Abstract
Quitting smoking is the most important element in the therapeutic management of chronic respiratory diseases. Combining pharmacotherapy with behavioral support increases smoking cessation success rates. In addition, hospitalized smokers have increased motivation to quit. We investigated the efficacy on smoking cessation, of varenicline in combination with behavioral support, in smokers hospitalized due to (a) acute exacerbation of chronic obstructive pulmonary disease (COPD), or (b) bronchial asthma attack, or (c) community-acquired pneumonia (CAP). The method used is prospective, open-label, preference-based, parallel group, 52-week trial. Patients chose the smoking cessation intervention they preferred: a standard regimen of varenicline combined with post-discharge advanced behavioral support (group A) or one private consultation session during hospitalization (group B). Follow-up phone calls were scheduled in weeks 1, 2, and 4 and months 3, 6, and 9. The final hospital visit was performed in week 52. Primary outcome was success rate defined as the percentage (%) of smoking abstinence at week 52 and secondary outcomes were (a) changes in quality of life (QoL) indicated by the scores on the Short Form 36 (SF36) questionnaire and (b) predictors of smoking abstinence investigated with multiple binary logistic regression. One hundred one patients were enrolled, 44 (43.6%) in group A and 57 (56.4%) in group B. Respective abstinence rates were 54.5% and 15.8% at week 12 and 52.3% and 14.0% at week 52. Scores on SF36 were statistically significantly increased in both groups. Predictors of smoking abstinence were varenicline (odds ratio (OR) 7.29; 95% confidence interval (CI) 2.15, 24.77; p = 0.001), age (OR 1.07; 95%CI 1.00, 1.15; p = 0.042), Fagerstrom score (OR 0.37; 95%CI 0.20, 0.68; p = 0.001), SF36 domains “vitality” (OR 1.12; 95%CI 1.04, 1.21; p = 0.003), and “social functioning” (OR 0.95; 95%CI 0.90, 1.00; p = 0.041). Varenicline in combination with behavioral support resulted in high abstinence rates inpatients hospitalized for exacerbation of COPD, asthma attack, or CAP, and improved QoL.
Keywords: Cessation, smoking, varenicline, COPD, asthma, pneumonia
Introduction
Tobacco smoking kills six million people around the world every year and is therefore the leading cause of preventable death.1,2 Greece has one of the highest prevalence rates for smoking, with 53.8% of men and 33.5% of women according to the WHO age-standardized estimate for 2013 in people older than 15 years.1,2 Every year, tobacco kills more than 25,400 people in Greece, and in 2010, 29.5% of deaths in men and 11.2% in women were caused by tobacco, the highest rates among other high-income countries.3,4
Tobacco is the major modifiable risk factor for noncommunicable diseases, that is, cardiovascular diseases, cancer, and chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma.1 Smoking causes symptoms to worsen, a rapid decline in pulmonary function, increased admissions to hospitals with prolonged hospitalizations, and death.5–10 For smokers, quitting smoking is the most important element in the therapeutic management of these conditions.5,6,11
A period of hospitalization offers the opportunity to encourage patients to stop.12–15 Hospitalization is “the ideal teachable moment”16 for smokers hospitalized for smoking-specific disease and shortens the time to quit because patients cannot smoke during their stay in hospital premises. Post-hospitalization follow-up support is an important component of counseling interventions and increases the smoking cessation rate after 12 months by 37%.12
A Cochrane meta-analysis of pharmacological interventions for smoking cessation showed that varenicline was superior to single forms of nicotine replacement therapy (odds ratio (OR) 1.57; 95%confidence interval (CI) 1.29,1.91) and to bupropion (OR 1.59; 95%CI 1.29, 1.96).17 A latest review of randomized controlled studies in smokers with COPD showed that pharmacotherapy plus high-intensity behavioral treatment was effective in helping these patients to quit smoking.18 Furthermore, combining pharmacotherapy with behavioral support increases smoking cessation success by 70–100% at 6 months compared to brief advice or support19 and by an additional 10–25% when increasing the amount of behavioral support by in-person or telephone contacts.20
Most data are derived from studies in healthy smokers and studies in patients with smoking-specific disease are scarce. In addition, current research needs include (a) the discovery and evaluation of the most effective combination of pharmacotherapy and behavioral support and (b) increasing rates of long-term smoking abstinence (i.e. beyond 6 months).21
Aims
The primary objective of the present study was to record smoking cessation rates induced by varenicline in combination with advanced behavioral support post-discharge in smokers hospitalized for exacerbation of COPD, bronchial asthma, or pneumonia and to compare this with the success rate of one private consultation session during hospitalization. The second aim was to record any changes in quality of life (QoL) assessed through the comparison of scores obtained on the domains of the Short Form 36 (SF36) questionnaire and possible predictors of successful smoking abstinence.
Methods
This open-label, nonrandomized, preference-based, prospective study was conducted between May 9,2012 and May 27,2015. Patients screened for eligibility were all the smokers who were hospitalized in the First Pulmonology Clinic of Kavala General Hospital from May 2012 to May 2014. Eligible patients, who agreed to participate and provided written informed consent, were assigned to two groups: those who wished to follow the “pharmacotherapy and behavioural support” (group A) and those who denied pharmacotherapy and agreed to participate in one consultation/advice session (group B). Patients in both groups quit smoking while they were still hospitalized and on the first day that followed the initial counseling session, which coincided with the day one of varenicline treatment in group A. Patients were followed up for 52 weeks after cessation. This study was approved by the Scientific Committee of the Kavala General Hospital, Greece (3/20-1-14).
Study outcomes
The primary outcome was the success rate defined as the percentage (%) of smoking abstinent patients at week 52. Abstinence is defined by exhaled carbon dioxide (CO) level of less than 9 parts per million (ppm).22 Secondary outcomes were (a) changes in QoL assessed through the comparison of scores obtained on the domains of the SF36 questionnaire and (b) possible predictors of successful smoking abstinence investigated with a multiple binary logistic regression.
Patient eligibility
Eligible patients were adult smokers (>100 cigarettes in their lifetime) and hospitalized due to either (a) acute exacerbation of COPD, or (b) acute exacerbation of bronchial asthma, or (c) community-acquired pneumonia. Only eligible patients who agreed to participate and provided written informed consent were recruited.
Interventions and assessments
Patients in both groups had an initial private consultation session and motivational interview while still in the hospital by the chest physician. This interview lasted for at least 60 minutes. We assessed tobacco use and readiness to quit via the 5A’s method: Ask about tobacco use, Advise to quit, Assess willingness to quit, Assist toward a successful quit attempt, and Arrange follow-up.23 We recorded the patients’ smoking history, as pack-years and start age. The degree of dependence was evaluated using the Fagerstrom Test, in which higher scores indicate higher nicotine dependence.24 We listen without judging patients’ concerns about quitting smoking. We informed them about all potential health risks of tobacco use and the benefits of quitting and we encouraged them to quit. To assess willingness to quit, patients rated from 1 (lowest degree of) to 10 (highest degree) their determination, readiness, and preparedness to quit. At this initial interview, the study investigator provided full information about the pharmacological properties of varenicline, efficacy and safety data, route of administration, dosage, and treatment duration. Exhaled CO was measured to determine the initial (and the final) smoking status and SF36 questionnaire was completed. Following this interview, patients decided whether they wanted to participate in group A or B and then gave written informed consent. All patients were advised to quit smoking on the next day to derive maximum benefit from the motivational interview, because they were still in hospital where smoking was not allowed. The schedule and type of the follow-up contacts and support was explained to the patients in detail.
The next day, patients in group A started varenicline treatment while still in hospital and they continued after their discharge. Follow-up phone calls with a minimum duration of 10 minutes were scheduled in weeks 1, 2, and 4 and months 3,6, and 9 following smoking cessation. The phone calls were used to assess the smoking cessation program and clarify any problems, adverse events, and treatment-related questions that the patients may have had.
Patients in group B were contacted by phone call in weeks 1, 2, and 4 and months 3, 6, and 9 to record adverse events and whether they were still abstinent.
Patients in both groups were asked to return to hospital in month 12 (week 52) for a final assessment. At this last visit, the SF36 was again completed and exhaled CO was measured.
Study medication
Varenicline was given for 12 weeks to all patients in group A. The initial dose was 0.5 mg/day for days 1–3 and was then up-titrated to 0.5 mg on days 4–7 and finally to 1 mg twice daily from day 8 until the end of treatment. All medication was dispensed free of charge to study participants by the study physician and it was provided by funds of the Department of Respiratory Medicine of the University of Thessaly and by a scholarship awarded to this research from the Hellenic Thoracic Society.
Statistical analyses
Normal distribution of continuous variables was assessed using the Kolmogorov–Smirnov test and normal probability plots. Associations between demographic and clinical indicators of the success of smoking cessation were assessed using the χ 2 test or Fisher’s exact test for categorical variables. Analysis of variance was used for continuous variables.
A multiple binary logistic regression using enter method with inclusion of all variables with a p value of <0.20 in univariate analysis was used to investigate prognostic factors of smoking cessation. The same model was also used with the forward selection Wald method to detect the most significant prognostic factors of smoking cessation. Kaplan–Meier survival analysis was used to estimate the duration of smoking abstinence. Log Rank test was used to compare the Kaplan–Meier curves of time to resume smoking between groups.
All tests were two-sided. The level of significance was set at p < 0.05. All analyzes were performed using the SPSS statistical package, version 17.00 (SPSS Inc., Chicago, Illinois, USA).
Results
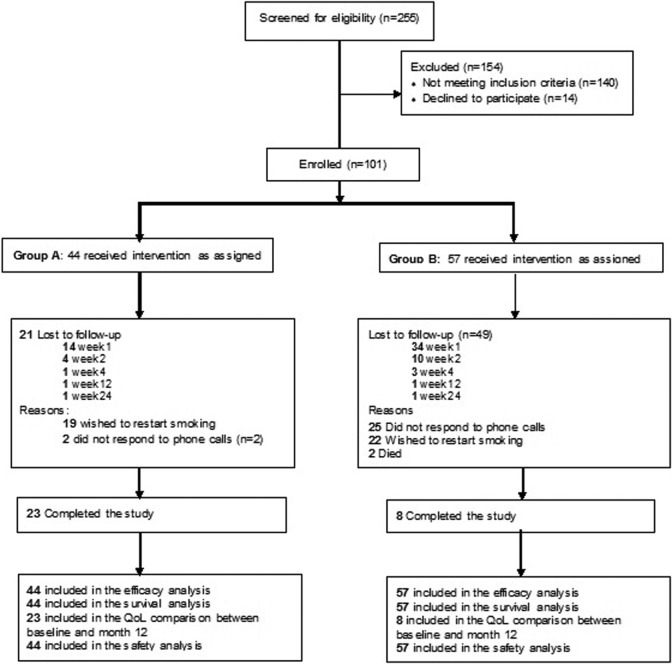
One hundred one patients were enrolled, 44 (43.6%) in the medication and behavioral support group A and 57 (56.4%) in group B. Patients were hospitalized for 4–10 days. Figure 1 shows a patient flow diagram. Baseline characteristics were similar between the two groups, since related statistical comparisons were not significant with all p > 0.05 as shown in Table 1. Seventy patients dropped out of the study, 48 (68.6%) of them during week 1 of smoking cessation. Most (49, 70.0%) were from group B. A major cause for dropping out (19/21, 90.5% in group A and 22/49, 44.9% in group B) was the wish to restart smoking (Figure 1).
Figure 1.
CONSORT 2010 flow diagram.
Table 1.
Patients’ baseline characteristics.
| All patients (n = 101) | Group A(n = 44) | Group B(n = 57) | p-Value for the comparison between groups | |
|---|---|---|---|---|
| Age, mean years (SD) | 50.86 (12.35) | 51.56 (11.55) | 50.32 (13.00) | 0.624 |
| Sex, male, n (%) | 69 (68.3) | 28 (63.6) | 41 (71.9) | 0.396 |
| Diagnosis, n (%) | ||||
| COPD | 38 (37.6) | 15 (34.1) | 23 (40.4) | 0.727 |
| Bronchial asthma | 17 (16.8) | 7 (15.9) | 10 (17.5) | |
| Pneumonia | 46 (45.5) | 22 (50.0) | 24 (42.1) | |
| Pack years, mean (SD) | 44.30 (31.02) | 43.02 (27.64) | 45.35 (33.79) | 0.724 |
| Previous quits, yes, n (%) | 54 (53.5) | 25 (56.8) | 29 (50.9) | 0.688 |
| Previous quits, mean (SD) | 2.73 (1.84) | 2.44 (1.38) | 2.97 (2.13) | 0.291 |
| Financial condition, n (%)a | ||||
| Very good | 2 (2.0) | 2 (4.5) | 0 (0.0) | 0.389 |
| Good | 43 (42.6) | 22 (50.0) | 21 (36.8) | |
| Medium | 33 (32.7) | 12 (27.3) | 21 (36.8) | |
| Bad | 15 (14.9) | 5 (11.4) | 10 (17.5) | |
| Very bad | 6 (5.9) | 2 (4.5) | 4 (7.0) | |
| No response | 2 (2.00) | 1 (2.3) | 1 (1.8) | |
| Fagerstrom score, mean (SD) | 6.14 (1.81) | 5.86 (1.49) | 6.35 (2.01) | 0.181 |
| CO, mean (SD) | 16.84 (6.53) | 15.66 (5.66) | 17.75 (7.04) | 0.110 |
| SF36 domains, mean score (SD) | ||||
| Physical functioning | 48.56 (24.72) | 47.73 (21.55) | 49.21 (27.09) | 0.760 |
| Rolephysical | 34.90 (28.74) | 36.36 (28.25) | 33.77 (29.31) | 0.655 |
| Bodily pain | 31.81 (17.72) | 33.18 (17.55) | 30.75 (17.93) | 0.498 |
| General health | 30.31 (17.65) | 31.80 (16.18) | 29.16 (18.76) | 0.459 |
| Vitality | 36.58 (12.90) | 38.98 (10.92) | 34.74 (14.06) | 0.102 |
| Social functioning | 41.34 (19.18) | 41.19 (16.30) | 41.45 (21.28) | 0.948 |
| Roleemotional | 34.65 (30.88) | 37.88 (33.40) | 32.16 (28.84) | 0.368 |
| Mental health | 37.66 (13.89) | 40.00 (12.38) | 35.86 (14.81) | 0.138 |
COPD: chronic obstructive pulmonary disease; SD: standard deviation; CO: carbon dioxide; SF36: Short Form 36.
aSelf-reported financial condition.
Smoking abstinence
All patients quit smoking as advised, from day 1 of varenicline administration (group A) or from the day after the day of the initial counseling session (group B). Figure 2 illustrates the percentage of abstinent patients by group; 54.5% of patients in group A and 15.8% in group B were still abstinent at week 12. At the end of the study follow-up (week 52), 31 patients were still smoking abstinent, 23 in group A (52.3%) and 8 in group B (14.0%) (Figure 2). Abstinence was verified by exhaled CO level that was much lower than 9 ppm as shown in Table 2: 5.61 ppm in group A and 6.00 ppm in group B.
Figure 2.
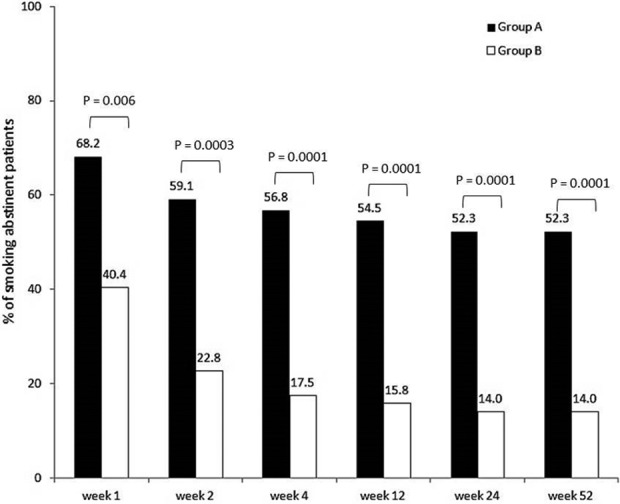
Percentage of smoking abstinent patients by group.
Table 2.
SF36 and exhaled CO mean values, for study completers, at baseline end at the end of the study.
| Group A(n = 23) | Group B(n = 8) | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 52 | p | Baseline | Week 52 | p | |
| SF36 domains, mean score (SD) | ||||||
| Physical functioning | 54.78 (18.98) | 79.78 (16.75) | <0.0005 | 64.38 (13.74) | 89.38 (8.63) | <0.0005 |
| Rolephysical | 39.13 (29.02) | 85.87 (19.69) | <0.0005 | 43.75 (29.12) | 81.25 (17.68) | 0.005 |
| Bodily pain | 38.48 (16.94) | 66.00 (18.58) | <0.0005 | 40.25 (17.89) | 84.50 (17.53) | <0.0005 |
| General health | 34.22 (14.51) | 59.13 (11.84) | <0.0005 | 42.88 (5.79) | 57.62 (7.29) | 0.001 |
| Vitality | 42.61 (8.77) | 61.74 (8.87) | <0.0005 | 47.50 (6.55) | 71.25 (9.16) | <0.0005 |
| Social functioning | 42.93 (15.91) | 77.17 (12.30) | <0.0005 | 50.00 (11.57) | 81.25 (16.37) | 0.001 |
| Roleemotional | 39.13 (35.75) | 89.86 (18.63) | <0.0005 | 33.33 (25.20) | 95.83 (11.79) | <0.0005 |
| Mental health | 42.61 (11.85) | 65.74 (7.42) | <0.0005 | 50.50 (7.69) | 77.50 (7.39) | <0.0005 |
| Exhaled CO, mean score (SD) | 14.74 (4.71) | 5.61 (2.10) | <0.0005 | 13.38 (2.92) | 6.00 (2.51) | <0.0005 |
SD: standard deviation; CO: carbon dioxide; SF36: Short Form 36.
Mantel–Cox Log Rank analysis showed that the mean time the patients remained abstinent from smoking was 26.5 weeks (95%CI 19.8, 33.2) for patients in group A and 8.5 weeks (95%CI 4.3, 12.7) in group B. This difference was statistically significant (χ 2: 17.2; df: 1; p < 0.0005). Figure 3 shows the Kaplan–Meier curve for time to resume smoking for both groups.
Figure 3.
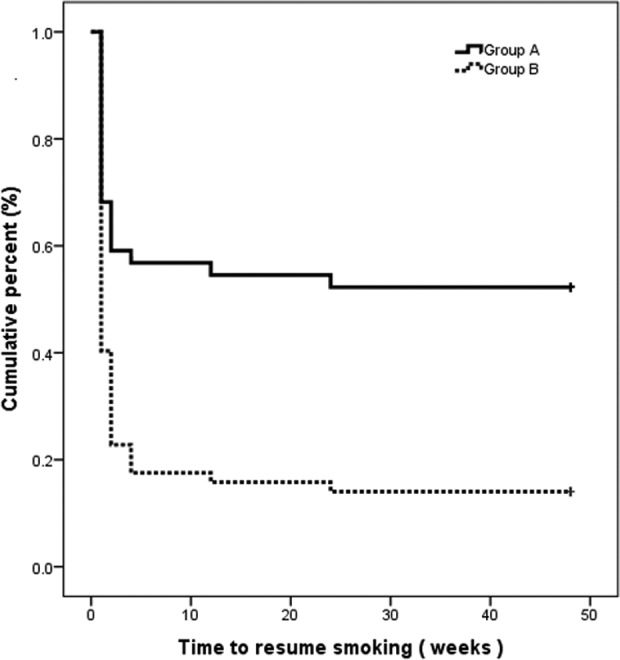
Kaplan–Meier curves of time to resume smoking for both groups.
The comparison of baseline characteristics between dropouts and completers showed that dropouts had significantly more pack years (49.7, standard deviation (SD) 33.4 vs. 32.2 SD 20.6, p = 0.003), previous attempts to quit (3.1, SD 2.0 vs. 2.2 SD 1.0, p = 0.028), a higher Fagerstrom score (6.6, SD 1.9 vs. 5.2 SD 1.2, p = 0.0005), higher exhaled CO levels (17.9, SD 7.1 vs. 14.4 SD 4.3, p = 0.003), and lower scores for five SF36 domains: physical functioning (44.7, SD 26.4vs. 57.3 SD 18.1, p = 0.007), bodily pain (28.7, SD 17.3 vs. 38.9 SD 16.9, p = 0.0007), general health (27.6, SD 18.7 vs. 36.5 SD 13.3, p = 0.008), vitality (33.4, SD 13.3 vs. 43.9 SD 8.4, p = 0.0005), and mental health (34.6, SD 13.9 vs. 44.6 SD 13.4, p = 0.001).
Factors predicting successful smoking abstinence
Multiple logistic regression with forward selection revealed five variables with a statistically significant impact on the probability of being able to quit smoking: being in group A, age, Fagerstrom score, and SF36 domains “vitality” and “social functioning”. Specifically, patients in group A were seven times more likely to quit smoking (OR 7.29; 95%CI 2.15, 24.77; p = 0.001). The probability of quitting smoking was also increased by 7% for each year of age (OR 1.07; 95%CI 1.00, 1.15; p = 0.042) and by 12% for each point on the SF36-vitality score (OR1.12; 95%CI 1.04, 1.21; p = 0.003). The probability was decreased by 63% with each point of Fagerstrom score (OR0.37; 95%CI 0.20, 0.68; p = 0.001) and by 5% with each point of the SF36-social functioning score (OR0.95; 95%CI 0.90, 1.00; p = 0.041).
QoL results
Smoking cessation improved QoL in both groups. The comparison of mean scores between baseline and week 52 showed statistically significant changes for all SF36 domains (Table 2). These figures must be viewed with caution due to the small number of eight completers in group B.
Adverse events
Weight gain was the most common adverse event reported in both groups (47.7% in group A vs. 21.1% in group B, p = 0.006), followed by nervousness (25.0% vs. 19.3%, p = 0.628), insomnia (18.2% vs. 0%, p = 0.001), nightmares (15.9% vs. 0%, p = 0.002), gastrointestinal disturbances (6.8% vs. 1.8%, p = 0.315), and dizziness (4.5% vs. 0%, p = 0.187).
Discussion
This 12-month, prospective, preference-based study evaluated the efficacy on smoking cessation of a 12-week course of varenicline combined with ongoing behavioral support in patients hospitalized due to acute exacerbation of COPD, or bronchial asthma, or due to community-acquired pneumonia and compared this with the success rate in patients given only one support session at baseline. We found that varenicline combined with behavioral support resulted in (a) high abstinence rates throughout 52 weeks of follow-up and (b) substantial improvements in all domains of the SF36 QoL questionnaire. We also showed that varenicline therapy can increase seven fold the probability of remaining abstinent for at least 12 months.
Combination of pharmacotherapy with behavioral support
Varenicline has both agonist and antagonist effects on the α4β2nACh receptor, thus decreasing nicotine withdrawal symptoms and also the rewarding effects of nicotine-induced dopamine release.25,26 Behavioral support in person or via telephone increases the anti-smoking effect of pharmacotherapy. It is believed that the two approaches complement each other and independently increase the chance of successfully stopping smoking.27,28 Two Cochrane reviews on the effects of behavioral support as an adjunct to pharmacotherapy trials have recently been published. They both found that the combination increases success rates and that this can be further increased by 10–25%, by intensifying the amount of behavioral support.19,20
Abstinence rates with varenicline combined with behavioral support
Most studies examining the efficacy of the same dosing schedule of varenicline as in our study have been conducted in nonhospitalized, generally healthy smokers attracted to participation through advertising.29–35 These trials report abstinence rates ranging between 32% and 56% at week 1231–33,35 and 19% and 26% at week 52.31–35 A study that enrolled patients with stable cerebrovascular disease resulted in similar success rates of 47.0% at week 12 and 19.2% at week 52. The same was observed in young asthma patients: 69% at week 12 and only 19% at week 24.36 Only the success rates at week 12, which was the last week of applying varenicline’s standard dosing schedule, are similar to our results. However, at week 52, our success rate was two times higher. In our opinion, this difference was because our patients were not healthy and were hospitalized, and therefore were more highly motivated to quit. Indeed, a study in hospitalized patients conducted by Eisenberg et al. in patients with acute coronary symptoms had abstinence rates similar to ours at weeks 4 (60.0%), 12 (57.7%), and 24 (47.3% end of follow-up).37
Furthermore, our success rates coincide with those reported when varenicline was administered for longer periods. Sansores et al. enrolled 30 outpatients with mild-to-moderate COPD and reported a 71% (20 patients) abstinence rate at 18 months.38 In the same study, participants were advised not to quit smoking until they lost the urge to smoke, up to which time they had been treated with varenicline, which was continued after stopping. The median duration of varenicline treatment was 6 months (range 3–24 months), far beyond the usual duration of 12 weeks. Smoking levels were evaluated using exhaled CO measurements at months 12 and 18 following declared voluntary abstinence.38 Similar high rates of abstinence were recorded in a retrospective review of patient records performed by Jimenez Ruiz et al. Patients had severe or very severe COPD and continuous abstinence rates at weeks 9–24 were 59.7% for varenicline standard duration of treatment (12 weeks) and 66.7% for long duration (24 weeks).39 In both these studies, various types of behavioral support accompanied varenicline.
Smoking abstinence rates in patients with pulmonary diseases
Most studies on smoking cessation in patients with pulmonary diseases have enrolled mainly COPD patients, regardless of the type of intervention. Our literature search did not return studies in patients with pneumonia, and only few in patients with asthma, two of which used pharmacological interventions to help quit smoking: the study by Tonnesen et al. with nicotine replacement therapy and a 53% abstinence rate at week 640 and the study we have already discussed of Westergaard et al.36 Current clinical guidelines on asthma management conclude that existing evidence on the efficacy of smoking cessation interventions is disappointing and that probably more intensive interventions should be evaluated.41
In COPD patients, randomized placebo-controlled trials on smoking cessation regardless of the type of intervention have reported lower success rates than ours. Behavioral support in combination with nicotine replacement resulted in a continuous abstinence rate of 14% (months 2–12).42 Bupropion resulted in 16% (weeks 7–26)43 and 28.6% (weeks 4–26) abstinence rates.44 In a further randomized, placebo-controlled trial, Tashkin and colleagues (2011) administered varenicline standard treatment, brief counseling sessions, and telephone calls in nonhospitalized patients with mild-to-moderate COPD. However, patients did not immediately quit smoking, as ours did. Instead, a target quit date on day 8 of treatment was scheduled. Continuous abstinence rates were 42.3%, for weeks 9–12, 25.8% for weeks 9–24, and 18.6% for weeks 9–52.45 Success rates in the study of Tashkin et al. are similar to those reported in healthy smokers, and the authors concluded that COPD patients respond as well as healthy smokers to effective pharmacotherapy, despite a higher assumed nicotine dependence that predisposes to less success in quitting.45 Our patients had more severe disease, since they were hospitalized for exacerbation of pulmonary diseases, but our higher success rates show that hospitalization is a “teachable moment” that can be exploited to reinforce the motivation to quit.16 A teachable moment occurs when health events motivate individuals to adopt risk-reducing health behaviors.16 In the case of our smokers hospitalized due to a worsening of their pulmonary disease, the heuristic model for a teachable moment is fully satisfied because three components come together at the same time: an increased perceived risk, redefinition of self-concept/social role, and increased emotion.16,46 In support of this notion, findings from the Framingham Heart Study showed that hospitalization in the previous 2 years increased the likelihood of smoking cessation by 30–40%.47 In addition, even without following a formal smoking cessation intervention, in a review of 20 controlled studies on inpatient hospital-based smoking cessation interventions, success rates of post-hospitalization at week 52 were between 15% and 78%, where the lower scores were more frequent in general admissions and the higher scores in cardiac and cancer admissions.16,48
QoL changes after quitting smoking
Smoking was negatively associated with QoL outcomes in 32 related studies conducted around the globe in smokers from different socioeconomic backgrounds.49 This negative association was dose dependent.49 Only few of these studies have been conducted in smokers with smoking-specific disease, and these studies also reported that, irrespective of disease severity, QoL outcome measurements were significantly worse in smokers than in nonsmokers with COPD50 or asthma.40,51 Our study showed that in hospitalized patients with COPD, bronchial asthma, or pneumonia, substantial improvement occurs in all domains of the SF36 following smoking cessation irrespective of the type of intervention. We believe that these results increase current knowledge for smokers with pulmonary disease, a population for whom existing literature data on QoL outcomes are scarce.
Predictors of smoking abstinence
Our multivariate results agree with findings reported by Ong et al., who investigated factors predicting the success of smoking abstinence in 248 hospitalized patients. The main predictors of success (OR) were sudden smoking cessation (7.19), low nicotine dependence score (Fagerstrom score < 5; 2.30), and first hospitalization (6.37).52
Limitations and strengths of the study
Our study’s main limitations are the lack of randomization, the absence of placebo control, and the open design. Because of nonrandomization, we cannot rule out the possibility that some patients who were more willing to quit chose the varenicline group or that others with fear or reluctance in taking another pill chose group B—both these possibilities are introducing bias to the interpretation of our results. However, preference-based design adds to existing evidence, the potential impact of patients’view toward their treatment, whereas an intention-to-treat analysis in randomized patients would underestimate the treatment effect in compliant patients.53 In addition, preference-based design by favoring compliance provides estimates on treatment benefit that might interest other patients willing and committed to adhere to a smoking cessation treatment.53 Such evidence would interest our future patients in search for an effective method to quit smoking. As Walter et al. have pointed out, “many physicians and patients want to know the expected benefit if they adhere to the therapy.”53 Furthermore, because we wanted to assess real-life outcomes in our patients, we were obliged to opt for a pragmatic design that did not alter routine clinical practice, and we therefore also did not exclude any patients who volunteered. These limitations do not allow for cause and effect conclusions, but certainly the results provide useful information for physicians and patients on smoking cessation practices.54 While a randomized trial can tell us whether a treatment is effective or better that another in ideal conditions, the pragmatic trial can tell us if this treatment will continue to work in normal settings.55,56 At last, the present results could serve as a starting point for the development of a randomized, placebo-controlled study in patients like ours, that is, hospitalized with severe pulmonary disease. A further limitation is that varenicline was administered free of charge to the participants, which might have influenced their decision to participate. This needs to be addressed in an appropriately designed study. If medication costs prove to be a consideration that prevents smokers from taking smoking cessation pharmacotherapy, then medical care professionals should press for free of charge administration of anti-smoking medications for obvious reasons: reduction in healthcare costs as a result of improved health in the general population, and reduced lost activity and working days, to name but two. Another important limitation is that group B received only one consultation session and not the behavioral support provided to group A. This again is in accordance with our standard routine clinical practice; however, a behavioral support like this provided in group A might have lowered the high dropout rates.
Among study’s strengths is the advantage of exploiting the “teachable moment” of hospitalization, and the secondary effect of this, that is, having to quit smoking from day 1 of varenicline administration, as patients were not allowed to smoke on hospital premises. Our study adds new findings to the very limited information available on smoking cessation success rates and QoL in patients with asthma or pneumonia.
Conclusions
Varenicline in combination with behavioral support can result in a seven fold increase in the probability of remaining smoking abstinent for at least 12 months. This smoking cessation intervention resulted in high smoking abstinence rates in patients hospitalized for exacerbation of COPD or bronchial asthma, or pneumonia, and significantly improved their QoL.
Footnotes
Authors’ note: The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was approved by the Scientific Committee of the Kavala General Hospital, Greece (3/20-1-14). Only eligible patients who agreed to participate and provided written informed consent were recruited.
Author contributions: AP, VI, and CH contributed to data collection and analysis. KIG, ZD, and CH, contributed to protocol writing. AP and CH contributed to manuscript preparation. KIG had the overall study’s supervision.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Respiratory Medicine of the University of Thessaly and by a scholarship awarded to this research from the Hellenic Thoracic Society. The funding bodies had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
References
- 1. World health Organization. WHO report on the global tobacco epidemic, 2015; raising taxes on tobacco. WHO, Geneva, Switzerland, 2015. [Google Scholar]
- 2. Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014; 311: 183–192 [DOI] [PubMed] [Google Scholar]
- 3. Erisken M, Mackay J, Schluger C, et al. The Tobacco Atlas Fact Sgeet – Greece. American Cancer Society, Inc Copyright© 2015 Tobacco Atlas, TobaccoAtlas.org http://www.tobaccoatlas.org/country-data/greece/ (accessed 6 September 2016).
- 4. Erisken M, Mackay J, Schluger N, et al. The Tobacco Atlas. American Cancer Society,Inc Copyright© 2015 Tobacco Atlas, TobaccoAtlas.org http://www.tobaccoatlas.org/country-data/greece/ (accessed 6 September 2016).
- 5. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med 2011; 24: 313–322. [DOI] [PubMed] [Google Scholar]
- 6. Hvidtfeldt UA, Rasmussen S, Gronbaek M, et al. Influence of smoking and alcohol consumption on admissions and duration of hospitalization. Eur J Public Health 2010; 20: 376–382. [DOI] [PubMed] [Google Scholar]
- 7. Siroux V, Pin I, Oryszczyn MP, et al. Relationships of active smoking to asthma and asthma severity in the EGEA study. Epidemiological study on the Genetics and Environment of Asthma. Eur Respir J 2000; 15: 470–477. [DOI] [PubMed] [Google Scholar]
- 8. Eisner MD, Yelin EH, Trupin L, et al. Asthma and smoking status in a population-based study of California adults. Public Health Rep 2001; 116: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casado JB. Asthma and smoking: an unfortunate combination. Arch Bronconeumol 2007; 43: 340–345. [PubMed] [Google Scholar]
- 10. Harrison B, Stephenson P, Mohan G, et al. An ongoing confidential enquiry into asthma deaths in the Eastern Region of the UK, 2001-2003. Prim Care Respir J 2005; 14: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The lung health study. Am J Respir Crit Care Med 2000; 161: 381–390. [DOI] [PubMed] [Google Scholar]
- 12. Rigotti NA, Clair C, Munafo MR, et al. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev 2012; 16: CD001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update – Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service, Rockville, MD: http://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf (accessed 2 September 2016). [Google Scholar]
- 14. Suplee PD. The importance of providing smoking relapse counseling during the postpartum hospitalization. J Obstet Gynecol Neonatal Nurs 2005; 34: 703–712. [DOI] [PubMed] [Google Scholar]
- 15. Houston TK, Allison JJ, Person S, et al. Post-myocardial infarction smoking cessation counseling: associations with immediate and late mortality in older Medicare patients. Am J Med 2005; 118: 269–275. [DOI] [PubMed] [Google Scholar]
- 16. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res 2003; 18: 156–170. [DOI] [PubMed] [Google Scholar]
- 17. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 31: CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Eerd EA, van der Meer RM, van Schayck OC, et al. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 8: CD010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stead LF, Koilpillai P, Fanshawe TR, et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016; 3: CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev 2015; CD009670. [DOI] [PubMed] [Google Scholar]
- 21. Elrashidi MY, Ebbert JO. Emerging drugs for the treatment of tobacco dependence: 2014 update. Expert Opin Emerg Drugs 2014; 19: 243–260. [DOI] [PubMed] [Google Scholar]
- 22. West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005; 100: 299–303. [DOI] [PubMed] [Google Scholar]
- 23. The Clinical Practice Guideline Treating Tobacco use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 2008; 35: 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 25. Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005; 48: 3474–3477. [DOI] [PubMed] [Google Scholar]
- 26. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 2007; 52: 985–994. [DOI] [PubMed] [Google Scholar]
- 27. Hughes JR. Combining behavioral therapy and pharmacotherapy for smoking cessation: an update. NIDA Res Monogr 1995; 150: 92–109. [PubMed] [Google Scholar]
- 28. Cofta-Woerpel L, Wright KL, Wetter DW. Smoking cessation 3: multicomponent interventions. Behav Med 2007; 32: 135–149. [DOI] [PubMed] [Google Scholar]
- 29. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 30. Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006; 166: 1571–1577. [DOI] [PubMed] [Google Scholar]
- 31. Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296: 47–55. [DOI] [PubMed] [Google Scholar]
- 32. Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296: 56–63. [DOI] [PubMed] [Google Scholar]
- 33. Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 2008; 63: 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015; 313: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 Weeks: a randomized clinical trial. JAMA 2016; 315: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Westergaard CG, Porsbjerg C, Backer V. The effect of Varenicline on smoking cessation in a group of young asthma patients. Respir Med 2015; 109: 1416–1422. [DOI] [PubMed] [Google Scholar]
- 37. Eisenberg MJ, Windle SB, Roy N, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation 2016; 133: 21–30. [DOI] [PubMed] [Google Scholar]
- 38. Sansores RH, Ramirez-Venegas A, Arellano-Rocha R, et al. Use of varenicline for more than 12 months for smoking cessation in heavy chronic obstructive pulmonary disease smokers unmotivated to quit: a pilot study. Ther Adv Respir Dis 2016; 10: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jimenez Ruiz CA, Ramos PA, Cicero GA, et al. Characteristics of COPD smokers and effectiveness and safety of smoking cessation medications. Nicotine Tob Res 2012; 14: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 40. Tonnesen P, Pisinger C, Hvidberg S, et al. Effects of smoking cessation and reduction in asthmatics. Nicotine Tob Res 2005; 7: 139–148. [DOI] [PubMed] [Google Scholar]
- 41. British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline, October 2014. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2014/ (accessed 7 September 2016).
- 42. Tonnesen P, Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006; 130: 334–342. [DOI] [PubMed] [Google Scholar]
- 43. Tashkin D, Kanner R, Bailey W, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001; 357: 1571–1575. [DOI] [PubMed] [Google Scholar]
- 44. Wagena EJ, Knipschild PG, Huibers MJ, et al. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med 2005; 165: 2286–2292. [DOI] [PubMed] [Google Scholar]
- 45. Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest 2011; 139: 591–599. [DOI] [PubMed] [Google Scholar]
- 46. Reid RD, Pipe AL, Quinlan B. Promoting smoking cessation during hospitalization for coronary artery disease. Can J Cardiol 2006; 22: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freund KMD’Agostino, RB, Belanger AJ, et al. III Predictors of smoking cessation: the Framingham Study. Am J Epidemiol 1992; 135: 957–964. [DOI] [PubMed] [Google Scholar]
- 48. France EK, Glasgow RE, Marcus AC. Smoking cessation interventions among hospitalized patients: what have we learned? Prev Med 2001; 32: 376–388. [DOI] [PubMed] [Google Scholar]
- 49. Goldenberg M, Danovitch I, IsHak WW. Quality of life and smoking. Am J Addict 2014; 23: 540–562. [DOI] [PubMed] [Google Scholar]
- 50. Joseph S, Pascale S, Georges K, et al. Cigarette and waterpipe smoking decrease respiratory quality of life in adults: results from a national cross-sectional study. Pulm Med 2012; 2012: 868294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sippel JM, Pedula KL, Vollmer WM, et al. Associations of smoking with hospital-based care and quality of life in patients with obstructive airway disease. Chest 1999; 115: 691–696. [DOI] [PubMed] [Google Scholar]
- 52. Ong KC, Cheong GN, Prabhakaran L, et al. Predictors of success in smoking cessation among hospitalized patients. Respirology 2005; 10: 63–69. [DOI] [PubMed] [Google Scholar]
- 53. Walter SD, Guyatt G, Montori VM, et al. A new preference-based analysis for randomized trials can estimate treatment acceptability and effect in compliant patients. J Clin Epidemiol 2006; 59: 685–696. [DOI] [PubMed] [Google Scholar]
- 54. Brass EP. The gap between clinical trials and clinical practice: the use of pragmatic clinical trials to inform regulatory decision making. Clin Pharmacol Ther 2010; 87: 351–355. [DOI] [PubMed] [Google Scholar]
- 55. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis 1967; 20:637–648. [DOI] [PubMed] [Google Scholar]
- 56. Lambert MF, Wood J. Incorporating patient preferences into randomized trials. J Clin Epidemiol 2000; 53: 163–166. [DOI] [PubMed] [Google Scholar]