Abstract
Statin-associated adverse effects, primarily muscle-related symptoms, occur in up to approximately one-third of patients in clinical practice. Recently, a Canadian Consensus Working Group outlined 6 key principles to assess and manage patients with goal-inhibiting statin intolerance, defined as a syndrome characterized by symptoms or biomarker abnormalities that prevent the long-term use of and adherence to indicated statin therapy, which includes a trial of at least 2 statins and precludes reversible causes of statin adverse effects. These principles ensure patients are appropriately receiving a statin and aware of both the benefits and risks of therapy. As well, they address factors that may increase the risk of statin-associated myopathy. A thorough assessment of patients’ clinical and laboratory history should be performed in any patient presenting with muscle symptoms on statin therapy, followed by a systematic dechallenge/rechallenge approach. In practice, most patients with statin intolerance due to muscle symptoms will be able to tolerate another statin. This is of particular importance because of the relative paucity of compelling evidence demonstrating a cardiovascular benefit with nonstatin therapies. Pharmacists are ideally situated to provide patient education, recommend changes to therapy and monitor patients with goal-inhibiting statin intolerance.
Knowledge Into Practice.
In 2016, a Canadian Consensus Working Group published the third iteration of a statement to evaluate the diagnosis, prevention and management of statin adverse effects and intolerance.
Goal-inhibiting statin intolerance is defined as a syndrome characterized by symptoms or biomarker abnormalities that prevent the long-term use of and adherence to indicated statin therapy, which includes a trial of at least 2 statins and precludes reversible causes of statin adverse effects.
There are 6 key principles in the management of patients with goal-inhibiting statin intolerance: ensuring a valid indication, identifying risk factors for intolerance, ensuring the patient is informed of the benefits and risks, encouraging nondrug therapies/not advocating for supplements to prevent statin-associated myopathy, using a systematic challenge/dechallenge/rechallenge approach and recommending nonstatin therapy, if necessary.
Mise En Pratique Des Connaissances.
En 2016, le Canadian Consensus Working Group (groupe de travail de consensus canadien) a publié la troisième version d’une déclaration sur la prévention, le diagnostic et la prise en charge de l’intolérance aux statines et des effets indésirables de ces médicaments.
L’intolérance aux statines (GISI) est un syndrome qui se caractérise par des symptômes ou des anomalies au niveau des biomarqueurs empêchant l’utilisation et l’observance à long terme d’un traitement par les statines, et qui inclut l’essai d’au moins deux statines et exclut les causes réversibles des effets indésirables des statines.
Six principes clés doivent guider la prise en charge des patients qui présentent une telle intolérance aux statines : s’assurer qu’il existe une indication valable pour la prescription de ces médicaments; déterminer les facteurs de risque d’intolérance; veiller à informer le patient des avantages et des risques du traitement; encourager l’utilisation de traitements non pharmacologiques/ne pas recommander la prise de suppléments pour prévenir la myopathie associée aux statines; suivre une approche systématique relativement à la séquence prise/arrêt/réadministration du médicament; et recommander un traitement sans statines, au besoin.
Introduction
Hydroxymethylglutaryl-coenzyme A reductase inhibitors (statins) are recommended in patients with established, or those at risk for, cardiovascular disease (CVD) to decrease the incidence of adverse cardiovascular events and mortality.1 Statin intolerance due to adverse effects, mostly muscle-related symptoms, is common in clinical practice. It is estimated that between 7% and 29% of patients taking statins experience some type of muscle-associated toxicity in real-world practice.2 This frequent clinical scenario led to the formation of a Canadian Consensus Working Group (CCWG) to evaluate the diagnosis, prevention and management of statin adverse effects and intolerance. In 2016, this group released the third iteration of a statement to inform and guide clinicians on how to approach patients with suspected statin-associated adverse effects.3
Terminology
The CCWG proposed terminology to provide consistency when describing muscle-related adverse effects3 (Table 1). Myopathy is a general term that applies to any disease of the muscle, which is further subcategorized depending on whether the patient is symptomatic. Myalgia refers to patients with symptoms of muscle aches or weakness in the absence of an elevated creatine kinase (CK), whereas myositis is the presence of symptoms with a CK elevation. Rhabdomyolysis is muscle aches or weakness with a CK more than 10 times the upper limit of normal and is typically associated with myoglobinuria. The term hyperCKemia refers to patients with an elevated CK without muscle-related symptoms and is graded based on the degree of elevation.
Table 1.
Terminology for statin-associated myopathy*
| Term | Laboratory characteristics | Clinical characteristics |
|---|---|---|
| Myalgia | CK ≤ ULN | Muscle ache or weakness |
| Myositis | CK > ULN | Muscle ache or weakness |
| Rhabdomyolysis | CK > 10× ULN | Muscle ache or weakness ± myoglobinuria |
| Mild (grade 1) hyperCKemia | CK > ULN but ≤ 5× ULN | May or may not have myositis |
| Mild (grade 2) hyperCKemia | CK > 5× ULN but ≤ 10× ULN | May or may not have myositis |
| Moderate hyperCKemia | CK > 10× ULN but ≤ 50× ULN | May or may not have rhabdomyolysis |
| Severe hyperCKemia | CK >50× ULN | May or may not have rhabdomyolysis |
Adapted from Mancini et al.3 with permission from Elsevier. CK, creatine kinase; ULN, upper limit of normal.
In the most recent version of the CCWG statement, the term goal-inhibiting statin intolerance (GISI) was introduced as a pragmatic way of defining statin intolerance. GISI was defined as a syndrome characterized by symptoms or biomarker abnormalities that prevent the long-term use of and adherence to indicated statin therapy, which includes a trial of at least 2 statins (including atorvastatin and rosuvastatin, as appropriate) and precludes reversible causes of statin adverse effects (e.g., drug interactions, untreated hypothyroidism). The working group also suggested the term goal-inhibiting statin resistance (GISR), which refers to patients who are unable to achieve the expected lipid-lowering effect with maximally tolerated statin dose. In other words, GISR refers to an inadequate response or lack of efficacy, as opposed to adverse effects. The use of lipid targets as a therapeutic goal is controversial. However, guidelines from both the Canadian Cardiovascular Society (CCS)1 and the American College of Cardiology/American Heart Association4 recommend a specific lipid goal or expected percentage reduction in lipid parameters—even the simplified lipid guidelines published in Canadian Family Physician endorse a specific statin dose based on the patient’s level of risk.5
Management of GISI
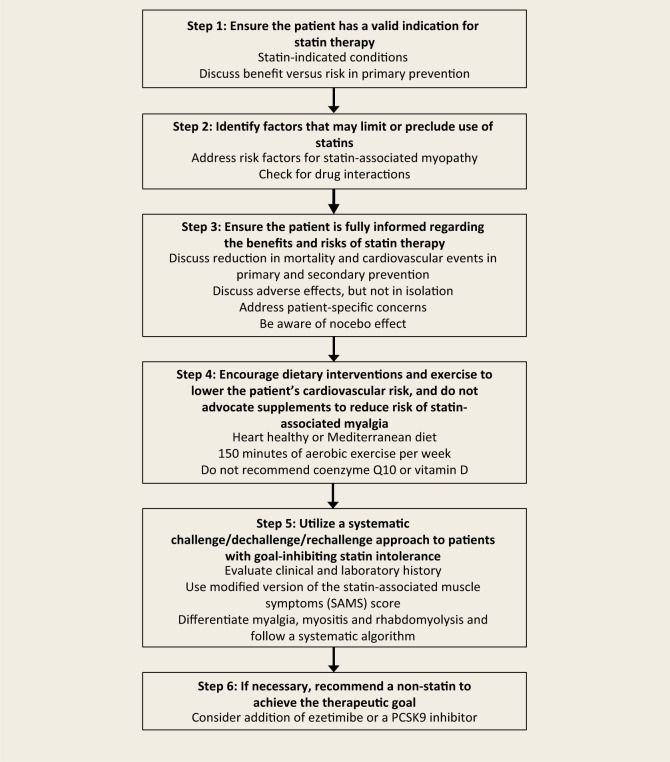
In the most recent statement, the CCWG advocate 6 key principles in the management of patients with GISI3 (Figure 1). The following is a fictional case vignette of a typical clinical scenario to help illustrate these concepts.
Figure 1.
Key principles in the management of patients with goal-inhibiting statin intolerance3
Case vignette
A 62-year-old man presents to your pharmacy to refill his medications but admits he is reluctant to refill his statin because he is having frequent leg pains and read on the Internet that this is a common side effect of statins. He is currently taking atorvastatin 20 mg daily. His medical history includes type 2 diabetes mellitus, hypertension, dyslipidemia and chronic back pain. His other medications include metformin, an angiotensin-converting enzyme inhibitor, dihydropyridine calcium channel blocker and acetaminophen as needed. He is wondering if diet and exercise can replace his statin. He also read on the Internet that coenzyme Q10 and vitamin D help to reduce muscle pain caused by statins and wonders if he should start supplementation.
Step 1: Ensure the patient has a valid indication for statin therapy
The first step in approaching a patient with suspected statin-associated muscle symptoms is to confirm the patient has a valid indication for therapy. The most recent CCS guidelines for the management of dyslipidemia describe 5 “statin-indicated conditions” in which statins have been demonstrated to be efficacious.1 These include patients with established CVD (i.e., secondary prevention), certain patients with diabetes mellitus or chronic kidney disease and patients with an aortic abdominal aneurysm or genetic dyslipidemia (i.e., low-density lipoprotein cholesterol >5 mmol/L). As well, statin therapy is recommended for high-risk primary prevention patients and certain patients at intermediate-risk for CVD depending on their risk factors, as well as their values and preferences. The CCS guidelines advocate the use of the Framingham Risk Score to determine a patient’s cardiovascular risk in the setting of primary prevention.1
Case
Your patient has a statin-indicated condition, as he has type 2 diabetes mellitus and is over 40 years of age.
Step 2: Identify factors that may limit or preclude use of statins
There are several factors that predispose patients to muscle-related adverse effects. These include advanced age (>80 years), female sex, Asian ethnicity, low body mass index, history of preexisting/unexplained muscle/joint pain, family history of myopathy, neuromuscular diseases (e.g., amyotrophic lateral sclerosis), severe renal/hepatic disease, untreated hypothyroidism and diabetes mellitus.3 In addition, there are several genetic polymorphisms that are associated with statin myopathy; however, these are not routinely investigated in clinical practice. There are several statin drug interactions that increase the risk of adverse effects.3,6 Atorvastatin, lovastatin and simvastatin are metabolized by the cytochrome P450 3A4 enzyme system and thus interact with strong inhibitors of this system (e.g., azole antifungals, large quantities of grapefruit juice, macrolide antibiotics, nondihydropyridine calcium channel blockers and protease inhibitors). As well, amiodarone may interact with any statin except pravastatin, and concomitant use of fibrates and niacin with a statin may also increase the risk of myopathy. High-dose statin therapy is more likely to be associated with statin muscle symptoms, although these symptoms can occur with any dose. Finally, red yeast rice supplements may increase statin toxicity, as they contain a compound called monacolin K, which is identical to lovastatin.
Case
Your patient has a history of preexisting muscle pain (i.e., chronic back pain) and diabetes mellitus, which are nonmodifiable risk factors for statin-associated myopathy. However, he is not taking any interacting medications (including supplements) and does not drink grapefruit juice.
Step 3: Ensure the patient is fully informed regarding the benefits and risks of statin therapy
There is an unmet opportunity for pharmacists to aid patients in making an informed decision about both the benefits and risks of statin therapy, as it was stated by the CCWG that this is “often poorly executed by busy physicians or inaccurately interpreted by the patient.”3 Pharmacists should be wary that focusing only on possible adverse effects without a discussion of the benefits is potentially detrimental. As well, patients often identify unsubstantiated and/or misleading information on the Internet, where there is a seemingly disproportionate amount of negative information about statins as compared with other medications. To facilitate shared decision-making, it is imperative to understand the numbers regarding the potential benefits and risks of statin therapy. A meta-analysis of 10 randomized controlled trials of >70,000 patients without established CVD (i.e., primary prevention) showed that statin therapy, as compared with placebo, reduced all-cause mortality by 0.6% (number needed to treat [NNT] of 167), major coronary events by 1.3% (NNT of 77) and stroke by 0.4% (NNT of 250) over approximately 4 years.7 The authors of this meta-analysis did not investigate safety outcomes other than the risk of cancer, which was not significantly different between groups. In patients with stable coronary heart disease (i.e., secondary prevention), high-dose atorvastatin, as compared with low dose, reduced death and adverse cardiovascular events by 2.2% (NNT of 46) over approximately 5 years.8 However, treatment-related adverse events and drug discontinuation due to treatment-related adverse events were higher with high-dose atorvastatin (number needed to harm [NNH] of 44 and 53, respectively). Treatment-related myalgia was similar between groups, but patients on high-dose atorvastatin had a higher rate of persistently elevated alanine aminotransferase and/or aspartate aminotransferase (NNH of 100). Of note, the total rates of adverse events (i.e., not just those categorized as related to treatment) were not reported. In patients with a recent acute coronary syndrome, high-intensity statin therapy reduced death and adverse cardiovascular events by 3.9% (NNT of 26) when compared with moderate-dose therapy over approximately 2 years.9 There were no significant between-group differences for any of the prespecified safety endpoints including liver enzyme elevation, myopathy or cancer. Although neither trial reduced the risk of all-cause mortality, this may have been due to the use of an active treatment in both groups.
There is a risk of a so-called “nocebo effect” with statins, where patients with negative expectations about a therapy are more likely to experience an adverse effect. A recent retrospective analysis of a large statin randomized controlled trial demonstrated that patients reported a higher incidence of muscle-related adverse events with atorvastatin compared with placebo during the unblinded extension phase.10 In other words, patients reported more muscle-related adverse effects when they knew they were taking a statin.
Case
You discuss with your patient the potential cardiovascular benefits of statin therapy compared with the potential risks. With respect to efficacy, you discuss with him the results of the Collaborative Atorvastatin Diabetes Study (CARDS), which compared atorvastatin 10 mg daily to placebo in patients aged 40 to 75 years with type 2 diabetes mellitus and another cardiovascular risk factor, such as hypertension.11 The results showed atorvastatin reduced the risk of adverse cardiovascular events (NNT of 32) and all-cause mortality (NNT of 67) compared with placebo, while about 5% of patients in each group reported muscle-related symptoms. He acknowledges these results seem important enough to continue treatment but not at the expense of having ongoing leg pain.
Step 4: Encourage dietary interventions and exercise to lower the patient’s cardiovascular risk and do not advocate supplements to reduce the risk of statin-associated myalgia
Lifestyle interventions, such as a heart healthy diet and exercise, are important components to reduce patients’ cardiovascular risk but are not as effective as and, in most cases, should not be viewed as a replacement for, statin therapy. Rather, these interventions should be used to augment therapy to minimize the statin dose or potentially avoid treatment in patients without established CVD. Recommendations should include a Mediterranean diet or increasing dietary intake of phytosterols or phytosterol-containing foods and 150 minutes of moderate-to-vigorous aerobic activity per week in bouts of 10 minutes or more.1
No vitamins or herbal supplements have definitively been shown to reduce the risk or improve the symptoms of statin-associated myopathy. The data for coenzyme Q10 supplementation in treating or preventing statin-associated muscle symptoms is inconsistent. A recent meta-analysis of 5 randomized controlled trials of patients with statin-associated myalgia did not demonstrate a significant improvement in pain scores with coenzyme Q10 vs placebo.12 As well, there are no data to support that coenzyme Q10 improves clinically meaningful outcomes, such as a reduction in incident myalgia or statin tolerability.13 Myalgias have been reported in patients on statin therapy with low serum levels of vitamin D. However, these data are heterogeneous, and there are no randomized controlled trials of vitamin D supplementation to prevent or treat statin-associated myalgia.3 However, if a patient perceives benefit with coenzyme Q10 or vitamin D, it is not unreasonable to continue therapy. As previously mentioned, red yeast rice should not be used concurrently with a statin, as this supplement often has variable concentration and purity and thus may increase the risk of statin toxicity.
Case
You recommend that your patient implement changes to his diet and exercise routine but explain these interventions are not adequate to replace his statin. You discuss with him the lack of evidence demonstrating a consistent benefit with coenzyme Q10 or vitamin D and that you would not recommend taking either supplement.
Step 5: Use a systematic challenge/dechallenge/rechallenge approach to patients with GISI
If statin-associated myopathy is suspected, it is essential to perform a thorough evaluation of the patient’s clinical and laboratory history and provide patient education as appropriate. Muscle symptoms are common and can be due to alternate causes such as exercise, soft-tissue disease, trauma or acute flu-like illness. Muscle complaints with statins are often described as a heaviness, stiffness, cramping or weakness during exertion, which are intermittent rather than continuous.14 These complaints are typically bilateral and affect multiple large muscle beds. If a patient has symptoms of isolated joint pain, it is unlikely to be secondary to the statin. Not all statins have the same incidence of myopathy—lipophilic statins (e.g., atorvastatin, fluvastatin, lovastatin, simvastatin) penetrate the muscle more readily and may have a greater potential for myopathy.15 The timing of muscle symptoms relative to when the statin was started or dose increased is an important component of the history. In a retrospective case series, patients were taking statins for an average of 6 months before developing muscle-related symptoms.16 The CCWG proposed a modified version of the statin-associated muscle symptoms (SAMS) score that can be used to assess patients’ symptoms3 (Table 2). Baseline bloodwork should be performed in all patients prior to initiating statin therapy—this should include lipid profile, glycosylated hemoglobin (to diagnose/rule out diabetes mellitus), CK (as some patients have an elevated CK based on their ethnicity, sex or physical activity), serum creatinine, alanine aminotransferase and thyroid-stimulating hormone (as kidney/liver disease or untreated hypothyroidism are risk factors for statin-associated myopathy).3 Routine monitoring of CK is not recommended; however, it is important to measure a baseline in case the patient develops muscle symptoms.
Table 2.
Simplified version of modified statin-associated muscle symptoms (SAMS) score*
| Parameter | Points |
|---|---|
| Distribution | |
| Symmetric hip or thigh aches | 3 |
| Symmetric calf or upper arm aches | 2 |
| Nonspecific, asymmetric or intermittent | 1 |
| Transient during statin use | 0 |
| Timing of symptom onset | |
| ≥2 days but <4 weeks | 3 |
| 4-12 weeks | 2 |
| >12 weeks | 1 |
| <2 days | 0 |
| Dechallenge | |
| Improves upon withdrawal in 2 days to <2 weeks | 2 |
| Improves upon withdrawal in 2-4 weeks | 1 |
| Does not improve upon withdrawal in >4 weeks† | 0 |
| Asymptomatic after 1 day | 0 |
| Rechallenge | |
| Same symptoms recur in ≥2 days but <4 weeks | 3 |
| Same symptoms recur in 4-12 weeks | 1 |
| Response to nonstatin therapy | |
| Same symptoms as with statin occur with nonstatin lipid-lowering drug | –5 |
| Probability | |
| Probable | ≥9 |
| Possible | 7-8 |
| Unlikely | <7 |
Adapted from Mancini et al.3 with permission from Elsevier.
Symptoms may persist or worsen despite discontinuation of statin in rare cases of immune-mediated necrotizing myopathy.
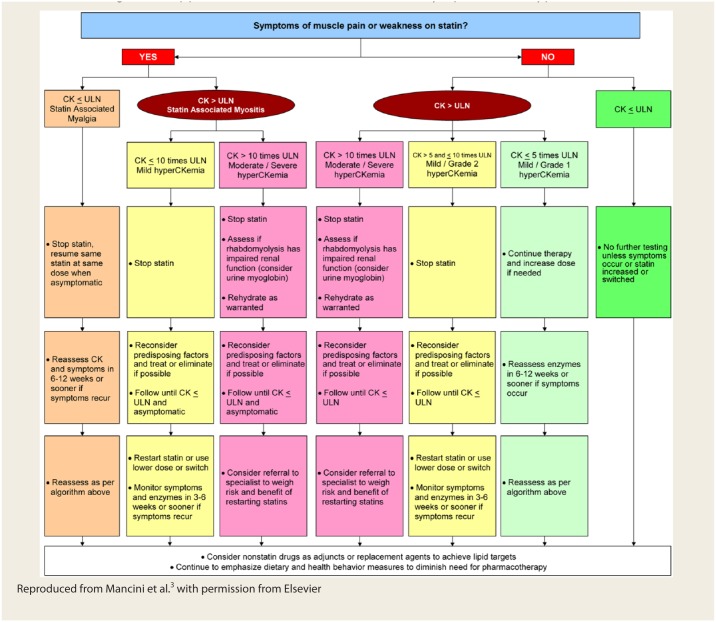
A variety of strategies can be used in the management of patients with statin-related myopathy. It is essential to balance the potential benefits and risks of continuing therapy on a case-by-case basis by incorporating the patient’s values and preferences. The CCWG created a systematic, algorithmic approach to managing statin-associated muscle symptoms or hyperCKemia3 (Figure 2). Systematically following this algorithm can be time-consuming and potentially frustrating for both patients and clinicians; however, it is important to stay resolute because of the paucity of therapeutic alternatives with evidence to support a reduction in adverse cardiovascular events. Based on published evidence, it is estimated that 70% to 90% of patients with statin intolerance will subsequently be able to tolerate another statin.3,17,18 In a retrospective case series of patients with statin-associated myopathy, 16 of 37 patients (43%) were able to tolerate another statin without recurrent symptoms.16 Some patients may actually tolerate a rechallenge of the same statin. A recent N-of-1 trial of 8 patients with statin-related myalgia were rechallenged with the same statin that was previously associated with myalgia vs placebo in a double-blind fashion.19 There was no significant difference in pain scores between the statin and placebo, and 5 patients (63%) were able to resume statin therapy after the trial. The appropriate time frame for follow-up is variable, depending on the patient and situation. Generally, it is recommended that patients be reassessed in 6 to 12 weeks if their CK is within normal limits or have mild (grade 1) hyperC- Kemia, although more frequent follow-up is likely warranted if the patient develops or has persistent symptoms.3 In cases of statin-associated myositis or moderate/severe hyperCKemia, the CCWG does not recommend a specific time frame for follow-up of CK and symptoms, but every 3 to 6 weeks is likely a reasonable time frame.3 In a retrospective case series of patients with statin-associated myopathy, resolution of muscle pain occurred an average of 2 months after discontinuing therapy.16
Figure 2.
Management approach for statin-associated muscle symptoms and hyperCKemia
Reproduced from Mancini et al.3 with permission from Elsevier
The CCWG outlined factors for which statin rechallenge may not be useful.3 Such cases include when the symptoms are plausible and resolve completely with cessation, are severe with objective weakness and/or hyperCKemia or if the patient refuses a rechallenge even at a low or intermittent dose. If the symptoms persist after discontinuing the statin for a reasonable period of time, this may be indicative of an underlying non–statin-related muscle condition, which should warrant further investigation but may not preclude future statin use.
Myalgia
Not all patients with myalgia will require cessation or reduction of their statin therapy. If the symptoms are tolerable, one could consider continuing therapy (if the patient is willing) and have them monitor their symptoms. If the symptoms are intolerable, the statin dose could be reduced (including intermittent or nondaily dosing), switched to a different statin or temporarily discontinued. Intermittent nondaily dosing strategies include every other day or weekly dosing, which some patients may prefer, although adherence could be more challenging. A review of nondaily statin dosing strategies showed that at least 70% of patients with statin-associated myopathy were able to tolerate an intermittent dosing strategy.20 Addressing any reversible risk factors for myopathy is also paramount. If a patient has myalgia with a lipophilic statin (e.g., atorvastatin, simvastatin), it may be reasonable to switch to a less lipophilic statin (e.g., pravastatin, rosuvastatin).
Myositis
The management of myositis follows the same principles as for myalgia regarding dechallenge/rechallenge and switching statins. In most cases, it is appropriate to hold the statin until the hyperCKemia resolves and the patient is asymptomatic.
Rhabdomyolysis
In patients presenting with suspected rhabdomyolysis, statin therapy should be discontinued with prompt assessment of the patient’s renal function (i.e., serum creatinine, urine myoglobin). Rhabdomyolysis is typically managed with intravenous rehydration and, in severe cases, dialysis. Previous rhabdomyolysis is not necessarily a contraindication to statin therapy, and patients should be assessed on a case-by-case basis based on their individualized benefit vs risk.3 There appears to be a dose-dependent relationship with statin-associated rhabdomyolysis.21 Therefore, if a statin is rechallenged, it should be started at a low dose.
Case
Your patient describes bilateral muscle cramping in his thighs. These symptoms started about 2 weeks ago. He has been taking atorvastatin for about 8 weeks, and there has been no recent change to his physical activity. His is referred for prompt bloodwork, which shows his CK is below the upper limit of normal. His muscle cramping is currently tolerable but quite bothersome. After discussing his options, he is willing to try a lower dose of atorvastatin at 10 mg daily. After 2 weeks, his muscle cramps are not improved. Therefore, you recommend he discontinue atorvastatin, and after a week his muscle cramps resolve. You then recommend he start rosuvastatin 10 mg daily, and after 6 weeks, he has had no recurrence of his muscle pain. Thus, his modified SAMS score is 7, which indicates that statin-associated myalgia was possible.
Step 6: If necessary, recommend nonstatin therapy to achieve the therapeutic goal
If a nonstatin drug is considered to achieve a lipid goal, the CCWG advocates that dual therapy is preferable to avoid unnecessary polypharmacy. Few therapeutic alternatives are available for patients with GISI or complete statin intolerance. While many nonstatin lipid-lowering agents have demonstrated a beneficial effect on lipid parameters, some have failed to show a reduction in adverse cardiovascular events when used in combination with a statin and thus should be avoided. Specifically, the combination of a fibrate or niacin with a statin has been demonstrated in large randomized controlled trials to be ineffective or, in the case of niacin, potentially harmful.22-25 On the other hand, there are recent data demonstrating that both ezetimibe and evolocumab (a novel proprotein convertase subtilisin/kexin type 9 [PCKS9] inhibitor), respectively, lower the risk of adverse cardiovascular events in combination with a statin in patients with established CVD.26,27 There are also short-term trials of ezetimibe vs a PCSK9 inhibitor in patients with complete statin intolerance. These trials were not adequately powered to evaluate cardiovascular events, and muscle-related symptoms were similar (approximately 20%-30% of patients) in each group.28,29
Case
Six weeks after initiating rosuvastatin, your patient has a nonfasting lipid profile that demonstrates a non–high-density lipoprotein cholesterol (non-HDL-C) level of 2.8 mmol/L, which is above the target recommended by the CCS (<2.6 mmol/L). He does not meet the inclusion criteria for the studies that investigated ezetimibe or PCSK9 inhibitors with a statin and is reluctant to start an additional therapy. However, he is willing to try a higher dose of rosuvastatin. Therefore, you recommend an increase in his rosuvastatin dose to 20 mg daily. Six weeks later, he reports no recurrent myalgias, and his non–HDL-C is now below target.
Conclusion
Statin-associated muscle symptoms are common, occurring in up to roughly 30% of patients in clinical practice. Recently, a Canadian working group outlined 6 key principles to manage patients with statin intolerance. This systematic approach ensures patients are appropriately receiving a statin and are aware of both the benefits and risks of therapy and addresses factors that may increase their risk of statin-associated myopathy. A thorough assessment of patients’ clinical and laboratory history should be performed in any patient presenting with muscle symptoms on statin therapy, followed by a systematic dechallenge/rechallenge approach. In practice, most patients with statin intolerance due to muscle symptoms will be able to tolerate another statin. This is of particular importance because of the absence of compelling evidence demonstrating a cardiovascular benefit with nonstatin therapies. Pharmacists are ideally situated to provide patient education, recommend changes to therapy and monitor patients with goal-inhibiting statin intolerance.
Footnotes
Author Contributions:A. Barry co-initiated the project, wrote the first draft of steps 1 to 4, 6 and the conclusion, supervised the manuscript writing, reviewed each draft and revised the final manuscript. J. Beach wrote the first draft of the manuscript including the introduction, terminology and step 5 and revised the final manuscript. G. Pearson co-initiated the project, supervised the manuscript writing, reviewed each draft and revised the final manuscript. All authors have approved the content. Each author meets all of the criteria for authorship, and all individuals who meet these criteria are listed as authors.
Declaration of Conflicting Interests:The authors have no real or potential financial conflicts of interest related to this work. Drs. Barry and Pearson were members of the primary panel and coauthors of the 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Dr. Pearson is a member of the Canadian Consensus Working Group and coauthor of the 2016 update for the diagnosis, prevention and management of statin adverse effects and intolerance.
Funding:This review was unfunded.
References
- 1. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–82. [DOI] [PubMed] [Google Scholar]
- 2. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention and management of statin adverse effects and intolerance: Canadian Consensus Working Group update. Can J Cardiol 2016;32:S35-65. [DOI] [PubMed] [Google Scholar]
- 4. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(suppl 2):S1-S45. [DOI] [PubMed] [Google Scholar]
- 5. Allan GM, Lindblad AJ, Comeau A, et al. Simplified lipid guidelines: prevention and management of cardiovascular disease in primary care. Can Fam Physician 2015;61:857–67. [PMC free article] [PubMed] [Google Scholar]
- 6. Wiggins BS, Saseen JJ, Page RL, II, et al. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease. A scientific statement for the American Heart Association. Circulation 2016;134:e468-95. [DOI] [PubMed] [Google Scholar]
- 7. Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504. [DOI] [PubMed] [Google Scholar]
- 10. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017;389:2473–81. [DOI] [PubMed] [Google Scholar]
- 11. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96. [DOI] [PubMed] [Google Scholar]
- 12. Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc 2015;90:24-34. [DOI] [PubMed] [Google Scholar]
- 13. Tan JT, Barry AR. Coenzyme Q10 supplementation in the management of statin-associated myalgia. Am J Health Syst Pharm 2017;74:786–93. [DOI] [PubMed] [Google Scholar]
- 14. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14. [DOI] [PubMed] [Google Scholar]
- 15. Rosenson RS. Current overview of statin-induced myopathy. Am J Med 2004;116:408–16. [DOI] [PubMed] [Google Scholar]
- 16. Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med 2005;165:2671-6. [DOI] [PubMed] [Google Scholar]
- 17. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med 2013;158:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mampuya WM, Frid D, Rocco M, et al. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J 2013;166:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joy TR, Monjed A, Zou GY, et al. N-of-1 (single-patient) trials for statin-related myalgia. Ann Intern Med 2014;160:301–10. [DOI] [PubMed] [Google Scholar]
- 20. Keating AJ, Campbell KB, Guyton JR. Intermittent nondaily dosing strategies in patients with previous statin-induced myopathy. Ann Pharmacother 2013;47: 398-404. [DOI] [PubMed] [Google Scholar]
- 21. Holbrook A, Wright M, Sung M, et al. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol 2011;27:146–51. [DOI] [PubMed] [Google Scholar]
- 22. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849–61. [DOI] [PubMed] [Google Scholar]
- 24. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- 25. HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12. [DOI] [PubMed] [Google Scholar]
- 26. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- 27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- 28. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580–90. [DOI] [PubMed] [Google Scholar]
- 29. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTER NATIVE randomized trial. J Clin Lipidol 2015;9:758–69. [DOI] [PubMed] [Google Scholar]