Abstract
We aimed to investigate (1) the relationship between cognitive impairment (CI) and disease severity and (2) the potential differences in exercise performance, daily activities, health status, and psychological well-being between patients with and without CI. Clinically stable chronic obstructive pulmonary disease (COPD) patients, referred for pulmonary rehabilitation, underwent a neuropsychological examination. Functional exercise capacity (6-minute walk test [6MWT]), daily activities (Canadian Occupational Performance Measure [COPM]), health status (COPD Assessment Test [CAT]) and St George’s Respiratory Questionnaire-COPD specific [SGRQ-C]), and psychological well-being (Hospital Anxiety and Depression Scale [HADS], Beck Depression Inventory [BDI], and Symptom Checklist 90 [SCL-90]) were compared between patients with and without CI. Of 183 COPD patients (mean age 63.6 (9.4) years, FEV1 54.8 (23.0%) predicted), 76 (41.5%) patients had CI. The prevalence was comparable across Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 1–4 (44.8%, 40.0%, 41.0%, 43.5%, respectively, p = 0.97) and GOLD groups A–D (50.0%, 44.7%, 33.3%, 40.2%, respectively, p = 0.91). Patients with and without CI were comparable for demographics, smoking status, FEV1% predicted, mMRC, 6MWT, COPM, CAT, HADS, BDI, and SCL-90 scores. Clinical characteristics of COPD patients with and without CI are comparable. Assessment of CI in COPD, thus, requires an active case-finding approach.
Keywords: COPD, cognitive impairment, functional status, health status, psychological well-being, pulmonary rehabilitation
Chronic obstructive pulmonary disease (COPD) is primarily a pulmonary disease characterized by a (usually) progressive, largely irreversible, airflow limitation.1 Although defined by an abnormal spirometry, COPD is well recognized as more than a respiratory disease. Indeed, extrapulmonary features and comorbidities occur frequently in patients with COPD and include, for example, decreased muscle mass, cardiovascular disease, anemia, and osteoporosis.2 Furthermore, psychological problems, such as symptoms of depression and anxiety, and cognitive impairment (CI), are common in patients with COPD.2,3
Patients with COPD can have either global impairment or deficits in specific cognitive domains, such as memory or cognitive flexibility.3 Impairment in memory and cognitive flexibility may lead to problems with adherence to treatment, educational achievement, self-management, and smoking abstinence,4–7 which are components of pulmonary rehabilitation (PR).8
Conventional COPD classification is mainly based on airflow limitation. In the Global initiative for chronic Obstructive Lung Disease (GOLD) document, the severity of airflow limitation is classified in four grades.1 Until now, the prevalence of CI among these traditional GOLD grades remains unknown. The updated COPD GOLD strategy 20141 recommends to grade disease severity into risk groups, based on symptoms and exacerbation risk. Also, the prevalence of CI among the GOLD groups remains unknown. Moreover, the relationship between CI and other patient-related outcomes is unknown. Nevertheless, identifying factors associated with CI in COPD patients can help clinicians to detect patients with possible CI for further cognitive assessment. Therefore, the first aim of this study was to study the relationship between CI and the severity of airflow limitation. The second aim was to compare functional status, disease-specific health status, and psychological well-being between COPD patients with and without CI. Because CI may be of special interest in educational components and behavior change interventions of PR, the current study focuses specifically on patients with COPD who are to start a comprehensive interdisciplinary PR program. A priori, we hypothesized that CI is most prevalent in GOLD grades 3–4 and in GOLD group D, having high risk and more symptoms. Furthermore, we hypothesized that functional status, disease-specific health status, and psychological well-being are worse in patients with CI compared to patients without CI.
Methods
Design
This cross-sectional observational study is part of a longitudinal study (COgnitive-PD study) concerning neuropsychological functioning in patients with COPD. Details of the methodology of this study and data concerning cognitive functioning have been published before.9 Moreover, 90 patients were part of a previous publication on the prevalence of general and domain-specific CI between patients with COPD and non-COPD controls.3
Study sample
Patients with clinically stable COPD were included. Diagnosis of COPD was based on GOLD criteria.1 All patients were admitted to CIRO, a tertiary referral center, for a 3-day assessment before the start of a comprehensive interdisciplinary PR program.10 Exclusion criteria were an exacerbation within the previous 4 weeks, insufficient comprehension of the Dutch language, or having a diagnosis of dementia. All participants gave informed consent. The Medical Ethics Committee of the University Hospital Maastricht and Maastricht University approved this study (NL45127.068.13).
Measures
Age, gender, educational level (according to The Dutch Standard Classification of Education [CBS]11), marital status, visual and hearing impairment, handedness, smoking behavior, and self-reported comorbidities (Charlson Comorbidity Index12) were recorded. The patient medical record was used to collect data on body mass index (BMI), long-term oxygen therapy, modified Medical Research Council (mMRC) dyspnea scale, BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index,13 post-bronchodilator spirometry (forced vital capacity [FVC], FVC% predicted, forced expiratory volume in the first second (FEV1), FEV1% predicted, and FEV1/FVC), exacerbation history, arterial blood gases, including arterial partial pressure of oxygen (PaO2), arterial partial pressure of carbon dioxide (PaCO2), and arterial oxygen saturation (SaO2), and single breath carbon monoxide diffusing capacity (DLCO% predicted) and level of hypoxemia (mild: PaO2 60–79 mmHg; moderate: PaO2 40–59 mmHg; and severe: PaO2 < 40 mm Hg).
Neuropsychological examination comprised a detailed neuropsychological testing battery from the Cognitive-PD study, namely, the Concept Shifting Test (CST); the Letter Digit Substitution Test (LDST); the Stroop Color-Word Test (SCWT); and the Visual Verbal Learning Task (VVLT); and a shortened version of the Groninger Intelligence Test (GIT) including subtasks vocabulary, mental rotation, figure discovery, doing sums, analogies, and fluency. For detailed information of the neuropsychological testing battery, see the Cognitive-PD study protocol.9
Two 6-minute walk tests (6MWT) were performed according to European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines to measure functional exercise capacity.14 The best 6MWT was expressed in percentage of predicted values. The Canadian Occupational Performance Measure (COPM) was used to assess the performance of daily activities and satisfaction with daily activities as described before.15 Problematic activities of daily living (ADLs) were categorized into four COPM domains (problems/no problems): “self-care,” “productivity,” “leisure,” and “mobility.”16
To assess disease-specific health status, participants completed the COPD Assessment Test (CAT) and the St George’s Respiratory Questionnaire-COPD specific (SGRQ-C). The CAT contains 8 items with a final single score ranging from 0 (mild) to 40 points (very severe).17 The SGRQ-C provides a total score and three domain scores: symptoms, activities, and impact (ranging from 0 [best] to 100 points [worst]).18
Symptoms of anxiety and depression were measured using the Hospital Anxiety and Depression Scale (HADS). The HADS is comprised of a Depression and Anxiety subscale. Scores per subscale range from 0 to 21 points. Scores of 0–7 points indicate normal levels of anxiety and depression; 8–10 points indicate borderline abnormal anxiety and depression levels; and 11–21 points suggest abnormal levels of anxiety and depression.19 The Beck Depression Inventory (BDI) was used to measure the intensity, severity, and depth of depression. The questionnaire consists of 21 items. Total score ranges from 0 to 63 points (0–9 points indicate that a person is not depressed; 10–18 points indicate mild-moderate depression; 19–29 points indicate moderate-severe depression; 30–63 points indicate severe depression).20 Psychological symptom intensity was measured using the Symptom Checklist 90 (SCL-90), a self-report inventory consisting of 90 items divided in 9 subscales. Items are scored on a 5-point Likert-type scale (ranging from 0 “not at all” to 4 points “extremely”), indicating the rate of occurrence of the symptom during the time reference.21
Statistics
All statistics were done using SPSS 21.0 (SPSS Inc. Chicago, IL). Because of multiple comparisons, a p-value ≤ 0.01 was considered as statistically significant. Categorical variables are described as frequencies, while continuous variables were tested for normality and are presented as mean and standard deviation (SD) or median and interquartile range (IQR).
Raw test scores on the neuropsychological testing battery were transformed into age, gender, and education corrected Z-scores based on normative data from the Maastricht Aging Study (MAAS).22 Six core tests used in MAAS to probe different cognitive domains,23 verbal memory (VVLT total, VVLT recall), verbal fluency (GIT animal naming), psychomotor speed (LDST 60 sec), and information processing (CST-C and SCWT card III), were used to distinguish between COPD patients with CI and COPD patients without CI (see Table E1). In accordance with Singh et al,24 Z-scores below −1.0 SD were considered as being impaired. Therefore, patients with general CI have a score less than 1.0 SD below the age-, gender-, and education-specific mean of the MAAS study population for at least two core tests.
Individual cognitive test measures were grouped into the following specific cognitive domains: psychomotor speed (SCWT card I, CST-A, and LDST 60 sec), planning (BADS key search and zoo map), working memory (VVLT trial 1 and DS backward), verbal memory (VVLT total recall, delayed recall, and retention), and cognitive flexibility (SCWT card III and CST-C). Within each cognitive domain, the scaled test Z scores were summed and averaged into one compound Z score. In each cognitive domain, a compound Z score of less than 1.0 SD below the age-, gender-, and education-specific mean of the MAAS study population was considered as impaired.24
Patients were categorized in severity grades 1–4 using spirometry (GOLD I: FEV1 ≥ 80%; GOLD II: FEV1 ≥ 50% and < 80%; GOLD III: FEV1 ≥ 30% and < 50%; GOLD IV: FEV1 < 30%). Additionally, patients were categorized into GOLD groups A–D combining symptom assessment by CAT (<10 or ≥10 points) and exacerbation risk (either by GOLD severity grades (1–2 or 3–4) or the number of exacerbations in the previous 12 months (≤1 or >1)) according to the 2011 GOLD strategy.1 The prevalence of general and domain-specific CI was compared between GOLD grades and GOLD groups using chi-square tests. A post hoc analysis was performed in order to compare clinical characteristics between COPD patients with and without impairments in the cognitive domains psychomotor speed, planning, working memory, verbal memory, and cognitive flexibility using independent sample t-test or Mann–Whitney U test, as appropriate. Furthermore, the relationship between FEV1 and the five cognitive domains and their individual cognitive test measures were analyzed using a Pearson’s correlation coefficient or Spearman’s rank correlation coefficient, depending on the variable distribution.
Patient characteristics, functional status, disease-specific health status, and psychological wellbeing were compared between COPD patients with and without CI using chi-square tests for categorical variables and independent sample t-test or Mann–Whitney U test, as appropriate for continuous variables.
Results
Patient characteristics
In total, 183 patients (53% men) with mild to very severe COPD were included (Table 1). In total, 29 (15.8%) patients were classified as GOLD grade 1, 70 (38.3%) patients as GOLD grade 2, 61 (33.3%) patients as GOLD grade 3, and 23 (12.6%) patients as GOLD grade 4. Furthermore, 6 (3.3%) patients were classified in GOLD group A, 47 (25.7%) patients in GOLD group B, 3 (1.6%) patients in GOLD group C, and 127 (69.4%) patients in GOLD group D. A majority of the patients had a lower general or vocational education level, were positioned in quartile I (0–2 points) of the BODE index, and a majority were nonsmokers (Table 1).
Table 1.
Patient characteristics of the study population.
| Total group (n = 183) | Patients with cognitive impairment (n = 76) | Patients without cognitive impairment (n = 107) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 63.3 (9.4) | 62.7 (8.7) | 63.7 (9.9) | 0.51 |
| Male, n (%) | 97 (53.0) | 46 (60.5) | 51 (47.7) | 0.09 |
| Lower general or vocational education, n (%) | 100 (54.6) | 42 (55.3) | 58 (54.2) | 0.89 |
| IQ, mean (SD) | 85.5 (14.9) | 77.5 (13.4) | 91.2 (13.2) | <0.01 |
| Married, n (%) | 111 (60.7) | 48 (63.2) | 63 (58.9) | 0.56 |
| Clinical characteristics | ||||
| Visual impairment, n (%) | 31 (16.9) | 12 (15.8) | 19 (17.8%) | 0.73 |
| Hearing impairment, n (%) | 45 (24.6) | 17 (22.4) | 28 (26.2) | 0.56 |
| Right-handed by nature, n (%) | 149 (81.4) | 63 (82.9) | 86 (80.4) | 0.13 |
| BMI (kg/m2), mean, (SD) | 27.1 (6.1) | 27.5 (7.2) | 26.8 (5.3) | 0.45 |
| Oxygen therapy, n (%) | 37 (20.2) | 15 (19.7) | 22 (20.6) | 0.98 |
| mMRC (points), mean (SD) | 2.2 (1.0) | 2.2 (1.0) | 2.3 (1.0) | 0.53 |
| BODE index | ||||
| 0–2 points, n (%) | 107 (58.5) | 47 (61.8) | 60 (56.1) | 0.33 |
| 2–3 points, n (%) | 27 (14.8) | 9 (11.8) | 18 (16.8) | |
| 4–5 points, n (%) | 33 (18.0) | 16 (21.1) | 17 (15.9) | |
| 6–10 points, n (%) | 16 (8.7) | 4 (5.3) | 12 (11.2) | |
| Exacerbation history in the previous 12 months | ||||
| 0 exacerbations, n (%) | 50 (27.9) | 23 (30.3) | 27 (26.0) | 0.19 |
| 1 exacerbation, n (%) | 32 (17.9) | 13 (17.3) | 19 (18.3) | |
| 2 exacerbations, n (%) | 30 (16.8) | 10 (13.3) | 20 (19.2) | |
| 3 exacerbations, n (%) | 21 (11.7) | 5 (6.7) | 16 (15.4) | |
| 4 ≥ exacerbations, n (%) | 46 (25.7) | 24 (32.0) | 22 (21.2) | |
| Spirometry and arterial blood gases | ||||
| FEV1/FVC, mean (SD) | 42.3 (14.8) | 43.7 (15.9) | 41.3 (14.0) | 0.28 |
| FEV1 (% predicted), mean (SD) | 54.8 (23.0) | 55.3 (24.8) | 54.4 (21.7) | 0.79 |
| SaO2 (%), median (IQR) | 94.6 (92.8–96.0)a | 94.4 (93.0–95.8)b | 94.8 (92.8–96.0)c | 0.98 |
| PaO2 (kPa), mean (SD) | 9.7 (1.6)a | 9.7 (1.5)b | 9.7 (1.6)c | 0.97 |
| PaCO2 (kPa), mean (SD) | 5.2 (0.8)a | 5.3 (0.9)b | 5.1 (0.6)c | 0.05 |
| DLCO (% predicted), median (IQR) | 49.3 (41.0-61.7)d | 48.3 (40.8-62.4)e | 49.6 (41.7-61.1)f | 0.61 |
| Hypoxemia | ||||
| Mild, n (%) | 178 (97.3) | 74 (97.4) | 104 (97.2) | 0.08 |
| Moderate, n (%) | 3 (1.6) | 0 (0.0) | 3 (2.8) | |
| Severe, n (%) | 2 (1.1) | 2 (2.6) | 0 (0.0) | |
| Smoking behavior | ||||
| Current smoker, n (%) | 24 (13.1) | 14 (18.4) | 10 (9.3) | 0.08 |
| Former smoker, n (%) | 156 (85.2) | 62 (81.6) | 94 (87.9) | |
| Never smoker, n (%) | 3 (1.6) | 0 (0.0) | 3 (2.8) | |
| Pack years, mean (SD) | 42.5 (24.6)g | 45.0 (24.7)h | 40.8 (24.6)i | 0.267 |
| Comorbidities | ||||
| Charlson comorbidity index score, mean, (SD) | 2.8 (1.8) | 2.9 (1.8) | 2.7 (1.9) | 0.44 |
COPD, chronic obstructive pulmonary disease; SD, standard deviation; IQR, interquartile range; BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; SaO2, oxygen saturation; PaO2, arterial oxygen partial pressure; PaCO2, arterial partial pressure of carbon dioxide; DLCO, single breath carbon monoxide diffusing capacity.
a n = 181. b n = 75. c n = 106. d n = 174. e n = 73. f n = 101. g n = 174. h n = 70. i n = 104.
Prevalence of cognitive impairment in different GOLD grades
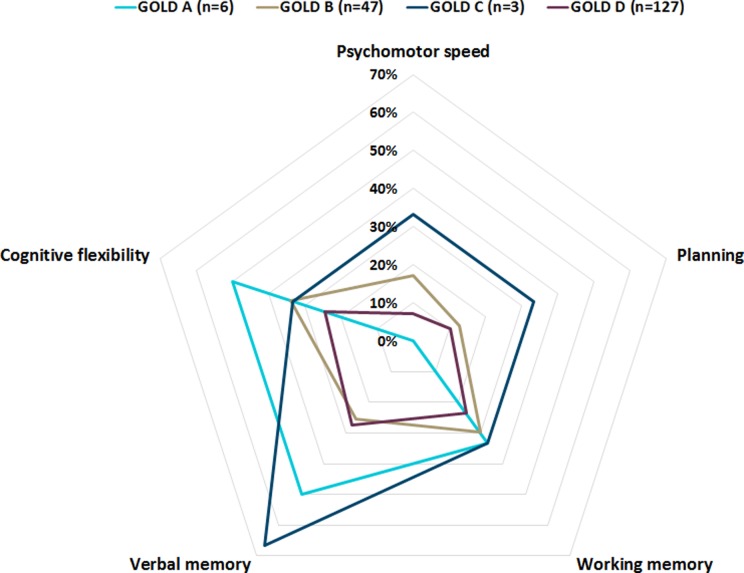
The prevalence of general CI did not differ between COPD patients in GOLD grades 1–4 and GOLD groups A–D (Figure 1). Also, impairments in specific cognitive domains (psychomotor speed, planning, working memory, verbal memory, and cognitive flexibility) did not differ between COPD patients in GOLD grades 1–4 (Figure 2) and GOLD groups A–D (Figure 3). Moreover, correlations between cognitive measures and FEV1 were weak (Table S1). Demographic and clinical characteristics, including smoking behavior, lung function, and results of arterial blood gases, were comparable between patients with and without CI (Table 1). The COPD patients with CI had a lower IQ compared to those without CI (Table 1).
Figure 1.
The prevalence of cognitive impairment in patients with COPD, stratified for GOLD grades (A) and GOLD groups (B). COPD: Chronic obstructive pulmonary disease; GOLD: The Global Initiative for Chronic Obstructive Lung Disease guidelines.
Figure 2.
Domain-specific cognitive impairment. Proportion of patients in GOLD grades 1–4 with impairments in the domains psychomotor speed (17.2%, 8.6%, 8.2%, and 8.7%, respectively; p = 0.544), planning (10.3%, 11.4%, 8.2%, and 17.4%, respectively; p = 0.686), working memory (34.5%, 28.6%, 19.7%, and 21.7%, respectively; p = 0.419), verbal memory (24.1%, 28.6%, 26.2%, and 39.1%, respectively; p = 0.636), and cognitive flexibility (37.9%, 28.6%, 27.9%, and 13.0%, respectively; p = 0.262) COPD: chronic obstructive pulmonary disease; GOLD: The Global Initiative for Chronic Obstructive Lung Disease guidelines.
Figure 3.
Domain-specific cognitive impairment. Proportion of patients in GOLD groups A–D with impairments in the domains psychomotor speed (0.0%, 17.0%, 33.3%, and 7.1%, respectively; p = 0.096), planning (0.0%, 12.8%, 33.3%, and 10.2%, respectively; p = 0.474), working memory (33.3%, 29.8%, 33.3%, and 23.6%, respectively; p = 0.808), verbal memory (50.0%, 25.5%, 66.7%, and 27.6%, respectively; p = 0.287), and cognitive flexibility (50.0%, 34.0%, 33.3%, and 24.4%, respectively; p = 0.369). GOLD: The Global Initiative for Chronic Obstructive Lung Disease guidelines.
Functional status, disease-specific health status, and psychological well-being in patients with and without cognitive impairment
Functional exercise capacity measured with the 6MWT was comparable between patients with and without CI (Table 2). The COPM domain “mobility” was most frequently scored followed by “productivity,” “leisure,” and “self-care.” The proportion of COPD patients scoring problematic ADLs within the COPM domains did not differ between patients with and without CI (Table 2). Also, performance of daily activities and satisfaction with performance of daily activities were comparable between the groups. Finally, patients with and without CI did not differ in disease-specific health status or psychological well-being (Table 2). When comparing COPD patients with and without impairments in specific cognitive domains, functional exercise capacity was worse in those with verbal memory impairments compared to those without verbal memory impairments (400.2 [111.1]) meters vs. 443.9 [110.1] meters; p = 0.018; Table S5). Regarding daily functioning, patients with impairments in psychomotor speed, planning, and cognitive flexibility had lower satisfaction scores on specific domains of the COPM (Tables S2, S3, and S6). Patients with and without domain-specific CI did not differ in disease-specific health status. As to psychological well-being, impairments in psychomotor speed, planning, and cognitive flexibility were related to somatization and impairments in planning, working memory, verbal memory, cognitive flexibility to symptoms of depression (Tables S2–S6). Moreover, patients with impairments in the domain cognitive flexibility had higher (worse) scores on the insufficiency (28.3 [9.2] and 25.4 [7.7], respectively) and psychoneuroticism (160.6 [44.1] and 143.9 [40.8], respectively) items of the SCL-90 compared to patients without impairments in this cognitive domain (Table S6).
Table 2.
Functional status, disease-specific health status, and psychological well-being.a
| Total group (n = 183) | Patients with cognitive impairment (n = 76) | Patients without cognitive impairment (n = 107) | p value | |
|---|---|---|---|---|
| Functional status | ||||
| Functional exercise capacity | ||||
| 6MWT (meters) | 432 (111.8) | 421.8 (112.2) | 439.2 (111.5) | 0.30 |
| 6MWT (% predicted) | 68.3 (16.6) | 66.1 (16.7) | 69.8 (16.4) | 0.14 |
| 6MWT < 350 meters, n (%) | 45 (24.6) | 22 (28.9) | 23 (21.5) | 0.16 |
| Daily activities | ||||
| COPM mobility domain, n (%) | 147 (80.3) | 64 (84.2) | 83 (77.6) | 0.27 |
| Performance score (points) | 3.8 (1.5) | 3.7 (1.7) | 3.8 (1.4) | 0.66 |
| Satisfaction score (points) | 2.8 (1.7) | 2.7 (1.9) | 2.9 (1.7) | 0.59 |
| COPM productivity domain, n (%) | 117 (63.9) | 50 (65.8) | 67 (62.6) | 0.66 |
| Performance score (points) | 3.9 (1.5) | 3.8 (1.5) | 3.9 (1.5) | 0.63 |
| Satisfaction score (points) | 3.3 (1.8) | 3.4 (1.9) | 3.2 (1.8) | 0.65 |
| COPM leisure domain, n (%) | 94 (51.4) | 38 (50.0) | 56 (52.3) | 0.76 |
| Performance score (points) | 3.4 (1.6) | 3.5 (1.5) | 3.4 (1.7) | 0.78 |
| Satisfaction score (points) | 2.8 (1.6) | 2.7 (1.6) | 2.9 (1.7) | 0.65 |
| COPM self-care domain, n (%) | 87 (47.5) | 39 (51.3) | 48 (44.9) | 0.39 |
| Performance score (points) | 4.2 (1.6) | 4.3 (1.5) | 4.0 (1.6) | 0.33 |
| Satisfaction score (points) | 3.2 (1.8) | 3.6 (1.9) | 2.7 (1.6) | 0.04 |
| Disease-specific health status | ||||
| CAT score (points) | 22.0 (6.5) | 22.7 (6.1) | 21.6 (6.7) | 0.27 |
| SGRQ-C symptom score (points) | 61.1 (19.9) | 60.1 (19.7) | 61.9 (20.1) | 0.55 |
| SGRQ-C activity score (points) | 80.5 (16.2) | 82.8 (14.0) | 78.9 (17.5) | 0.11 |
| SGRQ-C impact score (points) | 50.1 (19.0) | 53.2 (18.4) | 48.0 (19.3) | 0.07 |
| SGRQ-C total score (points) | 61.4 (15.4) | 63.5 (14.7) | 59.9 (15.8) | 0.13 |
| Psychological well-being | ||||
| HADS Depression score (points) | 7.4 (4.0) | 7.9 (4.0) | 6.9 (7.4) | 0.10 |
| HADS Anxiety score (points) | 8.0 (4.5) | 8.7 (4.7) | 7.4 (4.3) | 0.06 |
| BDI score score (points) | 15.5 (9.6) | 17.2 (9.6) | 14.3 (9.5) | 0.05 |
| SCL-90 Anxiety score (points) | 16.2 (6.2) | 16.5 (5.6) | 16.0 (6.6) | 0.54 |
| SCL-90 Agoraphobia score (points) | 10.1 (4.2) | 10.8 (4.2) | 9.6 (4.2) | 0.06 |
| SCL-90 Depression score (points) | 28.0 (10.7) | 28.7 (10.5) | 27.5 (10.8) | 0.46 |
| SCL-90 Somatization score (points) | 23.4 (7.6) | 24.0 (8.0) | 23.0 (7.2) | 0.40 |
| SCL-90 Insufficiency score (points) | 17.9 (5.9) | 18.9 (6.3) | 17.2 (5.4) | 0.05 |
| SCL-90 Sensitivity score (points) | 26.2 (8.2) | 27.2 (9.0) | 25.4 (7.5) | 0.15 |
| SCL-90 Hostility score (points) | 7.7 (2.3) | 7.8 (2.3) | 7.7 (2.3) | 0.69 |
| SCL-90 Insomnia score (points) | 6.6 (2.9) | 13.2 (4.5) | 12.2 (3.9) | 0.24 |
| SCL-90 Psychoneuroticism score (points) | 148.6 (42.3) | 153.9 (42.7) | 144.8 (41.8) | 0.15 |
6MWT, 6-min walk distance; BDI, Beck Depression Inventory; COPM, Canadian Occupational Performance Measure; HADS, Hospital Anxiety and Depression Scale; SCL-90, Symptom Checklist 90; SGRQ-C, COPD-specific version of the St. George respiratory questionnaire.
aData shown as mean (SD) or as n (%).
Discussion
In contrast to our hypotheses, we found a comparable prevalence of CI across traditional and revised GOLD grades. Additionally, patients with CI did not have worse functional status, disease-specific health status, or psychological well-being compared to patients without CI.
Cognitive impairment in different GOLD grades
The equal distribution of CI across GOLD grades demonstrates that CI is prevalent in COPD patients, independent of severity of airflow limitation. This is in line with the findings of Antonelli–Incalzi and colleagues who show that indexes of health status assessing cognitive status, affective status, and quality of sleep did not differ across GOLD grades.25 Additionally, the prevalence of CI did not differ across GOLD groups, which shows that CI is prevalent in all symptom groups and risk groups of COPD. Yet, due to the inclusion of patients entering PR, only a small number of patients in the low-symptom categories (GOLD A and C) were included. Moreover, FEV1% predicted did not differ significantly between patients with and without CI. Although the DS backward test and the domain cognitive flexibility correlated with FEV1, all correlations between domain-specific cognitive functioning, individual cognitive test measures, and FEV1 were weak. This is in line with the idea that complex, higher order cognitive functions, as reflected by cognitive flexibility, are more related to COPD-specific factors, such as lung function, than less complex cognitive functions. Indeed, in COPD patients without any comorbidities on the Charlson index compared to controls without COPD and other comorbidities on the Charlson index, worse scores for cognitive flexibility and planning, but not for lower order cognitive functions such as psychomotor speed and memory, have been demonstrated.3
However, another study, using a large sample size, did find an association between cognition and lung function, although associations were weak,26 and no association has been found between lung function and cognitive decline over time.27 Therefore, CI should be actively screened for in COPD patients admitted to PR, irrespective of airflow limitation. Cognitive impairment may provide additional clinical relevant information in order to optimize therapy which cannot be predicted from other clinical determinants.
Functional status, disease-specific health status, and psychological well-being in patients with and without cognitive impairment
No significant differences in clinical and patient-related outcomes were found between COPD patients with and without CI. For example, we found no statistically significant or clinically relevant difference14 in 6MWT. Similarly, Schure and colleagues found no association between cognitive impairment and decreased 6MWT after adjusting for disease severity, comorbidities, psychological functioning, and demographic characteristics.28 Moreover, Ozyemisci–Taskiran did not find an association between CI and functional capacity during an exacerbation of COPD.29 Yet, exacerbations itself have been shown to be correlated with cognitive decline30; nevertheless, cognitive performances improved after discharge from hospital following exacerbation.31 The proportion of patients reporting to experience problems in the four COPM domains did not differ between patients with and without CI. It has been shown to be problematic to use the COPM in patients with poor self-awareness.32 The self-reported nature of the COPM may lead to biased outcomes by lack of insight of the patient with COPD. Moreover, we did not find a difference in disease-specific health status between patients with and without CI. This is in contrast to Antonelli–Incalzi and colleagues33 who demonstrated a weak correlation between global cognitive functioning and domain scores of the SGRQ in 230 COPD patients. Also, Roncero and colleagues found an association between cognitive functioning, as measured with the Mini-Mental State Examination (MMSE), and health-related quality of life.34 This discrepancy may be explained by methodological differences. Our study used a more sensitive battery of cognitive tests, compared to the MMSE, a brief screening tool, of which the accuracy in detecting CI has been shown to have suboptimal specificity.35 Although CI is known to be associated with several psychopathological characteristics such as anxiety36 and depression,37 we did not find differences in anxiety and depression between patients with and without CI. Discrepancies may be explained by the fact that we assessed anxiety and depression symptoms by subjective questionnaires instead of the presence of an anxiety or major depression disorder diagnosed by a psychologist. Clinical characteristics and patient-related outcomes are not related to CI. Consequently, clinical characteristics of COPD patients are not able to differentiate COPD patients with and without CI.
However, functional exercise capacity, satisfaction scores regarding daily activities such as productivity and self-care, and symptoms of somatization, depression, and insufficiency as assessed during a 3-day assessment before the start of a comprehensive interdisciplinary PR program might be leads for clinicians to know whether or not they are dealing with COPD patients with domain-specific cognitive impairments. Consequently, they are warned for possible specific consequences during the PR program. For example, it is possible that a worse functional exercise capacity might be related to a tendency to ignore more often, given guidelines, requests, or instructions because we found that COPD patients with verbal memory impairments had lower 6MWT scores compared to those without. This needs to be verified in future research.
Potential clinical consequences
Previous studies showed that CI challenges adherence to complex medication regimens.5,38 Especially, learning and memory were associated with changes in medication adherence.39 Moreover, memory impairment is associated with poorer adherence to self-care practices and educational participation and achievement. For example, heart failure patients with CI reported poorer self-management behaviors such as recognizing symptoms of worsening compared to patients without CI.6 Cognitive impairment in executive functions among elderly has been associated with decreased levels of smoking cessation.4 Cognitive impairment also was predictive of negative outcomes in rehabilitation from hip fracture in elderly.7 The proportion of patients with CI varies in different studies due to differences in study population and neuropsychological assessment methods, ranging up to almost 80% in COPD patients.38,40,41 In non-COPD controls, matched for age, smoking status, and education, the prevalence rate of CI was 13.3%.3 Several facts underline the importance of active case finding of patient with COPD and CI. First, two of five COPD patients entering PR have CI. Second, CI is prevalent irrespective of disease severity and clinical outcomes. Third, and finally, CI may occur with above-mentioned clinical consequences. Yet, Dodd and colleagues42 did not find an association between executive functions and frequency of hospitalization, and a recent study showed no correlation between cognitive functioning and exacerbations, emergency room visits, hospitalizations, self-management skills, or quality of life.43 They conclude that an active screening approach for CI in COPD may not be indicated. However, due to the cross-sectional study design, the possible impact of CI on health or therapy outcomes could not be assessed in this study. In view of this, we believe that detecting patients with CI may improve overall care processes by for example including cognitive training strategies which can be divided into restorative and compensatory strategies.44 Restorative strategies attempt to improve functioning in specific cognitive domains or domains of functioning, such as ADLs, social skills, and behavioral disturbances. Compensatory cognitive strategies, such as incorporating daily routines with fewer tasks and/or pacing tasks, and providing the patient with electronic notes, reminders, calendars, or planners to keep track of activities and appointments,45 can be used to minimize functional and psychological problems experienced by patients. Health-care professionals should be aware of cognitive deficits in order to tailor clinical interventions to the individual patient and to determine the required type of assistance and environmental modification. Compensatory strategies have been applied in other patients, such as Alzheimer, multiple sclerosis, and patients with brain injuries.45,46 Evaluation of these cognitive training strategies in COPD is needed to explore which are beneficial and should be incorporated in the overall care.
Methodological considerations
A major strength of this study is the use of an extensive neuropsychological testing battery, which provides objective measures to target general and domain-specific CI. However, a comprise neuropsychological examination is time consuming. Future studies should assess how many neuropsychological tests are needed to do a first cognitive screening in clinical practice with a comparable sensitivity in order to detect both general and domain-specific CI. A further advantage of this study is the well-characterized study sample. Yet, in-depth analyses are needed to assess the impact of hypoxemia on cognitive impairment as hypoxemia30 has been proposed to be a factor, among multiple others, connecting COPD and cognitive impairment. Then again, the inclusion of patients from a tertiary referral center, including a majority of patients in high-symptom categories (GOLD B and D) and a minority in low-symptom categories (GOLD A and C), may limit the generalizability of our results. Performance and satisfaction of problematic ADLs were assessed with the COPM, which relies on the self-perception of the patient. It is possible that patients with CI less frequently report decreased performance of daily activities due to difficulties in seeing the relevance of daily life activities and problems with initiating activities. Instead of the COPM, The Perceive, Recall, Plan & Perform (PRPP) System of Task Analysis,47 The Arnadóttir OT-ADL Neurobehavioral Evaluation (A-ONE),48 or The Assessment of Motor and Process Skills (AMPS)49 can be administered in order to observe and analyze occupational activities. Specifically, the A-ONE directly links functional performance to cognitive–perceptual impairments, such as agnosia, body neglect, decreased organization, motor apraxia, and spatial neglect. Selection bias may have played a role in this study. Patients with subjective complaints of cognitive functioning might avoid participation due to anxiety or worry or may have been more willing to participate in the present study due to personal interest. Also, patients with severe CI or a diagnosis of dementia most likely will not be referred for PR. Future studies should include a test in order to detect poor testing motivation. Since we found that patients with COPD have a lower IQ compared to those without cognitive impairment, and an intellectually stimulating atmosphere may boost cognitive performance, longitudinal studies are needed to assess premorbid cognitive ability and to incorporate cognitive reserve and cognitive loss over time in relation to clinical characteristics of this study population.
Conclusion
Cognitive impairment in patients with COPD is prevalent, irrespective of clinical characteristics. Clinical characteristics are not able to differentiate patients with COPD with and without CI. An active case finding approach is required to assess CI in patients with COPD who are about to start a PR program. Future research should investigate whether and to what extent CI influences medication adherence, educational achievement, self-management, smoking cessation, and outcomes of PR in patients with COPD.
Supplementary material
Supplementary_Material for Cognitive impairment and clinical characteristics in patients with chronic obstructive pulmonary disease by Fiona A H M Cleutjens, Martijn A Spruit, Rudolf W H M Ponds, Lowie E G W Vanfleteren, Frits M E Franssen, Candy Gijsen, Jeanette B Dijkstra, Emiel F M Wouters, and Daisy J A Janssen in Chronic Respiratory Disease
Acknowledgements
Each author has participated sufficiently, intellectually or practically, in the work to take public responsibility for the content of the article, including the conception, design, and conduct of the experiment and for data interpretation. The contribution of the authors to the manuscript is as follows: FAHMC: study design, analyzing data, drafting the manuscript, primary responsibility of the final content; RWHMP, LEGWV, FMEF, CG, JBD, EFMW: reviewing the manuscript; MAS, DJAJ: study design, drafting the manuscript and primary responsibility of the final content, reviewing the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Weijerhorst Foundation, Maastricht, the Netherlands.
Supplementary material: Supplementary material for this article is available online.
References
- 1. Global initiative for chronic obstructive lung disease: pocket guide to COPD diagnosis, management, and prevention. Updated 2015. 2015 cited; http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_2015_Feb18.pdf (accessed 19 August 2016).
- 2. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Resp Crit Care 2013; 187(7): 728–735. [DOI] [PubMed] [Google Scholar]
- 3. Cleutjens FA, Franssen FM, Spruit MA, et al. Domain-specific cognitive impairment in patients with COPD and control subjects. Int J Chron Obstruct Pulmon Dis 2017; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brega AG, Grigsby J, Kooken R, et al. The impact of executive cognitive functioning on rates of smoking cessation in the san luis valley health and aging study. Age Ageing 2008; 37(5): 521–525. [DOI] [PubMed] [Google Scholar]
- 5. Campbell NL, Boustani MA, Skopelja EN, et al. Medication adherence in older adults with cognitive impairment: a systematic evidence-based review. Am J Geriatr Pharmacother 2012; 10(3): 165–177. [DOI] [PubMed] [Google Scholar]
- 6. Hajduk AM, Lemon SC, McManus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol 2013; 5: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lenze EJ, Munin MC, Dew MA, et al. Adverse effects of depression and cognitive impairment on rehabilitation participation and recovery from hip fracture. Int J Geriatr Psychiatr 2004; 19(5): 472–478. [DOI] [PubMed] [Google Scholar]
- 8. Spruit MA, Singh SJ, Garvey C, et al. An official american thoracic society/european respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Resp Crit Care 2013; 188(8): e13–64. [DOI] [PubMed] [Google Scholar]
- 9. Cleutjens FA, Wouters EF, Dijkstra JB, et al. The COgnitive-pulmonary disease (COgnitive-PD) study: protocol of a longitudinal observational comparative study on neuropsychological functioning of patients with COPD. BMJ Open 2014; 4(3): e004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spruit MA, Vanderhoven-Augustin I, Janssen PP, et al. Integration of pulmonary rehabilitation in COPD. Lancet 2008; 371(9606): 12–13. [DOI] [PubMed] [Google Scholar]
- 11. C.B.S. Standaard beroepenclassificatie 1992 – editie 2001. Den Haag: SDU, 2001. [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 13. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10): 1005–1012. [DOI] [PubMed] [Google Scholar]
- 14. Holland AE, Spruit MA, Troosters T, et al. An official European respiratory society/american thoracic society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1428–1446. [DOI] [PubMed] [Google Scholar]
- 15. Law M, Baptiste S, McColl M, et al. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther 1990; 57(2): 82–87. [DOI] [PubMed] [Google Scholar]
- 16. Annegarn J, Meijer K, Passos VL, et al. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J Am Med Dir Assoc 2012; 13(3): 284–290. [DOI] [PubMed] [Google Scholar]
- 17. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34(3): 648–654. [DOI] [PubMed] [Google Scholar]
- 18. Meguro M, Barley EA, Spencer S, et al. Development and validation of an improved, COPD-specific version of the St. george respiratory questionnaire. Chest 2007; 132(2): 456–463. [DOI] [PubMed] [Google Scholar]
- 19. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiat Scand 1983; 67(6): 361–370. [DOI] [PubMed] [Google Scholar]
- 20. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 21. Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull 1973; 9(1): 13–28. [PubMed] [Google Scholar]
- 22. Jolles J, van Boxtel MP, Ponds RW, et al. De maastricht aging study (MAAS). Het longitudinaal perspectief van cognitieve veroudering. [The maastricht aging study (MAAS). The longitudinal perspective of cognitive aging]. Tijdschr Gerontol Geriatr 1998; 29(3): 120–129. [PubMed] [Google Scholar]
- 23. Burgmans S, van Boxtel MP, Smeets F, et al. Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiol Aging 2009; 30(9): 1413–1419. [DOI] [PubMed] [Google Scholar]
- 24. Singh B, Parsaik AK, Mielke MM, et al. Chronic obstructive pulmonary disease and association with mild cognitive impairment: the mayo clinic study of aging. Mayo Clin Proc 2013; 88(11): 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antonelli-Incalzi R, Imperiale C, Bellia V, et al. Do GOLD stages of COPD severity really correspond to differences in health status? Eur Respir J 2003; 22(3): 444–449. [DOI] [PubMed] [Google Scholar]
- 26. Cleutjens FA, Spruit MA, Ponds RW, et al. Cognitive functioning in obstructive lung disease: results from the United Kingdom biobank. J Am Med Dir Assoc 2014; 15(3): 214–219. [DOI] [PubMed] [Google Scholar]
- 27. Pathan SS, Gottesman RF, Mosley TH, et al. Association of lung function with cognitive decline and dementia; the atherosclerosis risk in communities (ARIC) study. Eur J Neurol 2011; 18(6): 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schure MB, Borson S, Nguyen HQ, et al. Associations of cognition with physical functioning and health-related quality of life among COPD patients. Respir Med 2016; 114: 46–52. [DOI] [PubMed] [Google Scholar]
- 29. Ozyemisci-Taskiran O, Bozkurt SO, Kokturk N, et al. Is there any association between cognitive status and functional capacity during exacerbation of chronic obstructive pulmonary disease? Chron Respir Dis 2015; 12(3): 247–255. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Cai X, Shi X, et al. Chronic obstructive pulmonary disease as a risk factor for cognitive dysfunction: a meta-analysis of current studies. JAD 2016; 52(1): 101–111. [DOI] [PubMed] [Google Scholar]
- 31. Lopez-Torres I, Valenza MC, Torres-Sanchez I, et al. Changes in cognitive status in COPD patients across clinical stages. Copd 2016; 13(3): 327–332. [DOI] [PubMed] [Google Scholar]
- 32. Wressle E, Marcusson J, Henriksson C. Clinical utility of the canadian occupational performance measure--swedish version. Can J Occup Ther Revue canadienne d’ergotherapie 2002; 69(1): 40–48. [DOI] [PubMed] [Google Scholar]
- 33. Incalzi RA, Bellia V, Catalano F, et al. Evaluation of health outcomes in elderly patients with asthma and COPD using disease-specific and generic instruments: the salute respiratoria nell’Anziano (Sa.R.A.) study. Chest 2001; 120(3): 734–742. [DOI] [PubMed] [Google Scholar]
- 34. Roncero C, Campuzano AI, Quintano JA, et al. Cognitive status among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2016; 11: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009; 73(21): 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bierman EJ, Comijs HC, Rijmen F, et al. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Mental Health 2008; 12(4): 517–523. [DOI] [PubMed] [Google Scholar]
- 37. Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Ann Rev Clin Psychol 2010; 6: 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by COPD patients. Am J Resp Crit Care 2016; 195(10): 1333–1343. [DOI] [PubMed] [Google Scholar]
- 39. Becker BW, Thames AD, Woo E, et al. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav 2011; 15(8): 1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cleutjens FA, Janssen DJ, Ponds RW, et al. COgnitive-pulmonary disease. BioMed Res Int 2014; 2014: 697825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grant I, Heaton RK, McSweeny AJ, et al. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142(8): 1470–1476. [PubMed] [Google Scholar]
- 42. Dodd JW, Novotny P, Sciurba FC, et al. Executive function, survival, and hospitalization in chronic obstructive pulmonary disease. a longitudinal analysis of the national emphysema treatment trial (NETT). Ann Am Thorac Soc 2015; 12(10): 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dulohery MM, Schroeder DR, Benzo RP. Cognitive function and living situation in COPD: is there a relationship with self-management and quality of life? Int J Chron Obstruct Pulmon Dis 2015; 10: 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sitzer DI, Twamley EW, Jeste DV. Cognitive training in alzheimer’s disease: a meta-analysis of the literature. Acta psychiat Scand 2006; 114(2): 75–90. [DOI] [PubMed] [Google Scholar]
- 45. Fleming JM, Shum D, Strong J, et al. Prospective memory rehabilitation for adults with traumatic brain injury: a compensatory training programme. Brain Injury 2005; 19(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 46. Finlayson M. Multiple sclerosis rehabilitation: from impairment to participation. Boca Raton: CRC Press, 2012. [Google Scholar]
- 47. Aubin G, Chapparo C, Gelinas I, et al. Use of the perceive, recall, plan and perform system of task analysis for persons with schizophrenia: a preliminary study. Aust Occup Ther J 2009; 56(3): 189–199. [DOI] [PubMed] [Google Scholar]
- 48. Gardarsdottir S, Kaplan S. Validity of the arnadottir OT-ADL neurobehavioral evaluation (A-ONE): performance in activities of daily living and neurobehavioral impairments of persons with left and right hemisphere damage. Am J Occup Ther 2002; 56(5): 499–508. [DOI] [PubMed] [Google Scholar]
- 49. Asayama K. [Assessment of motor and process skills (AMPS)]. Nihon Rinsho Japanese J Clin Med 2004; 62(Suppl 4): 17–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Material for Cognitive impairment and clinical characteristics in patients with chronic obstructive pulmonary disease by Fiona A H M Cleutjens, Martijn A Spruit, Rudolf W H M Ponds, Lowie E G W Vanfleteren, Frits M E Franssen, Candy Gijsen, Jeanette B Dijkstra, Emiel F M Wouters, and Daisy J A Janssen in Chronic Respiratory Disease