Abstract
Introduction
It has not been reported how long the follow-up study after carotid artery stenting (CAS) should be continued. The purpose of the present study is to clarify the dynamic change of the in-stent neointimal layer and residual arterial lumen by two years following CAS using three-dimensional computed tomography angiography (3D CTA) with volume rendering.
Methods
Thirty-six stented carotid arteries in 34 consecutive patients were examined by 3D CTA with volume rendering at two weeks and 3, 6, 12, 24 months of follow-up.
Results
An in-stent hypodense area could be detected in 10 of 36 (27.8%) carotid arteries at two weeks after CAS. In-stent hypodense areas gradually declined thereafter by three months. In the course of longer follow-up, the layer of the in-stent hypodense area (neointimal hyperplasia) continued to grow in size for up to 24 months. Patients with an in-stent hypodense area at two weeks have a thicker layer of neointimal hyperplasia at 24 months than patients without in-stent hypodense area at two weeks’ follow-up. The predictive factors for growing neointimal hyperplasia at 24 months in multiple regression analysis are ulcer formation in pretreatment stenosis and the thickness of in-stent hypodense area at two weeks following CAS.
Conclusion
Our results suggest that follow-up study should be continued for a longer period even if in-stent restenosis could not be detected at one year following CAS. Especially in cases with ulcer formation in pretreatment stenosis and with a subacute in-stent hypodense area after CAS, longer follow-up is strongly recommended.
Keywords: Carotid artery stenting, in-stent thrombus, neointimal hyperplasia, neoatherosclerosis, restenosis
Introduction
The methods used in the treatment of carotid artery stenosis continue to evolve. Carotid artery angioplasty in combination with stent placement offers a promising alternative to carotid endarterectomy for certain patient populations.1–3 As a result, the number of patients receiving carotid artery stenting (CAS) is dramatically increasing. Hence, determining an appropriate radiographic follow-up period for such patients is important. It has been reported that in-stent restenosis after CAS is likely to occur within one year.4 Current restenosis rates are reported to be between 2% and 10%.2,5–7 However, systematic reports on long-term dynamic changes in the lumen of stents after CAS are still lacking.
It has previously been reported that carotid three-dimensional computed tomography angiography (3D CTA) with volume rendering is a reliable diagnostic modality in the evaluation of residual or recurrent stenosis and intraluminal thrombus formation in mid-term follow-up after CAS.7 Other reports have also shown the effectiveness of 3D CTA in evaluating carotid artery stenosis and residual arterial lumen after CAS.8,9
The purpose of the present study was to undertake a longer-term follow-up, evaluating the sequential changes in in-stent residual arterial lumen, stent diameter and in-stent hypodense area formation. Our study assessed these parameters for a period of two years after CAS using a multidetector 3D CTA with volume rendering. Our study also aimed to better clarify the dynamic changes in in-stent lumen assessed over a longer follow-up period than that in previous studies.
Materials and methods
Study design
The study included patients who had successfully undergone elective stent implantation in the carotid artery between January 2004 and June 2008. All patients were followed up for a period of two years after CAS using 3D CTA. Continual clinical and radiological follow-up examinations, including sequential 3D CTA, were performed at two weeks and 3, 6, 12, and 24 months after CAS. Axial and longitudinal reconstruction of CT imaging data was used to assess the diameter and configuration of the stent, in-stent hypodense area formation, the perfused lumen within the stent, and delineation of the stenosis.
All procedures were performed in accordance with a protocol approved by the institutional review board at the Murakami Memorial Hospital. Written informed consent was obtained from all patients.
Inclusion and exclusion criteria
Patients with symptomatic stenosis in the internal carotid artery (ICA) >50% according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria and asymptomatic patients with stenosis >80% were eligible for inclusion.1,10 In addition, any patients with impaired renal function (serum blood urea nitrogen (BUN) > 20 and/or creatinine > 2.0) were excluded from the 3D CTA follow-up.
Procedural technique of CAS
Patients were pretreated with aspirin (100 mg/day) and either ticlopidine (200 mg/day) or clopidogrel (75 mg/day) for a minimum of three days before undergoing the procedure. Carotid angioplasty and stenting were performed via transfemoral catheterization under local anesthesia with full heparinization. Either a PercuSurge GuardWire or an Angioguard XP was used throughout the procedure as a distal protection device. Predilatation was performed with a controlled semi-compliant balloon dilatation catheter. Balloon size was selected according to the normal luminal diameter of each ICA immediately distal to the stenotic segment. A self-expandable stent (Precise stent, Johnson & Johnson Cordis Endovascular Systems, NJ, USA) was then deployed covering the stenotic lesion with a sufficient margin. Post-stenting dilatation was omitted to prevent embolic complications. Intravenous argatroban was continued for 24 hours after the procedure (60 mg/24 hours). In some cases, because of the carotid sinus reflex, hypotension occurred with systolic blood pressure of less than 100 mmHg. Consequently, intravenous catecholamine was administered to maintain adequate blood pressure.
3D CTA
All patients underwent 3D CTA with a 16-channel multi-detector row spiral CT scanner (LightSpeed Ultra: General Electric Medical Systems, Milwaukee, WI, USA). Imaging parameters used were 120 kV, 250 mAs, 0.6 mm section thickness, 5 mm/s table feed, 0.6 second gantry rotation time, and a pitch of 1.3. The contrast agent used was Iopamiron (Ehzai, Tokyo, Japan), 370 mg of iodine/ml. The contrast volume used was 60 ml, without a saline chaser, at a flow rate of 4 ml/s. A 1.5-mm (20-gauge) cannula through the antecubital vein with a power injector was used to administer the contrast. For optimal intraluminal contrast enhancement, the delay time between the start of contrast material administration and the start of scanning was determined for each patient individually using a test-injection technique. The scan delay was determined by placing a region of interest in the lumen of the distal common carotid artery with an area of 3–5 mm2, followed by a 10 ml bolus of Iopamiron administered at 4 ml/s. The time after 2 seconds to reach the peak target based on a time-attenuation curve was used as the scanning delay. A total volume of 50 ml of Iopamiron was administered at a rate of 4 ml/s. Scanning delay ranged from 16 to 26 seconds. Spiral scanning included the volume between the sixth cervical vertebra and the foramen magnum. Patients were asked to breathe evenly and smoothly without swallowing or moving.
Image analysis
The axial images were loaded onto a separate workstation (ADW 4.0; GE Medical Systems, Milwaukee, WI, USA) for further analysis. A window width of 600–800 and window level of 200–400 were selected for the interactive interpretation of the carotid CTA studies and for the subsequent multiplane reformation measurements. Paraxial, paracoronal, and parasagittal planes were used in order to clearly visualize in-stent hypodense formation.
In-stent hypodense areas were measured at the thickest part in the paraxial image and at the narrowest part of the perfusion lumen in the parasagittal image according to the NASCET criteria methods. In-stent perfusion lumen diameter was measured as the shortest diameter on the same paraxial image as the in-stent hypodense area was measured. Stent diameter was also measured in the direction parallel to that used to measure it on the same paraxial image.
Statistical analysis
Statistical analysis was performed with descriptive statistics presented as the mean ± standard deviation (SD). Student’s t tests were used for the univariate analysis, and the chi-square test was applied for the univariate analysis of dichotomous variables. Fisher’s exact probability test was used for the proportion analysis. Independent predictive factors underwent multiple regression analysis. Values of p < 0.05 were considered to be significant.
Results
Between January 2004 and June 2008, 34 patients underwent extracranial carotid artery angioplasty and stent placement at the Murakami Memorial Hospital. These patients were followed up for a period of two years after CAS. Of these patients, two underwent bilateral stent placement. Therefore, the study consisted of 36 carotid arteries in 34 patients. Thirty-one of the 34 patients were male, and three were female. The mean age was 70.3 ± 6.42 years (range: 53–82 years). Mean stenosis was 80.2% ± 13.2% (range: 55%–99%), according to NASCET criteria.11 All carotid stenoses were thus successfully dilated. Carotid artery stenting achieved an increase in luminal caliber from 55% to 99% pretreatment to a residual stenosis of 0–40% post-stenting (mean residual stenosis 6.06 ± 12.2%), according to the NASCET criteria.
In addition, patients tolerated the procedures well and none of the patients exhibited any new ischemic symptoms. All stroke patients demonstrated a favorable functional recovery (modified Rankin disability score: 0, 1, or 2).
The use of a high-spatial-frequency convolution algorithm and appropriate windows allowed us to differentiate the wall of the stent from the enhanced arterial lumen (perfusion lumen) within the stent in all patients. Volume-rendered images showed an accurate delineation of the enhanced arterial lumen through the stent despite the thickness of the stent wall, which appeared to have increased to some degree. Moreover, in some cases, the hypodense area could be detected between the perfusion lumen and stent wall (in-stent hypodense area) during the follow-up period.
No significant in-stent restenosis (>50% diameter reduction) was observed in this study during the 24-month follow-up. Additionally, there were no cerebral ischemic events experienced by any of the patients during the follow-up.
Results throughout the two-year follow-up period after CAS
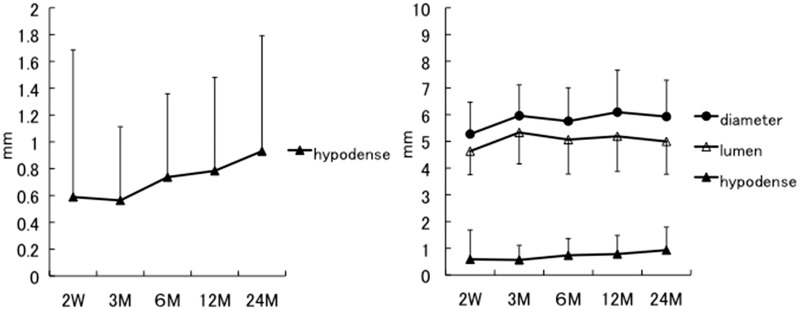
The sequential changes in in-stent hypodense area thickness, stent diameter, and perfusion lumen diameter in 36 carotid arteries following CAS are shown in Figure 1.
Figure 1.
These graphs demonstrate the sequential change in 36 carotid arteries using three-dimensional computed tomography angiography following carotid artery stenting. The left graph shows the sequential change of only the in-stent hypodense areas. The right graph shows the sequential change of the diameter of the stent (shaded circles), the perfusion lumen (unshaded triangles) and the thickness of the in-stent hypodense areas (shaded triangles), on the same scale.
Thickness of the in-stent hypodense area, which tended to be unevenly distributed within the stent at two weeks, was slightly decreased at three months on average. In detail, the 0.59 mm average thickness at two weeks decreased to 0.56 mm at three months. In the course of further follow-up, this thin layer increased to 0.74 mm at 6 months, 0.78 mm at 12 months, and 0.93 mm at 24 months (shaded triangles, Figure 1).
Stent diameter gradually increased during the initial three months after CAS because of the continuous self-expansion of the stent, after which time the diameter remained stable. In detail, the 5.27 mm average stent diameter at two weeks gradually increased to 5.96 mm by three months. In the course of further follow-up, the stent diameter did not change significantly (5.76 mm at 6 months, 6.09 mm at 12 months, and 5.93 mm at 24 months) (shaded circles on right graph, Figure 1).
Perfusion lumen diameter gradually increased over the first three months after CAS because of the continuous expansion of the stent and the decrease in the in-stent hypodense area thickness. After three months, perfusion lumen diameter decreased gradually because of thickening of the in-stent hypodense area. Specifically, the perfusion lumen averaged 4.63 mm at two weeks, and gradually increased to 5.33 mm at three months. In the course of further follow-up, the perfusion lumen decreased gradually to an average of 5.07 mm at 6 months, 5.19 mm at 12 months, and 5.00 mm at 24 months (unshaded triangles on right graph, Figure 1).
A typical case presentation
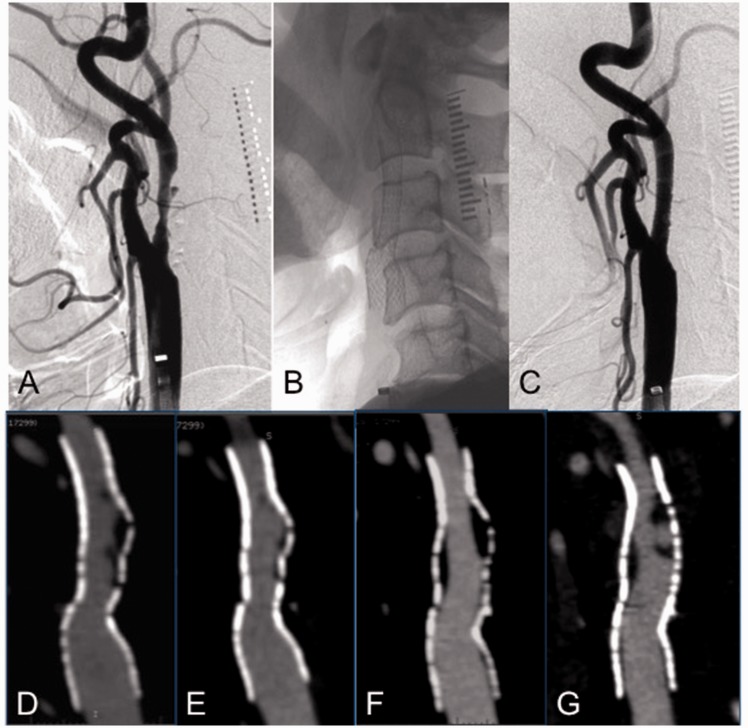
A 76-year-old man suffered from mild right hemiparesis and dysarthria. He had multiple small infarctions due to artery-to-artery embolisms caused by left ICA stenosis (approximately 60%) with ulcer formation (Figure 2(a)). He was treated with CAS. Predilatation was performed with a controlled semi-compliant balloon dilatation catheter (5 mm diameter, 4 cm length), with 7 atmospheres of pressure for a duration of 30 seconds. Subsequently, a Precise stent (10 mm diameter, 4 cm length) was deployed (Figure 2(b) and (c)). An in-stent hypodense area between the stent and the perfusion lumen was observed at two weeks following CAS (Figure 2(d)); however, the thickness of this area decreased remarkably at three months (Figure 2(e)). Conversely, the in-stent hypodense area appeared again at 12 months after CAS (Figure 2(f)) and continued to further increase in thickness with ulcer formation at 24 months (Figure 2(g)).
Figure 2.
A case of symptomatic left internal carotid artery stenosis (60%) with ulcer formation is shown. Upper images show lateral views of the digital subtraction angiography of left cervical carotid bifurcation. (a) Photo before carotid artery stenting. (b) Native image just after deployment of the stent. (c) Photo just after stenting. Lower images show the sequential change by parasagittal images of three-dimensional computed tomography angiography. (d) Parasagittal image at two weeks after stenting; the in-stent hypodense area is recognized between the stent and the perfusion lumen. (e) Parasagittal image at three months after stenting; the in-stent hypodense area is remarkably decreased. (f) Parasagittal image at 12 months after stenting; the in-stent hypodense area is increased again between the stent and the perfusion lumen. (g) Parasagittal image at 24 months after stenting; the in-stent hypodense area with ulcer formation is increasing again.
Follow-up results of the group with or without thrombus at two weeks after CAS
The significant change in morphology of the layer between the stent and perfusion lumen was sequentially assessed using 3D CTA. An in-stent hypodense area at two weeks after CAS was observed in 10 carotid arteries (27.8%).
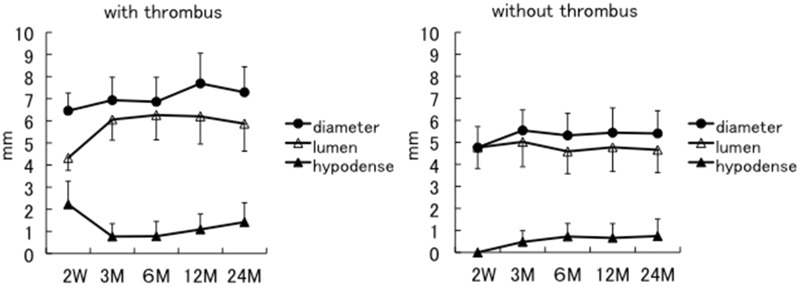
We analyzed the diameter of the stents and perfusion lumens as well as in-stent hypodense area thickness. The results were divided into two groups. One group (group with thrombus; N = 10), included the cases in which in-stent hypodense areas were recognized at two weeks after CAS (left graph in Figure 3). The other group (group without thrombus; N = 26), included the cases in which in-stent hypodense areas were not recognized at two weeks after CAS (right graph in Figure 3).
Figure 3.
The sequential change of the diameter of the stent (shaded circles), the perfusion lumen (unshaded triangles) and the thickness of in-stent hypodense areas (shaded triangles) are demonstrated. The left graph shows the sequential change in the group with thrombus: The in-stent hypodense areas were recognized at two weeks after carotid artery stenting. The right graph shows the sequential change in the group without thrombus: No hypodense areas were recognized at two weeks after stenting.
In the group with thrombus, the thickness of the in-stent hypodense area significantly decreased in the first three months after CAS; after that, the thickness gradually began to increase again (shaded triangles on left graph in Figure 3). Hence, perfusion lumen diameter remarkably increased in the first three months after CAS, and then gradually began to decrease again after that (unshaded triangles on left graphs in Figure 3). In detail, the average perfusion lumen diameter at two weeks was 4.32 mm and gradually increased to 6.06 mm at three months. In the course of further follow-up, the diameter decreased to 6.26 mm at 6 months, 6.20 mm at 12 months, and 5.87 mm at 24 months.
On the other hand, in the group without thrombus, the thickness of the in-stent hypodense area had increased at three months after CAS but did not change significantly after that (shaded triangles on right graph in Figure 3). The average perfusion lumen diameter also did not change significantly throughout the follow-up period (unshaded triangles on right graph in Figure 3). This is likely attributed to stent self-expansion occurring in the initial three months after CAS with little change after that (shaded circles on right graph in Figure 3). In detail, the 4.77 mm average perfusion lumen diameter at two weeks had only a minimal change to 5.02 mm at 3 months, 4.59 mm at 6 months, 4.78 mm at 12 months, and 4.66 mm at 24 months.
Differences between the group with and without thrombus at two weeks after CAS
The number of patients with hypertension, diabetes mellitus or hyperlipidemia was not significantly different between the group with and without thrombus. Likewise, the number of symptomatic cases, cases with calcification, or ulcer formation did not differ significantly between the two groups. Moreover, age, sex, rate of stenosis, stent diameter, stent length, stent position and residual stenosis after CAS were also not significantly different between the groups. The only parameter that showed a significant difference between the two groups was stenosis length (Table 1).
Table 1.
Risk factors of in-stent hypodense area at 2 weeks after carotid artery stenting.
| With thrombus | Without thrombus | P value | |
|---|---|---|---|
| Patient (number) (%) | 10 (27.8) | 26 (72.2) | – |
| Age (year) | 70.4 ± 6.95 | 70.2 ± 6.20 | 0.9311 |
| Male sex (number) (%) | 9 (90) | 24 (92.3) | 0.8224 |
| Hypertension (number) (%) | 8 (80) | 18 (69.2) | 0.581 |
| Diabetes mellitus (number) (%) | 3 (30) | 7 (26.9) | 0.8535 |
| Hyperlipidemia (number) (%) | 3 (30) | 5 (19.2) | 0.4863 |
| Symptomatic case (number) (%) | 6 (60) | 13 (50) | 0.5903 |
| Rate of stenosis (%) | 77.2 ± 12.8 | 81.3 ± 13.3 | 0.4148 |
| Length of stenosis (mm) | 22.0 ± 5.18 | 17.6 ± 5.71 | 0.049* |
| With calcification (number) (%) | 6 (60) | 10 (38.4) | 0.244 |
| With ulceration (number) (%) | 4 (40) | 3 (11.5) | 0.053 |
| Stent diameter (mm) | 9.2 ± 0.87 | 8.88 ± 0.85 | 0.3415 |
| Stent length (cm) | 3.8 ± 0.40 | 3.5 ± 0.69 | 0.2191 |
| Residual stenosis after carotid artery stenting (%) | 2.8 ± 8.40 | 7.3 ± 13.20 | 0.335 |
| Stent position from common carotid artery to internal carotid artery (number) (%) | 9 (90) | 20 (76.9) | 0.3745 |
Statistically significant
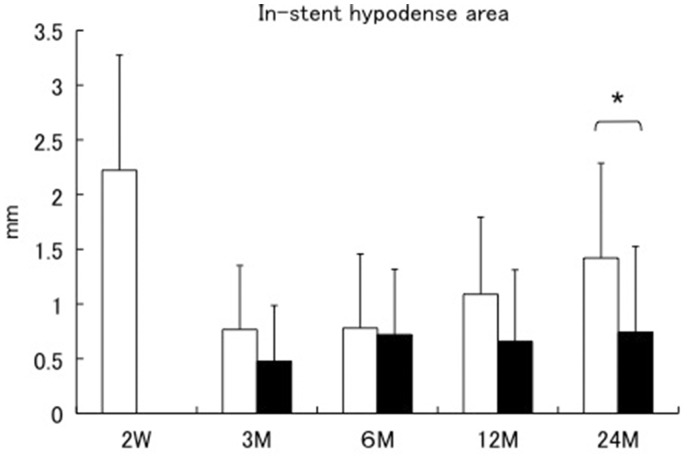
In the group with thrombus, the average thickness of the in-stent hypodense area (unshaded columns in Figure 4) continued to increase in the chronic phase. In comparison, the thickness of the in-stent hypodense areas within the group without thrombus (shaded columns in Figure 4) did not change significantly in the chronic phase. Moreover, there was a significant difference between the average thickness of the in-stent hypodense areas of the group with and without thrombus at 24 months after CAS (1.42 ± 0.87 mm vs 0.74 ±0.78 mm, respectively) (Figure 4).
Figure 4.
The sequential changes of the in-stent hypodense areas are separately demonstrated in the group with and without thrombus. Unshaded columns are the group with thrombus: These showed in-stent hypodense areas at two weeks after carotid artery stenting. Shaded columns are the group without thrombus: These did not show in-stent hypodense areas at two weeks after stenting. *Statistically significant.
Predicting factors for in-stent hypodense area at two years after CAS
Multivariate analysis revealed that pretreatment ulcer formation and thickness of the in-stent hypodense area at two weeks after CAS were independent predictive factors for the thickness of the in-stent hypodense area at 24 months after CAS (Table 2).
Table 2.
Predicting factors of the in-stent hypodense area at 2 years after carotid artery stenting.
| Statistically significant | ||
|---|---|---|
| Length of stenosis (mm) | 18.9 ± 5.90 | NS |
| With ulceration (number) (%) | 7 (19.4)* | P < 0.01 |
| Stent length (cm) | 3.6 ± 0.64 | NS |
| Thickness of the in-stent hypodense area at 2 weeks after carotid artery stenting (mm) | 0.59 ± 1.10* | P < 0.01 |
Statistically significant
Discussion
A major issue with CAS is the occurrence of in-stent neointimal growth, which may result in restenosis of the stented vessel.12,13 It has been reported that restenosis is likely to occur in 2%–10% of cases within 12 months after CAS.2,4–6,14 However, the present study showed restenosis is likely to occur after more than 12 months as the thickness of the in-stent hypodense area increases over time (Figure 1). This trend can especially be seen in the group with thrombus. The in-stent hypodense area of the group with thrombus was thicker than in the group without thrombus, remarkably, at 24 months after CAS (Figures 3 and 4). Recently, neointimal atherosclerotic changes inside the stent in the very late phase after coronary stenting have received significant attention in the field of coronary intervention.11,15,16 These findings of neointimal response, called “neoatherosclerosis,” have been assessed in coronary interventions by various imaging techniques and by the pathological features.
In our study, eccentric in-stent hypodense areas between the inner surface of a carotid stent and the perfusion lumen could be detected at two weeks after CAS. These hypodense areas decreased to undetectable levels for up to three months. After that, in-stent hypodense areas could again be detected at the chronic phase after CAS.
We believe that the eccentric in-stent hypodense area is thrombus in the subacute phase while it is neointimal hyperplasia in the chronic phase. This theory is supported by the fact that the hypodense area can be detected at an early phase after stent placement. Furthermore, the reduction in eccentric hypodense area to undetectable levels within three months may also reflect the removal of a thrombotic layer via endogenous thrombolysis. During this period, relative acute stent occlusions have been described in the literature.12 The experiences of other investigators may help to confirm our interpretation of these observations. Willfort-Ehringer et al. described the healing process following CAS with duplex sonography. According to their duplex data, an initial unstable phase was thus found in the early period starting one day after stent placement.13,17 The inner surface of the stent is covered by an echolucent layer, presumably a thrombotic layer, which diminishes to nearly zero or is no longer discernible at one month.13,17 In histological postmortem investigations, French and Rewcastle showed the site of the carotid endarterectomy to actually be covered by a thrombotic layer in the early phase and a neointimal layer thereafter.18 As a result, the substrate of the sonographic soft layer at the site of a recent thromboendoarterectomy may correspond with the thrombotic layer that has been documented histologically in postmortem specimens. Furthermore, the histological and immunohistochemical findings of Komatsu et al. in postmortem studies following coronary stenting are also worthy of mention. At 2–12 days after coronary stenting, the authors found thrombotic material without any cellular reaction “adjacent to the stent struts, and some cell mobilization in the deeper arterial layers.” From day 64 onward, a distinct layer of neointima was seen within the stents.19 On the other hand, Takano et al. reported that lipid-laden neointima, neointimal disruption, and thrombus as detected by optical coherence tomography (OCT) were more frequently seen in the late phase (≥5 years) compared to the early phase (<6 months) after bare metal stent implantation.16 Moreover, Matsumoto et al. has reported the neointimal atherosclerosis of very late (10-year) in-stent restenosis after CAS by OCT and plaque histology.20 Therefore, the in-stent hypodense areas at 24 months after CAS in our study may be “neoatherosclerosis.”
In the present study, the main risk factor for in-stent hypodense area formation at the acute phase (believed to be thrombus) following CAS was the presence of long stenotic lesions. The risk factors for in-stent hypodense chronic phase (believed to be neointimal hyperplasia) were ulcer formation within the stenotic lesion and in-stent thrombus formation following CAS. Moreover, Watarai et al. also reported that the risk factors for in-stent thrombus formation following CAS were patients with long stenotic lesions and long stents deployed across the carotid bifurcation. They reported that the eccentric in-stent hypodense areas, presumably the thrombotic layer, tended to occur on the dorsal surface at the carotid bifurcation level. They suggested that the site of predilatation for in-stent thrombus following CAS may correspond to the site of turbulence and/or reversal flow in the carotid artery, and that flow dynamics of the carotid artery may affect thrombus formation.7 The results that the ulcer formation of pretreatment plaque and the in-stent thrombus formation after CAS (which tends to occur in patients with long stenotic lesions) were risk factors for neointimal hyperplasia increasing in the present study may also lead to the hypothesis that the turbulent (or disturbed) flow in the carotid artery at the level of carotid bifurcation in which the stent has been deployed across the orifice of the external carotid artery causes not only subacute in-stent thrombus but also neointimal hyperplasia and in-stent restenosis via shear stress in the chronic phase. Several previous reports suggest endothelial shear stress may also contribute to the occurrence of complications upon stenting of atherosclerotic lesions in conjunction with other well-appreciated risk factors.21 Furthermore, such studies have reported that stenting induces changes in arterial geometry and consequently, in-flow and endothelial shear stress patterns modify the arterial response to endothelial injury, thereby increasing the risk of stent thrombosis and in-stent restenosis.21 We do not believe that turbulent or disturbed flow is the only mechanism leading to in-stent thrombus formation or neointimal hyperplasia. For example, plaque vulnerability may also contribute to in-stent thrombosis or neointimal hyperplasia. However, turbulent or disturbed flow is at least one of the factors associated with this.
Some of the limitations of this study are its small sample size, as well as the follow-up period being limited to only two years. These limitations do not allow us to draw any definitive conclusions regarding the dynamic changes in stent lumen at the acute, subacute, and chronic phases following CAS. Such dynamic changes still need to be confirmed in a larger study population with a longer follow-up period.
Conclusions
Pretreatment ulcer formation and the thickness of the in-stent hypodense area (thrombus) at two weeks after CAS were independent predictive factors for the thickness of the in-stent hypodense area (neointimal hyperplasia) at 24 months after CAS. Therefore, follow-up examinations should be continued even if in-stent restenosis is not detected at one year after CAS, especially in cases where there is ulcer formation in pretreatment stenosis or an in-stent hypodense area at the subacute phase after CAS.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 2.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 2008; 358: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 3.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gröschel K, Riecker A, Schulz JB, et al. Systematic review of early recurrent stenosis after carotid angioplasty and stenting. Stroke 2005; 36: 367–373. [DOI] [PubMed] [Google Scholar]
- 5.Wholey MH, Wholey M, Mathias K, et al. Global experience in cervical carotid artery stent placement. Catheter Cardiovasc Interv 2000; 50: 160–167. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol 2008; 7: 893–902. [DOI] [PubMed] [Google Scholar]
- 7.Watarai H, Kaku Y, Yamada M, et al. Follow-up study on in-stent thrombosis after carotid stenting using multidetector CT angiography. Neuroradiology 2009; 51: 243–251. [DOI] [PubMed] [Google Scholar]
- 8.Maintz D, Tombach B, Juergens KU, et al. Revealing in-stent stenoses of the iliac arteries: Comparison of multidetector CT with MR angiography and digital radiographic angiography in a Phantom model. AJR Am J Roentgenol 2002; 179: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 9.Leclerc X, Gauvrit JY, Pruvo JP. Usefulness of CT angiography with volume rendering after carotid angioplasty and stenting. AJR Am J Roentgenol 2000; 174: 820–822. [DOI] [PubMed] [Google Scholar]
- 10.North American Symptomatic Carotid Endarterectomy Trial Collaborators Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 11.Habara M, Terashima M, Nasu K, et al. Difference of tissue characteristics between early and very late restenosis lesions after bare-metal stent implantation: An optical coherence tomography study. Circ Cardiovasc Interv 2011; 4: 232–238. [DOI] [PubMed] [Google Scholar]
- 12.Robbin ML, Lockhart ME, Weber TM, et al. Carotid artery stents: Early and intermediate follow-up with Doppler US. Radiology 1997; 205: 749–756. [DOI] [PubMed] [Google Scholar]
- 13.Willfort-Ehringer A, Ahmadi R, Gschwandtner ME, et al. Single-center experience with carotid stent restenosis. J Endovasc Ther 2002; 9: 299–307. [DOI] [PubMed] [Google Scholar]
- 14.Heck D. Incidence and time course of carotid in-stent restenosis in a consecutive series of 295 patients. J Neurointerv Surg 2009; 1: 44–47. [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011; 57: 1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano M, Yamamoto M, Inami S, et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol 2009; 55: 26–32. [DOI] [PubMed] [Google Scholar]
- 17.Willfort-Ehringer A, Ahmadi R, Gruber D, et al. Arterial remodeling and hemodynamics in carotid stents: A prospective duplex ultrasound study over 2 years. J Vasc Surg 2004; 39: 728–734. [DOI] [PubMed] [Google Scholar]
- 18.French BN, Rewcastle NB. Sequential morphological changes at the site of carotid endarterectomy. J Neurosurg 1974; 41: 745–754. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu R, Ueda M, Naruko T, et al. Neointimal tissue response at sites of coronary stenting in humans: Macroscopic, histological, and immunohistochemical analyses. Circulation 1998; 98: 224–233. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto H, Yako R, Masuo O, et al. A case of in-stent neoatherosclerosis 10 years after carotid artery stent implantation: Observation with optical coherence tomography and plaque histological findings. Neurol Med Chir (Tokyo) 2014; 54: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koskinas KC, Chatzizisis YS, Antoniadis AP, et al. Role of endothelial shear stress in stent restenosis and thrombosis: Pathophysiologic mechanisms and implications for clinical translation. J Am Coll Cardiol 2012; 59: 1337–1349. [DOI] [PubMed] [Google Scholar]