Abstract
Study Design:
Cross-sectional study.
Objectives:
To continue the line of a previous publication using steroid for acute spinal cord injury (SCI) by spine surgeons from Latin America (LA) and assess the current status of methylprednisolone (MP) prescription in Europe (EU), Asia Pacific (AP), North America (NA), and Middle East (ME) to determine targets for educational activities suitable for each region.
Methods:
The English version of a previously published questionnaire was used to evaluate opinions about MP administration in acute SCI in LA, EU, AP, NA, and ME. This Internet-based survey was conducted by members of AOSpine. The questionnaire asked about demographic features, background with management of spine trauma patients, routine administration of MP in acute SCI, and reasons for MP administration.
Results:
A total of 2659 responses were obtained for the electronic questionnaire from LA, EU, AP, NA, and ME. The number of spine surgeons that treat SCI was 2206 (83%). The steroid was used by 1198 (52.9%) surgeons. The uses of MP were based predominantly on the National Acute Spinal Cord Injury Study III study (n = 595, 50%). The answers were most frequently given by spine surgeons from AP, ME, and LA. These regions presented a statistically significant difference from North America (P < .001). The number of SCI patients treated per year inversely influenced the use of MP. The higher the number of patients treated, the lower the administration rates of MP observed.
Conclusions:
The study identified potential targets for educational campaigns, aiming to reduce inappropriate practices of MP administration.
Keywords: spinal cord injury, treatment, corticosteroid, survey
Introduction
There has always been great interest in discovering effective treatments for spinal cord injury (SCI), since it is a debilitating pathology with considerable socioeconomic consequences.1–4 Over the years, publications involving the use of methylprednisolone (MP) after acute SCI have described conflicting results. While some articles demonstrated efficacy5–8 others showed no benefits.9,10
Currently, routine use of MP for SCI is not recommended because of a higher incidence of complications and no evident efficacy.11–14 Nevertheless, MP is still used worldwide to treat acute SCI.14,15 A recent publication about the use of MP by Latin American spine surgeons showed that surgeons reported using MP for acute SCI, mainly because of a belief in clinical benefits, fear of litigation, and because it was a protocol in the hospital.13 During the past years, several countries have evaluated patterns of clinical practices in patients with SCI through national surveys.4,12–23
The objective of this study was to continue the line of a previous publication involving spine surgeons from Latin America (LA) and assess the current status of worldwide MP prescription for acute SCI in Europe (EU), Asia Pacific (AP), North America (NA), and Middle East (ME). The worldwide overview on the use of MP, its reasons, and beliefs of different regions will provide a guide for educational activities.
Methods
Population Studied
E-mails were sent to AOSpine members from LA, EU, AP, NA, and ME with a cover letter explaining the objective and importance of this study and an attached link to SurveyMonkey. AOSpine is an internationally recognized professional community with 6403 members in LA (n = 1445), EU (n = 1528), AP (n = 2263), NA (n = 530), and ME (n = 637) with clinical and research interest in spine care (as of April 2017). The Portuguese-language and Spanish-language versions were applied to LA members and the English-language version to the other regions. The link was available for 15 days, and reminders were sent 3 times during this period. The LA results were reported in 2016.13
Assessment
The Hurlbert and Hamilton16 questionnaire was used to evaluate attitudes and thoughts of spine surgeons worldwide about MP administration after acute SCI. The electronic questionnaire contained topics regarding demographic features of participants, experience in spine trauma, routine administration of MP in acute SCI, reasons for MP administration, knowledge about the National Acute Spinal Cord Injury Study (NASCIS) results, and if their opinion about MP had changed in the past 5 years. The average time to complete the questionnaire was 5 minutes.
Statistical Analyses
Categorical data was presented as counts and percentages and were compared using χ2 test followed by Bonferroni adjustments for pairwise comparisons. Additionally, crude and adjusted odds ratios (ORs) and their respective 95% confidence intervals (CIs) were computed using multivariable logistic regression. There was no specific variable selection procedure. All variables of interest (region, specialty, time in practice, number of patients treated annually, and critical evaluation of NASCIS studies) were included in the model. Significance level was set at P < .05. Data was processed and analyzed using SPSS version 22.0.
Results
Contributions by Regions
A total of 2659 AOSpine members from LA, EU, AP, NA, and ME answered the electronic questionnaire (Figure 1). Table 1 lists the contributions by region and the 5 countries from which more answers were obtained. The majority of the answers came from surgeons of LA (n = 972, 36.6%) and EU (n = 718, 27%), and the country with the most questionnaires answered was Brazil (n = 252), followed by the United States and Mexico, both with 167 participants (Table 1).
Figure 1.

Percentage of respondents in each region based on number of AOSpine members.
Table 1.
List of the Top 5 Countries That Contributed to the Survey in Each Region.
| Regions (n, %) | Countries (n, %) |
|---|---|
| Latin America (972, 36.6%) | 1. Brazil (252, 26.0%) 2. Mexico (167, 17.1%) 3. Argentina (138, 14.2%) 4. Colombia (79, 8.1%) 5. Chile (54, 5.5%) |
| Europe (718, 27.0%) | 1. Italy (105, 14.6%) 2. United Kingdom (71, 9.8%) 3. Germany (62, 8.6%) 4. Spain (56, 7.8%) 5. Turkey (45, 6.2%) |
| Asia Pacific (529, 19.9%) | 1. India (122, 23.0%) 2. Japan (74, 14.0%) 3. Korea (52, 9.8%) 4. China (39, 7.3%) 5. Taiwan (32, 6.0%) |
| North America (228, 8.6%) | 1. USA (167, 73.2%) 2. Canada (48, 21.0%) |
| Middle East (212, 8.0%) | 1. Iraq (19, 9.0%) 2. Iran (18, 8.5%) 3. Pakistan (14, 6.6%) 4. Egypt (13, 6.1%) 5. Jordan (10, 4.7%) |
| Total (2,659, 100.0%) |
Baseline Characteristics
The number of spine surgeons that treat SCI was 2206 (83%), and steroid therapy was used by 1198 (52.9%) surgeons (Table 2, Figure 2). The uses of MP were based predominantly on the NASCIS III study (n = 595, 50%), mainly to improve patient recovery (n = 716, 49.3%; Table 2). Steroid therapy was considered a recommendation by 18.9% of spine surgeons and as an optional therapy by 40.7% (Table 2).
Table 2.
Baseline Characteristics of the Spinal Surgeonsa.
| Characteristics | Total (n = 2659) |
|---|---|
| Specialty | |
| Orthopedic | 1532 (57.6) |
| Neurosurgery | 1127 (42.4) |
| Practice in spine surgery | |
| <10 years | 1247 (46.9) |
| 10-20 years | 819 (30.8) |
| >20 years | 593 (22.3) |
| Yearly treatment of patients with traumatic SCI | |
| I don’t treat SCI | 453 (17.0) |
| <10 | 796 (29.9) |
| 10-20 | 744 (27.9) |
| 20-40 | 392 (14.7) |
| >40 | 273 (10.2) |
| MP administration after SCI | |
| I don’t use MP | 1008 (47.1) |
| NASCIS I study | 88 (4.0) |
| NASCIS II study | 357 (15.8) |
| NASCIS III study | 595 (26.0) |
| Different MP administration | 158 (7.1) |
| Reasons for MP administration | |
| Improve patient recovery | 716 (33.3) |
| Avoid legal problems | 336 (15.8) |
| Protocol in the department | 303 (14.3) |
| No adverse effects on patient | 87 (4.1) |
| MP therapy is considered | |
| Its use is not advised | 675 (27.1) |
| Conventional | 188 (7.6) |
| Recommended | 470 (18.9) |
| Optional | 1013 (40.7) |
| Experimental | 141 (5.3) |
Abbreviations: SCI, spinal cord injury; MP, methylprednisolone; NASCIS, National Acute Spinal Cord Injury Study.
aData is presented as counts (percentages).
Figure 2.

Percentage of methylprednisolone use per region.
Administration of Steroid Therapy Among Regions
Data of the routine administration of steroid therapy by spinal surgeons from different regions is provided in Table 3. MP administration to improve patient recovery was a common reason for the administration of the steroid by spinal surgeons from AP, ME, and LA but not from NA (P < .001; Figure 3). The justification for administration of MP in order to avoid legal problems was similar in all regions except in NA, where the percentage was lower (P = .001). Spinal surgeons from ME (26.1%), AP (21.0%), and LA (20.9%) usually classify MP therapy as recommended therapy, significantly different from NA (P < .001), where the recommendation rate was 4.7% and who usually labeled it as optional therapy (Table 3, Figure 4). The majority of surgeons modified their routine use of MP due to scientific publications and congresses (n = 1296, 62.9%).
Table 3.
Experience of Spinal Surgeons With Steroid Therapy in Different Regions*.
| Characteristics | Total (n = 2125) | LA (n = 814) | EU (n = 546) | NA (n = 192) | ME (n = 164) | AP (n = 409) | P |
|---|---|---|---|---|---|---|---|
| Reasons for MP administration | |||||||
| Improve patient recovery | 708 (33.3) | 257 (31.6)a | 166 (30.3)a,b | 39 (20.3)b | 65 (39.6)a | 181 (44.3)a | <.001 |
| Avoid legal problems | 336 (15.8) | 135 (16.6)a | 86 (15.8)a | 10 (5.2)b | 29 (17.7)a | 76 (18.7)a | .001 |
| Protocol in the department | 303 (14.3) | 123 (15.1)a | 74 (13.6)a | 9 (4.7)b | 29 (17.7)a | 68 (16.6)a | .001 |
| No adverse effects to patient | 87 (4.1) | 16 (2.0)a | 24 (4.4)a,b | 6 (3.1)a,b | 10 (6.1)b | 31 (7.6)b | <.001 |
| MP administration according to age | <.001 | ||||||
| Young patient | 298 (27.1) | 138 (30.7)a | 51 (20.5)b | 27 (58.7)c | 24 (23.5)a,b | 59 (22.7)a,b | |
| Young and older patients | 803 (73.1) | 312 (69.4)a | 196 (79.5)b | 19 (41.3)c | 78 (76.5)a,b | 198 (77.1)a,b | |
| Awareness of critical evaluation of NASCIS studies | 1652 (80.7) | 649 (81.5)a | 438 (83.6)a | 179 (93.7)b | 89 (58.6)c | 297 (77.4)a | <.001 |
| MP therapy is considered | <.001 | ||||||
| Its use is not advised | 589 (28.6) | 228 (28.5)a,b | 183 (34.7)b,c | 76 (39.7)c | 29 (18.4)a,d | 73 (19.0)d | |
| Conventional | 146 (7.1) | 47 (5.9)a,b,c | 38 (7.3)c,d | 2 (1.0)b | 18 (11.5)a,c,d | 41 (10.6)d | |
| Recommended | 377 (18.3) | 166 (20.9)a,b | 80 (15.4)b | 9 (4.7)c | 41 (26.1)a | 81 (21.0)a,b | |
| Optional | 830 (40.3) | 317 (39.7)a,b | 191 (36.2)b | 96 (50.3)a | 57 (36.3)a,b | 169 (43.9)a,b | |
| Experimental | 117 (5.7) | 41 (5.1)a | 35 (6.7)a | 8 (4.2)a | 12 (7.6)a | 21 (5.5)a | |
| In the last 5 years my MP indication has | <.001 | ||||||
| Not changed | 759 (36.9) | 219 (27.5)a | 221 (41.9)b | 100 (52.4)b | 64 (41.3)b | 155 (40.4)b | |
| Changed by update from articles | 851 (41.2) | 409 (51.3)a | 206 (39.1)b | 5663 (29.3)b | 56 (36.1)b | 124 (32.3)b | |
| Changed by update from Congress and colleagues | 445 (21.7) | 170 (21.3)a,b | 100 (19.0)b | 35 (18.3)a,b | 35 (22.6)a,b | 105 (27.3)a | |
Abbreviations: LA, Latin America; EU, European Union; NA, North America; ME, Middle East; AP, Asia Pacific; MP, methylprednisolone; NASCIS, National Acute Spinal Cord Injury Study.
*Data is presented as counts (percentages).
aDifferent letters indicate significant differences between groups (P < .05).
bDifferent letters indicate significant differences between groups (P < .05).
cDifferent letters indicate significant differences between groups (P < .05).
Figure 3.

Reasons to administer methylprednisolone in acute spinal cord injury according to country.
Figure 4.

Methylprednisolone therapy indication by region.
A multivariate logistic regression analysis of risk factors associated with routine administration of MP in different regions of the world is presented in Table 4. The LA, EU, AP, and ME regions had 2.63 to 5.56 more chances to use MP than NA. No difference was observed between specialties (P = .53). The use of steroid was not related to the time of practice. The number of patients treated annually between 10 and 20 (adjusted OR= 0.73, 95% CI = 0.56-0.96, P = .026) and between 20 and 40 (adjusted OR = 0.66, 95% CI = 0.49-0.89, P = .007) had a reduction of MP administration having as reference the number of patients treated annually were more than 40 (Table 4, Figure 5). Critical reading of the NASCIS studies showed avoidance of the use of MP and showed a significant association in crude analyses (P = .001) that disappears after adjustment of the risk factors (P = .067).
Table 4.
Logistic Regression Analysis With Factors Associated With Routine Administration of Methylprednisolone in Different Regions of the World.
| Characteristics | Use MP (%), n = 1122 | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| Regions | |||||
| Latin America | 56.0 | 4.06 (2.84-5.82) | <.001 | 4.08 (2.83-5.89) | <.001 |
| Europe | 46.9 | 2.80 (1.93-4.06) | <.001 | 2.63 (1.80-3.85) | <.001 |
| Asia Pacific | 64.1 | 5.68 (3.85-8.37) | <.001 | 5.56 (3.73-8.28) | <.001 |
| Middle East | 62.2 | 5.22 (3.30-8.25) | <.001 | 5.31 (3.28-8.61) | <.001 |
| North America | 24.0 | Ref. | Ref. | Ref | Ref. |
| Specialty | |||||
| Orthopedic | 54.6 | 1.17 (0.99-1.39) | .064 | 1.05 (0.88-1.27) | .539 |
| Neurosurgery | 50.7 | Ref | Ref | Ref | Ref |
| Practice in spine surgery | |||||
| <10 years | 51.7 | 1.13 (0.93-1.38) | .209 | 1.21 (0.98-1.49) | .075 |
| 10-20 years | 54.3 | 1.09 (0.87-1.36) | .425 | 1.12 (0.88-1.41) | .338 |
| >20 years | 53.5 | Ref. | Ref. | Ref. | Ref. |
| Number of patients with traumatic SCI treated annually | |||||
| <10 | 55.8 | 0.86 (0.70-1.06) | .170 | 0.89 (0.71-1.11) | .311 |
| 10-20 | 52.4 | 0.81 (0.63-1.04) | .110 | 0.73 (0.56-0.96) | .026 |
| 20-40 | 50.8 | 0.69 (0.52-0.91) | .010 | 0.66 (0.49-0.89) | .007 |
| >40 | 47.1 | Ref. | Ref. | Ref. | Ref. |
| Critical evaluation of NASCIS studies | |||||
| Yes | 50.8 | 0.69 (0.55-0.86) | .001 | 0.80 (0.63-1.01) | .067 |
| No | 60.0 | Ref. | Ref. | Ref. | Ref. |
Abbreviations: MP, methylprednisolone; OR, odds ratio; CI, confidence interval; SCI, spinal cord injury; NASCIS, National Acute Spinal Cord Injury Study.
Figure 5.

Significant influence of the number of patients treated with methylprednisolone administration in traumatic spinal cord injury patients.
Risk Factors Associated With Litigation
A multivariate logistic regression analysis of risk factors associated with litigation as an indication to use MP in order to avoid litigation is presented in Table 5. All regions had a significant increase of MP administration to avoid lawsuits, taking NA as reference (P < .001 for all regions except for ME where it was P = .001). Orthopedists use more MP to avoid legal problems when compared to neurosurgeons (adjusted OR = 1.55, 95% CI = 1.20-1.99, P < .001). The increasing numbers of years of practice and the number of patients treated annually had no association with the indication of MP to avoid lawsuits in the multiple regression analysis. However, a tendency to use less MP to avoid legal problems was observed in spinal surgeons who treat less than 10 and more than 10, 20, and 40 patients per year (55.8%, 52.4%, 50.8%, 47.1%, respectively). The use of MP to avoid litigation was more often observed in spine surgeons who did not critically evaluate the NASCIS studies (19.7%) when compared with those that critically evaluate the literature (14.8%) but without statistical significance.
Table 5.
Logistic Regression Analysis With Factors Associated With Litigation as Indication to Use Methylprednisolone in Traumatic Spinal Cord Injury.
| Characteristics | Litigation (%), n = 336 | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| Regions | |||||
| Latin America | 16.6 | 3.54 (1.83-6.85) | <.001 | 3.52 (1.80-6.86) | <.001 |
| Europe | 15.8 | 3.00 (1.53-5.88) | .001 | 3.39 (1.71-6.71) | <.001 |
| Asia Pacific | 18.6 | 3.77 (1.91-7.42) | <.001 | 3.95 (1.98-7.89) | <.001 |
| Middle East | 17.7 | 3.59 (1.71-7.54) | .001 | 3.70 (1.71-8.02) | .001 |
| North America | 5.2 | Ref. | Ref. | Ref | Ref. |
| Specialty | |||||
| Orthopedic | 19.0 | 1.36 (1.08-1.73) | .009 | 1.55 (1.20-1.99) | .001 |
| Neurosurgery | 12.3 | Ref. | Ref. | Ref. | Ref. |
| Practice in spine surgery | |||||
| <10 years | 15.8 | 1.04 (0.80-1.36) | .741 | 0.99 (0.74-1.31) | .957 |
| 10-20 years | 15.2 | 1.14 (0.85-1.52) | .368 | 1.04 (0.76-1.41) | .793 |
| >20 years | 16.8 | Ref. | Ref. | Ref. | Ref. |
| Number of patients with traumatic SCI treated annually | |||||
| <10 | 18.6 | 0.81 (0.61-1.06) | .131 | 0.89 (0.67-1.18) | .437 |
| 10-20 | 15.4 | 0.70 (0.49-0.99) | .049 | 0.74 (0.52-1.07 | .118 |
| 20-40 | 13.4 | 0.62 (0.42-0.93) | .023 | 0.71 (0.47-1.08) | .115 |
| >40 | 12.5 | Ref. | Ref. | Ref. | Ref. |
| Critical evaluation of NASCIS studies | |||||
| Yes | 14.8 | 0.88 (0.67-1.16) | .388 | 0.79 (0.59-1.06) | .121 |
| No | 19.7 | Ref. | Ref. | Ref. | Ref. |
Abbreviations: MP, methylprednisolone; OR, odds ratio; CI, confidence interval; SCI, spinal cord injury; NASCIS, National Acute Spinal Cord Injury Study.
Discussion
SCI is an extremely debilitating condition, leading to high rates of morbidity, decreased life expectancy, and socioeconomic problems.4,17,18 Different modalities of therapy were studied to change the morbidity and mortality that includes early spinal cord decompression, hypothermia, stem cell transplantation, hyperbaric therapy, and steroids.7,19,20,24–30 The use of MP in SCI was standardized worldwide19,20,28 based on the NASCIS.7,29,30 Despite concerns about weak evidence regarding MP administration, the 2002 AANS/CNS guideline recommended MP as an option for the treatment of acute SCI.31 The present study shows that the steroid was used by 1198 (52.9%) of surgeons who treat SCI. The main justification for MP administration was to improve patient recovery (n = 716, 49.3%), and it was based predominantly on the NASCIS III study (n = 595, 50%).
Contrary evidence for MP administration after acute SCI was published in the 2012 AANS/CNS guideline.11,12 The authors concluded that besides the fact that there was no Class I or Class II evidence supporting any clinical benefit, there was sufficient Class I, Class II, and Class III evidence showing harmful complications including death caused by MP administration. Finally, it was highlighted that the US Food and Drug Administration had not approved MP for use in acute SCI.11,12 Those statements were criticized in light of the absence of an injury heterogeneity assessment and a possible synergistic neuroprotective effect with the combination of steroids and early surgery observed in STASCIS results.32 The Canadian multicenter Spinal Cord Injury Registry showed no difference in motor recovery with the use of NASCIS-II MP compared with a matched-cohort controlled by age, time of neurological exam, neurological level, and baseline severity of injury, but a difference was encountered in the complications rate, which was significantly higher in the MP group (61% vs 36%; P = .02).33 This data supports current guidelines against the use of high-dose corticosteroids in acute SCI due to its inefficacy and potential harmful side effects. The present study showed that spine surgeons still have a higher index of recommendation of MP after acute SCI mainly because they believe that the steroid administration improves patient outcome (n = 716, 33.3%). Factors independently associated with the use of MP were the following: not being from North America, treating less than 20 patients annually, and not performing a critical evaluation of NASCIS studies.
International surveys have demonstrated progressive changes in patterns of practice in many countries according to information from the literature (Figure 6). For example, the proportion of specialists that reported routine use of MP in Canada was reduced from 76% in 200115 to 24% in 2006.16 In the United Kingdom, this proportion decreased from 68% in 200420 to 19% in 2012.23 In Switzerland, this proportion decreased from 96% in 2001 to 2003 to 23% in 2008 to 2010.28 The reduction of MP administration was related to the scientific publication of the critical analysis of NASCIS studies and new high level of evidence publications showing the potential harmful side effects of steroids and the inefficacy or controversial benefits. The literature can support the spine surgeon’s decision to not use MP. Despite that, the present study showed that the surgeons still recommend the use of steroid because of fear of lawsuit (n = 336, 15.8%). In other international surveys, fear of lawsuit is reported by 25% to 48%.14–16,19,21,22 The factors independently associated with litigation in this study were the following: not being from North America, being an orthopedist, and treating less than 10 patients annually. This scenario was more evident in spine surgeons that did not critically evaluate the NASCIS studies (19.7%) when compared with those that critically evaluate the literature (14.8%).
Figure 6.
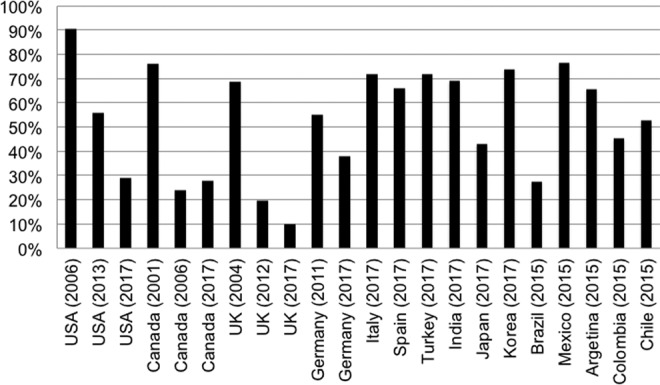
Percentage of steroid administration after acute spinal cord injury in international surveys.
The present study demonstrated that regions with less critical awareness of NASCIS studies such as LA, ME, and AP presented a greater tendency for administering MP to SCI when compared to NA. NA was the region with the highest percentage of critical awareness of the NASCIS studies (93.7%). It is noticeable that critical analysis of the literature leads to practices based on a more scientific background and reducing the influence of external factors, such as fear of litigation.
In the present study, all regions demonstrated the same response pattern of prescribing MP for both young and elderly patients, except for NA. Whenever surgeons from NA prescribe corticosteroids they have an inclination to prescribe it more for younger patients (58.7%).
Another factor that seemed to influence surgeons was that the use of MP for SCI is a protocol in some departments. The regions that were more influenced by this were ME (17.7%) and AP (16.6%). The fact that administration of MP is still a protocol in the service leads to its greater prescription.
Educational activities and clinical guidelines could help reduce the routine use of MP. There would be a greater impact and it would be more effective if these guidelines could be performed and consented to by the local entities.21 In the present study, the regions and the target population for educational campaigns were the independent factors associated with the use of MP: not being from NA treating less than 20 patients annually and not performing a critical evaluation of NASCIS studies.
It is recognized that this article has limitations. Even with a considerable number of questionnaire responses, this data may not represent the opinion of all orthopedists and neurosurgeons who deal with SCI in their regions. Furthermore, some countries were not covered or had very few responses. Despite those limitations, the methodology applied was similar to studies that were able to demonstrate changes in corticoid administration in SCI over the years.16 Therefore, our study will serve as a basis for future comparisons. This data will be important to AOSpine, a world-renowned organization that is constantly developing educational projects in spine care, in order to promote educational projects worldwide to reduce the routine use of MP.
Conclusion
This study found that more than half of the physicians who completed the questionnaires reported using MP for SCI (n = 1198, 52.9%). Factors independently associated with the use of MP were the following: not being from North America, treating less than 20 patients annually, and not performing a critical evaluation of NASCIS studies. The justification for administration of MP in order to avoid legal problems was observed in 15.8% of the surgeons with similar findings in the regions except in North America, where the lowest percentage was observed. The study identified potential targets for educational campaigns, aiming to reduce inappropriate practices of MP administration.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Dayan K, Keser A, Konyalioglu S, et al. The effect of hyperbaric oxygen on neuroregeneration following acute thoracic spinal cord injury. Life Sci. 2012;90:360–364. [DOI] [PubMed] [Google Scholar]
- 2. Hodgetts S, Plant G, Harvey A, Watson C, Paxinos G, Kayalioglu G. Spinal cord injury: experimental animal models and relation to human therapy In: Watson C, Paxinos G, Kayalioglu G, eds. The Spinal Cord. A Christopher and Dana Reeve Foundation Text and Atlas. London, England: Elsevier; 2009:209–237. [Google Scholar]
- 3. Rahimi-Movaghar V, Sayyah MK, Akbari H, et al. Epidemiology of traumatic spinal cord injury in developing countries: a systematic review. Neuroepidemiology. 2013;41:65–85. [DOI] [PubMed] [Google Scholar]
- 4. Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injuries. J Neurosurg. 1969;30:693–697. [DOI] [PubMed] [Google Scholar]
- 6. Brankman R, Schouten HJ, Blaauw-van Dishoeck M, Minderhoud JM. Megadose steroids in severe head injuries. Results of prospective double blind clinical trial. J Neurosurg.1983;58:326–330. [DOI] [PubMed] [Google Scholar]
- 7. Bracken MB, Shepard MJ, Collins WF, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. Results of the Second National Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. [DOI] [PubMed] [Google Scholar]
- 8. Bracken MB. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine (Phila Pa 1976). 2001;26(24 suppl):S47–S54. [DOI] [PubMed] [Google Scholar]
- 9. Bydon M, Lin J, Macki M, Gokaslan ZL, Bydon A. The current role of steroids in acute spinal cord injury. World Neurosurg. 2014;82:848–854. [DOI] [PubMed] [Google Scholar]
- 10. Readdy WJ, Chan AK, Matijakovich DJ, Dhall SD. A review and update on the guidelines for the acute non-operative management of cervical spinal cord injury. J Neurosurg Sci. 2015;59:119–128. [PubMed] [Google Scholar]
- 11. Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(suppl 2):93–105. [DOI] [PubMed] [Google Scholar]
- 12. Walters BC, Hadley MN, Hurlbert RJ, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(suppl 1):82–91. [DOI] [PubMed] [Google Scholar]
- 13. Teles AR, Cabrera J, Riew KD, Falavigna A. Steroid use for acute spinal cord injury in Latin America: a potentially dangerous practice guided by fear of lawsuit. World Neurosurg. 2016;88:342–349. [DOI] [PubMed] [Google Scholar]
- 14. Druschel C, Schaser KD, Schwab JM. Current practice of methylprednisolone administration for acute spinal cord injury in Germany: a national survey. Spine (Phila Pa 1976). 2013;38:E669–E677. [DOI] [PubMed] [Google Scholar]
- 15. Hurlbert RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci. 2002;29:236–239. [DOI] [PubMed] [Google Scholar]
- 16. Hurlbert RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41–45. [DOI] [PubMed] [Google Scholar]
- 17. Chamberlain JD, Meier S, Mader L, von Groote PM, Brinkhof MW. Mortality and longevity after a spinal cord injury: systematic review and meta-analysis. Neuroepidemiology. 2015;44:182–198. [DOI] [PubMed] [Google Scholar]
- 18. Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24:905–918. [DOI] [PubMed] [Google Scholar]
- 19. Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine (Phila Pa 1976). 2006;31:E250–E253. [DOI] [PubMed] [Google Scholar]
- 20. Frampton AE, Eynon CA. High dose methylprednisolone in the immediate management of acute, blunt spinal cord injury: what is the current practice in emergency departments, spinal units, and neurosurgical units in the UK? Emerg Med J. 2006;23:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miekisiak G, Kloc W, Janusz W, Kaczmarczyk J, Latka D, Zarzycki D. Current use of methylprednisolone for acute spinal cord injury in Poland: survey study. Eur J Orthop Surg Traumatol. 2014;24(suppl 1):S269–S273. [DOI] [PubMed] [Google Scholar]
- 22. Schroeder GD, Kwon BK, Eck JC, Savage JW, Hsu WK, Patel AA. Survey of Cervical Spine Research Society members on the use of high-dose steroids for acute spinal cord injuries. Spine (Phila Pa 1976). 2014;39:971–977. [DOI] [PubMed] [Google Scholar]
- 23. Werndle MC, Zoumprouli A, Sedgwick P, Papadopoulos MC. Variability in the treatment of acute spinal cord injury in the United Kingdom: results of a national survey. J Neurotrauma. 2012;29:880–888. [DOI] [PubMed] [Google Scholar]
- 24. Falavigna A, Teles AR, Velho MC, Kleber FD. Effects of hyperbaric oxygen therapy after spinal cord injury: systematic review. Coluna/Columna. 2009;8:330–336. [Google Scholar]
- 25. Yu Y, Matsuyama Y, Yanase M, et al. Effects of hyperbaric oxygen on GDNF expression and apoptosis in spinal cord injury. Neuroreport. 2004;15:2369–2373. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Zhang S, Luo M, Li Y. Hyperbaric oxygen therapy improves local microenvironment after spinal cord injury. Neural Regen Res. 2014;9:2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Felleiter P, Müller N, Schumann F, Felix O, Lierz P. Changes in the use of the methylprednisolone protocol for traumatic spinal cord injury in Switzerland. Spine (Phila Pa 1976). 2012;37:953–956. [DOI] [PubMed] [Google Scholar]
- 29. Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 30. Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 31. Apuzzo MLJ. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50(3 suppl):S63–S72. [DOI] [PubMed] [Google Scholar]
- 32. Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(suppl 1):36–42. [DOI] [PubMed] [Google Scholar]
- 33. Evaniew N, Noonan VK, Fallah N, et al. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian Multi-Center Spinal Cord Injury Registry. J Neurotrauma. 2015;32:1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]


