Abstract
Purpose
Repeat imaging in patients with non-aneurysmal subarachnoid hemorrhage (NASAH) remains controversial. We aim to report our experience with NASAH with different hemorrhage patterns, and to investigate the need for further diagnostic workup to determine the underlying cause of hemorrhage.
Method
We conducted a retrospective analysis of all spontaneous SAH with an initial negative computed tomography (CT) with angiography (CTA) and/or digital subtraction angiography (DSA) from October 2011 through May 2017. According to the bleeding pattern on the admission CT scan, NASAH was divided into two subgroups: (1) perimesencephalic SAH (PMSAH) and (2) non-perimesencephalic SAH (nPMSAH). Radiological data included the admission CT, CTA, DSA, and magnetic resonance imaging (MRI) with angiography (MRA).
Results
Seventy-four patients met the inclusion criteria. Thirty-nine (52.7%) patients had PMSAH on the initial CT scan, and 35 (47.3%) had nPMSAH. All underwent CTA and/or DSA revealing no vascular abnormalities. Forty-seven (63.5%) patients underwent subsequent diagnostic workup. DSA was performed in all patients at least once. No abnormalities were found on the repeat DSA or other radiological follow-up studies except in one (1.4%) patient with nPMSAH, in whom a follow-up DSA revealed a small saccular anterior choroidal artery aneurysm, considered to be the source of hemorrhage.
Conclusion
A repeat DSA may not be needed in case of PMSAH, if the initial negative DSA is technically adequate with absence of hematoma and vasospasm. In contrast, a follow-up DSA should be mandatory for confirming or excluding vascular pathology in case of nPMSAH in order to prevent rebleeding.
Keywords: Non-aneurysmal subarachnoid hemorrhage, perimesencephalic subarachnoid hemorrhage, cerebral angiography, digital subtraction angiography
Introduction
Spontaneous subarachnoid hemorrhage (SAH) is a devastating clinical entity, and is commonly caused by a rupture of an intracranial aneurysm. However, in approximately 15%–20% of patients with SAH, the cause of the hemorrhage cannot be detected despite extensive diagnostic imaging studies, and these are termed non-aneurysmal subarachnoid hemorrhage (NASAH).1 Depending on the pattern of blood distribution in the subarachnoid space on the initial computed tomography (CT) scan, NASAH is usually divided into two subgroups: (1) perimesencephalic subarachnoid hemorrhage (PMSAH) and (2) non-PMSAH (nPMSAH). PMSAH is a mild variant of NASAH with a characteristic radiographic pattern of hemorrhage restricted to the perimesencephalic or prepontine cistern, while nPMSAH is characterized by a more widespread distribution of SAH with a visible extension into the anterior part of the ambient cistern or to the basal part of the Sylvian fissures, mimicking an aneurysmal SAH (ASAH)1–3 (see the complete description of PMSAH and nPMSAH under the section “Materials and methods”). The origin of bleeding in both hemorrhage patterns remains uncertain. Several potential causes have been suggested such as tearing of the venous structures at the tentorial edge, or rupturing of the fine arterial vessels adjacent to the mesencephalon, which might cause the hemorrhage.3–5 However, the mechanism of bleeding often remains unidentified.
Although the clinical presentation at time of onset in patients with NASAH is similar to those with ASAH, e.g. sudden onset of headache, meningeal irritation, photophobia, nausea or vomiting, etc., the clinical course and outcome are different between these groups. Particularly patients with PMSAH seem to have an excellent clinical outcome with a substantially lower risk of vasospasm and hydrocephalus. In addition, they do not have a reduced life expectancy compared to patients with nPMSAH and ASAH.5–10 However, the distinction between PMSAH and nPMSAH is somewhat vague in some studies,11 leading to a possible ambiguity in the clinical outcome of the evaluated patients.
Traditionally, patients with NASAH and an initial negative CT angiogram (CTA) and/or digital subtraction angiography (DSA) at the time of admission often undergo subsequent radiological examinations including secondary DSA to identify the cause of bleeding. In the recent decade, however, there has been some debate regarding the need for further diagnostic workup in patients with NASAH.11,12 Currently, there are no internationally accepted guidelines for this particular group. Owing to the benign course and the favorable prognosis, skipping the repeat DSA has become a common practice in the management of these patients in most institutions worldwide, especially with PMSAH.13,14 At our institution, the repeat DSA is not routinely performed in patients with the perimesencephalic pattern, if the initial DSA is of high quality. The need for a repeat DSA also relies on the discretion of the treating neurosurgeon and neuroradiologist. Furthermore, recent advances in CTA have led to substantial increases in its use as a primary and often only technique for workup in patients with PMSAH, as well as a follow-up examination.15–17
In this retrospective study, we report our experience with patients with NASAH including perimesencephalic and non-perimesencephalic hemorrhage patterns, and we investigated the value of more extensive diagnostic workup in order to determine the cause of bleeding.
Materials and methods
Patient characteristics
In this retrospective, observational single-center study, a total of 639 patients with spontaneous SAH were admitted to the Department of Neurosurgery, Aarhus University Hospital, Denmark, from October 2011 through May 2017. Diagnosis of NASAH was confirmed by a typical history of sudden, severe headache and a positive cerebral CT scan with basal SAH, within 72 hours after onset of the ictus. Only patients with no vascular abnormality on the initial CTA and/or DSA were included in the study. Patients with NASAH over the cerebral convexities and negative CT scan with positive lumbar puncture (LP) were not included in the study. Neither SAH associated with arteriovenous malformation (AVM) nor traumatic SAH was included. Furthermore, patients with CT scans performed 72 hours after the ictus were excluded as well owing to possible washout and resorption of blood. The included patients were divided into two subgroups according to the blood distribution on the initial CT scan: (1) Group A with a hemorrhage pattern of PMSAH and (2) Group B with a hemorrhage pattern of nPMSAH. Patients’ medical data were extracted from the electronic patient charts, and were reviewed for demographic information, previous medical history, antithrombotic medication, and vascular risk factor. The neurological status at the time of admission was based on World Federation of Neurological Surgeon (WFNS) grade and Hunt and Hess (HH) grading systems. Initial CTA and DSA reports, clinical course with possible complications attributable to the hemorrhage (e.g. presence of vasospasm, acute/late hydrocephalus) and subsequent investigation (e.g. repeat CTA or DSA, magnetic resonance angiography (MRA) of the brain and/or cervical spine) were recorded. The long-term clinical outcome was assessed according to the Glasgow Outcome Scale (GOS) and the modified Rankin Scale (mRS) scores after three months, obtained from the patient charts.
Approvals
This study was approved by the Danish Data Protection Agency (1-16-02-609-16) and the Local Health Research Ethical Committee (3-3013-1928/1). Verbal and written informed consent was obtained from all patients included in the study as part of the clinical routine before the diagnostic workup.
Definition of PMSAH and nPMSAH
Both hemorrhage patterns of NASAH were defined according to the criteria described by Rinkel et al.:1–3
PMSAH was classified as hemorrhage located immediately anterior to the midbrain, with or without extension of blood to the anterior part of the ambient cistern or to the basal part of the Sylvian fissures, and no complete filling of the anterior interhemispheric fissure and extension to the lateral Sylvian fissures with no more than a minute amount of blood. The sedimentation of a small amount of intraventricular blood in the occipital horns was allowed, but not frank intraventricular hemorrhage.1
nPMSAH was classified as a SAH that did not meet all of abovementioned criteria, and had a more widespread distribution of the subarachnoid blood resembling an ASAH.
Cerebral and catheter angiographic techniques
All CTA examinations included bilateral internal carotid arteries and vertebral arteries, and were performed on a multidetector CT scanner (a Philips Brilliance 40 (Philips Medical System, Eindhoven, Holland) from August 2006 until February 2012, and SOMATOM Definition Flash (Siemens Healthcare, Forchheim, Germany) from February 2012 onward). The used intravenous contrast agent was primarily Iomeron® (Iomeprol) 400 mg/ml, and was administered by using an injector coupled to the scanner with an injection rate of 6 ml/second, and a total amount adjusted to the weight of the patient. Thickness of slices was 0.9 mm with an increment of 0.45 mm, and images were reformatted in axial, sagittal, and coronal projections.
DSA was performed on a Philips Integris Allura 15″/12″, biplane (Philips Medical System, Eindhoven, Holland). After femoral catheterization using the Seldinger technique, a diagnostic catheter was advanced via the aorta, and selective four-vessel angiography including internal carotid and vertebral arteries and additional common or selective external carotid series on both sides was performed. Rotational three-dimensional angiography was performed at the discretion of the treating neuroradiologist.
Patient monitoring and management
All patients were treated symptomatically, initially in the intensive care unit. Prophylactic nimodipine, a calcium channel blocker, was administered at a dose of 60 mg orally every four hours according to a standardized SAH protocol at the time of diagnosis. Clinical condition was assessed by continuous neurological examinations including Glasgow Coma Scale (GCS) scores. Any clinical deterioration was carefully documented and evaluated by CT scan.
Hydrocephalus was registered only if it was clinically significant and required a temporary external drainage of cerebrospinal fluid. The rate of vasospasm was noted as well by measuring blood flow velocities using transcranial Doppler (TCD) ultrasound; however, the degree of vasospasm was not recorded, as it was not the focus of the study.
Statistics
Data analysis was performed with the statistical software package IBM SPSS version 20.0. Descriptive data are presented as median values with interquartile range (IQR) in parentheses. Non-parametric Mann-Whitney U test was used to assess the differences between groups, e.g. PMSAH versus nPMSAH. A p value less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 74 (11.6%) of 639 patients fulfilled the criteria for NASAH and were included in the study. They consisted of 48 males and 26 females. The median age at the time of admission was 57 (IQR, 49–62). Thirty-nine (52.7%) patients had PMSAH on the initial CT scan, while 35 (47.3%) had nPMSAH. In the latter group, five (14.3%) patients were on antithrombotic therapy, and six (17.1%) on dietary supplements containing fish oil and garlic, etc. Six (8.1%) patients underwent LP at the time of clinical workup in the emergency department. All but one (6.8%) demonstrated a perimesencephalic hemorrhage pattern on CT scan. Neurological findings at the time of admission were unremarkable, corresponding with HH grade 1 in 58 (78.4%) patients and WFNS grade 1 in 62 (83.8%) patients, except for nuchal rigidity. HH and WFNS grades 3 or above were mostly seen in patients with nPMSAH (p = 0.027 and p = 0.014, respectively). Seven (9.5%) patients briefly lost consciousness at the time of ictus; among them one (1.4%) had a seizure. Two (2.7%) patients presented with abducens nerve palsy, and one (1.4%) with oculomotor nerve palsy. Twenty-two (29.7%) patients had hydrocephalus at the time of admission, which was significantly more common in patients with nPMSAH (p < 0.0001). Patient characteristics including medical history and neurological condition at the time of admission are listed in Table 1.
Table 1.
Comparison of possible demographic and clinical parameters of patients with perimesencephalic and non-perimesencephalic patterns of hemorrhage.
| PMSAH | nPMSAH | p value | |
|---|---|---|---|
| Total (%) | 39 (53) | 35 (47) | |
| Demographic (%) | |||
| Age (median, years) | 55 (47–62) | 60 (53–65) | 0.103 |
| Gender | 0.53 | ||
| Male | 24 (32) | 24 (32) | |
| Female | 15 (20) | 11 (15) | |
| Risk factors (%) | |||
| Hypertension | 8 (21) | 7 (20) | 0.957 |
| Smoking | 12 (31) | 5 (14) | 0.095 |
| Coagulation-influencing drugs | 0.020c | ||
| aAntiplatelet therapy | 3 (8) | 5 (14) | 0.246 |
| Dietary supplements | 1 (6) | 6 (17) | 0.025c |
| Alcohol | 1 (6) | 0 (0) | – |
| bDrug use | 0 (0) | 1 (3) | – |
| Clinical condition at admission (%) | |||
| HH | 0.027c | ||
| Grade 1 or 2 | 38 (97) | 26 (74) | |
| Grade 3, 4 or 5 | 1 (6) | 9 (26) | |
| WFNS | 0.014c | ||
| Grade 1 or 2 | 39 (100) | 28 (80) | |
| Grade 3, 4 or 5 | 0 (0) | 7 (20) | |
| Clinical manifestations (%) | |||
| Nausea/vomiting | 22 (56) | 19 (54) | – |
| Loss of consciousness | 1 (8) | 6 (17) | – |
| Ocular nerve palsy | 0 (0) | 3 (9) | – |
| Seizure | 0 (0) | 1 (3) | – |
| Clinical events attributable to SAH (%) | |||
| Hydrocephalus | 2 (5) | 20 (57) | <0.0001d |
| Vasospasms | 3 (8) | 9 (26) | 0.069 |
| VP-shunt | 2 (5) | 9 (26) | 0.014c |
| Outcomes at discharge (%) | |||
| GOS | 0.073 | ||
| 1–2 | 39 (100) | 32 (91) | |
| 3–5 | 0 (0) | 3 (9) | |
| mRS | 0.267 | ||
| 0–2 | 39 (100) | 31 (89) | |
| 3–6 | 0 (0) | 4 (11) | |
PMSAH: perimesencephalic subarachnoid hemorrhage; nPMSAH: non-perimesencephalic subarachnoid hemorrhage; HH: Hunt-Hess; WFNS: World Federation of Neurological Surgeons; VP: ventriculoperitoneal; GOS: Glasgow Outcome Scale; mRS: modified Rankin Scale.
Coagulation-influencing drugs included aspirin and clopidogrel.
Cocaine abuse.
Significant p value below 0.05.
Significant p value below 0.01.
Imaging investigation
All but five (93.2%) patients underwent CTA at the time of admission, as indicated by the current European Stroke guidelines.18 The CTA scans were negative regarding structural pathology, and another 73 (98.6%) underwent DSA. None of the initial DSAs demonstrated the origin of the hemorrhage. Forty-seven (63.5%) patients subsequently underwent further diagnostic procedures (Table 2). Among them, 14 (29.8%) and 33 (70.2%) had shown PMSAH and nPMSAH on their initial CT scan, respectively. In the rest of the patients (36.5%), no further examinations were performed, as the initial DSA was technically satisfactory and without evidence of vasospasm or severe hematoma. Repeat DSA was performed on median day 9 (IQR, 7–10 days) in 27 (36.5%) patients (e.g. seven patients with PMSAH and 20 with nPMSAH). One (2.9%) patient in the nPMSAH subgroup demonstrated severe vasospasm on the repeat DSA, and further underwent a third and fourth DSA on day 11 and day 23, respectively. No complications occurred during DSA examinations. Fifteen (27%) patients also underwent MRA of the brain and cervical spine. No abnormalities were found on the repeat DSA examination or other follow-up angiographic studies except in one (1.4%) case. In this case, a female patient, who was previously diagnosed with nPMSAH by two negative CTA and one DSA, was readmitted nine days later because of rebleeding. DSA following another negative CTA revealed a causative aneurysm, considered to be the source of hemorrhage (see the section “Illustrated case” below).
Table 2.
The number and types of follow-up imaging studies including CTA, DSA, and MRI including MRA.
| Follow-up imaging studies | PMSAH (n = 14) | nPMSAH (n = 33) |
|---|---|---|
| aRepeat CTA | 8 | 15 |
| bRepeat DSA | 7 | 20 |
| cMRI + MRA | 3 | 11 |
| No follow-up | 23 | 3 |
PMSAH: perimesencephalic subarachnoid hemorrhage; nPMSAH: non-perimesencephalic subarachnoid hemorrhage; CTA: computed tomography angiography; DSA: digital subtraction angiography; MRI: magnetic resonance imaging; MRA: MR angiography.
Four of CTAs were performed for vasospasms (n = 1 with PMSAH; and n = 3 with nPMSAH).
Three of DSAs were performed for vasospasms (n = 2) and intra-arterial nimodipine (n = 1).
Two patients underwent MRI + MRA twice.
The functional outcome
The average hospital stay was 11 days (IQR, 5–14), with a significantly longer stay for the patients with nPMSAH (p < 0.0001). One (2.9%) patient in the nPMSAH subgroup demonstrated moderate to severe vasospasm that required endovascular treatment with intra-arterial nimodipine. In 13 (17.6%) patients with hydrocephalus, the external ventricular drain (EVD) was weaned and eventually removed, while 11 (14.9%) patients subsequently needed a permanent ventriculoperitoneal shunt (VPS) because of refractory hydrocephalus, nine (81.8%) of them with nPMSAH. Twenty-three (31.1%) patients were transferred to the local Neurological Department for further observation or rehabilitation. Among them, two (8.7%) were connected to a mechanical ventilator. Twelve (52.2%) of them were diagnosed with nPMSAH, including the patients with assisted ventilation. All patients with PMSAH and the majority of patients with nPMSAH showed an excellent recovery around three months after discharge from the hospital (Table 1). However, four (10.3%) patients with PMSAH still had residual complaints such as headache, forgetfulness, weariness and reduced endurance. No deaths had occurred during the hospital stay. Only one (1.4%) event of rebleeding was reported after discharge, as illustrated below.
Illustrative case
History and examination
A 55-year-old woman was admitted to the Neurosurgical Department after sudden onset of severe headache associated with nausea, photophobia, and meningismus. She did not lose consciousness at the time of ictus. She was a former smoker with no significant past medical history beside hypertension, and she was on relevant antihypertensive therapy. In addition, she was not on any kind of antiplatelet therapy or dietary supplements. At the time of admission, she had no neurological deficits expect for nuchal rigidity, and she was alert, thus mildly disoriented with a GCS score of 14.
First hemorrhage and clinical course
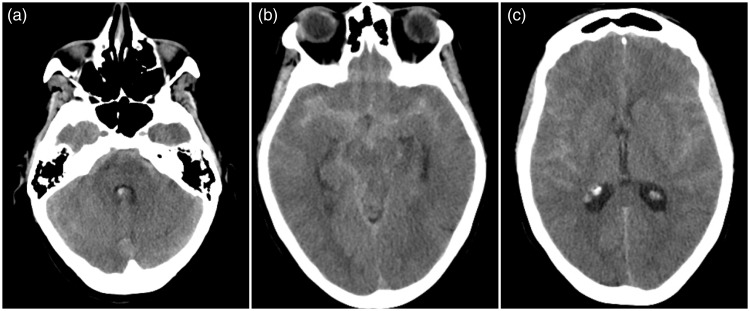
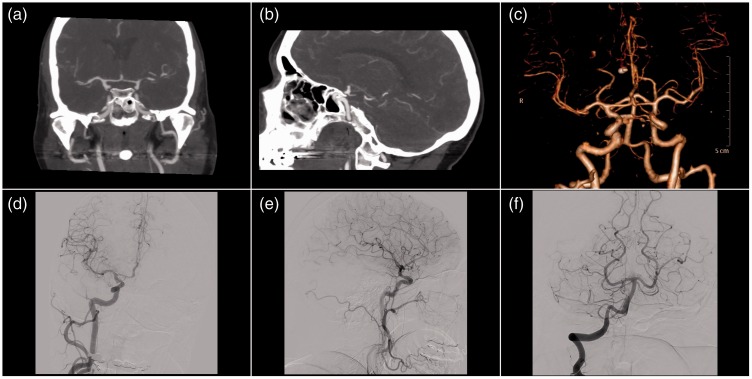
A non-contrast CT scan of the brain, obtained three hours after ictus, revealed a moderate to severe SAH in the basal cisterns and Sylvian fissures bilaterally (Figure 1). An initial CTA was negative regarding intracranial aneurysm or AVM, which was verified by DSA two days later (Figure 2). She was treated with bed rest, analgesia for headache, prophylactic nimodipine and fluid intake of at least three liters per day. During her hospital stay she remained stable, although she suffered from a headache with an alternating character. A repeat CTA performed seven days later showed no source of bleeding, hydrocephalus or vasospasms. In addition, the SAH was reabsorbed. CTA was considered to be of a good quality. The patient was discharged from the hospital to home on day 13.
Figure 1.
Initial computed tomography scan.
Images 1(a)–(c) show a non-contrast computed tomography scan with a moderate to severe subarachnoid hemorrhage in the basal cisterns and Sylvian fissures bilaterally and a small amount of intraventricular blood in the posterior part of the lateral horns.
Figure 2.
Initial computed tomography angiography and digital subtraction angiography.
Images 2(a) and (b) show computed tomography angiography with normally calibrated vessels with no vascular pathology, which was verified by digital subtraction angiography (Images 2(c)–(f)).
Second hemorrhage and clinical course
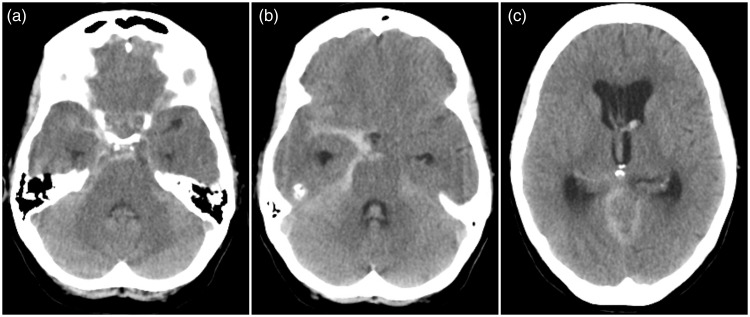
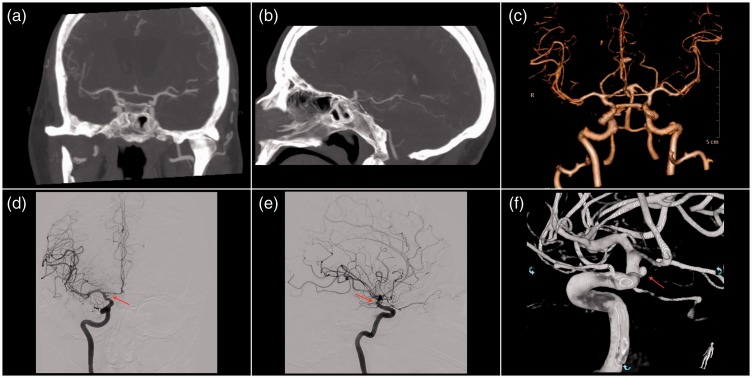
Nine days later, the patient experienced another sudden headache, photophobia, and meningismus, and she was readmitted to the Neurosurgical Department. A CT scan demonstrated a severe rebleeding located occipitally and along the tentorium cerebelli and right posterior communicating artery (Figure 3). The following CTA, however, showed no aneurysm, nor AVM (Figures 4(a) and (b)). DSA was then performed demonstrating a small right-sided saccular anterior choroidal aneurysm measuring 2 × 3.6 mm (Figures 4(c)–(f)), considered to be the source of hemorrhage. The patient underwent an uneventful surgical clipping the same day. However, she subsequently developed hydrocephalus later the same evening requiring placement of an EVD. The day after, high blood flow velocities (i.e. >170 cm/s) were demonstrated by TCD. On day 5, she was extubated, and the neurological examination revealed left-sided paralysis and central facial paresis with a GCS score of 13. Because of refractory hydrocephalus, she received a VPS 14 days later. She was then transferred to a local Neurological Department on day 20 for further rehabilitation.
Figure 3.
Computed tomography scan at second admission.
Images 3(a)–(c) show a non-contrast computed tomography scan that demonstrated a severe rebleeding located occipitally and along the tentorium cerebelli and right posterior communicating artery. Increasing hydrocephalus.
Figure 4.
Computed tomography angiography and digital subtraction angiography at second admission.
Images 4(a) and (b) show computed tomography angiography with normally calibrated vessels with no sign of aneurysms or arteriovenous malformation. Images 4(c)–(f) reveal a small right-sided saccular anterior choroidal aneurysm measuring 2 × 3.6 mm.
Discussion
This present retrospective study was performed in order to estimate the incidence of occult aneurysms, which might have been missed on the initial DSA, and because there is still no consensus among colleagues as to when to perform a follow-up DSA. All initial imaging studies were of high quality, revealing no vascular abnormalities. More than 60% of patients underwent subsequent follow-up imaging. Among them, unfortunately, we had one false-negative initial DSA with a 3.6 mm aneurysm, which was seen only on a follow-DSA, considered to be the source of hemorrhage.
Multiple studies have been carried out over the past two decades assessing the utility of repeat DSA.12,19–22 In one of the largest studies of 254 patients by Dalyai et al.,12 the authors found no angiographic abnormalities at follow-up DSA examinations in a subgroup of 118 patients with PMSAH. Conversely, they reported a vascular source in 12.5% of 136 patients with nPMSAH. In two other studies, repeat DSA examinations were positive in two patients, both with nPMSAH on the admission CT.20,21 Current European Guidelines18 suggest performing DSA in case of PMSAH only if the initial CTA is not considered to be sufficient or if there is doubt as to the perimesencephalic pattern of the SAH, while in case of nPMSAH, CTA or DSA must be repeated. The patient in the present case presented with nPMSAH on both the first- and second-admission CT scans, and in total demonstrated three false-negative CTAs and one-false DSA. Only the second DSA at the second admission was able to detect the bleeding source. This observation is in line with the study by Westerlaan et al.,23 in which the authors reported four patients experiencing rebleeding after initial negative findings on CTA and DSA. Topcuoglu et al.22 investigated the diagnostic yield of repeat DSA and other neuroimaging studies in 70 patients with NASAH and negative initial neuroimaging studies, and reported the source of bleeding in four patients with nPMSAH, all revealed by repeat DSA. The authors concluded that DSA was the only subsequent imaging that could reveal the cause of hemorrhage, especially in patients with nPMSAH. Our study therefore reinforces these findings and supports the recommendations of Rinkel et al.3 that repeat angiography, especially DSA, must be warranted in cases of diffuse aneurysmal pattern of hemorrhage in order to reduce the risk of rebleeding, even if the initial DSA is negative and of high quality.
It is documented that CTA might be a useful adjunct procedure for subsequent investigation following a negative initial DSA, with a reported pooled sensitivity and specificity up to 97%.24 CTA has some advantages over DSA, as it is a less invasive, widely available technique, and requires shorter examination time. In several studies, authors have investigated its use as the primary and/or only angiographic modality for investigation of patients with PMSAH.12,13,15,25 In a recently published meta-analysis of 40 studies with 1031 patients with PMSAH and initial negative DSA or CTA,11 the rate of aneurysm detection on follow-up DSA and/or CTA was 0.78% (8/1031; 95% confidence interval, 0.23–1.32%). Comparison of different imaging strategies did not show any statistically significant benefit of performing DSA during initial or at follow-up evaluation of these patients. The authors concluded in the end that patients with typical PMSAH and presentations compatible with a non-aneurysmal etiology could undergo initial evaluation with CTA alone, and they did not require an additional DSA at the time of diagnosis or any angiographic follow-up imaging. However, this meta-analysis was somewhat limited due to inconsistent definitions of PMSAH used in the included studies. In rare cases as in the present case study, CTA may yield false-negative as well as false-positive results, even when it is performed with a modern multidetector CT.26 McKinney et al.26 reported one false-negative CTA in which a 2 mm aneurysm was missed on a 64-channel multidetector CT scanner. In the literature, the repetition of angiographic studies has reported positive pathologic findings in up to 35% of cases.26,27 Especially small aneurysms (<4 mm in diameter) and those localized in the posterior circulation are more likely to be missed by CTA.17,26 For this reason, some authors continue to advocate for DSA studies.23,28,29
Repeat diagnostic workup is controversial in patients, who are fully awake with a normal neurological examination and an initial negative invasive workup. There is considerable variability from center to center in regard to what proportion of patients with NASAH should undergo repeat DSA. At our institution, repeat DSA is seldom needed in cases with a typical perimesencephalic pattern, and is only performed in selected cases for which there is a strong suspicion of ASAH (e.g. vasospasm, intraventricular bleeding, significant hydrocephalus). In our series of 39 patients with PMSAH, the cause of bleeding was not detected through repeated DSA. Therefore, if the negative initial DSA is of high quality without evidence of severe hemorrhage, vasospasm, and thrombosis concealing the aneurysm or AVM, a repeat DSA may not be needed, as we believe that the procedural risks of DSA (e.g. the arterial puncture site or intravascular emboli resulting in infarction and/or death) arguably exceed the benefits of carrying out this procedure.19,30 In contrast, the present case shows that the benefit of repeat DSA should not be underestimated, especially in cases with nPMSAH.
There is a distinct difference between PMSAH and extended nPMSAH regarding the risk of vasospasm and hydrocephalus, clinical outcome and quality of life.6,7,10 Angiographic vasospasm, which is mild and focal, has been reported more frequently than clinical vasospasm. One study reported angiographic vasospasm in 33.3% patients with nPMSAH versus 13.8% patients with PMSAH (p = 0.073).31 This is in line with the present study, which showed angiographic vasospasm in 25.7% patients with nPMSAH versus 7.7% patients with PMSAH (p = 0.069). In addition, hydrocephalus was mainly seen in patients with nPMSAH (57.1%) as well, compared to PMSAH (5.1%) (p < 0.0001), which is in accord with the literature as well.8,31 Nine (25.7%) patients in the nPMSAH group subsequently required a placement of VPS, compared to two (5.1%) in the group with PMSAH (p = 0.014).
Finally, there was a significantly higher number of patients with nPMSAH who took antithrombotic drugs and dietary supplements (p = 0.020), which could explain the diffuse pattern of hemorrhage. One study suggested an increased risk of rebleeding early after PMSAH in patients with antiplatelet drugs.32 Another recent study (2015) reported a growing number of patients with nPMSAH due to an increased use of anticoagulant drugs.33 There are no data regarding dietary supplements and NASAH. In the present study, six (85.7%) of seven patients who used dietary supplements presented with nPMSAH (p = 0.025). These remedies are known to affect platelet function, and may have the potential to increase the risk of diffuse hemorrhagic pattern as nPMSAH. However, this needs to be investigated further in future studies.
Limitations
The main limitation of this study is its retrospective design and the relatively small sample size. However, the latter is of course due to the natural low incidence of this entity. Since there is no established local diagnostic regimen at our institution, the number of follow-up studies per patient, the timing and the imaging modality vary from case to case, dependent on the referring neurosurgeon. Only 20 of 33 patients with nPMSAH underwent repeat DSA studies. In the remaining patients, the decision of no need for further imaging was made on a case-by-case basis by a multidisciplinary neurovascular team, and was in accordance with the European Stroke guidelines.18
Conclusion
In summary, we recommend that the diagnosis of PMSAH should continue to be confirmed with an initial DSA, as CTA at this point in time is inadequate for gold standard scientifically, despite the fact that some physicians are comfortable with this as a sole diagnostic approach. If the primary DSA is technically adequate, and severe hematoma and vasospasm are absent, and the clinical scenario is consistent, a repeat DSA is not needed in most cases with PMSAH. A conservative approach with follow-up using CTA may be an alternative option in cases of some uncertainty. In cases with nPMSAH, a more aggressive approach is needed to exclude an aneurysm and thus the risk of rebleeding, even if the initial DSA is negative and of high quality. The present case shows that repeat CTA, even when performed with a modern multidetector CT scanner, is inadequate to exclude aneurysms as the source of hemorrhage, especially in cases with a diffuse hemorrhagic pattern and aneurysms less than 4 mm. Although CTA has a high negative predictive value according to the literature, it does not rule out 100% of cases, which is mandatory to avoid the life-threatening conditions that follow a ruptured cerebral aneurysm. Therefore, a repeat DSA must still remain the gold standard for confirming or excluding aneurysms in patients with nPMSAH.
Funding
This research received no specific grant from any finding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Rinkel GJ, Wijdicks EF, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. Am J Neuroradiol 1991; 12: 829–834. [PMC free article] [PubMed] [Google Scholar]
- 2.Rinkel GJ, Wijdicks EF, Vermeulen M, et al. The clinical course of perimesencephalic nonaneurysmal subarachnoid hemorrhage. Ann Neurol 1991; 29: 463–468. [DOI] [PubMed] [Google Scholar]
- 3.Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm. A review of the causes. Stroke 1993; 24: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 4.van der Schaaf IC, Velthuis BK, Gouw A, et al. Venous drainage in perimesencephalic hemorrhage. Stroke 2004; 35: 1614–1618. [DOI] [PubMed] [Google Scholar]
- 5.Rinkel GJ, Wijdicks EF, Hasan D, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet 1991; 338: 964–968. [DOI] [PubMed] [Google Scholar]
- 6.Beseoglu K, Pannes S, Steiger HJ, et al. Long-term outcome and quality of life after nonaneurysmal subarachnoid hemorrhage. Acta Neurochir 2010; 152: 409–416. [DOI] [PubMed] [Google Scholar]
- 7.Hui FK, Tumialán LM, Tanaka T, et al. Clinical differences between angiographically negative diffuse subarachnoid haemorrhage and perimesencephalic subarachnoid haemorrhage. Neurocrit Care 2009; 11: 64–70. [DOI] [PubMed] [Google Scholar]
- 8.Canneti B, Mosqueira AJ, Nombela F, et al. Spontaneous subarachnoid hemorrhage with negative angiography managed in a stroke unit: Clinical and prognostic characteristics. J Stroke Cerebrovasc Dis 2015; 24: 2484–2490. [DOI] [PubMed] [Google Scholar]
- 9.Hermann LL, Zabramski JM. Nonaneurysmal subarachnoid hemorrhage: A review of clinical course and outcome in two hemorrhage patterns. J Neurosci Nurs 2007; 39: 135–142. [PubMed] [Google Scholar]
- 10.Greebe P, Rinkel GJ. Life expectancy after perimesencephalic subarachnoid hemorrhage. Stroke 2007; 38: 1222–1224. [DOI] [PubMed] [Google Scholar]
- 11.Kalra VB, Wu X, Matouk CC, et al. Use of follow-up imaging in isolated perimesencephalic subarachnoid hemorrhage: A meta-analysis. Stroke 2015; 46: 401–406. [DOI] [PubMed] [Google Scholar]
- 12.Dalyai R, Chalouhi N, Theofanis T, et al. Subarachnoid hemorrhage with negative initial catheter angiography: A review of 254 cases evaluating patient clinical outcome and efficacy of short- and long-term repeat angiography. Neurosurgery 2013; 72: 646–652. [DOI] [PubMed] [Google Scholar]
- 13.Huttner HB, Hartmann M, Köhrmann M, et al. Repeated digital subtraction angiography after perimesencephalic subarachnoid hemorrhage? J Neuroradiol 2006; 33: 87–89. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Shand Smith JD, Kirkman MA, et al. Angiogram negative subtraction haemorrhage: Outcomes and the role of repeat angiography. Clin Neurol Neurosurg 2013; 115: 1470–1475. [DOI] [PubMed] [Google Scholar]
- 15.Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, et al. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis—systematic review and meta-analysis. Radiology 2011; 258: 134–145. [DOI] [PubMed] [Google Scholar]
- 16.Chappell T, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysm: A meta-analysis. Neurosurg 2003; 52: 624–631. [DOI] [PubMed] [Google Scholar]
- 17.Menke J, Larsen J, Kallenberg K. Diagnosing cerebral aneurysms by computed tomographic angiography: Meta-analysis. Ann Neurol 2011; 69: 646–654. [DOI] [PubMed] [Google Scholar]
- 18.Steiner T, Juvela S, Unterberg A, et al. European stroke organisation guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerbrovasc Dic 2013; 35: 93–112. [DOI] [PubMed] [Google Scholar]
- 19.Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: When is digital subtraction angiography still needed? Am J Neuroradiol 2013; 31: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akcakaya MO, Aydoseli A, Aras Y, et al. Clinical course of nontraumatic nonaneurysmal subarachnoid hemorrhage: A single institution experience over 10 years and review of the contemporary literature. Turk Neurosurg 2017; 27: 732–742. [DOI] [PubMed] [Google Scholar]
- 21.Yu DW, Jung YJ, Choi BY, et al. Subarachnoid hemorrhage with negative baseline digital subtraction angiography: Is repeat digital subtraction angiography necessary? J Cerebrovasc Endovasc Neurosurg 2012; 14: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westerlaan HE, Gravendaal J, Fiore D, et al. Multislice CT angiography in the selection of patients with ruptured intracranial aneurysms suitable for clipping or coiling. Neuroradiology 2007; 49: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topcuoglu MA, Ogilvy CS, Carter BS, et al. Subarachnoid hemorrhage without evident cause on initial angiography studies: Diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg 2003; 98: 1235–1240. [DOI] [PubMed] [Google Scholar]
- 24.Ruigrok YM, Rinkel GJ, Buskens E, et al. Perimesencephalic hemorrhage and CT angiography: A decision analysis. Stroke 2000; 31: 2976–2983. [DOI] [PubMed] [Google Scholar]
- 25.Kershenovich A, Rappaport ZH, Maimon S. Brain computed tomography angiographic scans as the sole diagnostic examination for excluding aneurysm in patients with perimesencephalic subarachnoid hemorrhage. Neurosurgery 2006; 59: 798–802. [DOI] [PubMed] [Google Scholar]
- 26.McKinney AM, Palmer CS, Truwit CL, et al. Detection of aneurysm by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. Am J Neuroradiol 2008; 29: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galal A, ElSerry TH, Aziz MM. Is catheter diagnostic cerebral angiography still essential for patients with spontaneous perimesencephalic subarachnoid hemorrhage and negative computed tomography angiogram? Neurosurgery 2016; 63: 188. [Google Scholar]
- 28.Ausman JI. Perimesencephalic nonaneurysmal subarachnoid hemorrhage: What is it? What are we missing? Surg Neurol 2002; 57: 211. [DOI] [PubMed] [Google Scholar]
- 29.Andaluz N, Zuccarello M. Yield of further diagnostic work-up of cryptogenic subarachnoid hemorrhage based on bleeding patterns on computed tomographic scans. Neurosurgery 2008; 62: 1040–1046. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann TJ, Huston J, 3rd, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: Evaluation of 19826 consecutive patients. Radiology 2007; 243: 812–819. [DOI] [PubMed] [Google Scholar]
- 31.Coelho LG, Costa JM, Silva EI. Non-aneurysmal spontaneous subarachnoid hemorrhage: Perimesencephalic versus non-perimesencephalic. Rev Bras Ter Intensiva 2016; 28: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Worp HB, Fonville S, Ramos LM, et al. Recurrent perimesencephalic subarachnoid hemorrhage during anti-thrombotic therapy. Neurocrit Care 2009; 10: 209–212. [DOI] [PubMed] [Google Scholar]
- 33.Konczalla J, Schuss P, Platz J, et al. Clinical outcome and prognostic factors of patients with angiogram-negative and non-perimesencephalic subarachnoid hemorrhage: Benign prognosis like perimesencephalic SAH or same risk as aneurysmal SAH? Neurosurg Rev 2015; 38: 121–127. [DOI] [PubMed] [Google Scholar]