Abstract
Study Design:
Cross-sectional study.
Objectives:
The aim of this study was to evaluate cervical disc degeneration on magnetic resonance imaging (MRI) in a large population of symptomatic patients and to provide baseline data on the pattern of degeneration in order to understand how the cervical spine ages.
Methods:
We performed a cross-sectional study of 1059 patients who underwent upright cervical MRI for neck pain with and without neurological symptoms. A total of 6354 cervical discs from C2/3 to C7/T1 were evaluated. Cervical disc degeneration was evaluated on T2-weighted MRI and graded into 4 categories (Grades 0-III). Positive degeneration was defined as greater than Grade II. The correlation between age and total grade of degeneration of each patient was evaluated, as well as the prevalence and pattern of degeneration.
Results:
The average number of degenerated disc levels and the total grade of cervical disc degeneration significantly increase with age. In the patient group with 1-level degeneration, C5/6 was the most common degenerated level followed by C4/5 and C6/7. In the group with 2-level degeneration, C5/6 & C6/7 was most common followed by C4/5 & C5/6 and C3/4 & C4/5. Skip level degeneration was significantly rarer than contiguous level degeneration, and C7/T1 and C2/3 were the most unlikely to degenerate in multilevel degeneration.
Conclusion:
Disc degeneration is most common in the middle cervical spine (C5/6) and progresses to contiguous levels, except for C7/T1 and C2/3. This pattern may play a role in adjacent-level disc degeneration associated with spinal fusion.
Keywords: cervical spine, MRI, degeneration pattern, disc degeneration, symptomatic patient, grading system, aging
Introduction
Cervical intervertebral disc degeneration is a common finding on imaging studies, and it is well known that disc degeneration prevalence increases in accordance with age. Boden et al conducted a magnetic resonance imaging (MRI) study of the cervical spine in 63 asymptomatic volunteers and reported that abnormal findings, including disc degeneration and disc space narrowing, were found in 14% of volunteers aged less than 40 years and in 28% of those aged over 40 years.1 Several other studies also have shown a significant correlation between age and disc degeneration,2–7 but the specific pattern of cervical disc degeneration with age has not been well studied.
Anterior cervical discectomy and fusion (ACDF) is currently the gold standard surgical treatment for cervical radiculopathy and myelopathy but adjacent segmental disease or degeneration after ACDF is an ongoing problem.8 In an effort to preserve motion, cervical disc replacement (CDR) was developed. A number of clinical studies comparing CDR with ACDF have been reported; however, none of the studies to date suggest that CDR decreased the rate of symptomatic adjacent segment disease (ASD) when compared with ACDF.9–11 It is still controversial whether ASD is related to biomechanical changes after fusion surgery or if it represents the natural history of cervical spondylosis. In order to understand the mechanism of ASD, it is important to know the natural pattern of cervical disc degeneration in symptomatic patients.
At present, MRI is the most sensitive method for the assessment of intervertebral disc pathology. In the early stage of disc degeneration, loss of water and proteoglycan content is represented as loss of signal intensity on T2-weighted image.12 Disc bulging and loss of disc height in the latter stage are also well detected on MRI.13 Previously, we developed a new classification system for cervical disc degeneration based on detailed analysis of the changes seen on MRI,14 which is simple, reproducible, and related to functional parameters. The purpose of this study was to evaluate cervical disc degeneration on MRI using the classification system in a large population of symptomatic patients in order to provide baseline data on the pattern of cervical disc degeneration and to describe how the spine ages.
Methods
Sample Population
From March to September 2011, 1059 symptomatic patients (493 males, 566 females) with an average age of 48.1 ± 10.0 years (range 15-79 years) underwent upright cervical spine MRI in the neutral position. The sample consisted of consecutive patients reporting neck pain or radiculopathy with or without neurologic deficits. Exclusion criteria consisted of previous spine surgery or trauma. The institutional review board at our institution approved this study (Approval Number: 10-000 968), and informed consent was obtained from every subject by written form.
Magnetic Resonance Imaging
MRIs of the cervical spine were acquired using a 0.6-Tesla MRI scanner (Upright Multi-Position; Fonar Corp, New York, NY) with subjects in an upright, weight-bearing, neutral position using a flexible surface coil.15 The magnets are separated by a 0.5-mm gap. A standard imaging protocol was used, which included sagittal T1-weighted spin-echo sequences (repetition time [TR]/echo time [TE], 671/17 milliseconds; slice thickness, 3.0 mm; field of view, 24 cm; matrix, 256 200; and number of excitations [NEX], 2) and T2-weighted fast spin-echo sequences (TR/TE, 3432/160 milliseconds; slice thickness, 3.0 mm; field of view, 24 cm; and NEX, 2). Axial T2-weighted spin-echo sequences were only acquired with fat suppression.
Grading System for Cervical Disc Degeneration
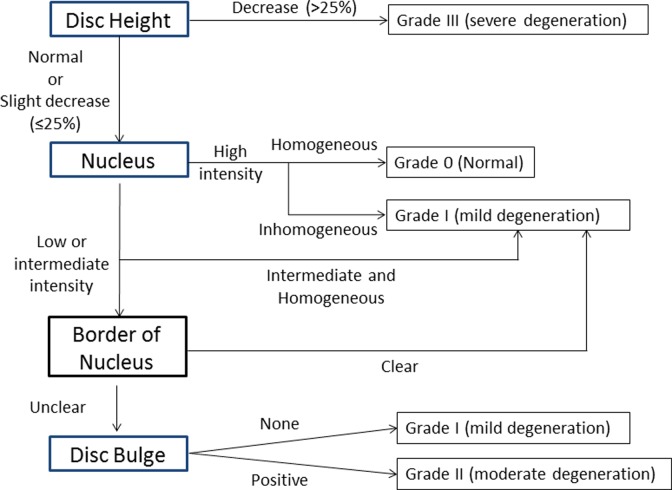
On the basis of our previous grading system13 and literature review,5,16–21 we developed a comprehensive grading system and an algorithm for cervical disc degeneration (Figure 1 and Table 1).14 The grading system consists of 4 factors: disc height, nucleus intensity and structure, distinction between nucleus and annulus, and disc bulge/herniation. The nucleus structure and signal intensity was categorized into 3 grades: (1) homogeneous high intensity, (2) inhomogeneous high intensity or homogeneous intermediate intensity, and (3) inhomogeneous intermediate intensity or low intensity. These were defined as follows: high intensity—similar intensity to cerebrospinal fluid (CSF) and/or bone marrow; intermediate intensity—similar intensity to the spinal cord; and low intensity—similar intensity to endplate. The distinction of nucleus and annulus was categorized into 2 grades: clear and unclear. Loss of disc height was defined as more than 25% decrease compared with normal disc height. The category of disc bulge/herniation was graded as positive or negative. All assessments of disc degeneration were made with T2-weighted images in the neutral position. The most medial slice was chosen to determine the score of disc height, nucleus intensity and structure, and distinction between nucleus and annulus. Several parasagittal slices were checked to determine disc bulge/herniation. According to the algorithm, the grade of cervical disc degeneration was categorized into 4 grades: Grade 0 (no degeneration), Grade I (mild degeneration), Grade II (moderate degeneration), and Grade III (severe degeneration). Briefly, Grade I indicates low-intensity change or structural change of the nucleus pulposus; Grade II indicates disc bulge or herniation with degeneration of the annulus fibrosus; and Grade III indicates further degeneration with disc height decrease of more than 25%.
Figure 1.
Algorithm for the grading system and for the assessment of cervical disc degeneration grade.
Table 1.
Cervical Disc Degeneration Grading System.
| Disc Heighta | Intensity and Structure of Nucleusb | Distinction Between Nucleus and Annulus | Disc Bulge/Herniation | |
|---|---|---|---|---|
| Grade 0 (no degeneration) | Normal | Hyperintense and homogeneous | Clear | None |
| Grade 1 (mild degeneration) | Normal | Hyperintense and inhomogeneous, or decrease of signal intensity | Clear or unclear | None |
| Grade 2 (moderate degeneration) | Normal to slight decrease | Decrease of signal intensity | Unclear | Positive |
| Grade 3 (severe degeneration) | Decrease | Decrease of signal intensity | Unclear | Positive |
aHigh intensity is defined as similar intensity of cerebrospinal fluid and/or bone marrow; decrease of signal intensity is defined as lower intensity than bone marrow.
bAs for disc height, slight decrease is defined as less than 30% decrease of normal disc height, and decrease is defined as more than 30% decrease of normal disc height.
Magnetic resonance images of 50 cases were randomly selected from the 1059 cases, and the disc degeneration grades for 300 levels (6 levels from C2/3 to C7/T1) were measured twice by 4 experienced spine surgeons. Kappa values for intra- and interobserver error were calculated using computer software (SPSS version 19.0; IBM Co, New York, NY).
Analysis of Cervical Disc Degeneration Grade
After the confirmation of reliability, a total of 6354 cervical discs from C2/3 to C7/T1 were evaluated by the same 4 spine surgeons. The total grade of cervical disc degeneration was calculated as the sum of the degenerative disc scores of all cervical discs from C2/3 to C7/T1 in each patient. The correlation between age and the total grade was evaluated using Pearson’s correlation test using computer software (SPSS version 19.0). In this study, we defined positive degeneration as greater than Grade II. The prevalence of degeneration was assessed in different age groups. The patients were then grouped according to the number of degenerated discs.
Statistical Analysis
All statistical analysis was performed using computer software (SPSS version 19.0). For the correlation between age and the total grade, Pearson’s correlation was used. The trend of the disc degeneration by age group was analyzed using linear regression analysis. The pattern of degeneration was analyzed using χ2 test. Values of P < .05 were considered statistically significant.
Results
The Intra- and Interobserver Reliability of the Grading System for Cervical Disc Degeneration
The average Kappa values for intra- and interobserver agreement of overall grade were 0.96 ± 0.01 (range = 0.942-0.964) and 0.89 ± 0.03 (range = 0.857-0.923), respectively, and the reproducibility of the new grading system was considered “excellent.”
The Correlation Between Age and Cervical Disc Degeneration
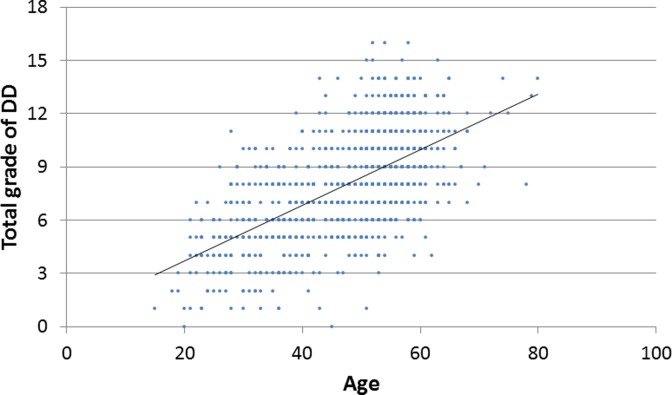
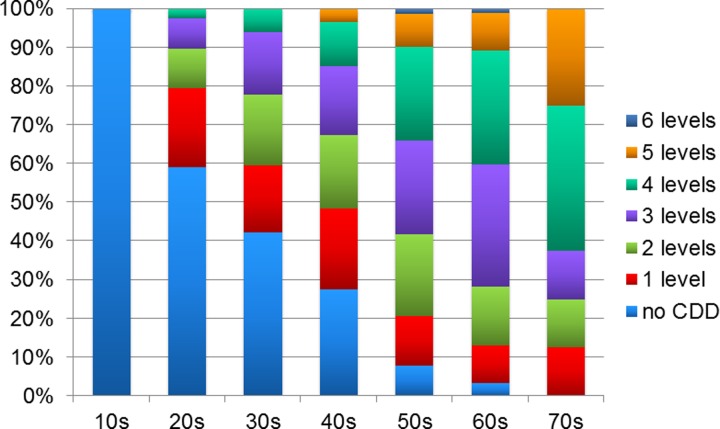
The total grade of cervical disc degeneration was significantly correlated with age (P < .01, R = 0.603; Figure 2), and the average number of degenerated disc levels also increased with age (10s: 0.0, 20s: 0.7, 30s: 1.3, 40s: 1.7, 50s: 2.8, 60s: 3.1, 70s: 3.5 levels; P < .001). In this study population, none of the patients in the teenage years had cervical disc degeneration more than grade II, whereas all the patients in the 70s age range had cervical disc degeneration at more than 1 level (Figure 3). The prevalence of cervical disc degeneration at more than 1 level was 41.0% in patients in their 20s; 57.8% in patients in their 30s; 72.4% in the 40s; 92.1% in the 50s; and 96.7% in the 60s (P < .001). In terms of disc level, the prevalence of disc degeneration at C5/6 was highest in all decades (Figure 4). Disc degeneration at the levels from C3/4 to C6/7 was found in more than 50% of the patient who were older than 50 years of age. However, the prevalence of disc degeneration at C2/3 or C7/T1 was less than 15%, even in the elderly population (Figure 4).
Figure 2.
The relationship between age and mean disc degeneration grade of each patient.
Figure 3.
Number of degenerated disc levels according to age.
Figure 4.
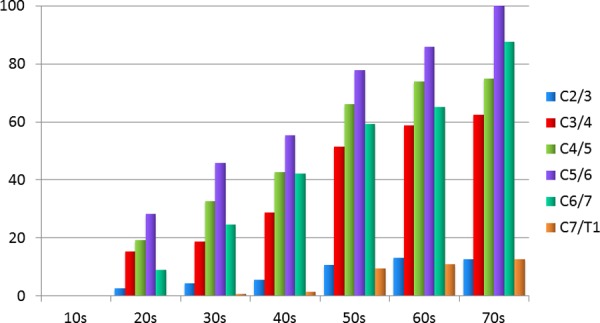
Percentage of degenerated disc according to age and levels.
The Pattern of Degeneration Level
The number of degenerated levels greater than Grade II and the average age is shown in Table 2. In the patient group with 1-level degeneration, C5/6 was the most common degenerated level (51.2%), followed by C4/5 (19.8%) and C6/7 (16.7%; Table 3). In the groups with 2-level degeneration, C5/6 & C6/7 was most common (40.7%) followed by C4/5 & C5/6 (27.6%) and C3/4 & C4/5 (14.1%; Table 4). In the group with 3-level degeneration, C4/5 & 5/6 & C6/7 was most common (41.4%), followed by C3/4 & 4/5 & C5/6 (33.3%; Table 5). Even in the group with 4-level degeneration, degeneration at C2/3 and C7/T1 was rare (C2/3, 12.8%; C7/T1, 11.2%; Table 6).
Table 2.
The Number of Degenerated Levels and the Average Age.
| n | Mean Age ± SD | |
|---|---|---|
| None | 219 | 38.5 ± 10.8 |
| 1 level | 162 | 46.1 ± 10.6 |
| 2 levels | 199 | 49.4 ± 9.6 |
| 3 levels | 222 | 50.9 ± 9.0 |
| 4 levels | 188 | 53.6 ± 7.7 |
| 5 levels | 61 | 56.0 ± 6.9 |
| 6 levels | 8 | 56.4 ± 3.8 |
| Total | 1059 | 48.1 ± 10.0 |
Table 3.
The Prevalence of Disc Degeneration Level in the Patients With 1-Level Degeneration (Sorted by Frequency).
| Prevalence, n (%) | |
|---|---|
| C5/6 | 83 (51.2) |
| C4/5 | 32 (19.8) |
| C6/7 | 27 (16.7) |
| C3/4 | 18 (11.1) |
| C2/3 | 2 (1.2) |
| C7/T1 | 0 (0) |
| Total | 162 (100) |
Table 4.
The Prevalence of Disc Degeneration Levels in the Patients With 2-Level Degeneration (Sorted by Frequency).
| Prevalence, n (%) | |
|---|---|
| C5/6 and C6/7 | 81 (40.7) |
| C4/5 and C5/6 | 55 (27.6) |
| C3/4 and C4/5 | 28 (14.1) |
| C3/4 and C5/6 | 15 (7.6) |
| C4/5 and C6/7 | 10 (5.0) |
| C3/4 and C6/7 | 5 (2.5) |
| C2/3 and C3/4 | 1 (0.5) |
| C2/3 and C4/5 | 1 (0.5) |
| C2/3 and C5/6 | 1 (0.5) |
| C2/3 and C6/7 | 1 (0.5) |
| C4/5 and C7/T1 | 1 (0.5) |
| Total | 199 (100) |
aDegeneration at contiguous level in boldface.
bOther combinations of levels were not found in this study population.
Table 5.
The Prevalence of Disc Degeneration Levels in the Patients With 3-Level Degeneration (Sorted by Frequency).
| Prevalence, n (%) | |
|---|---|
| C4/5, C5/6, and C6/7 | 92 (41.4) |
| C3/4, C4/5, and C5/6 | 74 (33.3) |
| C3/4, C5/6, and C6/7 | 25 (11.3) |
| C3/4, C4/5, and C6/7 | 11 (5.0) |
| C2/3, C3/4, and C4/5 | 5 (2.3) |
| C2/3, C5/6, and C6/7 | 4 (1.8) |
| C4/5, C5/6, and C7/T1 | 3 (1.4) |
| C2/3, C4/5, and C5/6 | 2 (0.9) |
| C2/3, C3/4, and C5/6 | 2 (0.9) |
| C5/6, C6/7, and C7/T1 | 2 (0.9) |
| C2/3, C4/5, and C6/7 | 1 (0.5) |
| C4/5, C6/7, and C7/T1 | 1 (0.5) |
| Total | 222 (100) |
aDegeneration at contiguous level in boldface.
bOther combinations of levels were not found in this study population.
Table 6.
The Prevalence of Disc Degeneration Levels in the Patients With 4-Level Degeneration (Sorted by Frequency).
| Prevalence, n (%) | |
|---|---|
| C3/4, C4/5, C5/6, and C6/7 | 143 (76.1) |
| C4/5, C5/6, C6/7, and C7/T1 | 74 (8.0) |
| C2/3, C3/4, C4/5, and C5/6 | 11 (5.9) |
| C2/3, C3/4, C5/6, and C6/7 | 6 (3.2) |
| C3/4, C4/5, C5/6, and C7/T1 | 5 (2.7) |
| C2/3, C4/5, C5/6, and C6/7 | 4 (2.1) |
| C2/3, C3/4, C4/5, and C6/7 | 3 (1.6) |
| C3/4, C5/6, C6/7, and C7/T1 | 1 (0.5) |
| Total | 188 (100) |
aDegeneration at contiguous level in boldface.
bOther combinations of levels were not found in this study population.
Skip-level degeneration was found in 17% of patients with 2-level degeneration; 22% with 3-level degeneration; 10% with 4-level degeneration; and 1.6% with 5-level degeneration. In all the groups with 2 or more levels of degeneration, contiguous patterns were significantly more common than skip-level patterns (P < .001).
Discussion
MRI provides a noninvasive and accurate morphologic evaluation of the spine and is considered to be the most sensitive method for the assessment of disc degeneration. There are few studies that examine cervical disk degeneration in a large population. To the best of our knowledge, this is the largest study in symptomatic patients focused on cervical disc degeneration. We believe that the study of symptomatic patients is important for delineating the natural history of disk degeneration given that surgeons primarily see symptomatic patients.
In agreement with previous reports,1,5 we confirmed the correlation between the number and degree of disc degeneration with age. Matsumoto et al5 conducted an MRI study with 497 asymptomatic volunteers and reported an increase in abnormal findings of cervical discs significantly increased with age such as low signal intensity change, disc protrusion, and disc height loss. The percentage of degenerated discs with low signal intensity changes in our symptomatic patient group was slightly higher than the asymptomatic volunteer group in their study (14.5% vs 66.8% in 20s, 87.6% vs 98.7% in 60s and 70s). This discrepancy in the prevalence may be attributable to the differences in patient population or method; however, it is important to note that both studies showed low signal intensity changes starting in the teenage years, although disc protrusion/bulging was rare in that age group. Both studies also demonstrated that the most common degenerated level was C5/6 followed by C6/7 and C4/5, and that disc protrusion was rare at C2/3. These results may suggest that the pattern of disc degeneration in symptomatic patients is similar to that in asymptomatic subjects.
In the present study, we investigated the pattern of disc degeneration in a large cross-sectional study by grouping patients by the number of degenerated discs found on MRI. In the group with 1-level degeneration, mid-low cervical disc levels (C4/5, C5/6, C6/7) tended to be the first to degenerate. In the group with 2- or 3-level degeneration, degeneration tended to be present at mid-low and contiguous level in 80% of the patients (83% in the group with 2-level degeneration and 78% in the group with 3-level degeneration). Skip-level degeneration was much less common than contiguous-level degeneration. These results suggest that subsequent degeneration is likely to occur at an adjacent level, except at C2/3 and C7/T1. Why does degeneration usually occur at the adjacent level? Simpson et al,6 by using multivariate analysis, demonstrated that disc degeneration was significantly associated with decrease of motion at that level. Our previous study, using kinetic MRI and the earlier grading system of cervical disc degeneration, also showed that sagittal angular motion decreased as degeneration became more severe. Hussain et al22 performed biomechanical analysis using a finite element model and reported that motion decreased at C5/6 with progressive disc degeneration at that level, and that it affects adjacent level motion especially in flexion and extension. This suggests that disc degeneration at one level may induce disc degeneration at adjacent levels by changing mechanical stress on the disc. The mechanism may be similar to adjacent segmental degeneration after fusion surgery, although the effect of fusion surgery may be much more immediate than natural disc degeneration.
There are limitations to this study. This study is a cross-sectional study and not a longitudinal study. Longitudinal studies will be necessary to elucidate the pattern of degeneration more accurately. Second, the population of this study was predominantly middle-aged; therefore, the prevalence of disc degeneration in this study may not represent the prevalence in the general population of symptomatic patients. Last, this study did not have clinical data available regarding the degree of patient symptoms that could be correlated with MRI findings. Despite these limitations, this article provides valuable data on the natural pattern of cervical disc degeneration in symptomatic patients that will be useful for follow-up articles to compare their data with as a baseline.
Conclusion
The present MRI study reveals the prevalence of cervical disc degeneration and its natural pattern in symptomatic middle-aged patients. Disc degeneration is most common in the mid-cervical level (C5/6) and contiguously progresses from that level to adjacent levels, except at C7/T1 and C2/3. Skipped-level disc degeneration is rarely observed in patients with multilevel disc degeneration. This disc degeneration pattern may affect the development of adjacent-level disc degeneration associated with spinal fusion surgery.
Footnotes
Authors’ Note: This study’s protocol was approved by the Institutional Review Board of the University of California, Los Angeles (Approval Number: 10-000 968).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 2. Gore DR. The arthrodesis rate in multilevel anterior cervical fusions using autogenous fibula. Spine (Phila Pa 1976). 2001;26:1259–1263. [DOI] [PubMed] [Google Scholar]
- 3. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976). 1986;11:521–524. [DOI] [PubMed] [Google Scholar]
- 4. Lehto IJ, Tertti MO, Komu ME, Paajanen HE, Tuominen J, Kormano MJ. Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36:49–53. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19–24. [DOI] [PubMed] [Google Scholar]
- 6. Simpson AK, Biswas D, Emerson JW, Lawrence BD, Grauer JN. Quantifying the effects of age, gender, degeneration, and adjacent level degeneration on cervical spine range of motion using multivariate analyses. Spine (Phila Pa 1976). 2008;33:183–186. [DOI] [PubMed] [Google Scholar]
- 7. Wilder FV, Fahlman L, Donnelly R. Radiographic cervical spine osteoarthritis progression rates: a longitudinal assessment. Rheumatol Int. 2011;31:45–48. [DOI] [PubMed] [Google Scholar]
- 8. Cho SK, Riew KD. Adjacent segment disease following cervical spine surgery. J Am Acad Orthop Surg. 2013;21:3–11. [DOI] [PubMed] [Google Scholar]
- 9. Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine. 2011;15:348–358. [DOI] [PubMed] [Google Scholar]
- 10. Maldonado CV, Paz RD, Martin CB. Adjacent-level degeneration after cervical disc arthroplasty versus fusion. Eur Spine J. 2011;20(suppl 3):403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nunley PD, Jawahar A, Kerr EJ, 3rd, et al. Factors affecting the incidence of symptomatic adjacent-level disease in cervical spine after total disc arthroplasty: 2- to 4-year follow-up of 3 prospective randomized trials. Spine (Phila Pa 1976). 2012;37:445–451. [DOI] [PubMed] [Google Scholar]
- 12. Weidenbaum M, Foster RJ, Best BA, et al. Correlating magnetic resonance imaging with the biochemical content of the normal human intervertebral disc. J Orthop Res. 1992;10:552–561. [DOI] [PubMed] [Google Scholar]
- 13. Miyazaki M, Hong SW, Yoon SH, Morishita Y, Wang JC. Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech. 2008;21:288–292. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki A, Daubs MD, Hayashi T, et al. Magnetic resonance classification system of cervical intervertebral disc degeneration: its validity and meaning. Clin Spine Surg. 2017;30(5):E547–E553. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki A, Daubs MD, Inoue H, et al. Prevalence and motion characteristics of degenerative cervical spondylolisthesis in the symptomatic adult. Spine (Phila Pa 1976). 2013;38:E1115–E1120. [DOI] [PubMed] [Google Scholar]
- 16. Abdulkarim JA, Dhingsa R, Finlay DBL. Magnetic resonance imaging of the cervical spine: frequency of degenerative changes in the intervertebral disc with relation to age. Clin Radiol. 2003;58:980–984. [DOI] [PubMed] [Google Scholar]
- 17. Christe A, Laubli R, Guzman R, et al. Degeneration of the cervical disc: histology compared with radiography and magnetic resonance imaging. Neuroradiology. 2005;47:721–729. [DOI] [PubMed] [Google Scholar]
- 18. Kettler A, Rohlmann F, Neidlinger-Wilke C, Werner K, Claes L, Wilke HJ. Validity and interobserver agreement of a new radiographic grading system for intervertebral disc degeneration: part II. Cervical spine. Eur Spine J. 2006;15:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kettler A, Wilke HJ. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur Spine J. 2006;15:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kolstad F, Myhr G, Kvistad KA, Nygaard OP, Leivseth G. Degeneration and height of cervical discs classified from MRI compared with precise height measurements from radiographs. Eur J Radiol. 2005;55:415–420. [DOI] [PubMed] [Google Scholar]
- 21. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873–1878. [DOI] [PubMed] [Google Scholar]
- 22. Hussain M, Natarajan RN, An HS, Andersson GB. Motion changes in adjacent segments due to moderate and severe degeneration in C5-C6 disc: a poroelastic C3-T1 finite element model study. Spine (Phila Pa 1976). 2010;35:939–947. [DOI] [PubMed] [Google Scholar]