Abstract
Background:
Both activation of monocytes and increased serum fatty acid binding protein-4 (FABP4) occur in diabetes and are associated with increased atherosclerosis. The oxidized lipid, 9-hydroxyoctadecadienoic acid (9-HODE) increases FABP4 in macrophages, and is a ligand for G protein-coupled receptor 132 (GPR132). We investigated the involvement of GPR132 in mediating the 9-, 13-HODE stimulation of FABP4 secretion, and whether GPR132 expression is increased in monocytes from patients with type 2 diabetes.
Methods:
The effects of siRNA silencing of GPR132 gene and of the PPAR-γ antagonist T0070907 were studied in THP-1 cells. Serum levels of FABP4 and other adipokines were measured in patients with diabetes, and monocyte subpopulations were analyzed using flow cytometry. GPR132 mRNA was quantified in isolated CD14+ cells.
Results:
9-HODE and 13-HODE increased FABP4 expression in THP-1 monocytes and macrophages, and also increased GPR132 expression. Silencing of GPR132 did not influence the increase in FABP4 with 9-HODE, 13-HODE, or rosiglitazone (ROSI). By contrast, T0070907 inhibited the effect of all three ligands on FABP4 expression. Diabetic subjects had increased serum FABP4, and activated monocytes. They also expressed higher levels of GPR132 mRNA in CD14+ cells.
Conclusions:
We conclude that GPR132 is an independent monocyte activation marker in diabetes, but does not contribute to PPAR-γ-mediated induction of FABP4 by HODEs.
Keywords: FABP4, HODEs, macrophages
Introduction
Circulating levels of fatty acid binding protein-4 (FABP4) are increased in those who have, or are at risk of, metabolic syndrome, including obese subjects and those with macrovascular disease.1–3 Also referred to as adipocyte lipid binding protein (ALBP or aP2), FABP4 belongs to a group of low molecular weight (approximately 15 kDa) lipid chaperone molecules that solubilize fatty acids, allowing them to participate in intracellular metabolic and signaling processes. FABP4 regulates increased lipid trafficking and metabolism in adipocytes and macrophages and is involved in conditions associated with insulin resistance and increased cardiovascular risk.4 Targeted disruption of the gene for FABP4 in mice protects them against the detrimental effects of a high-fat diet,5 and knockout of the FABP4 gene inhibits foam cell formation when macrophages are exposed to oxidized LDL (oxLDL).6 In addition, small molecule inhibitors of FABP4 have been proposed as a therapeutic approach for patients with type 2 diabetes and atherosclerosis.7 Atherosclerosis and arterial plaque formation are increased in diabetic subjects. However, the mechanisms through which this occurs are not completely understood.8 Increase in lipid accumulation in the arterial wall and oxidative stress make substantial contributions to the increased vascular risk in diabetes.9 These processes lead to accumulation of oxLDL in the arterial wall, and enhance macrophage differentiation into lipid-laden foam cells. The latter process is partly mediated through increased FABP4 expression and is regulated by the nuclear transcription factor peroxisome proliferator activated receptor-γ (PPAR-γ).10
Hydroxyoctadecadienoic acids (HODEs) are lipid moieties reported to increase FABP4 expression and foam cell formation in macrophage cultures.11 HODEs are stable oxidation products of linoleic acid (LA), and are the most abundant oxidized fatty acids in oxLDL and atherosclerotic plaque.12–14 Two isomers of HODE, 9-HODE and 13-HODE, have been reported to be PPAR-γ ligands.15 However, studies have demonstrated that 9-HODE has distinct actions from 13-HODE on macrophages, acting through a mechanism that is independent of PPAR-γ.16 In keratinocytes, 9-HODE has pro-inflammatory effects that are mediated through the G protein-coupled receptor GPR132 (also known as G2A).12,14,17 In contrast, 13-HODE is not a ligand for GPR132, and its effects appear to be predominantly protective in relation to inflammation and atherosclerosis.18,19 GPR132 is also known to be involved in regulating atherosclerosis from studies in atherosclerosis-prone mouse models,20,21 although its precise role has yet to be determined. It is highly expressed in macrophages,22 and has been demonstrated to be a high-affinity receptor for 9-HODE.18,19 Tissue-resident macrophages, including those that accumulate in the arterial wall during atherogenesis, are derived from circulating monocytes. Monocyte receptors CD36 and CD11b have been associated with oxLDL interaction,23 while increased levels of receptor CD54 are thought to increase inflammatory state among diabetic subjects.24 Monocyte activation is the preliminary step in differentiation into macrophages and finally into foam cells, and these processes are accelerated in subjects with diabetes.25,26 Although GPR132 is a receptor for 9-HODE, and its expression in monocyte/macrophage cells has been documented, it has not previously been investigated as a potential marker of human monocyte activation.
The aim of this study was to determine if GPR132 is a marker of monocyte activation in diabetes and if it plays a role in transducing the pro-atherogenic actions of HODEs. The human monocytic leukemia cell line THP-1 was utilized as an in vitro model to investigate regulation of FABP4 expression by HODEs, and to determine the relative involvement of GPR132 and PPAR-γ. We hypothesized that GPR132 was elevated in the monocyte fraction of type 2 diabetic subjects, and that it played a role in mediating the increase in serum FABP4. We measured levels of serum FABP4 and adipokines in type 2 diabetic subjects. Subpopulations of activated monocytes were quantified in these patients, and a correlation with GPR132 expression in CD14+ monocytes was sought.
Materials and methods
Materials
Roswell Park Memorial Institute (RPMI) medium, penicillin, streptomycin, glutamine and fetal bovine serum (FBS) were purchased from Invitrogen (Mulgrave, VIC, Australia). Ficoll-Paque was purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). CD14-coated magnetic beads and MS MACS columns were purchased from Miltenyi Biotec (North Ryde, NSW, Australia). α-linolenic acid (ALA), LA, ROSI, 9-HODE and 13-HODE were purchased from Cayman Chemicals (Ann Arbor, MI, USA). 2-chloro-5-nitro-N-4-pyiridnyl-benzamide (T0070907) was purchased from Sapphire Biosciences (NSW, Australia). Flow cytometry antibodies CD14-PE-Cy7, CD11b-PE, CD36-FITC, CD54-APC and isotype controls were purchased from BD Australia (North Ryde, NSW, Australia). Rabbit polyclonal GPR132, isotype controls, biotinylated rabbit IgG and HRP-streptavidin were purchased from Perkin Elmer (Melbourne, VIC, Australia). Phorbol myristate acetate (PMA), 3,3′-diaminobenzidine (DAB) chromogen were purchased from Vector Labs, Abacus ALS (Brisbane, QLD, Australia).
Subjects
Patients (n = 51, diabetic cohort n = 31, control n = 20) with type 2 diabetes who were over the age of 18 years and were not treated with any thiazolidinediones, were recruited from the diabetes clinics at the Townsville Hospital, Queensland. From each subject (patient and control), 24 ml of heparinized blood was collected for cell preparation, and 10 ml of clotted blood from which serum was stored at −80°C pending cytokine measurement.
Ethics statement
Human peripheral blood was obtained with informed, written consent from all patients and controls. Institutional approval was obtained from the Human Research Ethics Committees of Townsville Health Service District (HREC/09/QTHS/115) and James Cook University (H3992).
Patient cell preparation
Peripheral blood mononuclear cells (PBMCs) were prepared from heparinized blood samples by Ficoll-Paque density gradient centrifugation. GPR132 gene expression was determined in CD14+ cells positively selected from PBMC using CD14-coated magnetic beads. Magnetic separation was carried out on an MS MACS column according to the manufacturer’s instructions.
THP-1 cell culture
The human monocytic leukemia cell line THP-1 was cultured in RPMI medium containing 10% FBS, 4 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were maintained at a density of 2 × 105–1 × 106 cells/ml. For all experiments, 1 × 106 THP-1 cells were cultured in triplicate in six-well plates. THP-1 cells were differentiated into macrophages by treatment with 100 nM PMA for 36 h followed by 24 h in low serum RPMI medium (0.4% FBS) with antibiotics and L-glutamine. The long-chain fatty acids ALA, LA, 9-HODE and 13-HODE were incubated for 24–48 h with THP-1 monocytes or macrophages at a concentration of 30 µM. For some experiments the PPAR-γ antagonist, 2-chloro-5-nitro-N-4-pyiridnyl-benzamide (10 µM) was incubated with cells for 2 h, after which cells were washed with Dulbecco’s phosphate-buffered saline (DPBS) three times, and then treated with 9-HODE (30 µM), 13-HODE (30 µM) or ROSI (0.1 µM) for 24 h. Control cells were maintained without addition of the above ligands for all experiments.
Adipokine and protein measurement
Serum and tissue culture-derived cytokines were quantified using the Multiplex FlowCytomix system (Bendermedsystems, Jomar Diagnostics Pty Ltd, Adelaide, Australia). Antibody-coated beads were incubated with either patient serum or cell supernatant from THP-1 cells, followed by a biotin-conjugated secondary antibody and finally streptavidin-PE. Analysis was performed using software provided by the manufacturer. The multiplex immunoassay measured inflammatory and obesity markers (sCD40L, sICAM-1, IL-6, leptin, MCP-1, myeloperoxidase (MPO), osteoprotegerin (OPG), resistin and soluble TNF receptor). FABP4 was measured using a commercially available enzyme-linked immunosorbent assay (ELISA; Cayman Chemicals, Ann Arbor, MI, USA).
Flow cytometry analysis of activated monocytes
PBMCs were stained with the monocyte antibody cocktail CD14-PE-Cy7, CD11b-PE, CD36-FITC and CD54-APC, or isotype controls, and analyzed using flow cytometry (FACS Calibur, Cell Quest Pro software, BD Australia, North Ryde, NSW, Australia). The cells were gated to CD14+, and expression of the various activation markers determined in this population. Mean fluorescence intensity was used to determine differences between the diabetic and control samples.
Immunocytochemical analyses
Indirect immunoperoxidase cytochemistry (Sapphire Biosciences) was used to determine the proportion of THP-1 monocytes and macrophages expressing GPR132. THP-1 monocytes (1 × 105) were cytospun onto sialinized coverslips (Cytospin 4 centrifuge, Thermo Fischer Scientific, Scoresby, VIC, Australia). THP-1 derived macrophages were cultured on sterile coverslips by treating THP-1 monocytes with 100 nM PMA for 36 h in 10% FBS RPMI medium. Coverslips were then air-dried and fixed in 75% ethanol in DPBS prior to incubation with rabbit polyclonal GPR132 or GPR120 antibody or isotype control (45 min), followed by biotinylated rabbit IgG (30 min) and, finally, HRP-streptavidin (30 min). Bound antibody was detected by the addition of DAB chromogen. Cells were counterstained with Mayer’s hematoxylin.
Real-time RT-PCR analysis
RNA was extracted from PBMCs from diabetic and control subjects using RNeasy extraction kits (Qiagen Pty Ltd, Melbourne, VIC, Australia). Total RNA was quantified using a Nanodrop spectrophotometer (Thermo Fischer Scientific, Australia). Samples were assessed for DNA contamination by running a no reverse transcriptase reaction. One-step real-time reverse transcriptase polymerase chain reation (RT-PCR) was performed on a Corbett Roto-gene 6000 (Qiagen) with signal acquired at the SYBR green channel. Reactions were performed in duplicate and samples were normalized and quantified against the reference gene peptidylpropyl isomerase A (PPIA) to determine the expression of FABP4, GPR132, resistin, MAPK and PPAR-γ. All primers were obtained commercially (SABiosciences, Melbourne, VIC, Australia) with the exception of GPR132, with the following sequences: sense 5′-ACGAGATGAGACGGAACTG-3′ and anti-sense 5′-CTGCCTCTGTGCCTTAGC-3′.
GPR132 gene silencing
THP-1 derived macrophages (5 × 105) were cultured for 24 h in RPMI medium with 0.4% FBS devoid of antibiotics. Immediately prior to transfection, the medium was replaced with 900 µl of serum-free RPMI without antibiotics. siRNA oligonucleotides for the silencing control MAPK, scrambled oligos as negative control or for GPR132 (Qiagen) were prepared by incubating 1 µl of 10 µM siRNA with 2 µl of lipofectomine RNAiMAX transfection reagent for 15 min at RT in 97 µl of RPMI medium. Transfection complex was then added drop by drop into treatment wells and mixed, providing a final concentration of 10 nM. The transfection complex medium was replaced after 6 h with 1 ml RPMI medium (10% FBS without antibiotics) and incubated for 18 h. A combination of oligonucleotide sequences used for GPR132 silencing are as follows:
5′-CAGGATTGCCGGGTACTACTA-3′
5′-ACGGACCATTCCCGCCAAGAA-3′
5′-CTGGGTCACCATCGAGATCAA-3′
5′-TACCAATTTCTCGTTCCTGAA-3′
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM) where applicable. Differences between groups of measurements were analyzed using the nonparametric Mann–Whitney U test. Differences between groups were examined using either one-way analysis of variance (ANOVA) followed by Fisher’s multiple comparison test, two-way ANOVA, followed by Tukey’s post hoc test, or unpaired t test. Correlations between monocyte activation markers and serum adipokines were performed using Pearson’s correlation coefficient. Statistical significance was set at p < 0.05.
Results
FABP4 gene expression is induced by 9-HODE and 13-HODE but not by ALA or LA
THP-1 cells were employed as a model to investigate the induction of FABP4 in monocytes. They were either maintained in culture as monocytes or differentiated into macrophage-like cells following exposure to PMA. Neither the C18 n-3 fatty acid ALA, nor the C18 n-6 fatty acid LA affected FABP4 mRNA expression in monocytes or macrophages (Figure 1(a)). In contrast, both 9-HODE and 13-HODE markedly increased FABP4 expression in monocytes and macrophages (Figure 1(b), p < 0.001). Increase in the levels of FABP4 expression were similar in monocytes and macrophages. Absolute levels of FABP4 mRNA in macrophages were higher than in monocytes (data not shown). There was a concentration-dependent increase in FABP4 expression following PMA treatment of monocytes (Figure 1(c), p < 0.001). Activation of THP-1 monocytes by 1 nM PMA in conjunction with treatment with ALA or LA did not alter expression of FABP4 (Figure 1(c)). In contrast, there was a marked increase in FABP4 expression when activated monocytes were co-treated with either 9-HODE or 13-HODE (Figure 1(c), p < 0.001).
Figure 1.
FABP4 gene expression is induced by 9-HODE and 13-HODE but not by ALA or LA in THP-1 monocytes and differentiated macrophages. Gene expression of FABP4 was determined and results expressed as percentage of control, mean ± SEM (n = 3 replicates per group); representative data from three independent experiments. (a) There was no change to FABP4 expression in either THP-1 monocytes or differentiated macrophages treated with ALA and LA. (b) 9-HODE and 13-HODE both upregulated FABP4 gene expression in both THP-1 monocytes and differentiated macrophages (*p < 0.001, two-way ANOVA, *p < 0.001 (compared against control, Tukey’s post-test corrected for multiple comparisons). (c) Co-treatment of 1 nM PMA with either 9-HODE or 13-HODE dramatically increased FABP4 expression in THP-1 monocytes (*p < 0.001, one-way ANOVA), while there was no change with the combination of PMA with ALA or LA, or PMA alone.
GPR132 is expressed in THP-1 cells and mRNA is upregulated by 9-HODE and augmented by PMA
Having observed induction of FABP4 in THP-1 cells, GPR132 mRNA expression was investigated in THP-1 monocytes and macrophages (induced by exposure to PMA) following exposure to 9-HODE and 13-HODE. PMA alone increased expression of GPR132 mRNA (Figure 2(a), p < 0.001). Only 9-HODE upregulated GPR132 mRNA expression and this was augmented by addition of PMA (p < 0.001) (Figure 2(a)). GPR132 protein expression was demonstrated in monocytes with moderate levels of expression (~30% of cells), while staining was more widespread in the macrophages (~90% of cells; Figure 2(b)).
Figure 2.

GPR132 mRNA and protein expression in THP-1 cells is upregulated by 9-HODE, and augmented by PMA. Expression of GPR132 mRNA in THP-1 monocytes was assessed by real-time RT-PCR. (a) In the absence of PMA, GPR132 mRNA was increased by treatment with 9-HODE (30 µM), and PMA (1 nM) augmented this effect. Results are expressed as a percentage of control, mean ± SEM (n = 3 separate experiments). Groups with different annotations are significantly different; *p < 0.001, two-way ANOVA general linear model; Tukey pair-wise comparisons. (b) Immunocytochemical staining of GPR132 in: (i) THP-1 monocytes – isotype control, (ii) THP-1 monocytes – anti-GPR132 rabbit polyclonal antibody, (iii) THP-1 differentiated macrophages – isotype control, (iv) THP-1 differentiated macrophages – anti-GPR132 rabbit polyclonal antibody.
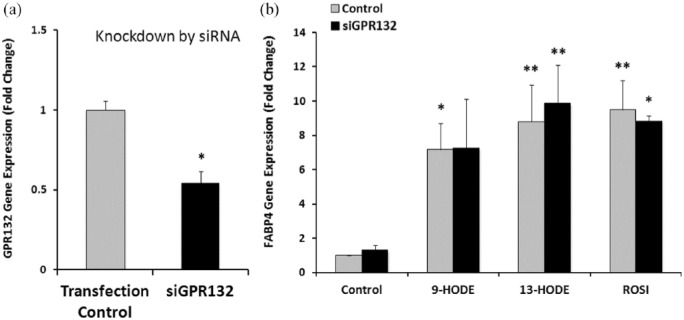
Gene silencing of GPR132 does not affect the induction of FABP4 gene expression by HODEs
Silencing of the gene for MAPK was performed to establish optimum conditions for transfection and gene silencing (data not shown). Effective silencing of GPR132 mRNA was only possible when THP-1 cells were first differentiated into macrophages by exposure to PMA. GPR132 mRNA level decreased by 46% compared to control (Figure 3(a)). As demonstrated previously, both 9-HODE and 13-HODE markedly increased expression of FABP4, as did the PPAR-γ agonist ROSI (Figure 3(b), p < 0.01). Partial silencing of GRPR132 did not affect FABP4 expression.
Figure 3.
Gene silencing of GPR132 does not affect the induction of FABP4 gene expression by HODEs. THP-1 differentiated macrophages were transfected with siGPR132 and the expression of GPR132 assessed by real-time PCR. GPR132 mRNA expression decreased 46% by siGPR132. Results are expressed as a percentage of control, mean ± SEM of triplicates from a representative experiment. (*p < 0.05, t test). B. THP-1 differentiated macrophages were treated with 9-HODE, 13-HODE or ROSI, and the gene expression of FABP4 assessed by real-time PCR. FABP4 expression was responsive to 9-HODE, 13-HODE and ROSI. The silencing of GPR132 (siGPR132) did not affect this induction. Results are expressed as a percentage of control, mean ± SEM [n = 3 separate experiments; *p < 0.01, two-way ANOVA, *p < 0.05, **p < 0.01 (compared against control, Tukey’s post-test corrected for multiple comparisons)].
HODE-stimulated FABP4 mRNA expression and protein secretion by THP-1 cells is mediated by PPAR-γ
To investigate the potential role of transcription factor PPAR-γ in HODE-induced FABP4 expression, THP-1 cells were co-treated with PPAR-γ antagonist, T0070907. HODEs and ROSI increased gene expression of FABP4 (Figure 4(a)). T0070907 significantly decreased induction of FABP4 mRNA by ROSI, 9-HODE and 13-HODE (66%, 75% and 66%, respectively; Figure 4(a), p < 0.001). Secretion of FABP4 protein by ROSI-treated cells and inhibition by T0070907 was comparable to that of mRNA expression. This pattern was similar for 9-HODE and 13-HODE, although these trends did not reach significance (Figure 4(b)).
Figure 4.

Increased mRNA expression and protein secretion of FABP4 by THP-1 cells treated with HODEs is partially mediated by PPAR-γ. THP-1 cells were pre-treated with either the ROSI antagonist T0070907 or vehicle for 2 h, after which cells were washed and cultured in the presence of 9-HODE (30 µM), 13-HODE (30 µM) or ROSI (0.1 µM) for 24 h. (a) FABP4 mRNA levels were determined by real-time PCR. Treatment with 9-HODE, 13-HODE and ROSI induced an increase in FABP4 gene expression while pre-treatment with T0070907 significantly reduced this effect. (b) FABP4 protein concentration in tissue culture medium was measured by ELISA. Treatment with 9-HODE, 13-HODE and ROSI induced an increase in FABP4 gene expression while pre-treatment with T0070907 significantly reduced this effect. Results are expressed as mean ± SEM (n = 3 replicates per group). Groups with different superscripts are significantly different; p < 0.001; two-way ANOVA; Fisher’s multiple comparison test; representative data from three independent experiments.
Serum levels of FABP4, resistin and leptin are increased in diabetic patients
Diabetic cohort data: 31 patients with type 2 diabetes attending a community-based diabetes clinic and 20 healthy controls who consented to participate were included in the study. The diabetic cohort was further divided into well controlled (HbA1c ⩽7.5%, n = 16) and poorly controlled (HbA1c >7.5%, n = 15). Demographic and clinical characteristics of these patients are shown in Table 1(a,b). All had established diabetes (mean duration 5 years, range 1–15), and all were treated either with oral hypoglycemic agents, insulin or both. All measures were compared between well and poorly controlled diabetic cohorts (Table 1(c)). Serum FABP4 concentration was measured using a specific ELISA in subjects with well-controlled type 2 diabetes, poorly controlled diabetes subjects and in healthy controls. FABP4 levels were significantly higher in the diabetic patients (Figure 5(a); control versus well-controlled diabetes cohort, p < 0.05; control versus poorly controlled diabetes, p < 0.01; and well- versus poorly controlled diabetes cohort, p > 0.05). A number of obesity-related proteins were measured by flow cytometry using bead arrays. Both well- and poorly controlled diabetic patients had increased concentrations of resistin (Figure 5(b)) compared (of minimum 1.5-fold) against controls (well controlled versus control, p < 0.05; poorly controlled versus control, p < 0.05). Leptin levels in the well-controlled diabetes cohort was twofold higher compared against control (p < 0.01) (Figure 5(c)). Tumor necrosis factor receptor (sTNF-R, p < 0.01) and osteoprotegerin (OPG, p < 0.05) was significant higher in the poorly controlled diabetes cohort compared against control. There was no significant difference in concentration of soluble CD40 ligand (sCD40L), MPO, monocyte chemoattractant protein-1 (MCP-1) or soluble intercellular adhesion molecule-1 (sICAM-1) (Figure 5(D)).
Table 1.
Patient characteristics: (a,b) the clinical features of the 31 patients with type 2 diabetes that were included in the study. (c) Overview of the patient pool.
| Overview | No. | % | |
|---|---|---|---|
| A. Diabetic cohort (n = 31) | |||
| Gender (female) | 17 | 54.8 | |
| Gender (male) | 14 | 45.2 | |
| Using insulin | 15 | 48.4 | |
| Nonsmokers | 21 | 67.7 | |
| Lipid-lowering drugs | 21 | 67.7 | |
| Antihypertensives | 19 | 61.3 | |
| Abnormal LFTs | 12 | 38.7 | |
| Medication profile | |||
| Insulin | 75.0 | ||
| Metformin | 50.0 | ||
| Gliptins | 16.7 | ||
| Statins | 45.8 | ||
| Comorbidities | |||
| Hypertension | 57.9 | ||
| Dyslipidemia | 47.4 | ||
| Cardiovascular disease | 31.6 | ||
| B. Control cohort (n = 20) | |||
| Gender (female) | 9 | 45 | |
| Gender (male) | 11 | 55 | |
| Age (mean, range) male | 38 (24–54) | ||
| Age (mean, range) female | 42 (34–58) | ||
| C. Characteristics | Mean (range) well controlled (HbA1c ⩽7.5%) (n = 16) | Mean (range) poorly controlled (HbA1c >7.5%) (n = 15) | p value |
| Age (years) | 58.6 (41–71) | 56.9 (32–78) | 0.65 |
| BMI (kg/m2) | 33.2 (22–51) | 31.2 (22–44) | 0.50 |
| Estimated GFR | 82.9 (32–90) | 72.5 (29–90) | 0.28 |
| Cholesterol (mmol/l) | 4.2 (3–5.8) | 4.3 (3.1–7.1) | 0.90 |
| Triglycerides | 1.7 (0.5–3.3) | 2.0 (0.5–6.5) | 0.96 |
| HDL | 1.1 (0.5–1.8) | 1.0 (0.7–1.7) | 0.60 |
| LDL | 2.4 (1.1–3.7) | 2.5 (1.1–5) | 0.70 |
| ESR (mm/h) | 17.1 (5–97) | 32.5 (6–101) | 0.04* |
| CRP (mg/l) | 3.9 (1–9) | 6.9 (2–23) | 0.20 |
Data represent mean (range). Comparisons between well-controlled (n = 16) and poorly controlled (n = 15) groups were performed; nonparametric Mann–Whitney U test performed except for variables age, BMI and LDL, for which a parametric t test was performed.
BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LFT, liver function test.
Figure 5.
Serum levels in study cohort. (a) Serum FABP4 was measured by ELISA following manufacturer’s standard protocol for the groups; well- (n = 16), poorly controlled (n = 15) type 2 diabetes patients and healthy controls (n = 20). Results are expressed as mean ± SEM (p < 0.01, one-way ANOVA followed by Holm–Sidak multiple comparisons test); *p < 0.05, **p < 0.01 compared against control. Cytokines were quantified using flow cytometry-based bead arrays following manufacturer’s protocol. Serum from poorly controlled diabetic (n =15), well-controlled diabetic (n = 16) and control (n = 20) subjects was assessed for Th1/Th2 and obesity-related markers. (b) Resistin (c) leptin. Results presented as a box plot (min–max) (p < 0.001 Kruskal–Wallis one-way ANOVA for both resistin and leptin levels, followed by Dunn’s post-test corrected for multiple comparisons). (d) Cytokines levels in the groups (mean ± SEM) [Kruskal–Wallis one-way ANOVA, *p < 0.05, **p < 0.01 (compared against control, Dunn’s post-test corrected for multiple comparisons)].
GPR132 mRNA is elevated in activated monocytes from diabetic patients
Diabetic patients, in general, had increased numbers of activated monocytes. Among the diabetic cohort, the well-controlled diabetic cohort had increased numbers of circulating CD14+ monocytic cells (p < 0.05) and monocyte subpopulations, CD14+CD36+ (p < 0.05), CD14+CD11b+ (p < 0.05) and CD14+CD54+ cells (p < 0.05, Figure 6(a)–(d)) compared against control. These markers of oxLDL interaction (CD36 and CD11b) and inflammation (CD54) were increased in well-controlled diabetic subjects. Purity of MACs-isolated CD14+ cells was assessed by flow cytometry and was found to be >95%. Levels of mRNA for GPR132 were increased by 50% in CD14+ cells from well-controlled diabetic patients compared with controls (p < 0.05, Figure 6(e)). Normalized expression of GPR132 mRNA correlated with total lymphocyte count (Pearson correlation coefficient 0.47, p = 0.01), but not with the number of CD14+ cells (−0.10, p = 0.61) or with any of the other cell populations. Compared with controls, there was no difference in expression of mRNA for either FABP4 or resistin in circulating CD14+ cells (data not shown). mRNA correlation analysis (Spearman’s rank correlations) revealed positive correlation between GPR132 and resistin expression in both well- (p < 0.05) and poorly controlled (p < 0.01) diabetic cohorts (Figure 6(f)), while no correlations were observed between GPR132 and FABP4 mRNA expression.
Figure 6.
Monocyte activation markers and GPR132 mRNA are increased in diabetic patients. Peripheral blood monocytes were prepared from control (n = 20) and diabetic (n = 29) subjects. Activation markers were measured using flow cytometry, and GPR132 expression was measured in CD14+ cells using real-time RT-PCR. (a) Total circulating CD14+ cells; (b) CD14+CD36+ cells; (c) CD14+CD11+ cells; (d) CD14+CD54+ cells. Results are expressed as a box plot (min–max). (e) GPR132 mRNA expression. mRNA expression from poorly controlled diabetic (n =15), well-controlled diabetic (n = 16) and control (n = 20) subjects was assessed for GPR132 expression using real-time RT-PCR. p < 0.05 (a–e), ordinary one-way ANOVA followed by Tukey’s post-test corrected for multiple comparisons; *p < 0.05 compared against control. (f) Correlation analysis was performed between GPR132 gene expression in monocytes and resistin and FABP4 in circulation. ρ = Spearman’s rank correlation coefficient, *p < 0.05 and **p < 0.01 (two-tailed).
Discussion
We show here that 9-HODE and 13-HODE increase FABP4 expression in THP-1 monocytes and macrophages. The increase in FABP4 is mediated by PPAR-γ, but not through GPR132, for which 9-HODE is known to be a ligand. We have also demonstrated that GPR132 is increased in the activated monocytes of subjects with type 2 diabetes. GPR132 has not previously been documented as a monocyte activation marker in diabetes, although it is known to be involved in the atherosclerotic process.
The THP-1 monocytic cell line is a suitable model to study events involved in monocyte activation, differentiation into macrophages and subsequent foam cell formation.10 The present study utilized both unstimulated THP-1 cells (quiescent monocytes) and THP-1 cells treated with PMA (differentiated macrophage) to determine whether the HODE stimulated FABP4 expression was mediated via GPR132. First, we showed that GPR132 was expressed in THP-1 monocytes and macrophages, although basal expression levels were considerably higher in macrophages. GPR132 has been shown to be expressed in immune cells (including macrophages), and is thought to have diverse roles in regulating chemotaxis and immune function;22 however, GPR132 has not previously been studied in THP-1 cells. Interestingly, the GPR132 receptor has been shown to be highly expressed in macrophages localized in atherosclerotic plaques in mouse and rabbit models,22 but it is not clear from available studies in mice whether GPR132 is pro-atherogenic or protective. The studies of Bolick and colleagues20 using GPR132 knockout mice suggest a protective effect, whereas Parkes and colleagues21 showed that mice doubly deficient in GPR132 and the LDL receptor had attenuated lesion progression. In vitro studies with cultured cells and cell lines may help to better define how GPR132 expression is regulated and the role it has in cellular processes that influence atherogenesis. In the current study, GPR132 mRNA levels were increased when THP-1 monocytes were exposed to PMA, 9-HODE and 13-HODE, but not ALA nor LA. This supports a hypothesis that HODEs have specific signaling functions.16
9-HODE (but not 13-HODE) displays a high affinity for GPR132.18,19 However, in our study 9- and 13-HODE had similar effects on expression of both GPR132 and FABP4 in THP-1 monocytes. This implies that this phenomenon is not mediated through GPR132, a conclusion supported by evidence that partial gene silencing of GPR132 did not diminish the effect of 9-HODE. However, this does not rule out an effect of 9-HODE acting through GPR132 in other aspects of atherosclerosis, and it is noted that the 9-HODE/GPR132 axis has been shown to mediate pro-inflammatory effects in keratinocytes.17 In the present study we demonstrated that the effects of 9-HODE and 13-HODE are reproduced by ROSI, and inhibited by the PPAR-γ antagonist, T0070907. It is known that PPAR-γ-mediated actions on macrophages are generally protective in relation to atherosclerosis,27 with PPAR-γ activation leading to differentiation of monocytes through the alternative pathway into M2 macrophages, which have an anti-inflammatory phenotype.28 T0070907 is a specific and potent inhibitor of PPAR-γ, but did not completely abolish the stimulatory effects of 9-HODE, 13-HODE or ROSI on FABP4 expression. It is well recognized that many PPAR-γ agonists, including PGJ2, have additional effects that are independent of PPAR-γ.29 Recently, the stimulatory effect of 13-HODE in macrophages, increasing CD36 expression and foam cell formation has been shown to involve the transcription factor testicular orphan receptor-4 (TR4).30 Given that FABP4 is a potential therapeutic target for insulin resistance and atherosclerosis,5–7 further studies are required to determine how its production in macrophages is regulated and its role in regulating atherogenesis.
Our finding of increased levels of FABP4 in the serum of patients with type 2 diabetes supported previous studies that demonstrated elevated FABP4 predicts development of metabolic syndrome,3 correlates with the presence of metabolic syndrome variables,2 relates to increased risk of coronary artery disease1 and is high in patients with type 2 diabetes.31 FABP4 is known to be involved in differentiation of monocytes into macrophages, and macrophages into foam cells. We also documented increased serum levels of inflammatory markers sTNF-R, OPG, MCP-1, and obesity markers resistin and leptin in diabetic patients. Circulating levels of one of these proteins, OPG, has recently been reported by a number of investigators to correlate with vascular inflammation and other features of developing atherosclerosis.32 Taken together, the lipid and adipokine profile observed in these diabetic patients indicated a heightened level of inflammatory activity associated with activation of various cell populations.
Activated circulating monocytes are the precursors of tissue-resident macrophages. These include macrophages in the arterial wall that contribute to atherogenesis and macrophages in adipose tissue that interact with adipocytes. Once residing in tissue they stimulate secretion of mediators of inflammation and insulin resistance that accompanies obesity, with a predisposition to vascular disease.33 Increased expression of activation markers on circulating monocytes has been well documented in subjects with diabetes,25,26 and may occur in response to hyperglycemia or to increased levels of free fatty acids.34 Consistent with previous studies,35 we confirmed increased expression of monocyte markers associated with oxLDL interaction (scavenger receptor CD36 and CD11b), as well as a marker of inflammation (CD54) in the diabetic patients.23,24 Further to this, we report for the first time that gene expression of GPR132 was increased in these activated diabetic monocytes. We observed a direct correlation between GPR132 expression and serum resistin. Given the known involvement of GPR132 in atherogenesis,20 further studies are warranted to assess whether the increased expression of GPR132 relates to a risk of vascular complications. Recent observations showing that monocyte activation in diabetes is decreased in patients taking thiazolidinedione36 or statin37 drugs, both of which favorably modify cardiovascular risk, supports the need for further studies on the role of GPR132 as a monocyte activation marker or in atherosclerosis-prone states. We did not find increased mRNA for either FABP4 or resistin in circulating monocytes from diabetic subjects, suggesting that these cells were not the source of increased circulating FABP4 or resistin.
Oxidized lipids, predominantly as oxLDL, accumulate at sites of tissue injury, including atherosclerotic plaque,12,38 and regulate expression of FABP4 in resident macrophages as part of a process that ultimately leads to foam cell formation.10 The stimulatory effect of oxLDL on foam cell formation is at least partially mediated through PPAR-γ.28 LA and its derivatives are the most abundant unsaturated fatty acids in plaque, with about 30% being present as oxidized derivatives – mainly HODEs.9 The predominant isoform of HODE in early atherosclerosis is 13-HODE, produced enzymatically by 15-lipoxygenase-1.38 In later lesions both 9-HODE and 13-HODE are present in approximately equal quantities and are formed non-enzymatically.9 Like the oxLDL, both 9-HODE and 13-HODE can act as PPAR-γ agonists and mediate the increase in expression of FABP4, CD36 and other lipid mediators involved in foam cell formation.11,39 To date, published studies of the actions of HODEs on monocytic cells have suggested that the two HODE isomers behave similarly. Our recent study also demonstrated both HODEs inducing apoptosis in THP-1 monocytic cells in a PPAR-γ-dependent manner.40 Jostarndt and colleagues showed that 13-HODE upregulated CD36 expression, but they did not study the effects of 9-HODE.41 However a study by Hampel and colleagues using U937 cells suggested that 9-HODE had actions distinct from 13-HODE in relation to regulation of expression of PPAR-γ2, cell cycle progression and apoptosis, and that these effects of 9-HODE were not PPAR-γ mediated.16
In summary, HODEs, a stable oxidation product of LA that has pro-inflammatory effects in other cells, increased FABP4 expression, although this effect was not mediated through the GPR132 receptor, but was regulated through PPAR-γ signaling. We have also demonstrated that GPR132 is a potential activation marker for monocytes in type 2 diabetes, and its increased expression may reflect an increased risk of vascular disease. There were a number of limitations in this study. Difference between age and BMI in the diabetic group versus the controls is to be taken into account while interpreting the results for increased levels of some of the cytokines observed in the diabetic cohort. Further work to determine the role of HODEs and their effect on FABP4 secretion in vitro in primary monocytes, and determining levels of HODEs in circulation among the well- and poorly controlled diabetic subjects and its correlation with FABP4 and other markers in circulation is needed. The suggested work would help us understand the role of HODEs in relation to lipid metabolism among the diabetic patients who are at high risk of developing cardiovascular disorders.
Acknowledgments
We are grateful to Linda Thomas, Stephen Garland and Frances Woods for their technical help during the early phases of this project.
Footnotes
Funding: Our work was funded by the Private Practice Trust Fund of the Townsville Hospital, Queensland and by James Cook University.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Venkat Vangaveti  https://orcid.org/0000-0002-4363-309X
https://orcid.org/0000-0002-4363-309X
Contributor Information
Venkat Vangaveti, Room 114, Building 47, College of Medicine and Dentistry, James Cook University, Townsville, Queensland, Australia 4814, Australia.
Venkatesh Shashidhar, College of Medicine and Dentistry, James Cook University, Queensland, Australia.
Fiona Collier, School of Medicine, Deakin University, Victoria, Australia.
Jason Hodge, School of Medicine, Deakin University, Victoria, Australia.
Catherine Rush, College of Public Health, Medical & Vet Sciences, James Cook University, Queensland, Australia.
Usman Malabu, College of Medicine and Dentistry, James Cook University, Queensland, Australia.
Bernhard Baune, Department of Psychiatry, University of Adelaide, South Australia.
Richard Lee Kennedy, School of Medicine, Deakin University, Victoria, Australia.
References
- 1. Hsu BG, Chen YC, Lee RP, et al. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ J 2010; 74: 327–331. [DOI] [PubMed] [Google Scholar]
- 2. Karakas SE, Almario RU, Kim K. Serum fatty acid binding protein 4, free fatty acids, and metabolic risk markers. Metabolism 2009; 58: 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu A, Tso AW, Cheung BM, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation 2007; 115: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 4. Makowski L, Brittingham KC, Reynolds JM, et al. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem 2005; 280: 12888–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao H, Gerhold K, Mayers JR, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008; 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 2001; 7: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furuhashi M, Tuncman G, Gorgun CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007; 447: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nandish S, Wyatt J, Bailon O, et al. Implementing cardiovascular risk reduction in patients with cardiovascular disease and diabetes mellitus. Am J Cardiol 2011; 108: 42B–51B. [DOI] [PubMed] [Google Scholar]
- 9. Waddington EI, Croft KD, Sienuarine K, et al. Fatty acid oxidation products in human atherosclerotic plaque: an analysis of clinical and histopathological correlates. Atherosclerosis 2003; 167: 111–120. [DOI] [PubMed] [Google Scholar]
- 10. Fu Y, Luo N, Lopes-Virella MF. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res 2000; 41: 2017–2023. [PubMed] [Google Scholar]
- 11. Fu Y, Luo N, Lopes-Virella MF, et al. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 2002; 165: 259–269. [DOI] [PubMed] [Google Scholar]
- 12. Jira W, Spiteller G, Carson W, et al. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids 1998; 91: 1–11. [DOI] [PubMed] [Google Scholar]
- 13. Vangaveti V, Baune BT, Kennedy RL. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab 2010; 1: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vangaveti V, Shashidhar V, Jarrod G, et al. Free fatty acid receptors: emerging targets for treatment of diabetes and its complications. Ther Adv Endocrinol Metab 2010; 1: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol 2008; 15: 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hampel JK, Brownrigg LM, Vignarajah D, et al. Differential modulation of cell cycle, apoptosis and PPARgamma2 gene expression by PPARgamma agonists ciglitazone and 9-hydroxyoctadecadienoic acid in monocytic cells. Prostaglandins Leukot Essent Fatty Acids 2006; 74: 283–293. [DOI] [PubMed] [Google Scholar]
- 17. Hattori T, Obinata H, Ogawa A, et al. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol 2008; 128: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 18. Obinata H, Izumi T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat 2009; 89: 66–72. [DOI] [PubMed] [Google Scholar]
- 19. Yin H, Chu A, Li W, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 2009; 284: 12328–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolick DT, Skaflen MD, Johnson LE, et al. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circ Res 2009; 104: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parks BW, Srivastava R, Yu S, et al. ApoE-dependent modulation of HDL and atherosclerosis by G2A in LDL receptor-deficient mice independent of bone marrow-derived cells. Arterioscler Thromb Vasc Biol 2009; 29: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rikitake Y, Hirata K, Yamashita T, et al. Expression of G2A, a receptor for lysophosphatidylcholine, by macrophages in murine, rabbit, and human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002; 22: 2049–2053. [DOI] [PubMed] [Google Scholar]
- 23. Rios FJO, Jancar S, Melo IB, et al. Role of PPAR-gamma in the modulation of CD36 and FcgammaRII induced by LDL with low and high degrees of oxidation during the differentiation of the monocytic THP-1 cell line. Cell Physiol Biochem 2008; 22: 549–556. [DOI] [PubMed] [Google Scholar]
- 24. Ruetten H, Thiemermann C, Perretti M. Upregulation of ICAM-1 expression on J774.2 macrophages by endotoxin involves activation of NF-KappaB but not protein tyrosine kinase: comparison to induction of iNOS. Mediators Inflamm 1999; 8: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cipolletta C, Ryan KE, Hanna EV, et al. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes 2005; 54: 2779–2786. [DOI] [PubMed] [Google Scholar]
- 26. Devaraj S, Glaser N, Griffen S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006; 55: 774–779. [DOI] [PubMed] [Google Scholar]
- 27. Gerry JM, Pascual G. Narrowing in on cardiovascular disease: the atheroprotective role of peroxisome proliferator-activated receptor gamma. Trends Cardiovasc Med 2008; 18: 39–44. [DOI] [PubMed] [Google Scholar]
- 28. Bouhlel MA, Derudas B, Rigamonti E, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 2007; 6: 137–143. [DOI] [PubMed] [Google Scholar]
- 29. Castrillo A, Mojena M, Hortelano S, et al. Peroxisome proliferator-activated receptor-gamma-independent inhibition of macrophage activation by the non-thiazolidinedione agonist L-796,449: comparison with the effects of 15-deoxy-delta(12,14)-prostaglandin J(2). J Biol Chem 2001; 276: 34082–34088. [DOI] [PubMed] [Google Scholar]
- 30. Xie S, Lee YF, Kim E, et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci USA 2009; 106: 13353–13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bagheri R, Qasim AN, Mehta NN, et al. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am J Cardiol 2010; 106: 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vik A, Mathiesen EB, Brox J, et al. Relation between serum osteoprotegerin and carotid intima media thickness in a general population: the Tromso Study. J Thromb Haemost 2010; 8: 2133–2139. [DOI] [PubMed] [Google Scholar]
- 33. Constant VA, Gagnon A, Yarmo M, et al. The antiadipogenic effect of macrophage-conditioned medium depends on Erk1/2 activation. Metabolism 2008; 57: 465–472. [DOI] [PubMed] [Google Scholar]
- 34. Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via toll-like receptors. Am J Physiol Endocrinol Metab 2011; 300: E145–E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sampson MJ, Davies IR, Braschi S, et al. Increased expression of a scavenger receptor (CD36) in monocytes from subjects with type 2 diabetes. Atherosclerosis 2003; 167: 129–134. [DOI] [PubMed] [Google Scholar]
- 36. Pitocco D, Giubilato S, Zaccardi F, et al. Pioglitazone reduces monocyte activation in type 2 diabetes. Acta Diabetol 2009; 46: 75–77. [DOI] [PubMed] [Google Scholar]
- 37. Mandosi E, Fallarino M, Gatti A, et al. Atorvastatin downregulates monocyte CD36 expression, nuclear NFkappaB and TNFalpha levels in type 2 diabetes. J Atheroscler Thromb 2010; 17: 539–545. [DOI] [PubMed] [Google Scholar]
- 38. Kuhn H, Belkner J, Zaiss S, et al. Involvement of 15-lipoxygenase in early stages of atherogenesis. J Exp Med 1994; 179: 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagy L, Tontonoz P, Alvarez JG, et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998; 93: 229–240. [DOI] [PubMed] [Google Scholar]
- 40. Vangaveti V, Shashidhar V, Rush C, et al. Hydroxyoctadecadienoic acids regulate apoptosis in human THP-1 cells in a PPARγ-dependent manner. Lipids 2014; 49: 1181–1192. [DOI] [PubMed] [Google Scholar]
- 41. Jostarndt K, Rubic T, Kuhn H, et al. Enzymatically modified low-density lipoprotein upregulates CD36 in low-differentiated monocytic cells in a peroxisome proliferator-activated receptor-gamma-dependent way. Biochem Pharmacol 2004; 67: 841–854. [DOI] [PubMed] [Google Scholar]