cardiology 2005. 45 1997–2003. ( 10.1016/j.jacc.2005.01.059) [DOI] [PubMed] [Google Scholar]
- 26.Rousseau MF, Gurné O, Duprez D, Van Mieghem W, Robert A, Ahn S, Galanti L, Ketelslegers JM. & Belgian RALES Investigators. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. Journal of the American College of Cardiology 2002. 40 1596–1601. ( 10.1016/S0735-1097(02)02382-3) [DOI] [PubMed] [Google Scholar]
- 27.Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction clinical perspective: a Simultaneous Invasive-Echocardiographic Study. Circulation 2017. 135 825–838. ( 10.1161/CIRCULATIONAHA.116.024822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2016. 29 277–314. ( 10.1016/j.echo.2016.01.011) [DOI] [PubMed] [Google Scholar]
- 29.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. Journal of the American College of Cardiology 2014. 63 447–456. ( 10.1016/j.jacc.2013.09.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biering-Sørensen T, Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. Left ventricular deformation at rest predicts exercise-induced elevation in pulmonary artery wedge pressure in patients with unexplained dyspnoea. European Journal of Heart Failure 2017. 19 101–110. [DOI] [PubMed] [Google Scholar]
- 31.Lam Carolyn SP, Solomon Scott D. The middle child in heart failure: heart failure with mid‐range ejection fraction (40–50%). European Journal of Heart Failure 2014. 16 1049–1055. ( 10.1002/ejhf.159) [DOI] [PubMed] [Google Scholar]
- 32.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Annals of Internal Medicine 2002. 137 631–639. ( 10.7326/0003-4819-137-8-200210150-00006) [DOI] [PubMed] [Google Scholar]
- 33.Hwang S-J, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. Journal of the American College of Cardiology 2014. 63 2817–2827. ( 10.1016/j.jacc.2014.03.034) [DOI] [PubMed] [Google Scholar]
- 34.Tribouilloy C, Rusinaru D, Mahjoub H, Goissen T, Lévy F, Peltier M. Impact of echocardiography in patients hospitalized for heart failure: a prospective observational study. Archives of Cardiovascular Diseases 2008. 101 465–473. ( 10.1016/j.acvd.2008.06.012) [DOI] [PubMed] [Google Scholar]
- 35.Agha SA, Kalogeropoulos AP, Shih J, Georgiopoulou VV, Giamouzis G, Anarado P, Mangalat D, Hussain I, Book W, Laskar S, Smith AL, et al. Echocardiography and risk prediction in advanced heart failure: incremental value over clinical markers. Journal of Cardiac Failure 2009. 15 586–592. ( 10.1016/j.cardfail.2009.03.002) [DOI] [PubMed] [Google Scholar]
- 36.Folse R, Braunwald E. Determination of fraction of left ventricular volume ejected per beat and of ventricular end-diastolic and residual volumes. Experimental and clinical observations with a precordial dilution technic. Circulation 1962. 25 674–685. ( 10.1161/01.CIR.25.4.674) [DOI] [PubMed] [Google Scholar]
- 37.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. Journal of the American College of Cardiology 2003. 42 736–742. ( 10.1016/S0735-1097(03)00789-7) [DOI] [PubMed] [Google Scholar]
- 38.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. European Heart Journal 2006. 27 65–75. ( 10.1093/eurheartj/ehi555) [DOI] [PubMed] [Google Scholar]
- 39.Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K, Biering-Sørensen T. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC: Cardiovascular Imaging 2015. 8 1351–1359. ( 10.1016/j.jcmg.2015.07.013) [DOI] [PubMed] [Google Scholar]
- 40.Hasselberg NE, Haugaa KH, Sarvari SI, Gullestad L, Andreassen AK, Smiseth OA, Edvardsen T. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. European Heart Journal: Cardiovascular Imaging 2015. 16 217–224. ( 10.1093/ehjci/jeu277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. New England Journal of Medicine 2009. 361 1329–1338. ( 10.1056/NEJMoa0906431) [DOI] [PubMed] [Google Scholar]
- 42.Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Current Opinion in Cardiology 2006. 21 20–26. ( 10.1097/01.hco.0000198983.93755.99) [DOI] [PubMed] [Google Scholar]
- 43.Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, Ferguson TB Jr, Hammill SC, Karasik PE, Link MS, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society (corrected). Circulation 2012. 126 1784–1800. ( 10.1161/CIR.0b013e3182618569) [DOI] [PubMed] [Google Scholar]
- 44.Risum N, Williams ES, Khouri MG, Jackson KP, Olsen NT, Jons C, Storm KS, Velazquez EJ, Kisslo J, Bruun NE, et al. Mechanical dyssynchrony evaluated by tissue Doppler cross-correlation analysis is associated with long-term survival in patients after cardiac resynchronization therapy. European Heart Journal 2013. 34 48–56. ( 10.1093/eurheartj/ehs035) [DOI] [PubMed] [Google Scholar]
- 45.Kosiuk J, Dinov B, Bollmann A, Koutalas E, Mussigbrodt A, Sommer P, Arya A, Richter S, Hindricks G, Breithardt OA. Association between ventricular arrhythmias and myocardial mechanical dispersion assessed by strain analysis in patients with nonischemic cardiomyopathy. Clinical Research in Cardiology 2015. 104 1072–1077. ( 10.1007/s00392-015-0875-7) [DOI] [PubMed] [Google Scholar]
- 46.Haugaa KH, Goebel B, Dahlslett T, Meyer K, Jung C, Lauten A, Figulla HR, Poerner TC, Edvardsen T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. Journal of the American Society of Echocardiography 2012. 25 667–673. ( 10.1016/j.echo.2012.02.004) [DOI] [PubMed] [Google Scholar]
- 47.Witkowski FX, Leon LJ, Penkoske PA, Giles WR, Spano ML, Ditto WL, Winfree AT. Spatiotemporal evolution of ventricular fibrillation. Nature 1998. 392 78 ( 10.1038/32170) [DOI] [PubMed] [Google Scholar]
- 48.Biering-Sorensen T, Knappe D, Pouleur AC, Claggett B, Wang PJ, Moss AJ, Solomon SD, Kutyifa V. Regional longitudinal deformation improves prediction of ventricular tachyarrhythmias in patients with heart failure with reduced ejection fraction: a MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). Circulation: Cardiovascular Imaging 2017. 10 e005096 ( 10.1161/CIRCIMAGING.116.005096) [DOI] [PubMed] [Google Scholar]
- 49.Biering-Sørensen T, Olsen FJ, Storm K, Fritz-Hansen T, Olsen NT, Jøns C, Vinther M, Søgaard P, Risum N. Prognostic value of tissue Doppler imaging for predicting ventricular arrhythmias and cardiovascular mortality in ischaemic cardiomyopathy. European Heart Journal: Cardiovascular Imaging 2016. 17 722–731. [DOI] [PubMed] [Google Scholar]
- 50.Kotecha D, Mohamed M, Shantsila E, Popescu BA, Steeds RP. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. EP Europace 2017. 19 1427–1438. ( 10.1093/europace/eux027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? European Heart Journal 2015. 36 3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olsen FJ, Jørgensen PG, Dons M, Svendsen JH, Køber L, Jensen JS, Biering-Sørensen T. Echocardiographic quantification of systolic function during atrial fibrillation: probing the ‘ten heart cycles’ rule. Future Cardiology 2016. 12 159–165. ( 10.2217/fca.15.77) [DOI] [PubMed] [Google Scholar]
- 53.Modin D, Sengeløv M, Jørgensen PG, Bruun NE, Olsen FJ, Dons M, Fritz Hansen T, Jensen JS, Biering-Sørensen T. Global longitudinal strain corrected by RR interval is a superior predictor of all-cause mortality in patients with systolic heart failure and atrial fibrillation. ESC Heart Failure 2017. 5 311–318. ( 10.1002/ehf2.12220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinamonti B, Di Lenarda A., Sinagra G, Camerini F. Restrictive left ventricular filling pattern in dilated cardiomyopathy assessed by Doppler echocardiography: clinical, echocardiographic and hemodynamic correlations and prognostic implications. Heart Muscle Disease Study Group. Journal of the American College of Cardiology 1993. 22 808–815. ( 10.1016/0735-1097(93)90195-7) [DOI] [PubMed] [Google Scholar]
- 55.Xie GY, Berk MR, Smith MD, Gurley JC, DeMaria AN. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. Journal of the American College of Cardiology 1994. 24 132–139. ( 10.1016/0735-1097(94)90553-3) [DOI] [PubMed] [Google Scholar]
- 56.Hamdan A, Shapira Y, Bengal T, Mansur M, Vaturi M, Sulkes J, Battler A, Sagie A. Tissue Doppler imaging in patients with advanced heart failure: relation to functional class and prognosis. Journal of Heart and Lung Transplantation 2006. 25 214–218. ( 10.1016/j.healun.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 57.Acil T, Wichter T, Stypmann J, Janssen F, Paul M, Grude M, Scheld HH, Breithardt G, Bruch C. Prognostic value of tissue Doppler imaging in patients with chronic congestive heart failure. International Journal of Cardiology 2005. 103 175–181. ( 10.1016/j.ijcard.2004.08.048) [DOI] [PubMed] [Google Scholar]
- 58.Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, Klein AL, Marsan NA, Prior DL, Yu CM, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). European Journal of Heart Failure 2009. 11 929–936. ( 10.1093/eurjhf/hfp112) [DOI] [PubMed] [Google Scholar]
- 59.Hsiao S-H, Chiou K-R. Left atrial expansion index predicts all-cause mortality and heart failure admissions in dyspnoea. European Journal of Heart Failure 2013. 15 1245–1252. ( 10.1093/eurjhf/hfbib87) [DOI] [PubMed] [Google Scholar]
- 60.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. Journal of the American College of Cardiology 2001. 37 183–188. ( 10.1016/S0735-1097(00)01102-5) [DOI] [PubMed] [Google Scholar]
- 61.Breiman L. Classification and Regression Trees. New York, NY, USA: Chapman & Hall, 1993. [Google Scholar]
- 62.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017. 136 e137–e161. ( 10.1161/CIR.0000000000000509) [DOI] [PubMed] [Google Scholar]
- 63.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005. 330 625 ( 10.1136/bmj.330.7492.625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. Journal of the American College of Cardiology 2013. 61 1498–1506. [DOI] [PubMed] [Google Scholar]
- 65.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002. 105 2392–2397. ( 10.1161/01.CIR.0000016642.15031.34) [DOI] [PubMed] [Google Scholar]
- 66.Campana C, Pasotti M, Klersy C, Alessandrino G, Albertini R, Magrini G, Ghio S, Tavazzi L. Baseline and 6-month B-type natriuretic peptide changes are independent predictors of events in patients with advanced heart failure awaiting cardiac transplantation. Journal of Cardiovascular Medicine 2009. 10 671–676. ( 10.2459/JCM.0b013e328329346a) [DOI] [PubMed] [Google Scholar]
- 67.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014. 370 1383–1392. ( 10.1056/NEJMoa1313731) [DOI] [PubMed] [Google Scholar]
- 68.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricle. New England Journal of Medicine 2004. 350 1953–1959. ( 10.1056/NEJMoa032566) [DOI] [PubMed] [Google Scholar]
- 69.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008. 117 2051–2060. ( 10.1161/CIRCULATIONAHA.107.716886) [DOI] [PubMed] [Google Scholar]
- 70.Yip G, Wang M, Zhang Y, Fung JWH, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart 2002. 87 121–125. ( 10.1136/heart.87.2.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation 2012. 125 e244-248. ( 10.1161/CIRCULATIONAHA.111.086348) [DOI] [PubMed] [Google Scholar]
- 72.Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ, Tajik JA, Seward JB, Khandheria BK, Belohlavek M. Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. Journal of the American Society of Echocardiography 2007. 20 539–551. ( 10.1016/j.echo.2006.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Streeter DD, Spotnitz HM, Patel DP, Ross J, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circulation Research 1969. 24 339–347. ( 10.1161/01.RES.24.3.339) [DOI] [PubMed] [Google Scholar]
- 74.Biering-Sørensen T, Hoffmann S, Mogelvang R, Zeeberg Iversen A, Galatius S, Fritz-Hansen T, Bech J, Jensen JS. Myocardial strain analysis by 2-dimensional speckle tracking echocardiography improves diagnostics of coronary artery stenosis in stable angina pectoris. Circulation: Cardiovascular Imaging 2014. 7 58–65. [DOI] [PubMed] [Google Scholar]
- 75.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. Journal of the American College of Cardiology 2009. 54 36–46. ( 10.1016/j.jacc.2009.03.037) [DOI] [PubMed] [Google Scholar]
- 76.Hung C-L, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. Journal of the American College of Cardiology 2010. 56 1812–1822. ( 10.1016/j.jacc.2010.06.044) [DOI] [PubMed] [Google Scholar]
- 77.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. European Heart Journal 2012. 33 1716–1717. ( 10.1093/eurheartj/ehs124) [DOI] [PubMed] [Google Scholar]
- 78.Biering-Sørensen T, Solomon SD. Assessing contractile function when ejection fraction is normal: a case for strain imaging. Circulation: Cardiovascular Imaging 2015. 8 e004181. [DOI] [PubMed] [Google Scholar]
- 79.Kuznetsova T, Herbots L, Richart T, D’hooge J, Thijs L, Fagard RH, Herregods MC, Staessen JA. Left ventricular strain and strain rate in a general population. European Heart Journal 2008. 29 2014–2023. ( 10.1093/eurheartj/ehn280) [DOI] [PubMed] [Google Scholar]
- 80.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circulation: Cardiovascular Imaging 2009. 2 382–390. ( 10.1161/CIRCIMAGING.108.811620) [DOI] [PubMed] [Google Scholar]
- 81.Jensen MT, Sogaard P, Andersen HU, Bech J, Fritz Hansen T, Biering-Sørensen T, Jørgensen PG, Galatius S, Madsen JK, Rossing P, et al. Global longitudinal strain is not impaired in type 1 diabetes patients without albuminuria: the Thousand & 1 study. JACC: Cardiovascular Imaging 2015. 8 400–410. ( 10.1016/j.jcmg.2014.12.020) [DOI] [PubMed] [Google Scholar]
- 82.Wong CY, O’Moore-Sullivan T., Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004. 110 3081–3087. ( 10.1161/01.CIR.0000147184.13872.0F) [DOI] [PubMed] [Google Scholar]
- 83.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. American Journal of Cardiology 2009. 104 1398–1401. ( 10.1016/j.amjcard.2009.06.063) [DOI] [PubMed] [Google Scholar]
- 84.Sengupta SP, Caracciolo G, Thompson C, Abe H, Sengupta PP. Early impairment of left ventricular function in patients with systemic hypertension: New insights with 2-dimensional speckle tracking echocardiography. Indian Heart Journal 2013. 65 48–52. ( 10.1016/j.ihj.2012.12.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. European Heart Journal 2008. 29 1283–1289. ( 10.1093/eurheartj/ehn141) [DOI] [PubMed] [Google Scholar]
- 86.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. Journal of the American Society of Echocardiography 2008. 21 1138–1144. ( 10.1016/j.echo.2008.07.016) [DOI] [PubMed] [Google Scholar]
- 87.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. Journal of the American College of Cardiology 1995. 26 195–202. ( 10.1016/0735-1097(95)00153-Q) [DOI] [PubMed] [Google Scholar]
- 88.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005. 112 3738–3744. ( 10.1161/CIRCULATIONAHA.105.561423) [DOI] [PubMed] [Google Scholar]
- 89.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015. 132 402–414. ( 10.1161/CIRCULATIONAHA.115.015884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang W, Chai SC, Lee SGS, MacDonald MR, Leong KTG. Prognostic factors after index hospitalization for heart failure with preserved ejection fraction. American Journal of Cardiology 2017. 119 2017–2020. ( 10.1016/j.amjcard.2017.03.032) [DOI] [PubMed] [Google Scholar]
- 91.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. Journal of the American College of Cardiology 2005. 46 1883–1890. ( 10.1016/j.jacc.2005.07.051) [DOI] [PubMed] [Google Scholar]
- 92.Wang J, Fang F, Wai-Kwok Yip G, Sanderson JE, Feng W, Xie JM, Luo XX, Lee AP, Lam YY. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. International Journal of Cardiology 2015. 178 131–135. ( 10.1016/j.ijcard.2014.10.130) [DOI] [PubMed] [Google Scholar]
- 93.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation: Heart Failure 2014. 7 104–115. ( 10.1161/CIRCHEARTFAILURE.113.000887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 2007. 116 637–647. ( 10.1161/CIRCULATIONAHA.106.661983) [DOI] [PubMed] [Google Scholar]
- 95.Borlaug BA, Jaber WA, Ommen SR, Lam CSP, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 2011. 97 964–969. ( 10.1136/hrt.2010.212787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okura H, Kubo T, Asawa K, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Elevated E/E′ predicts prognosis in congestive heart failure patients with preserved systolic function. Circulation Journal 2009. 73 86–91. [DOI] [PubMed] [Google Scholar]
- 97.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. European Journal of Echocardiography 2009. 10 165–193. ( 10.1093/ejechocard/jep007) [DOI] [PubMed] [Google Scholar]
- 98.Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. E/e′ ratio in patients with unexplained dyspnea: lack of accuracy in estimating left ventricular filling pressure. Circulation: Heart Failure 2015. 8 749–756. ( 10.1161/CIRCHEARTFAILURE.115.002161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsang TSM, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. American Journal of Cardiology 2002. 90 1284–1289. ( 10.1016/S0002-9149(02)02864-3) [DOI] [PubMed] [Google Scholar]
- 100.Linssen GCM, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. European Journal of Heart Failure 2011. 13 1111–1120. ( 10.1093/eurjhf/hfr066) [DOI] [PubMed] [Google Scholar]
- 101.Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA strain categorization of LV diastolic dysfunction. JACC: Cardiovascular Imaging 2017. 10 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circulation: Heart Failure 2016. 9 e002763 ( 10.1161/CIRCHEARTFAILURE.115.002763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. Journal of the American College of Cardiology 2009. 53 1119–1126. ( 10.1016/j.jacc.2008.11.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. American Journal of Cardiology 2010. 106 284–286. ( 10.1016/j.amjcard.2010.02.039) [DOI] [PubMed] [Google Scholar]
- 105.Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014. 130 2310–2320. ( 10.1161/CIRCULATIONAHA.113.008461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circulation: Cardiovascular Imaging 2013. 6 711–721. ( 10.1161/CIRCIMAGING.113.000640) [DOI] [PubMed] [Google Scholar]
- 107.Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circulation: Heart Failure 2011. 4 569–577. ( 10.1161/CIRCHEARTFAILURE.111.962654) [DOI] [PubMed] [Google Scholar]
- 108.Moons KGM, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, Grobbee DE. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012. 98 683–690. ( 10.1136/heartjnl-2011-301246) [DOI] [PubMed] [Google Scholar]
- 109.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. European Heart Journal 2003. 24 987–1003. [DOI] [PubMed] [Google Scholar]
- 110.Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC: Heart Failure 2014. 2 440–446. ( 10.1016/j.jchf.2014.04.008) [DOI] [PubMed] [Google Scholar]
- 111.Moons KGM, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, Woodward M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012. 98 691–698. ( 10.1136/heartjnl-2011-301247) [DOI] [PubMed] [Google Scholar]
- 112.Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, Riley RD, Hemingway H, Altman DG. & PROGRESS Group. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Medicine 2013. 10 e1001381 ( 10.1371/journal.pmed.1001381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakatani S. Left ventricular rotation and twist: why should we learn? Journal of Cardiovascular Ultrasound 2011. 19 1–6. ( 10.4250/jcu.2011.19.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haugaa KH, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH, Florian A, Sjøli B, Brunvand H, Køber L, et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC: Cardiovascular Imaging 2013. 6 841–850. ( 10.1016/j.jcmg.2013.03.005) [DOI] [PubMed] [Google Scholar]
- 115.Melenovsky V, Hwang S-J, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circulation: Heart Failure 2015. 8 295–303. ( 10.1161/CIRCHEARTFAILURE.114.001667) [DOI] [PubMed] [Google Scholar]
- 116.Melenovsky V, Hwang S-J, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. European Heart Journal 2014. 35 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a