Abstract
Background and purpose
Vascular risk factors have been associated with decreased cerebral blood flow (CBF) but this is etiologically nonspecific and may result from vascular insufficiency or a response to decreased brain metabolic activity. We apply new MRI techniques to measure oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen consumption (CMRO2), hypothesizing that decreased CBF related to these vascular risk factors will be associated with increased OEF, confirming a primary vascular insufficiency.
Methods
3T MRI was obtained on 70 community-based participants in this IRB-approved study with informed consent, with previous assessment of systolic blood pressure, hypertension medication, elevated serum triglycerides, low serum HDL, and diabetes mellitus. CBF was measured using phase contrast adjusted for brain volume (ml/100 g/min), OEF (%) was obtained from T2-Relaxation-Under-Spin-Tagging (TRUST), and CMRO2 (μmol/100 g/min) was derived using the Fick principle. Stepwise linear regression identified optimal predictors of CBF with age, sex, and hematocrit included for adjustment. This predictive model was then evaluated against OEF and CMRO2.
Results
Hypertriglyceridemia was associated with low CBF and high OEF. High systolic blood pressure was associated with high CBF and low OEF, which was primarily attributable to those with pressures above 160 mmHg. Neither risk factor was associated with significant differences in cerebral metabolic rate.
Conclusion
Low CBF related to hypertriglyceridemia was accompanied by high OEF with no significant difference in CMRO2, confirming subclinical vascular insufficiency. High CBF related to high systolic blood pressure likely reflected limitations of autoregulation at higher blood pressures.
Keywords: MRI, CBF, OEF, hypoperfusion, hypertriglyceridemia, hypertension
Introduction
Many uncertainties remain about the nature of the link between vascular risk factors, cerebral blood flow (CBF), and subsequent risk of dementia. Decreased CBF has been noted in association with vascular risk factors including increased blood pressure, diabetes, low high-density lipoprotein (HDL), and high triglycerides.1–5 As a result, CBF has been proffered as an imaging biomarker of cardiovascular risk. Neuronal metabolic demand and CBF are linked by neurovascular coupling,6 however, so diminished CBF may be due either to a primary hemodynamic impairment or secondary to brain hypo-metabolism due to neurologic insult.7 Little is known about the impact of vascular risk factors on brain metabolic rate. A multifactorial data-driven analysis of more than 1100 individuals from the Alzheimer's Disease Neuroimaging Initiative suggested vascular dysregulation may be the earliest and strongest pathologic insult in development of dementias such as late-onset Alzheimer's disease, preceding amyloid deposition and decreased metabolism.8 Insults to the brain vasculature may also result in neuronal injury without causing hypoperfusion by damaging the blood-brain barrier and exposing the brain parenchyma to neurotoxic elements in the blood.9 Characterization of brain vascular function beyond assessment of CBF alone can better specify the presence and severity of primary vascular insult.
Cerebral hemodynamic impairment progresses in stages that have characteristic alterations of different vascular parameters.10 Arteriosclerotic remodeling that diminishes luminal area and increases vascular resistances is initially countered by autoregulation, which maintains CBF by dilating smooth muscle in resistance vessels. With progressive insult, there is a loss of reserve mechanisms and autoregulatory capacity is exhausted. As CBF declines, oxygen tension in the tissues decreases and more oxygen is extracted from the blood within the capillaries, which maintains the cerebral metabolic rate. The amount of oxygen extracted as it passes from arteries into the veins may be quantified as the oxygen extraction fraction (OEF). After this mechanism is also overwhelmed, the ability to maintain tissue metabolism is compromised and cell injury and death may ensue. The amount of oxygen used by the tissue provides a measure of the cerebral metabolic rate (CMRO2), which can be quantified on magnetic resonance imaging (MRI) from the product of CBF, OEF, and the oxygen-carrying capacity of blood using the Fick principle. With a decrease in CMRO2 related to diminished brain activity, there is less utilization of oxygen and OEF does not increase as neurovascular coupling reduces CBF.
New techniques have now been developed to permit a similar, more comprehensive profiling with MRI. In this study, we sought to clarify the associations between vascular risk factors previously shown to correlate with decreased CBF using MRI while also evaluating its relation to OEF and CMRO2 using new techniques. The presence of vascular risk factors in mid-life more strongly confers risk for dementia than risk factors assessed later in life according to a systematic review of longitudinal population-based studies.11 Unfortunately, to observe meaningful differences within a reasonable observation period most studies of dementia enroll individuals later in life and reliable risk factor data from mid-life are not accessible. We therefore assessed individuals from a community-based cohort with vascular risk factors assessed in mid-life against subsequent vascular function on MRI as they transition to late life.
Objective
We test the hypothesis that mid-life vascular risk factors associated with a decrease in CBF reflect a primary vascular insult as evidenced by a paired increase in OEF. A secondary aim was to determine the impact, if any, of these risk factor(s) on CMRO2. We therefore evaluated the relationships between vascular risk factors among 70 participants from a multi-ethnic community-based cohort with CBF, OEF, and CMRO2 on MRI.
Participants and methods
Participants
We evaluated the relationship between presence of mid-life vascular risk factors among 70 participants, 36 female, drawn from a previously established multi-ethnic community-based cohort12 with CBF, OEF, and CMRO2 on MRI acquired six years later. Demographic characteristics are given in Table 1, with depiction of mean and standard deviation for continuous data. At time of exam median age was 67 years (interquartile range (Q1–Q3): 64–71, minimum 56, maximum 82). The University of Texas Southwestern (UTSW) Alzheimer's Disease Center initiated a follow-up study, mailing invitations to participants currently over the age of 55 with targeted enrollment of 150. Grant support was provided to obtain MRI for 70 individuals, a number shown sufficient in prior publications to identify associations between vascular risk factors we study here with CBF. Participants were recruited for this imaging study in a sequential fashion as individuals were scheduled for follow-up evaluation by the Alzheimer's Disease Center. Individuals in this study were non-demented, per formal cognitive testing by the UTSW Alzheimer's Disease Center, and had no history of heart attack or evidence of large-vessel stroke on prior brain MRI. These findings were infrequent (<5%) in the overall cohort but will result in a healthier sample in this imaging study. The UTSW institutional review board approved the study and all participants provided written informed consent.
Table 1.
Demographic characteristics of the 70 participants.
| Number (%) | |
|---|---|
| Female | 36 (51) |
| Diabetic | 8 (11) |
| Hypertriglyceridemia | 16 (23) |
| Low high-density lipoprotein | 18 (26) |
| Hypertension medication | 40 (57) |
| Mean ± SD | |
| Systolic blood pressure, mmHg | 132 ± 14 |
| Hematocrit | |
| Female | 39 ± 3 |
| Male | 44 ± 4 |
| Age | |
| At vascular risk assessment | 61 ± 5 |
| At magnetic resonance imaging | 68 ± 5 |
Vascular risk assessment
Vascular risk factors were selected based on prior reported associations with CBF1–5 and included hypertension, broken down into observed blood pressure and presence of hypertension treatment to evaluate associations with treated hypertension and persistent elevations in blood pressure regardless of treatment, presence of diabetes, and dyslipidemia broken down into low HDL and high triglycerides. Systolic blood pressure (mmHg) was measured by an automated oscillometric device (Welch Allyn Inc, Skaneateles Falls, NY) as the mean of the third to fifth recordings. The following risk factors were binary indicator variables operationalized as the presence or absence of the condition (with “absence of the condition” being the reference group): Elevated serum triglycerides (fasting triglycerides ≥ 150 mg/dl or treatment) and low serum HDL (men < 40 mg/dl and women < 50 mg/dl or treatment) were calculated according to National Institutes of Health Cholesterol Education Program Adult Treatment Panel III Guidelines.13 Diabetes mellitus was defined by either self-report accompanied by use of anti-hyperglycemic medication or by elevated serum glucose (fasting > 126 mg/dl (7.0 mmol/l) or by non-fasting glucose > 200 mg/dl (11.1 mmol/l)).14 Hypertension treatment was self-reported.
Imaging details
Imaging was performed on a 3T MRI scanner (Achieva, Philips Medical Systems, the Netherlands) using a body coil for radiofrequency transmission and an eight-channel sensitivity encoding head coil for receiving. Time of flight angiogram was performed to localize the arteries in the neck: repetition time (TR)/echo time (TE)/flip angle = 20 ms/3.45 ms/18 degrees, field of view (FOV) = 160 × 160 × 70.5 mm3, voxel size = 1.0 × 1.0 × 1.5 mm3, number of slices = 47, one 60 mm saturation (sat) slab positioned above the imaging slab, scan duration = 1.4 minute. Total CBF was measured using four-phase contrast MRI of each of the internal carotid arteries (at the level of the foramen magnum) and vertebral arteries (at the C1–C2 level): single slice, voxel size = 0.45 × 0.45 × 5 mm3, FOV =230 × 230 × 5 mm3, maximum velocity encoding =80 cm/s, four averages, scan duration of one-phase contrast MRI scan is 0.5 minutes. Regions of interest were manually drawn. Brain volumes were measured from T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequences with voxel size = 1 ×1 × 1 mm3 with quantitation using the software FSL (FMRIB Software Library, Oxford University).
Blood T2 values are derived from T2-Relaxation-Under-Spin-Tagging15,16 (TRUST) MRI and are used to derive oxygenation level via reference to a calibration plot obtained from in vitro experiments with blood of varying oxygenation.16 TRUST imaging was acquired from a slice parallel to the anterior commissure-posterior commissure line roughly perpendicular to the superior sagittal sinus approximately 20 mm above the confluence of sinuses. Post-sat TRUST sequence parameters: TR = 3000 ms, inversion time (TI) = 1200 ms, voxel size = 3.44 × 3.44 × 5 mm3, four different T2-weightings with estimated TEs of 0 ms, 40 ms, 80 ms, and 160 ms, inter-echo spacing in Carr-Purcell-Meiboom-Gill sequence (τCPMG) = 10 ms, scan duration = 1.2 minutes. The labeling slab was 100 mm in thickness and was positioned 22.5 mm above the imaging slice.
CBF is total CBF adjusted for brain volume with units of ml/100 g/min. Ya−Yv gives the difference in % oxygen saturation between the (systemic) arterial input and (sagittal sinus) venous blood outflow. The OEF may be derived as OEF = (Ya−Yv)/ Ya ⋅ 100% where Ya−Yv gives the difference in % oxygen saturation between the (systemic) arterial input and (sagittal sinus) venous blood outflow. Ya is measured using pulse oximetry. Yv was determined using TRUST.15,16 Our MRI technique for measuring CMRO2 has been previously described17,18 and is based on the Fick principle of arteriovenous oxygen difference: CMRO2 =CBF × (Ya – Yv) × Ca,19 where Ca is the oxygen carrying capacity of blood, which is almost entirely determined by hemoglobin concentration20 with 55.6 μmol of oxygen per gram of hemoglobin.19,21 For one male and one female hematocrit was not available and values were imputed based on mean for gender. Hemoglobin was derived as one-third the hematocrit value.
Data analysis
Statistical analyses were performed using JMP Pro 12.1.0 (SAS Institute Inc, Cary, NC). Demographic and clinical characteristics for the overall sample were described using the sample mean and standard deviation for continuous variables and the frequency and percentage for categorical variables. A sex difference in hematocrit in our sample was confirmed by t-test. Vascular risk factors previously associated with CBF alterations in the literature were tested to identify those that best predicted CBF differences in our cohort. Age, sex, and hematocrit were included as adjustment variables. Age, systolic blood pressure, and hematocrit were evaluated as continuous variables. Binary variables included female sex, diabetes, hypertriglyceridemia, low HDL, and presence of hypertension medication, which were operationalized as the presence or absence of the condition (with “absence of the condition” being the reference group). We performed a forward stepwise regression model predicting CBF that minimized the Akaike information criterion value. We then evaluated the associations for the same predictive model with OEF as the primary and CMRO2 as exploratory dependent variables.
In post-hoc analysis, we further explored the relationship between CBF and systolic blood pressure by comparing models of their relationship with simple linear correlation and using a least squares spline fit (with variable smoothness by manipulating lambda factors to best fit the association without excessive local variability). Since female sex was a significant adjustment factor in our CBF model, and sex differences have been noted in CBF and cerebral autoregulation,22 we added interaction terms for female sex with systolic blood pressure and high triglycerides to our selective fit model of CBF. For significant interactions, we further explored the difference in their prediction of CBF for women and men separately with simple linear correlation. To assist in interpreting the relationships between hemodynamic parameters, we explored the associations of CBF with OEF and CMRO2 in linear correlation; for CMRO2 we repeated the analysis with one outlier having extreme high CBF (and systolic blood pressure).
Results
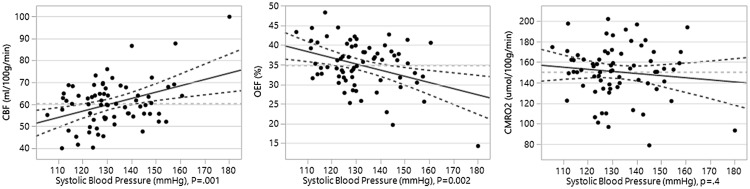
Characteristics of study participants used in linear regression modelling are given in Table 1. Participants were 61.2 ± 5.0 years of age at vascular risk assessment and 67.5 ± 5.1 years at MRI. Hematocrit was 38.8 ± 2.8 in women and 43.6 ± 3.7 in men, which was a significant difference ( p < 0.001). Hemodynamic parameters for the overall population were: CBF 60 ± 13 ml/100 g/min, OEF 35 ± 6%, and CMRO2 166 ± 33 μmol/100 g/min. The stepwise linear regression optimizing Akaike information criteria predicting CBF was significant with model adjusted R-squared 0.36 and p value < 0.0001 (model equation: CBF = 53.8 ± 19.2 +7.3 ± 3.1*(female sex) – 8.0 ± 2.9*(high triglycerides) +0.29 ± 0.09*(systolic blood pressure) – 0.8 ± 0.4*(hematocrit)). Adjusted significance values for each factor selected in the model are female sex ( p = 0.02), high triglycerides ( p = 0.009), systolic blood pressure ( p = 0.001), and hematocrit ( p = 0.03). Levels of significance for excluded variables adjusted for variables included in the model are diabetes ( p = 0.6), low HDL ( p = 0.8), and age ( p = 0.9). The CBF model was then evaluated for associations with OEF and CMRO2 using standard least squares. The model for OEF was significant with adjusted R-squared 0.17 and p value < 0.003, (model equation: OEF = 68.1 ±11.0 – 2.8 ± 1.8*(female) +3.7 ± 1.7*(high triglycerides) – 0.16 ± 0.05*(systolic blood pressure) – 0.30 ±0.22*(hematocrit)). Adjusted significance values for each factor are female sex ( p = 0.1), high triglycerides ( p = 0.03), systolic blood pressure ( p = 0.002), and hematocrit ( p = 0.2). For CMRO2, the model was not significant ( p = 0.8) and none of the component factors were significantly associated ( p = 0.4 or greater). We compare the values for systolic blood pressure and high triglycerides in predicting CBF, OEF, and CMRO2 in Table 2. The plots of CBF, OEF, and CMRO2 for a given systolic blood pressure adjusted for other variables in the model are shown in Figure 1; values of CBF, OEF, and CMRO2 with and without hypertriglyceridemia adjusted for other model variables are shown in Table 3.
Table 2.
Optimal vascular risk factor associations with brain hemodynamic measures.
| CBF (ml/100g/min) |
OEF (%) |
CMRO2 (μmol/100 g/min) |
||||
|---|---|---|---|---|---|---|
| Parameter estimate | p value | Parameter estimate | p value | Parameter estimate | p value | |
| Hypertriglyceridemia | –8.0 ± 2.9 | 0.009 | 3.7 ± 2.2 | 0.03 | – | .9 |
| Systolic blood pressure | 0.29 ± 0.09 | 0.001 | –0.17 ± 0.05 | 0.002 | – | .4 |
Regression line of fit (solid dark gray), 0.05 significance boundary curves (dashed dark gray), line of mean (dashed light gray).
CBF: cerebral blood flow; OEF: oxygen extraction fraction; CMRO2: cerebral metabolic rate of oxygen consumption.
Figure 1.
Leverage plot showing hemodynamic values for systolic blood pressure adjusting for other predictors in the model (hypertriglyceridemia, sex, and hematocrit). CBF: cerebral blood flow; OEF: oxygen fraction extraction; CMRO2: cerebral metabolic rate of oxygen consumption.
Table 3.
Mean hemodynamic measures for those with and without hypertriglyceridemia adjusting for other variables in the model (systolic blood pressure, sex and hematocrit).
| CBF (ml/100 g/min) |
OEF (%) |
CMRO2 (μmol/100 g/min) |
||||
|---|---|---|---|---|---|---|
| Adjusted mean | p value | Adjusted mean | p value | Adjusted mean | p value | |
| Hypertriglyceridemia | 54 ± 2.6 | 0.009 | 38 ± 1.5 | 0.03 | 148 ± 5.1 | 0.9 |
| Normal triglycerides | 62 ± 1.4 | 34 ± 0.8 | 153 ± 6.0 | |||
CBF: cerebral blood flow; OEF: oxygen extraction fraction; CMRO2: cerebral metabolic rate of oxygen consumption.
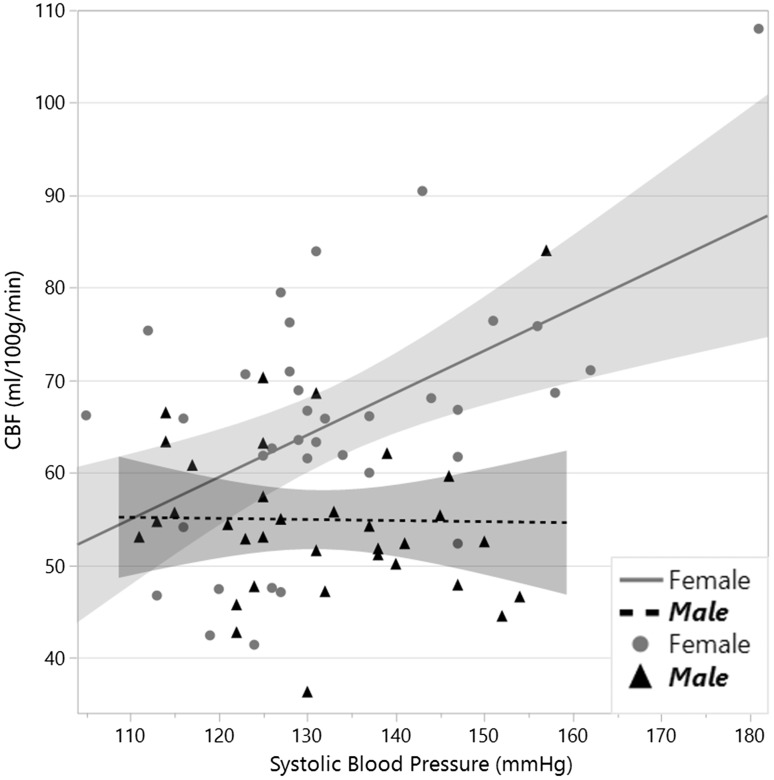
The linear fit between systolic blood pressure and CBF appeared suboptimal because of a steeper slope for CBF at the higher levels of systolic blood pressure as demonstrated in Figure 1. Exploring this relationship, we found a simple linear correlation of CBF and systolic blood pressure resulted in a model fit ( p = 0.004) with adjusted R-squared of 0.10 compared with 0.25 for a cubic spline (lambda smoothness parameter of 10,000), which followed the data points more closely with an apparent inflection point at around systolic blood pressures of 150–160 mmHg as shown in Figure 2. We evaluated for an interaction for female sex with systolic blood pressure and high triglycerides in a regression model predicting CBF based on optimizing Akaike information criterion: CBF = 93.1 – 53.4*(female) – 7.2*(high triglycerides) – 0.85*(hematocrit) + 0.46*(female systolic blood pressure); adjusted R-squared 0.43; model p < 0.0001. Significance for each factor chosen in the model was systolic blood pressure in women ( p < 0.0001), hematocrit ( p = 0.02), high triglycerides ( p = 0.01), and female sex ( p = 0.0004). Probability for factors not selected in the model with interaction terms adjusted for other terms in the model was blood pressure in men and women ( p = 0.9), and high triglycerides in women ( p = 0.2). To better understand the relationship between blood pressure and CBF for women and men, we then plotted linear correlations for each group and found a highly significant association for systolic blood pressure in women (parameter estimate (p.e.) 0.46 ± 0.13; p = 0.0009) and no significant association for systolic blood pressure in men (p.e. –0.012 ± 0.12; p = 0.9) as shown in Figure 3.
Figure 2.
Linear and smoothing spline fit of cerebral blood flow (CBF) by systolic blood pressure. The linear fit (p = 0.004) had an adjusted R-squared of 0.10 compared with 0.25 for a cubic spline. The spline shows a more rapid rise in CBF per systolic blood pressure among the cohort past 150–160 mmHg.
Figure 3.
Linear fit of cerebral blood flow (CBF) by systolic blood pressure for female and male sex.
Exploring associations among our hemodynamic parameters, we observed a positive trend between CBF and CMRO2 (p.e. 0.47 ± 0.25, p = 0.06), which became highly significant (p.e. 0.92 ± 0.25, p = 0.0005) with exclusion of one outlier with CBF > 100 and CMRO2 below 100. CBF was highly negatively correlated with OEF (p.e. –0.25 ± 0.05, p < 0.0001), and this significance persisted with exclusion of the outlier with high CBF (p.e. –0.19 ± 0.06, p = 0.001).
Discussion
Hypertriglyceridemia was associated with decreased CBF and increased OEF, which is consistent with suboptimal blood flow necessitating greater utilization of oxygen reserve capacity in the blood. While this compensatory mechanism allowed CMRO2 to be preserved, it may still indicate a susceptibility for ischemic insult as lower oxygen pressures in the tissues are needed for greater extraction from the blood. Lower oxygen tension in the tissues may confer risk for chronic ischemic insult. Increased systolic blood pressure was associated with a relative excess of CBF resulting in decreased OEF and no change in CMRO2. This association was particularly prominent above systolic blood pressures 150–160 mmHg, at which an inflection point was seen in a cubic spline fit. This suggests imperfect cerebral autoregulation in our sample among those with systolic blood pressure in hypertension ranges above 150–160 mmHg.
Triglycerides, also known as fats, are an important energy source that are stored in adipose tissue. High triglycerides are often found in association with other vascular risk factors including obesity, elevated fasting blood glucose, increased total cholesterol, and inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate.23 In addition to indicating the likely presence of other vascular risk factors, increased triglyceride levels are an independent risk factor for atherosclerotic disease including coronary heart disease23 and ischemic stroke.24,25 High triglyceride levels can result in increased effective vascular resistance by increasing the viscosity of blood26 and have been associated with decreased cerebral perfusion pressure5 and presence of leukoaraiosis,27 a type of microvascular brain insult. In a cohort with metabolic syndrome, diminished CBF related to hypertriglyceridemia and obesity was shown to mediate memory impairment.28 In our study we showed that diminished CBF in hypertriglyceridemia likely reflects a primary vascular phenomenon with increased OEF, which carries risk of ischemic insult. Even while CMRO2 is preserved there may remain tissues with dangerously low oxygen tension due to diffusion and solubility limitations of oxygen in brain tissue; large, possibly exponential, increases in CBF may be needed to ensure proper oxygenation throughout all the brain tissue including within “lethal corners” as oxygen content drops along a capillary and radially outward into the tissue.29 This has potential implications for understanding therapies as apparently “adequate” perfusion may yet confer risk in some regions. Fortunately, treatment to lower serum triglycerides has been shown to increase CBF with potential benefits in preserving cognitive function. In asymptomatic middle-aged individuals, treatment with atorvastatin—which decreases cholesterol and triglycerides—resulted in an increase in CBF.30 In an elderly cohort with symptoms of occlusive cerebrovascular disease, participants were randomly assigned to gemfibrozil to lower triglycerides.31 At six-month follow-up those in the treatment group showed an increase in CBF and preservation of cognitive function whereas those in the control group had no change in CBF and a decrease in cognitive function.
Hypertension is associated with decreased CBF in most studies, though some have shown a similar increase as seen in our study.32 The difference is likely attributed to an interplay between a short-term impact of elevated blood pressure to increase perfusion pressure and CBF and a long-term remodeling that increases vascular resistance and decreases CBF. The elevated CBF in our study exceeded metabolic demand as indicated by the diminished OEF. Indeed, we noted a strong negative correlation overall in the study between CBF and OEF. The elevation in CBF with higher systolic blood pressure in our study likely indicates imperfect cerebral autoregulation, which is recognized to occur even within the normally accepted range of 60 to 150 mm Hg cerebral perfusion pressure.6 Cerebral perfusion pressure is the difference between mean arterial pressure ((systolic blood pressure + 2 × diastolic blood pressure)/3) minus intracranial pressure33 (normal 7–15 mmHg supine).34 The highest mean arterial pressure in our sample at time of MRI was 131 mmHg, which is within the classic autoregulation threshold for cerebral perfusion pressure even without accounting for intracranial pressure. Failure of autoregulation is important as increased CBF beyond what is needed may be harmful as it exposes the microvasculature to excess pulsatility, which promotes vascular remodeling that increases resistance35,36 and leads to hypoperfusion.37–39 In further exploring our data, we found a significant interaction for female sex. Splitting the analysis into men and women, we found that increased CBF with greater systolic blood pressure was significant only for women ( p = 0.0009), not for men ( p = 0.9). In looking at Figure 3, only one man had CBF above 75 compared with eight women. The women in our study were all post-menopausal and this may suggest they are susceptible to hypertension-induced failure of autoregulation. The women in our group had a wider range of blood pressures than men, and it is possible that sex differences we observed in autoregulation may be due to sex differences in hypertension prevalence in our sample and not an underlying susceptibility to hypertensive damage; further prospective studies are needed for confirmation.
While CBF is known to be responsive to changes in neuronal metabolic demand,6 a recent study by Cha et al. showed limited correlation between CBF and CMRO2 between individuals at rest.40 In distinction to that study, we did observe a trend ( p = 0.06) between resting CBF and CMRO2 that became significant ( p = 0.0005) with the exclusion of an outlier with systolic blood pressure of 181 mmHg and CBF above 100 ml/100 g/min. Despite the importance of neurovascular coupling, factors aside from metabolic rate may influence CBF through direct insults to the cerebral vasculature. To date, only a few studies with modest sample sizes have studied the impact of vascular risk factors on CBF and shown how this may affect metabolic rate. Of these, several studies were performed in patients with history of stroke or transient ischemic attack and the associations may not be widely generalizable to asymptomatic individuals. These prior studies used invasive and costly nuclear medicine imaging approaches not widely available; similar assessments have not previously been feasible with MRI. The findings to date, particularly among asymptomatic individuals, are inconsistent. A positron emission tomography (PET) study by Mentis et al. of 42 men (average age 68 years), found that those with well-controlled hypertension had decreased metabolic rate in the anterior circulation.41 A small retrospective PET study by Fujii et al. of 15 patients (average age 61) with transient neurologic symptoms showed decreased CBF and CMRO2 and increased OEF in those with hypertension.42 A PET study by Nakane et al. also demonstrated decreased CBF and CMRO2 among those with hypertension among a group of 22 (average age 58) with cerebral infarction.43 In a smaller group of 13 without brain infarction (average age 62), Nakane et al. saw decreased CBF but no change in CMRO2 in those with hypertension.43 To our knowledge, no prior studies have shown changes in brain metabolic rate related to hypertriglyceridemia, though it has been shown to affect CBF. It is hoped that the advent of noninvasive MRI methods to obtain a more complete characterization of vascular hemodynamics will shed further insights into the impact of systemic vascular risk factors on brain health.
There were several limitations to our study. We evaluated only global measures of brain hemodynamics, which may not identify impacts, like a decrease in CMRO2, in at-risk regions such as vascular watersheds. We assessed CBF, OEF, and CMRO2 only once and were therefore not able to evaluate for determinants of change in these brain vascular parameters or assess causality. We focused on the impact of mid-life vascular risk factors that have been more closely linked with subsequent risk for dementia, which may differ from associations in other studies in which brain vascular measures are assessed at the same time as brain assessment, typically later in life. CMRO2 was derived from measurements of CBF and OEF, and as previously described this showed good agreement with the existing PET literature.17 Nonetheless, the sensitivity of this MRI method with those of PET requires further study; intrinsic errors and noise both in CBF and OEF measures may be compounded and result in less-accurate assessments. Finally, lack in alteration of CMRO2 does not exclude the presence of brain insult. Early insults to the brain parenchyma may not result in hypometabolism. Further, the brain may also initially compensate for damage to specialized brain regions by recruiting additional areas to maintain cognitive performance, increasing metabolic rate.44
Conclusions
Decreased CBF related to hypertriglyceridemia was accompanied by increased OEF without significant difference in CMRO2, confirming an association with subclinical vascular insufficiency. Increased CBF related to systolic blood pressure was associated with decreased OEF, and no change in CMRO2, indicating a relative excess in perfusion; the CBF association was most prominent for those with systolic blood pressures above 150–160 mmHg, suggesting limitations in autoregulation with more severe hypertension.
OEF can be obtained noninvasively using MRI without need for intravenous contrast agents and can help to provide specificity to the nature of alterations in CBF when the etiology may be due to a primary vascular etiology or secondary to hypometabolism from neuronal insult. Assessing CMRO2 further informs the degree of metabolic insult present by indicating whether reserve mechanisms have been exhausted.
Funding
This work was supported by the Friends of the UT Southwestern Alzheimer's Disease Center, and UL1TR001105 and KL2TR000453 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Friedman JI, Tang CY, de Haas HJ, et al. Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC Cardiovasc Imaging 2014; 7: 1039–1053. [DOI] [PubMed] [Google Scholar]
- 2.Jennings JR, Heim AF, Kuan DC, et al. Use of total cerebral blood flow as an imaging biomarker of known cardiovascular risks. Stroke 2013; 44: 2480–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Káplár M, Paragh G, Erdei A, et al. Changes in cerebral blood flow detected by SPECT in type 1 and type 2 diabetic patients. J Nucl Med 2009; 50: 1993–1998. [DOI] [PubMed] [Google Scholar]
- 4.Wakisaka M, Nagamachi S, Inoue K, et al. Reduced regional cerebral blood flow in aged noninsulin-dependent diabetic patients with no history of cerebrovascular disease: Evaluation by N-isopropyl-123I-p-iodoamphetamine with single-photon emission computed tomography. J Diabet Complications 1990; 4: 170–174. [DOI] [PubMed] [Google Scholar]
- 5.Meyer JS, Rogers RL, Mortel KF, et al. Hyperlipidemia is a risk factor for decreased cerebral perfusion and stroke. Arch Neurol 1987; 44: 418–422. [DOI] [PubMed] [Google Scholar]
- 6.Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapoport SI, Hatanpää K, Brady DR, et al. Brain energy metabolism, cognitive function and down-regulated oxidative phosphorylation in Alzheimer disease. Neurodegeneration 1996; 5: 473–476. [DOI] [PubMed] [Google Scholar]
- 8.Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun 2016; 7: 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derdeyn CP, Videen TO, Yundt KD, et al. Variability of cerebral blood volume and oxygen extraction: Stages of cerebral haemodynamic impairment revisited. Brain 2002; 125: 595–607. [DOI] [PubMed] [Google Scholar]
- 11.Kloppenborg RP, van den Berg E, Kappelle LJ, et al. Diabetes and other vascular risk factors for dementia: Which factor matters most? A systematic review. Eur J Pharmacol 2008; 585: 97–108. [DOI] [PubMed] [Google Scholar]
- 12.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 14.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183–1197. [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med 2008; 60: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Xu F, Grgac K, et al. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med 2012; 67: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med 2009; 62: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P, Xu F, Lu H. Test-retest reproducibility of a rapid method to measure brain oxygen metabolism. Magn Reson Med 2013; 69: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kety SS, Schmidt CF. Measurement of cerebral blood flow and cerebral oxygen consumption in man. Fed Proc 1946; 5: 264. [PubMed] [Google Scholar]
- 20.Pittman RN. Oxygen transport. In: Pittman RN. (ed). Regulation of tissue oxygenation, San Rafael, CA: Morgan & Claypool Life Sciences, 2011. [PubMed] [Google Scholar]
- 21.Guyton AC, Hall JE. Respiration. In: Guyton AC, Hall JE. (eds). Textbook of medical physiology, 11th ed Amsterdam: Elsevier, 2005. [Google Scholar]
- 22.Deegan BM, Sorond FA, Lipsitz LA, et al. Gender related differences in cerebral autoregulation in older healthy subjects. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 2859–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007; 115: 450–458. [DOI] [PubMed] [Google Scholar]
- 24.Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007; 298: 309–316. [DOI] [PubMed] [Google Scholar]
- 25.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 2008; 300: 2142–2152. [DOI] [PubMed] [Google Scholar]
- 26.Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: Triglycerides and blood viscosity. Atherosclerosis 2002; 161: 433–439. [DOI] [PubMed] [Google Scholar]
- 27.Leistner S, Koennecke HC, Dreier JP, et al. Clinical characterization of symptomatic microangiopathic brain lesions. Front Neurol 2011; 2: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birdsill AC, Carlsson CM, Willette AA, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring) 2013; 21: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mintun MA, Lundstrom BN, Snyder AZ, et al. Blood flow and oxygen delivery to human brain during functional activity: Theoretical modeling and experimental data. Proc Natl Acad Sci 2001; 98: 6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson CM, Xu G, Wen Z, et al. Effects of atorvastatin on cerebral blood flow in middle-aged adults at risk for Alzheimer's disease: A pilot study. Curr Alzheimer Res 2012; 9: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers RL, Meyer JS, McClintic K, et al. Reducing hypertriglyceridemia in elderly patients with cerebrovascular disease stabilizes or improves cognition and cerebral perfusion. Angiology 1989; 40: 260–269. [PubMed] [Google Scholar]
- 32.van Laar PJ, van der Graaf Y, Mali WP, et al. Effect of cerebrovascular risk factors on regional cerebral blood flow. Radiology 2008; 246: 198–204. [DOI] [PubMed] [Google Scholar]
- 33.Phillips SJ, Whisnant JP. Hypertension and the brain. The National High Blood Pressure Education Program. Arch Intern Med 1992; 152: 938–945. [PubMed] [Google Scholar]
- 34.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma 2007; 24(Suppl 1): S55–S58. [DOI] [PubMed] [Google Scholar]
- 35.Clark LR, Nation DA, Wierenga CE, et al. Elevated cerebrovascular resistance index is associated with cognitive dysfunction in the very-old. Alzheimers Res Ther 2015; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pires PW, Dams Ramos CM, Matin N, et al. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 2013; 304: H1598–H1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility-Reykjavik Study. Brain 2011; 134: 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crane DE, Black SE, Ganda A, et al. Gray matter blood flow and volume are reduced in association with white matter hyperintensity lesion burden: A cross-sectional MRI study. Front Aging Neurosci 2015; 7: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uh J, Yezhuvath U, Cheng Y, et al. In vivo vascular hallmarks of diffuse leukoaraiosis. J Magn Reson Imaging 2010; 32: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cha YH, Jog MA, Kim YC, et al. Regional correlation between resting state FDG PET and pCASL perfusion MRI. J Cereb Blood Flow Metab 2013; 33: 1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mentis MJ, Salerno J, Horwitz B, et al. Reduction of functional neuronal connectivity in long-term treated hypertension. Stroke 1994; 25: 601–607. [DOI] [PubMed] [Google Scholar]
- 42.Fujii K, Sadoshima S, Okada Y, et al. Cerebral blood flow and metabolism in normotensive and hypertensive patients with transient neurologic deficits. Stroke 1990; 21: 283–290. [DOI] [PubMed] [Google Scholar]
- 43.Nakane H, Ibayashi S, Fujii K, et al. Cerebral blood flow and metabolism in hypertensive patients with cerebral infarction. Angiology 1995; 46: 801–810. [DOI] [PubMed] [Google Scholar]
- 44.Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol 2009; 60: 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]