Abstract
The objective of this study is to evaluate whether a chronic obstructive pulmonary disease (COPD) self-management education program with coaching of a case manager improves patient-related outcomes and leads to practice changes in primary care. COPD patients from six family medicine clinics (FMCs) participated in a 1-year educational program offered by trained case managers who focused on treatment adherence, inhaler techniques, smoking cessation, and the use of an action plan for exacerbations. Health-care utilization, health-related quality of life (HRQL), treatment adherence, inhaler technique, and COPD knowledge were assessed at each visit with validated questionnaires. We also evaluated whether the use of spirometry and the assessment of individual patient needs led to a more COPD-targeted treatment by primary care physicians, based on changes in prescriptions for COPD (medication, immunization, and written action plan). Fifty-four patients completed the follow-up visits and were included in the analysis. The number of unscheduled physician visits went from 40 the year before intervention to 17 after 1 year of educational intervention (p = 0.033). Emergency room visits went from five to two and hospitalizations from two to three (NS). Significant improvements were observed in HRQL (p = 0.0001), treatment adherence (p = 0.025), adequate inhaler technique (p < 0.0001), and COPD knowledge (p < 0.001). Primary care physicians increased their prescriptions for long-acting bronchodilators with/without inhaled corticosteroid, flu immunizations, and COPD action plans in the event patient had an exacerbation. The COPD self-management educational intervention in FMCs reduced unscheduled visits to the clinic and improved patients’ quality of life, self-management skills, and knowledge. The program had a positive impact on COPD-related practices by primary care physicians in the FMCs.
Keywords: COPD, self-management education, health-related quality of life, inhaler technique, treatment adherence, COPD knowledge
Introduction
Self-management interventions in patients with chronic obstructive pulmonary disease (COPD) improve health-related quality of life (HRQL) and reduce the rate of hospital readmissions.1,2 However, self-management education alone is not effective neither it should be recommended. Instead, education together with a written action plan and the coaching of a case manager are beneficial and can be recommended to prevent severe exacerbations in COPD.3
Although supported by the most recent recommendations,4 primary care physicians often have insufficient time and infrastructure to meet the needs for self-management interventions.5,6 As a result, many facets of patient coaching, including motivational communication, reinforcement, and follow-up, cannot be carried out. Furthermore, there is little information regarding the impact of COPD educational interventions on patient-related outcomes and on physician practice changes in the primary care setting.
Recently, the Quebec government has deployed several resources to offer asthma and COPD education services in family medicine clinics (FMCs). As part of its mission, the Quebec Respiratory Health Education Network (QRHEN) has evaluated the use of these resources in formalized programs of asthma and COPD education. Importantly, patients participating in the asthma education program reduced respiratory-related unscheduled medical visits.7
In the present study, we evaluate the implementation of a self-management education program with written action plans and coaching support by trained respiratory therapists for COPD patients in six FMCs. Our primary outcome was patients’ HRQL at 1 year. Secondary patient-related outcomes included the impact of the COPD self-management education program on health-care utilization, self-management skills, and knowledge. Finally, we wanted to determine whether the use of spirometry, together with the assessment of individual patient needs, leads to practice changes in primary care physicians (i.e. a more COPD-targeted treatment plan).
Methods
Patient recruitment and eligibility criteria
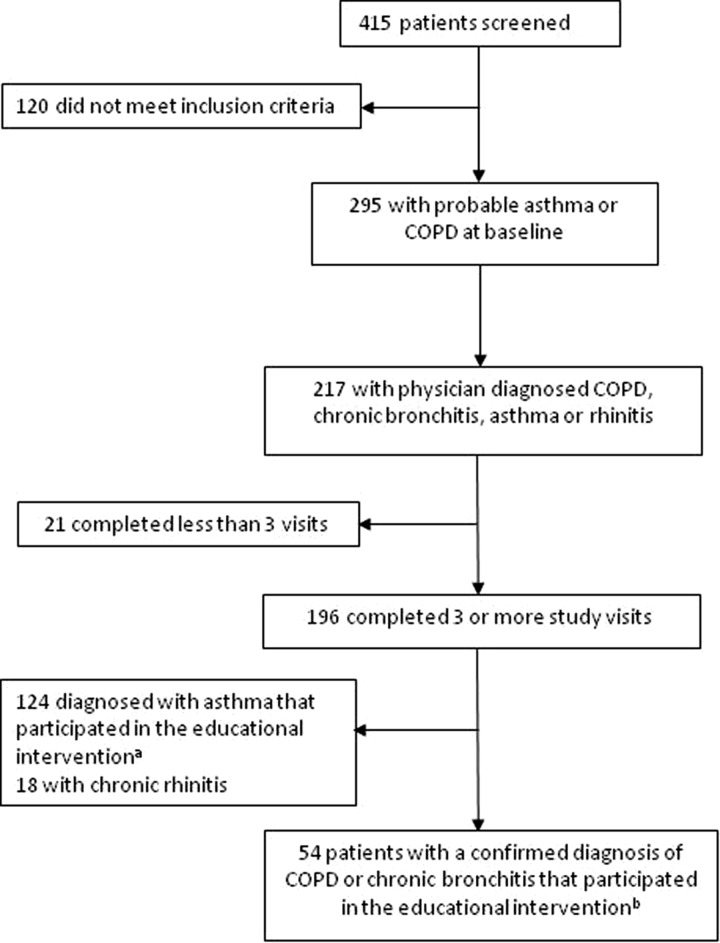
Patients with a probable diagnosis of asthma or COPD were referred to the program by general practitioners from six FMCs in Quebec. The COPD and asthma self-management educational programs were done separately. Results from patients in the asthma component of the program have been reported elsewhere.7 Patients were eligible to participate in the COPD component if they met one of the following criteria: (1) patient with a probable diagnosis of COPD consulting the family doctor due to an exacerbation of symptoms; (2) patients with an uncertain diagnosis of COPD (COPD vs. asthma); (3) patients ≥40 years with a history of smoking and symptoms of chronic bronchitis; and (4) patients to be evaluated for their disease severity. Only patients who signed a consent form and who completed at least three of the four follow-up visits were included. Out of the 415 patients who were initially screened, 295 had a probable diagnosis of COPD or asthma at baseline. The diagnosis of COPD, chronic bronchitis, asthma, or rhinitis was confirmed by primary care physicians for 217 patients at the third visit. Out of the 196 patients who completed at least three visits, 54 were diagnosed with COPD, 124 with asthma, and 18 with rhinitis by their family physicians. Only patients with a physician diagnosis of COPD were included in our analysis (Figure 1). This project received ethics approval and was supervised by a coordinating committee that included representatives from the QRHEN, FMCs, patient groups, health ministry, and private partners.
Figure 1.
Patient recruitment flowchart. (a) Results from the asthma educational intervention reported in the manuscript by Boulet et al.7 (b) Results from the COPD educational intervention reported in this article. COPD: chronic obstructive pulmonary disease.
Self-management education program
The two respiratory therapists involved in the study have been previously trained in asthma and COPD education (5 and 15 years before the intervention) and attended yearly updates offered by the QRHEN. The educator COPD training was based on the Living Well with COPD platform (www.livingwellwithcopd.com).8 Throughout the intervention, respiratory therapists acted as both educators and case managers. The program administered in the FMCs consisted of an initial visit and three follow-ups at 4–6 weeks, 4–6 months, and 1 year. The initial visit lasted around 90 minutes and consisted of an assessment of educational needs, a spirometry, and a 1-hour encounter with the educator (respiratory therapist). During this encounter, the following topics were covered: COPD etiology and pathophysiology, COPD control, smoking cessation, use of a written action plans for acute exacerbations, adequate inhaler technique, and medication adherence.7,9 Follow-up visits lasted around 60 minutes in which pre-bronchodilator spirometries were performed and educational needs were assessed. Based on individual patient needs, specific self-management skills were addressed (exacerbation recognition, action plan use, inhaler technique, among others). Visits took place at each of the FMCs between January 7, 2013, and March 14, 2014. In addition to the education sessions, respiratory therapists acted as case managers, coaching the patients with continuous support throughout the 1-year study period.
Procedures and measurements
Patient characteristics, health-care utilization, and patient-reported outcomes
Baseline characteristics, diagnosis of airflow obstruction, and disease severity
During the initial visit, demographic data including sex, age, body mass index (BMI), previous diagnosis of COPD (COPD, bronchitis, and emphysema), smoking status, and co-morbidities were recorded. At baseline, pre- and post-bronchodilator spirometries were performed to measure forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) values according to the American Thoracic Society (ATS) guidelines.10 Percent of predicted values were calculated using data from the European Respiratory Society for patients ≤65 years and from Knudson for patients >65 years old. The presence of obstructive airway disease was defined as a ratio of FEV1/FVC < 0.7 in post-bronchodilator spirometry. Disease severity was classified as follows: (1) global initiative for chronic obstructive lung disease (GOLD1), FEV1 ≥ 80% of the predicted value; (2) GOLD2, 50% ≤ FEV1 > 80% predicted; (3) GOLD3, 30% ≤ FEV1 > 50% predicted; and (4) GOLD4, FEV1 < 30% of predicted.4 Patients with post-bronchodilator FEV1/FVC values <0.70 with a history of smoking and with symptoms compatible with chronic bronchitis were considered to be at risk to develop COPD.11
Health service utilization and treatment use
Data concerning emergency room (ER) visits, hospital admissions, and unscheduled physician visits to the clinic for the year prior to enrollment were self-reported by patients. The use of antibiotics and oral corticosteroids during the year prior to the study was self-reported and verified with pharmacy records. Throughout the study, health-care utilization was reported at each visit as the number of ER visits, hospital admissions, and unscheduled visits to the clinic since the last visit. Antibiotic and oral corticosteroid use was reported as the number of treatments per patient since the last visit.
Patient reported outcomes
Health-related quality of life (HRQL) was assessed at baseline and at each follow-up visit with the COPD assessment test (CAT). This brief 8-item questionnaire provides a reliable and valid measure of the health status of COPD patients.12,13 The lower the score, the lower the impact of COPD on patients’ quality of life, with scores below 10 indicating a low impact, scores between 10 and 20 a moderate impact, and scores above 20 a high impact of COPD. In addition to measuring the general impact of COPD with the CAT, the disability associated with dyspnea was evaluated using the Medical Research Council scale.14
Patient self-management skills and knowledge (process measures)
Inhaler technique
This evaluation was performed at baseline and at each follow-up visit by the respiratory therapist with different placebo devices of different types of inhalers filling a checklist of steps based on current guidelines. Patients were asked to use their inhalers as they normally did at home. Technique was qualified as good if no error was made during demonstration and as corrected if one or more errors were made.
Treatment adherence
This was assessed at baseline and at each follow-up visit by the respiratory therapist based on a questionnaire in which patients were asked about their current respiratory medication, its use, and frequency. This information was then compared to patients’ pharmacy records to determine whether or not patients filled and use their prescriptions as indicated. Treatment adherence categories were qualified as good (missing maintenance medication once per week or less), partial (missing doses 2–3 days per week), or inadequate (not taking medication ≥4 days per week).
COPD knowledge
Knowledge was assessed at baseline and at each follow-up with the shortened version of the Bristol COPD Knowledge Questionnaire (BCKQ). The original version consists of 65 questions for which there is a right or wrong answer. This questionnaire has demonstrated good consistency and test–retest reliability and has been used to evaluate the impact of COPD-specific education on primary care.15,16 For the present study, we used a shortened version of 34 questions covering the following topics: (1) general concepts and epidemiology, (2) etiology, (3) common symptoms, (4) dyspnea, (5) respiratory infections and exacerbations, (6) smoking and vaccination, and (7) pharmacological treatment. Items are scored +1 if the correct answer is given and 0 otherwise. A score of 34 corresponds to 100% of correct answers. The shortened version of the Bristol Questionnaire was adapted by the QRHEN from the original document with permission from the authors.15
Smoking state of change
This was assessed at baseline and at each follow-up visit with a 7-point scale based on Prochaska’s model.17,18 This evaluates patients’ level of readiness to quit smoking.
Physician practice changes
Practice changes in physicians of each of the FMCs were compared at baseline (patient enrollment) and at 1 year. This was done to determine whether an increased awareness of individual patient needs impacts the clinical practice of primary care physicians. We focused in comparing changes in treatment that best align with the most recent recommendations in COPD care4 including (1) prescription of inhaled long-acting bronchodilators with or without inhaled corticosteroids (maintenance medication); (2) prescription of a rescue bronchodilator, antibiotic, and/or oral corticosteroid to be used with a written action plan to manage exacerbations; and (3) vaccination against pneumococcus and seasonal influenza.
Statistical analysis
Continuous numerical data across different GOLD categories at baseline were compared with univariate analysis of variance for normally distributed variables or the Kruskal–Wallis test for not normally distributed data. Categorical data between GOLD categories at baseline were compared using Fisher’s exact test.
Continuous numerical data at baseline and at 1-year follow-up were compared with paired t-tests for normally distributed data and with Wilcoxon’s rank signed test for data not following the normal distribution. McNemar’s test was used to compare categorical data 1 year before and 1 year after the first visit. Treatment adherence categories at baseline visit and at 1-year follow-up were compared with Bowker’s test of symmetry.
Generalized estimating equation (GEE) Poisson models were used to compare categorical outcomes across time (treatment adherence, smoking state of change, and inhaler technique). Continuous numerical outcomes across time were compared with repeated-measures general mixed-models followed by Bonferroni’s posttest.
Results were considered to be statistically significant if p values were ≤0.05. Data were analyzed using SAS v9.4 software.
Results
Patients’ characteristics
Table 1 presents the characteristics of the study patients from the six FMCs at baseline for all the patients as well as stratified by disease severity. Fifty-four patients (32 male and 22 female) aged 66.2 ± 9 years were included in the analysis. No significant differences in age, sex, BMI, smoking status, dyspnea scores, comorbidities, and medication use are observed between the different disease severity groups. CAT scores, post-bronchodilator spirometry results, and patients with one or more visits to the ER were significantly different among disease severity groups. Notably, 13 patients (24.1%) did not have evidence of airway obstruction (FEV1/FVC ratio < 0.70) following post-bronchodilator spirometry. However, these patients had an important history of smoking and symptoms compatible with chronic bronchitis and were therefore considered to be at risk to develop COPD and retained in the study.
Table 1.
Patient characteristics at baseline for all the patients and according to the GOLD disease severity scale.a,b
| Variable | Total | At risk for COPD | GOLD1 | GOLD2 | GOLD3+ | |
|---|---|---|---|---|---|---|
| n = 54 | n = 13 (24.1%) | n = 5 (7.4%) | n = 25 (46.3%) | n = 11 (20.4%) | p-Value | |
| Age (years) | 66.2 ± 9.0 | 63.5 ± 8.3 | 60.4 ± 7.2 | 68.2 ± 9.1 | 67.4 ± 9.3 | 0.207 |
| Sex (male) | 32 (59.3) | 6 (11.1) | 4 (7.4) | 13 (24.7) | 9 (16.6) | 0.21 |
| BMI | 27.5 ± 5.7 | 28.9 ± 5.2 | 27.8 ± 5.6 | 27.9 ± 5.4 | 24.8 ± 6.8 | 0.23 |
| Smoking status | ||||||
| Never smoked | 2 (3.7) | 1 (1.9) | 0 (0) | 1 (1.9) | 0 (0) | 1 |
| Current smoker | 32 (59.3) | 9 (16.7) | 30 (5.6) | 15 (27.8) | 5 (9.3) | 0.67 |
| Smoking pack-years | 44.2 ± 18.8 | 46.75 ± 21.6 | 37.2 ± 25.3 | 40.6 ± 17.4 | 50.38 ± 16.2 | 0.82 |
| MRC | 2.5 ± 1.4 | 2.4 ± 1.6 | 1.6 ± 0.9 | 2.6 ± 1.3 | 2.8 ± 1.4 | 0.321 |
| MRC ≥ 3 | 21 (38.8) | 5 (9.7) | 1 (1.9) | 9 (16.7) | 6 (11.1) | 0.59 |
| CAT | 15.1 ± 6.8 | 10.5 ± 5.2 | 12.6 ± 5.5 | 16.1 ± 6.7 | 19.5 ± 6.3 | 0.01b |
| Post-bronchodilator spirometry | ||||||
| FEV1/FVC | 60.1 ± 13.2 | 76.5 ± 7.4 | 64.5 ± 5.1 | 58.1 ± 7.6 | 43.4 ± 6.0 | <0.001b |
| FEV1 (L) | 1.6 ± 0.6 | 1.9 ± 0.6 | 2.5 ± 0.4 | 1.6 ± 0.5 | 1.1 ± 0.3 | <0.001b |
| FEV1 (% of predicted) | 65.5 ± 20.3 | 75.1 ± 21.5 | 95.8 ± 18.7 | 65.5 ± 9.0 | 40.6 ± 5.4 | <0.001b |
| Co-morbidities | ||||||
| Allergies | 20 (37.4) | 4 (7.4) | 3 (5.6) | 11 (20.4) | 2 (3.7) | 0.33 |
| Asthma | 16 (29.6) | 3 (5.6) | 1 (1.9) | 10 (18.5) | 2 (3.7) | 0.59 |
| Diabetes | 9 (16.7) | 3 (5.6) | 0 (0) | 6 (11.1) | 0 (0) | 0.2627 |
| Cardiovascular disease | 18 (33.3) | 2 (3.7) | 1 (1.9) | 12 (22.2) | 3 (5.6) | 0.21 |
| GERD | 17 (31.5) | 4 (7.4) | 2 (3.7) | 10 (18.5) | 1 (1.9) | 0.29 |
| Medication usec | ||||||
| Antibiotic | 22 (40.7) | 5 (9.3) | 2 (3.7) | 9 (16.6) | 6 (11.11) | 0.79 |
| Oral corticosteroid | 11 (21.2) | 1 (1.9) | 0 (0) | 6 (11.5) | 4 (7.7) | 0.31 |
| Health-care utilizationd | ||||||
| Hospitalization | 2 (3.7) | 0 (0) | 0 (0) | 0 (0) | 2 (3.7) | 0.083 |
| ER visits | 3 (5.6) | 0 (0) | 0 (0) | 0 (0) | 3 (5.6) | 0.017b |
| Patients with ≥1 unscheduled visits | 22 (40.7) | 4 (7.4) | 3 (5.6) | 10 (18.5) | 5 (9.3) | 0.699 |
GOLD: global initiative for chronic obstructive lung disease; BMI: body mass index; MRC: Medical Research Council; CAT: COPD assessment test; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; GERD: gastroesophageal reflux disease; ER: emergency room; SD: standard deviation.
aValues are expressed as mean ± SD or as n (%).
bDifferences between the disease severity groups are considered significant if p values are <0.05.
cResults presented as the number of patients (%) with self-reported antibiotic or oral corticosteroid treatments in the year prior to the study.
dResults presented as the number of patients (%) with self-reported hospitalizations, ER visits, and/or one or more unscheduled visits to the clinic in the year prior to the study.
Changes in outcomes 1 year after the self-management education
A comparison of changes in health service utilization, lung function, and patient reported outcomes at baseline/1 year before the intervention and 1 year after the intervention is presented in Table 2. Overall, the educational intervention resulted in a significant reduction in the number of unscheduled visits to FMCs. ER visits were reduced but not statistically significant and hospitalizations were not reduced although these were very infrequent. We also observed an improvement of pre-bronchodilator FEV1 values at 1-year follow-up. The number of supplementary antibiotic treatments was significantly reduced; however, we did not observe a decrease in oral corticosteroid treatments. Symptom burden was not different but HRQL significantly improved throughout the education program (Figure 2).
Table 2.
Changes in COPD outcomes after 1 year of self-management educational intervention (n = 54).
| Baseline/1 year before the intervention | 1-year follow-up | p-Values | |
|---|---|---|---|
| Health-care utilization and treatment use | |||
| Unscheduled visits to the clinica | 40 | 17 | 0.033b |
| ER visitsa | 5 | 2 | 0.64 |
| Hospitalizationsa | 2 | 3 | 0.99 |
| Supplementary antibiotic treatmentsc | 22 | 14 | 0.033b |
| Supplementary oral corticosteroid treatmentsc | 11 | 9 | 0.48 |
| Spirometry valuesd | |||
| FEV1 (L) | 1.54 ± 0.57 | 1.63 ± 0.59 | 0.0029b |
| FVC (L) | 2.6 ± 0.85 | 2.68 ± 0.86 | 0.21 |
| FEV1/FVC (%) | 59.25 ± 12.07 | 60.92 ± 12.01 | 0.067 |
| Patient reported outcomes | |||
| MRC dyspnea ≥ 3 | 21 | 17 | 0.24 |
| CAT score | 15 ± 6.8 | 11 ± 6.4 | 0.0001b |
ER: emergency room; CAT: COPD assessment test; MRC: Medical Research Council; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity.
aResults presented as the total number of events for the year prior to the intervention and at 1-year follow-up.
bDifferences are considered to be statistically significant if p < 0.05.
cResults presented as the number of patients with self-reported antibiotic or oral corticosteroid treatments (1 or more) in the year prior to the study (baseline) and during the intervention (1-year follow-up).
dPre-bronchodilator spirometry values.
Figure 2.
Changes in patients’ HRQL throughout the intervention. CAT score values for each visit are presented in box plots as follows: whiskers, minimum and maximum values, respectively; bottom border, first quartile; horizontal line within box plots, median; diamonds within box plots, mean; upper border, third quartile; circles, outliers. Data were analyzed with mixed linear models with repeated measures (classed by patient ID and visit) followed by Bonferroni’s posttest to detect differences between follow-ups; *p < 0.05, ***p < 0.001. Lower values reflect a lower impact of COPD on patients’ HRQL. HRQL: health-related quality of life; CAT: COPD assessment test.
Patient skills and adherence (process measures)
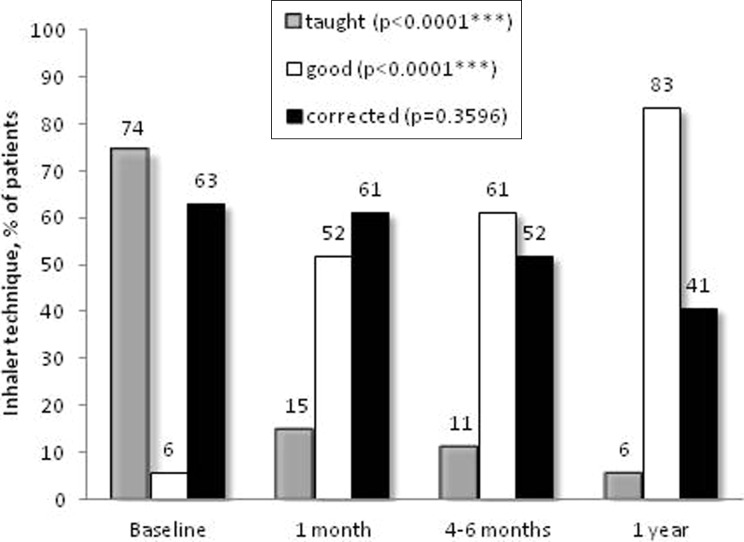
The education program significantly improved patients’ self-management skills and treatment adherence. Regarding inhaler skills, most patients initially on a metered dose inhaler (MDI) were switched and taught the appropriate technique for an MDI with aerochamber (data not shown). Ten patients were taught the appropriate use of the Turbuhaler® device, 2 patients of the Diskus® device, 19 patients the Handihaler®, and 4 patients the Breezhaler® at the initial visit. Three-quarter of patients needed to be taught at least one inhaler device utilization technique at the initial visit (Figure 3). Only three patients (6%) had a good inhaler technique at baseline and this number significantly increased to 45 (83%) at 1-year follow-up (Figure 3). Good adherence to the inhalers increased from 1/3 to 3/4 of the participants at the end of the intervention (4–6 weeks) and that was maintained at long term, that is, 4–6 months and 1 year (Figure 4).
Figure 3.
Inhaler technique. The proportion of patients belonging to each inhaler technique category at each visit is presented as follows: one or more techniques taught, gray bars; good technique for one or more devices, white bars; technique requiring corrections for one or more devices; black bars. Data for each inhaler technique category throughout the study were analyzed with Poisson regression models; ***p < 0.001.
Figure 4.
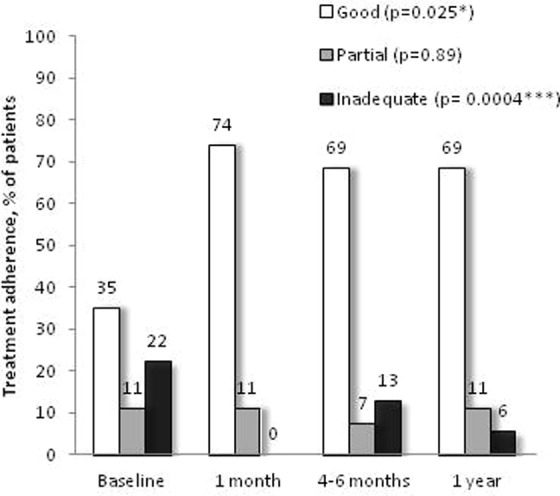
COPD treatment adherence. The proportion of patients in each treatment adherence category at each visit is presented as follows: good adherence, white bars; partial adherence, gray bars; inadequate adherence, black bars. Data for each adherence category throughout the study were analyzed with Poisson regression models; *p < 0.05, ***p < 0.001. COPD: chronic obstructive pulmonary disease.
COPD-specific knowledge also significantly improved after the intervention since mean scores of the modified BCKQ increased from 16.8 (49.5% correct answers) at baseline to 20.5 (60.2% correct answers) at 1-year follow-up (Figure 5). The mean scores steadily increased throughout the intervention, with significant differences observed between baseline and 1 month, 4–6 months, and 1-year follow-ups.
Figure 5.
COPD knowledge. Modified BCKQ scores are presented as percent of correct answers for each visit in box plots as follows: whiskers, minimum and maximum values, respectively; bottom border, first quartile; horizontal line within box plots, median; diamonds within box plots, mean; upper border, third quartile. Data were analyzed with mixed linear models with repeated measures (classed by patient ID and visit) followed by Bonferroni’s posttest to detect differences between follow-ups; ***p < 0.001. COPD: chronic obstructive pulmonary disease; BCKQ: Bristol COPD knowledge questionnaire.
Thirty-two patients were active smokers at the beginning of the study (Table 1). By the end of the study, only two patients successfully quit smoking. Although there were variations among the proportion of patients in the different state of change categories throughout the study, these changes were not statistically significant (Figure 6).
Figure 6.
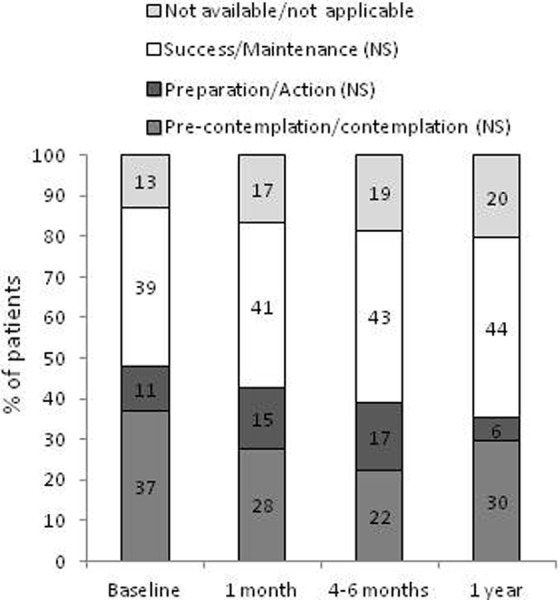
Smoking state of change. The distribution of patients belonging to different smoking state of change categories at each visit is shown in the different boxes within each bar (from bottom to top: pre-contemplation/contemplation; preparation/action; success/maintenance and not available/not applicable). Data for each smoking state of change category throughout the study were analyzed with Poisson regression models. NS: nonsignificant.
Primary care physician practice changes
Table 3 presents the changes in COPD treatment made by primary care physicians in FMCs. This educational intervention significantly impacted COPD management by primary care physicians. This is evidenced by the significant increase in the number of prescriptions for short-acting beta agonist (SABA), long-acting beta agonist (LABA), long-acting anti-cholinergic (LAAC), and inhaled corticosteroid (ICS) + LABA treatments. Additionally, the number of patients receiving vaccination against seasonal flu and Streptococcus pneumoniae significantly increased by the end of the intervention. Finally, the number of patients with a written action plan (in most cases with a prescription for rescue bronchodilator and only a few patients with standing prescriptions for antibiotics and oral corticosteroid) went from 0 before the intervention to 35 at the end of the study.
Table 3.
Primary care physician practice changes after 1-year follow-up.
| Baseline | One-year follow-up | p-Values | |
|---|---|---|---|
| Pharmacological treatment | |||
| SABA | 27 | 37 | 0.0016a |
| LABA | 2 | 10 | 0.0047a |
| SAAC | 1 | 0 | 0.99 |
| LAAC | 20 | 48 | <0.0001a |
| ICS | 11 | 6 | 0.0253a |
| ICS + LABA | 20 | 33 | 0.0003a |
| Vaccination | |||
| Vaccine against influenza | 28 | 43 | 0.0006a |
| Vaccine against S. pneumoniae | 22 | 24 | 0.025a |
| Written action plan | 0 | 35 | <0.0001a |
SABA: short-acting beta agonist; LABA: long-acting beta agonist; SAAC: short-acting anti-cholinergic; LAAC: long-acting anti-cholinergic; ICS: inhaled corticosteroid.
aDifferences are considered to be statistically significant if p < 0.05.
Discussion
Summary of main findings
Our study evaluated the effects of a COPD self-management educational intervention (Living Well with COPD) on patient and health-care-related outcomes and on COPD management by primary care physicians. Even though patients had more scheduled visits to the clinic as a result of the education program, the number of yearly respiratory-related unscheduled visits to the clinic significantly decreased when comparing to the year prior to the intervention. Similarly, we observed a significant decrease in supplementary antibiotic treatments. These reductions in acute care use and supplementary treatments may reflect an improvement in the way health care is delivered to patients, which in turn results in a better control of their COPD. This notion is further supported by the significant improvement in patients’ HRQL. Patients also learned how to appropriately use inhaler devices and improved their techniques throughout the study and the number of patients who were adherent to their maintenance respiratory medication significantly increased. Moreover, this educational intervention significantly improved patients’ understanding of their COPD. The challenge remains with smoking cessation since only few patients tried to quit smoking and only two patients successfully quit at 1 year.
This intervention also had a significant impact on COPD management by primary care physicians. This is evidenced by the increase in prescriptions for SABAs, LABAs, LAACs, and ICS + LABAs that are recommended in symptomatic patients or those who have a history of exacerbation.4 As well, there was an increase in immunizations against seasonal flu and S. pneumoniae. Finally, primary care doctors were more at ease with prescribing COPD written action plans, the great majority including a prescription for rescue bronchodilator and instructing patients to consult a health-care professional during exacerbations.
Our study in the context of existing literature
Currently, COPD care is transitioning from the traditional model of patient care, in which disease-related decision-making is completely assumed by the physician, to a patient-centered model in which physicians, patients, and health-care professionals are all partners in the health-related decision-making process.19,20 Patient-centered interventions aim to achieve behavioral changes in which patients will apply a set of skills to better control their disease.21 In our study, we observed improvements in COPD knowledge which are relevant since poor health literacy has been associated with poor outcomes.22 However, knowledge alone is insufficient to affect patient behavior.21 In a previous study, we have systematically evaluated the impact of case manager coaching on health-related behaviors, particularly COPD action plan adherence. We were able to show that patients’ self-management skills improved progressively throughout the intervention. This, in turn, resulted in a reduction of acute care use in a tertiary care center, with patients with moderate to severe disease.23 With the present study, we have demonstrated that, similar to what was observed in specialized respiratory clinics,23 educational interventions with the coaching of a case manager in primary care clinics can also improve self-management skills and reduce acute care use.
Unfortunately, the intervention was not successful in changing patients’ attitude toward smoking. Although disappointing and despite evidence to support very brief interventions,24 this could be due to the fact that a different approach to counseling is required to impact patients’ level of readiness to quit smoking.25
Finally, the comprehensive evaluation of patient health status and individual needs impacted the practice of primary care physicians. Even though it could be argued that these changes might reflect overtreatment of the disease, evidence supports the use of long-acting bronchodilators, immunization, and the use of COPD action plans to improve HRQL and to reduce acute care use in COPD.26–28 Taking the latter factors into consideration, we consider that primary care practice changes in our study reflect an improvement in COPD treatment that better aligns with the most recent recommendations.
Strengths and limitations of the study
The main strength of the study is that it represents findings from a “real-life” intervention in mild to moderate COPD patients in a family medicine setting. The study design enabled us to assess how COPD is managed by family physicians in primary care. The diagnosis of COPD in subjects without spirometric evidence of airflow obstruction is common in primary care.29 As such, we consider that our results reflect the reality of COPD management in primary care and further support the importance of the use and appropriate interpretation of spirometry in the diagnosis of COPD. On the other hand, we have demonstrated that education programs by trained health-care professionals, acting as case manager, are feasible to implement in FMCs. Furthermore, the frequent follow-ups allowed to monitor changes in COPD self-management skills and knowledge over time and to determine the impact of these changes on health-related outcomes. Another important strength of this study is the evaluation of process measures such as treatment adherence, inhaler technique, COPD knowledge, and physician practice changes, which are key to determine if the intervention was properly constructed to impact the desired outcomes. This will allow for improvements in design and implementation of future educational self-management interventions in similar settings or to make appropriate adaptations as needed.
The main limitations of the study are mainly related to its design. First, the diagnosis of COPD by family physicians was not always based on post-bronchodilator spirometries, which resulted in the inclusion of 13 patients who did not have COPD according to the GOLD criteria. However, these patients had an important history of smoking and symptoms compatible with chronic bronchitis (chronic cough with sputum production) that moderately impacted their quality of life and were therefore considered to be at risk to develop COPD.11 We thought that these patients would benefit from the educational intervention particularly for smoking cessation, COPD awareness, and healthy habits. Another limitation of our study was the lack of randomization and of a control group; therefore, the effects of the intervention could not be compared to those of regular care. Finally, we acknowledge the potential for recall bias, particularly for the baseline visit in which patients might have underestimated their medication and acute health-care use in the year prior to the study and might have been more precise in recalling medication and health-care utilization during the study because visits were more frequent. Notwithstanding the latter limitations, our results demonstrate that integrated care can improve outcomes of COPD patients in primary care.
Implications for clinical practice
In conclusion, the educational self-management intervention in FMCs was very successful in improving patient and health-care professional-related outcomes. Results from this real-life study should encourage and facilitate the implementation of similar self-management educational programs with coaching of trained health-care professionals, acting as case manager, in FMCs treating COPD patients across Canada. This in turn should allow for further evaluation of such programs to determine the cost–benefit and long-term impact of patient self-management education on COPD.
Acknowledgements
The authors thank the six family medicine clinics (GMF Ancienne-Lorette/Les Saules, GMF Beauport, GMF Charlesbourg, GMF Cité Verte, GMF Duberger, and GMF Quatre-Bourgeois).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Canada, Novartis and Takeda.
References
- 1. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 3: CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casas A, Troosters T, Garcia-Aymerich J, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J 2006; 28(1): 123–130. [DOI] [PubMed] [Google Scholar]
- 3. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American college of chest physicians and Canadian thoracic society guideline. Chest 2015; 147(4): 894–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Report 2017, Global Initiative for Chronic Obstructive Lung Disease, inc.
- 5. Yarnall KS, Østbye T, Krause KM, et al. Family physicians as team leaders: “time” to share the care. Prev Chronic Dis 2009; 6(2): A59. [PMC free article] [PubMed] [Google Scholar]
- 6. Peterson LE, Phillips RL, Puffer JC, et al. Most family physicians work routinely with nurse practitioners, physician assistants, or certified nurse midwives. J Am Board Fam Med 2013; 26(3): 244–245. [DOI] [PubMed] [Google Scholar]
- 7. Boulet LP, Boulay M, Gauthier G, et al. Benefits of an asthma education program provided at primary care sites on asthma outcomes. Respir Med 2015; 109(8): 991–1000. [DOI] [PubMed] [Google Scholar]
- 8. Living Well With COPD. www.livingwellwithcopd.com/en/home.html (accessed 19 April 2017).
- 9. Boulet LP, Dorval E, Labrecque M, et al. Towards excellence in asthma management: final report of an eight-year program aimed at reducing care gaps in asthma management in Quebec. Can Respir J 2008; 15(6): 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease. Report. 2016, update 2016, Global Initiative for Chronic Obstructive Lung Disease, inc. [Google Scholar]
- 11. Guerra S, Sherrill DL, Venker C, et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009; 64(10): 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34(3): 648–654. [DOI] [PubMed] [Google Scholar]
- 13. Jones P, Harding G, Wiklund I, et al. Improving the process and outcome of care in COPD: development of a standardised assessment tool. Prim Care Respir J 2009; 18(3): 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FLETCHER CM. The clinical diagnosis of pulmonary emphysema: an experimental study. Proc R Soc Med 1952; 45(9): 577–584. [PubMed] [Google Scholar]
- 15. White R, Walker P, Roberts S, et al. Bristol COPD knowledge questionnaire (BCKQ): testing what we teach patients about COPD. Chron Respir Dis 2006; 3(3): 123–131. [DOI] [PubMed] [Google Scholar]
- 16. Hill K, Mangovski-Alzamora S, Blouin M, et al. Disease-specific education in the primary care setting increases the knowledge of people with chronic obstructive pulmonary disease: a randomized controlled trial. Patient Educ Couns 2010; 81(1): 14–18. [DOI] [PubMed] [Google Scholar]
- 17. Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol 1994; 13(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 18. Rustin TA, Tate JC. Measuring the stages of change in cigarette smokers. J Subst Abuse Treat 1993; 10(2): 209–220. [DOI] [PubMed] [Google Scholar]
- 19. Roberts NJ, Kidd L, Dougall N, et al. Measuring patient activation: the utility of the patient activation measure within a UK context-Results from four exemplar studies and potential future applications. Patient Educ Couns 2016; 99(10): 1739–1746. [DOI] [PubMed] [Google Scholar]
- 20. Tiedeman ME, Lookinland S. Traditional models of care delivery: what have we learned? J Nurs Adm 2004; 34(6): 291–297. [DOI] [PubMed] [Google Scholar]
- 21. Bourbeau J, Lavoie KL, Sedeno M. Comprehensive self-management strategies. Semin Respir Crit Care Med 2015; 36(4): 630–638. [DOI] [PubMed] [Google Scholar]
- 22. Omachi TA, Sarkar U, Yelin EH, et al. Lower health literacy is associated with poorer health status and outcomes in chronic obstructive pulmonary disease. J Gen Intern Med 2013; 28(1): 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bourbeau J, Saad N, Joubert A, et al. Making collaborative self-management successful in COPD patients with high disease burden. Respir Med 2013; 107(7): 1061–1065. [DOI] [PubMed] [Google Scholar]
- 24. Dawson GM, Noller JM, Skinner JC. Models of smoking cessation brief interventions in oral health. N S W Public Health Bull 2013; 24(3): 131–134. [DOI] [PubMed] [Google Scholar]
- 25. Mulhall P, Criner G. Non-pharmacological treatments for COPD. Respirology 2016; 21(5): 791–809. [DOI] [PubMed] [Google Scholar]
- 26. Bourbeau J. Disease-specific self-management programs in patients with advanced chronic obstructive pulmonary disease. A comprehensive and critical evaluation. Dis Manage Health, 2003. pp. 311–319. [Google Scholar]
- 27. Bourbeau J, Collet JP, Schwartzman K, et al. Economic benefits of self-management education in COPD. Chest 2006; 130(6): 1704–1711. [DOI] [PubMed] [Google Scholar]
- 28. Gadoury MA, Schwartzman K, Rouleau M, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005; 26(5): 853–857. [DOI] [PubMed] [Google Scholar]
- 29. Gershon AS, Hwee J, Chapman KR, et al. Factors associated with undiagnosed and overdiagnosed COPD. Eur Respir J 2016; 48(2): 561–564. [DOI] [PubMed] [Google Scholar]