Abstract
Aim
Flow diverters are increasingly used to treat aneurysms, but treatment is not always effective. The management of aneurysms that fail to occlude following flow diversion is problematic. We aimed to reproduce failures in an animal model and study re-treatment with additional flow diverters alone or with flow diverters and liquid embolic agent.
Material and methods
Twenty wide-necked aneurysms were created at the carotid-lingual bifurcation in 10 dogs, and were treated with flow diverters 4–6 weeks later. Follow-up angiography was performed at three months. Suitable residual aneurysms were randomly allocated: re-treatment with flow diverters alone (n = 6), or with the injection of liquid embolic between two layers of flow diverters (n = 4) or no re-treatment (n = 2). Angiography was repeated three months later, followed by euthanasia, photography and pathology.
Results
Patent wide-necked aneurysms were produced in 17/20 attempts (85%); three months after flow diversion there were 15/17 (88%) residual aneurysms. In three cases, re-treatment was not possible because the flow diverter had prolapsed into the aneurysm, leaving 12 aneurysms to study. Re-treated aneurysms showed improved angiographic results at six months (median score of 2; P = 0.03), but residual aneurysms were present in all cases. Parent artery occlusion occurred in two aneurysms treated with flow diverter plus liquid embolic. At pathology, aneurysms were only partially filled with thrombus; leaks through the flow diverters were found in the neointima connecting the arterial lumen to residual aneurysms.
Conclusion
Re-treatment of residual flow-diverted experimental aneurysms with additional flow diverters did not lead to aneurysm occlusion.
Keywords: Experimental aneurysm, flow diversion, failure, re-treatment, animal models
Introduction
Flow diverters (FDs) are increasingly used to treat intracranial aneurysms. Case series and systematic reviews have reported incomplete aneurysm occlusion after flow diversion in 20–30% of cases.1,2 The endovascular management of post-flow diversion residual aneurysms is problematic.3 FDs are braided stents characterised by low porosity; after deployment, FD pores are too small to cross to allow catheterisation of the aneurysm with a microcatheter for further coiling.4 Thus re-treatment often involves deploying another telescoping FD to cover the aneurysm neck area with an additional layer of FD mesh.5 Other re-treatment options may include parent vessel occlusion, with or without surgical creation of a bypass, or other surgical management of the residual.6,7 Aneurysm re-treatment after failure of flow diversion has never been studied in an animal model.
Residual aneurysms after flow diversion can be reproduced in some animal models.8–10 Residual aneurysms are associated with persistent ‘leaks’ in the neointima that does not completely cover the FD struts to reconstruct the parent vessel fully.8,9 We aimed to create large bilateral canine bifurcation aneurysms that would fail to occlude after flow diversion,9 to study re-treatment with additional FDs. We also explored a novel approach, the use of a liquid embolic agent (LEA) injected between two layers of FDs, attempting to occlude the residual leaks in the neointima after failure of flow diversion.
Materials and methods
Surgical aneurysm creation
The present report was prepared in accordance with the ARRIVE guidelines for animal research.11 The principles of laboratory animal care (NIH publication no. 86-23, revised 1985) were followed, as well as the guidelines of the Canadian Council on Animal Care. The protocol for animal experimentation was approved by the institutional animal care committee of the authors institution (CIPA). All procedures were performed in 22–32 kg dogs under general anaesthesia. Large wide-necked aneurysms were created at the bifurcation of the carotid and lingual artery in 10 animals (two aneurysms per animal), according to a previously published surgical model.9 Briefly, through a paramedian cervical incision, the left external jugular vein was harvested, inverted to remove valves and placed in heparinised saline. The distal carotid at the level of the lingual artery was exposed. Temporary clips were applied and a large arteriotomy was created on the medial aspect of the distal carotid artery, extending to the origin of the lingual artery. The vein graft was anastomosed to the carotid-lingual artery bifurcation using continuous 7.0 Prolene sutures (Ethicon, Cincinnati, OH, USA). The procedure was repeated on the contralateral carotid artery, to construct bilateral wide-necked lingual artery aneurysms. Incisions were closed in multiple layers over a drain left in place for 48 hours. Further details regarding animal anaesthesia, housing, husbandry conditions and welfare are available on request.
Endovascular treatment
Animals were prepared with acetylsalicylic acid (ASA) 325 mg and clopidogrel 75 mg for four days prior to flow diversion treatment.12 Clopidogrel was discontinued 10 days post-implantation, while ASA was continued until euthanasia. Angiography was performed 4–6 weeks after aneurysm construction, using a surgical transfemoral approach. Aneurysm and arterial dimensions were measured on a Siemens Leonardo workstation, using Syngo software. FDs were deployed through Marksman (Covidien/Medtronic, Dublin, Ireland) or Headway 27 microcatheters (Microvention, Tustin, CA, USA) from the distal to the proximal carotid artery to span the aneurysm neck with sufficient proximal and distal landing zones (5–10 mm). The size of the FD was selected according to the diameter of the proximal landing zone of the recipient vessel (3.5–4.0 mm in this model). In four aneurysms with very wide necks, a high-porosity stent (low-profile visualised intraluminal support device (LVIS; Microvention, Tustin, CA, USA) was necessary to anchor the FD into the parent artery. In one aneurysm, two overlapping FDs were used to ensure coverage of a very wide neck and sufficient landing zones. FDs were flow redirection endoluminal devices (FREDs) (gifts from Microvention, Tustin, CA, USA) 3.25–4.50 mm in diameter, 16–42 mm in length.
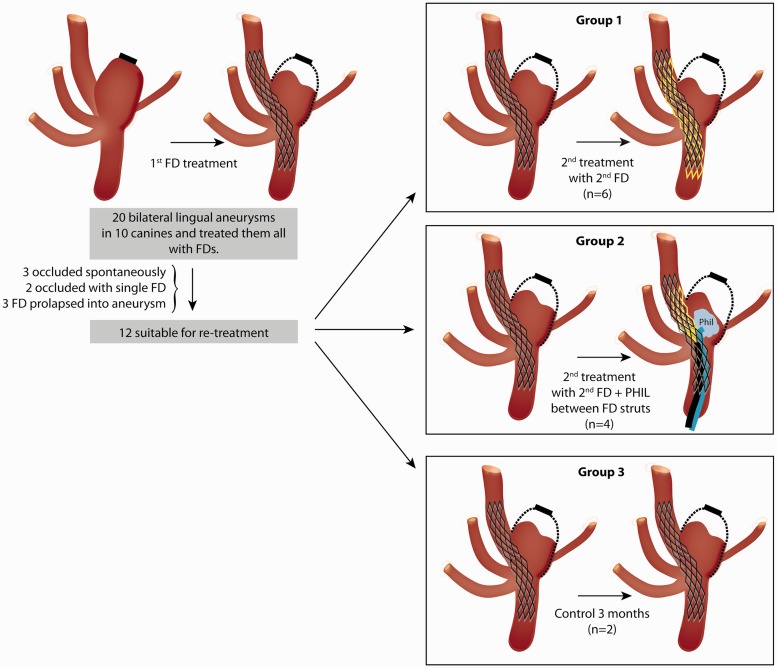
Follow-up angiography was performed three months after the first procedure and results were scored according to an ordinal scale.13 A score of 0 indicated no change, 1 indicated residual filling of more than 50%, 2 indicated residual filling of less than 50% of the pre-treatment aneurysm volume, 3 indicated a residuum confined to the neck region, and 4 indicated complete occlusion. One animal without residual aneurysms was euthanised at three months; all other animals had follow-up angiography three months following the second treatment (total of six months follow-up). Suitable residual aneurysms (scores 0–2) were then randomly allocated by drawing lots to one of the following treatments (show in a schematic flow chart, Figure 1): (a) re-treatment with one or two telescoping FDs (n = 6); (b) re-treatment with injection of 0.2–0.6 ml of a LEA (precipitating hydrophobic injectable liquid 35% (PHIL)) (Microvention, Tustin, CA, USA) by way of a microcatheter (Headway 17; Microvention, Tustin, CA, USA) trapped between the initial FD and a partially deployed second FD, which was then fully deployed to sandwich the liquid embolic between the two layers of FD mesh (n = 4); or (c) no additional treatment (n = 2). The number of animals per group was selected to give priority to the re-treatment method most frequently used in patients (additional FDs in six aneurysms), explore the use of a novel method which had never been used in patients in a smaller number of aneurysms (n = 4), and observe a minimal number of controls to show that post-FD residual aneurysms would not occlude with time (n = 2), while minimising the total number of animals used for experiments (n = 10).
Figure 1.
Schematised flow chart to show disposition of 20 experimental aneurysms, with randomised allocation of 12 suitable residual aneurysms to various re-treatment groups.
Parent artery stenosis, if present, was measured using the formula 1 – N/D, where N = diameter at most stenosed region, and D = diameter of the distal normal artery.
Pathology
Euthanasia by barbiturate overdose was performed at six months. Carotid aneurysm constructs were harvested en bloc, fixed in 10% formalin for 5–7 days, dissected and photographed, using a computerised imaging system (Motic Images Advanced 3.2, Hong Kong, China). Residual aneurysms were sectioned longitudinally, to photograph the aneurysmal cavity and the FD visible through the neck and the ostium of the lingual artery. The carotid artery was then longitudinally opened under loupe magnification, to photograph the luminal surface of the FD and aneurysm neck. The degree of neointimal coverage of the device(s) at the level of the aneurysm neck was scored independently by two observers (JR and IS) according to a semiquantitative scale using percentiles, from 0% to 100% coverage (in 10% increments), with disagreements resolved in a consensus session, as previously described.12,14 The macroscopic status of aneurysms at pathology was examined and classified according to a three-grade ordinal scale: complete or near complete disappearance of the aneurysmal sac (1); aneurysm sac partially filled with thrombus (2); residual aneurysm without thrombus (3). Selected aneurysms (with or without thrombus) were sectioned, embedded in paraffin, and stained with haematoxylin, phloxine and saffron (HPS) or Movat’s pentachrome for microscopic study. Biopsies of the neointima covering FDs were treated in the same fashion.
Statistics
Aneurysm dimensions were compared using analysis of variance (ANOVA). Aneurysm occlusion scores were compared using the Mann–Whitney U test/Kruskal–Wallis test for non-parametric data. The significance level was set at P = 0.05.
Results
A schematised flow chart of the aneurysms to show allocation to the different experimental groups is presented in Figure 1.
Initial aneurysm construction and first flow diversion treatment
After 4–6 weeks, surgical construction yielded wide-necked lingual aneurysms in 17/20 attempts (85%, 95% confidence interval (CI) 61–96%), with three aneurysms found to be spontaneously occluded.
Aneurysm dimensions, device characteristics, and angiographic occlusion scores three months following initial flow diversion are presented in Table 1.
Table 1.
Aneurysm characteristics and outcomes after initial treatment with flow diversion.
| Aneurysm | Aneurysm dimensions (mm) |
FRED dimensions (diameter × length) | Use of additional LVIS for FRED anchoring | Angiographic score at 3 months | Parent vessel stenosis (%) | Randomised re-treatment group allocation | ||
|---|---|---|---|---|---|---|---|---|
| Length | Width | Neck | ||||||
| 1 | 12.2 | 13.8 | 7.6 | 4.5 × 25 mm | – | 2 | 60 | Repeat flow diversion |
| 2 | 11.2 | 9.1 | 7.6 | (2) 3.5 × 20 mm 3.25 × 16 mm | – | 2 | 65 | Repeat flow diversion |
| 3 | 13.3 | 10.9 | 11.2 | 4.5 × 20 mm | – | 0 | 60 | Repeat flow diversion |
| 4 | 19.3 | 14.3 | 11.8 | 4.0 × 23 mm | – | 0 | 30 | Repeat flow diversion |
| 5 | 27.4 | 10.7 | 13.1 | 4.0 × 23 mm | Yes | 0 | 20 | Repeat flow diversion |
| 6 | 16.1 | 10.1 | 11.6 | 3.75 × 42 mm | – | 0 | 60 | Repeat flow diversion |
| 7 | 13.2 | 11.7 | 9.5 | 4.5 × 20 mm | – | 1 | 50 | Repeat flow diversion + LEA |
| 8 | 23.6 | 8.3 | 13.1 | 4.5 × 25 mm | – | 0 | 10 | Repeat flow diversion + LEA |
| 9 | 18.4 | 11.2 | 14.8 | 4.0 × 23 mm | Yes | 0 | 0 | Repeat flow diversion + LEA |
| 10 | 15.9 | 8.8 | 10.1 | 3.5 × 22 mm | – | 1 | 60 | Repeat flow diversion + LEA |
| 11 | 8.7 | 6.7 | 7.1 | 3.5 × 22 mm | 2 | 10 | Control | |
| 12 | 11.3 | 7.9 | 8.9 | 3.5 × 22 mm | Yes | 1 | 10 | Control |
| 13a | 32.2 | 17.3 | 11.3 | 4.5 × 25 mm | – | 0 | 60 | / |
| 14a | 11.9 | 9.1 | 9.6 | 4.0 × 20 mm | – | 0 | 10 | / |
| 15a | 19.9 | 10.2 | 13.6 | 4.5 × 25 mm | Yes | 0 | 20 | / |
| 16b | 15.6 | 9.1 | 6.4 | 4.5 × 25 mm | – | 3 | 35 | / |
| 17b | 13.5 | 12.0 | 9.0 | 4.5 × 25 mm | – | 3 | 40 | / |
| Mean ± SD | 16.7 ± 6.3 | 10.7 ± 2.6 | 10.4 ± 2.5 | 0.88 ± 1.11 | 34.71 ± 22.39 | |||
| Median (IQR) | 0 (0–2) | 40 (10–60) | ||||||
Excluded from study; FD prolapsed into aneurysm at 3 months.
Excluded from study; aneurysms too well occluded at 3 months to be re-treated.
FRED: flow-redirection endoluminal device; LVIS: low-profile visualised intraluminal support; LEA: liquid embolic agent; IQR: interquartile range; /: not applicable.
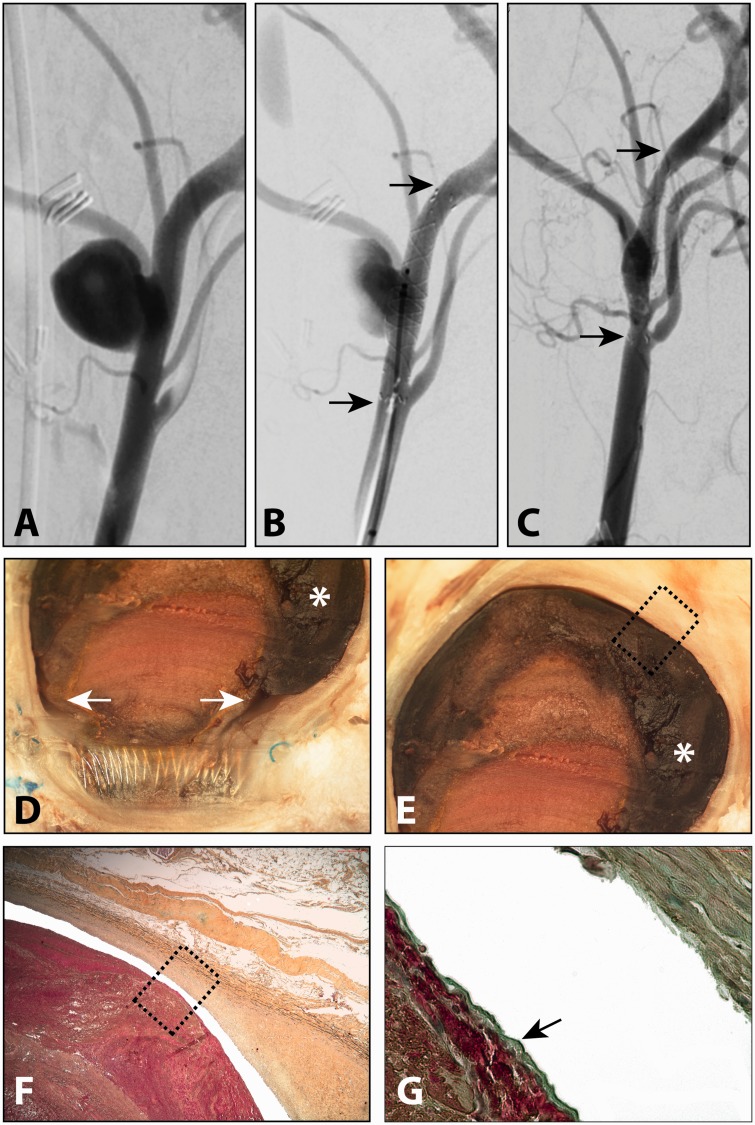
There were no significant differences in aneurysm dimensions in the different groups (P > 0.05, ANOVA). Treatment with a single FD was sufficient to occlude two out of 17 (11.8%, 95% CI 1.5–36.4%) aneurysms by three months (Figure 2). These two adequately occluded aneurysms were excluded from the study on re-treatment, but pathological analysis was still performed: aneurysms were filled with incompletely organised but endothelial cell-covered thrombus. A thin circumferential crescentic space persisted between the aneurysm wall and the endothelialised thrombus. Each FD was almost completely covered with mature neointima (0.38 mm in thickness), except for small leaks connected to the crescentic endothelialised residual spaces.
Figure 2.
Successful angiographic but not pathological occlusion of a lingual aneurysm (a) treated with a single flow diverter (FD) (b) after three months (c). Pathological analysis shows small leaks in the neointimal coverage of the FD, with clefts (arrows) leading to poorly unorganised thrombus in the fundus (asterisk in d and e). The thrombus was dissociated from the aneurysm wall (f), with evidence of endothelialisation (arrow in g) (Movat’s pentachrome stain, original magnification × 400).
In three other aneurysms, at three-month angiography the FD was found to have prolapsed into the aneurysmal cavity, and the distal parent vessel could not be re-catheterised; these aneurysms were also excluded. The total yield was therefore 12/20 (60%) constructed aneurysms which were suitable (median angiographic score of 0.5) for re-treatment, having failed initial flow diversion by three months.
Re-treatment of residual aneurysms after flow diversion
The dimensions of the residual aneurysms included in the re-treatment portion of the study, details of re-treatment, and final angiographic and pathological outcomes are shown in Table 2. All re-treated aneurysms remained patent in spite of repeat treatments (Figures 3 and 4). Re-treatment did improve angiographic results, and over a three-month period the median angiographic scores improved from 0.5 to 2 (P = 0.03) but no aneurysms became completely or near-completely occluded (no scores of 3 or 4). Angiographic scores at three and six months are presented in Table 3. Re-treatment with additional flow diversion alone led to improved angiographic scores at six months in three out of six (50%) aneurysms (median angiographic score from 0 to 2; P = 0.23). Stenosis of the parent artery, when present, was limited to less than 70% and was not haemodynamically significant. The degree of parent vessel stenosis decreased over time in four animals, even with the addition of a second intraluminal device.
Table 2.
Residual aneurysms characteristics and outcomes after re-treatment.
| Re-treatment group | Residual aneurysm dimensions (mm) |
FRED dimensions (diameter × length) + volume PHIL | Angiographic score 3 months later | Parent vessel stenosis (%) | Neointimal score | % Neointimal coverage | ||
|---|---|---|---|---|---|---|---|---|
| Length | Width | Neck | ||||||
| Repeat flow diversion | 11 | 4.5 | 7.6 | 4.0 × 23 mm | 2 | 40 | 2 | 90 |
| Repeat flow diversion | 6 | 5.5 | 7.6 | 4.0 × 13 mm | 2 | 50 | 3 | 100 |
| Repeat flow diversion | 13.3 | 10.9 | 11.2 | (2) 4.0 × 13 mm 4.0 × 13 mm | 0 | 50 | 3 | 90 |
| Repeat flow diversion | 19.3 | 14.3 | 11.8 | (2) 4.0 × 13 mm 4.0 × 13 mm | 2 | 40 | 2 | 90 |
| Repeat flow diversion | 27.4 | 10.7 | 13.1 | (2) 4.0 × 13 mm 4.0 × 13 mm | 1 | 30 | 2 | 30 |
| Repeat flow diversion | 16.1 | 10.1 | 11.6 | 4.0 × 13 mm | 2 | 60 | 3 | 60 |
| Repeat flow diversion + LEA | 10.9 | 10.2 | 9.5 | (2) 4.0 × 13 mm 4.0 × 13 mm + 0.50 cc PHIL | 2a | 100a | 2 | N/A |
| Repeat flow diversion + LEA | 23.6 | 8.3 | 13.1 | 4.5 × 23 mm + 0.20 cc PHIL | 2 | 20 | 2 | 90 |
| Repeat flow diversion + LEA | 18.4 | 11.2 | 14.8 | 4.5 × 23 mm + 0.20 cc PHIL | 1a | 100a | 3 | N/A |
| Repeat flow diversion + LEA | 14.1 | 8.1 | 10.1 | (2) 4.0 × 13 mm 4.0 × 13 mm + 0.05 cc PHILb | 1 | 50 | 3 | 90 |
| Control | 5.1 | 5.2 | 7.1 | / | 2 | 10 | 3 | 90 |
| Control | 9.1 | 5.9 | 8.9 | / | 1 | 10 | 3 | 90 |
| Mean ± SD | 14.5 ± 6.8 | 8.7 ± 3.0 | 10.5 ± 2.5 | |||||
| Median (IQR) | 2 (1–2) | 45 (27.5–52.5) | 3 (2–3) | 90 (90–90) | ||||
Parent vessel completely occluded 3 months following re-treatment; retrograde aneurysm filling.
Microcatheter occluded before injection completed.
FRED: flow-redirection endoluminal device; LVIS: low-profile visualised intraluminal support; LEA: liquid embolic agent; IQR: interquartile range; /: not applicable.
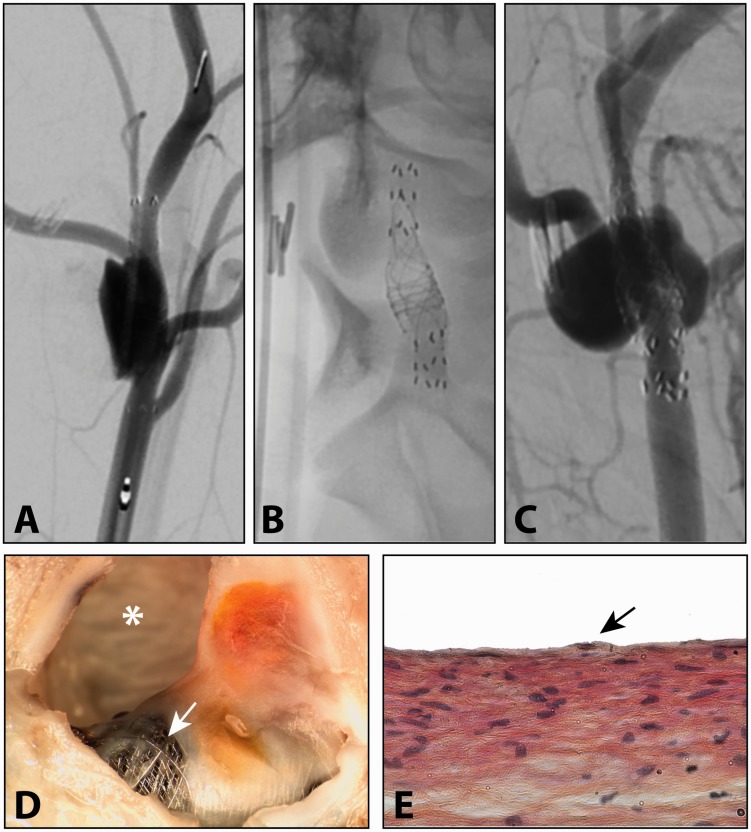
Figure 3.
Residual aneurysm three months after treatment with single flow diverter (FD) (a), which was re-treated with a second FD (b), with persistent failure to occlude the aneurysm (c). Pathological analysis (d) showed bare areas of FD mesh (arrow) with partially organised intra-aneurysmal thrombus. Biopsy of the neointima over the device showed endothelialisation (arrow in e) (haematoxylin, phloxine and saffron stain, original magnification × 400).
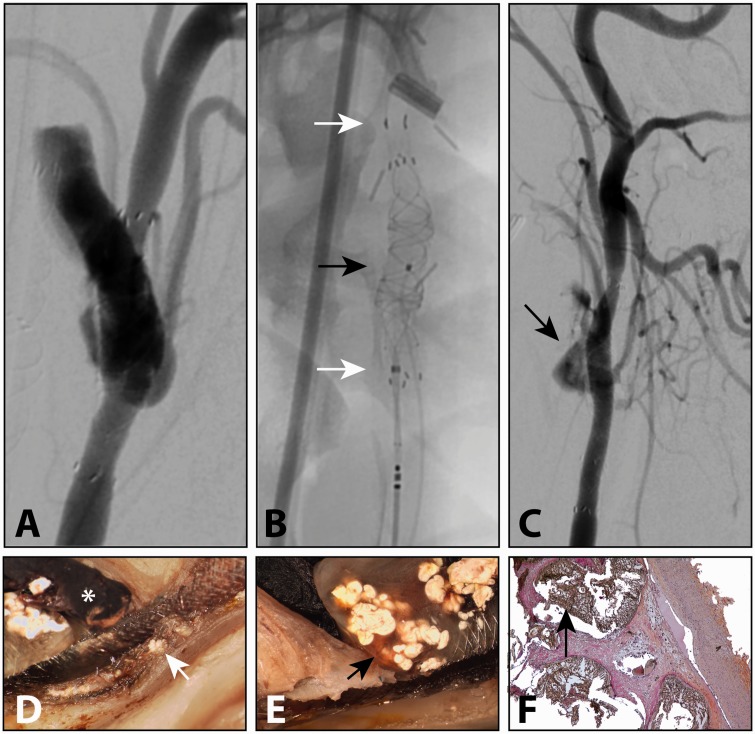
Figure 4.
Residual aneurysm three months after treatment with single flow diverter (FD) (a), which was re-treated (b) with a second FD (white arrows) and PHIL liquid embolic delivered through a microcatheter (black arrow). At three months, persistent aneurysm filling was observed (arrow in c). Magnified photography showed white liquid embolic on either side of the FD mesh (arrow in d), with persistent leak into the aneurysms at (black arrow in e). Histopathology showed minimal reaction to the liquid embolic (black arrow in f) inside the aneurysm fundus (haematoxylin, phloxine and saffron stain, original magnification × 50).
Table 3.
Change in angiographic scores and parent vessel stenosis with re-treatment.
| Re-treatment group | Angiographic score 3 months after initial flow diversion | Angiographic score at 6 months (3 months following re-treatment) | Change in angiographic score | Parent vessel stenosis at 3 months (%) | Parent vessel stenosis at 6 months (%) | Change in parent vessel stenosis with re-treatment |
|---|---|---|---|---|---|---|
| Repeat flow diversion | 2 | 2 | 0 | 60 | 40 | −20 |
| Repeat flow diversion | 2 | 2 | 0 | 65 | 50 | −15 |
| Repeat flow diversion | 0 | 0 | 0 | 60 | 50 | −10 |
| Repeat flow diversion | 0 | 2 | +2 | 30 | 40 | +10 |
| Repeat flow diversion | 0 | 1 | +1 | 20 | 30 | +10 |
| Repeat flow diversion | 0 | 2 | +2 | 60 | 60 | 0 |
| Repeat flow diversion + LEA | 1 | 2a | +1 | 50 | 100a | +50 |
| Repeat flow diversion + LEA | 0 | 2 | +2 | 10 | 20 | +10 |
| Repeat flow diversion + LEA | 0 | 2a | +2 | 0 | 100a | +100 |
| Repeat flow diversion + LEA | 1 | 1 | 0 | 60 | 50 | −10 |
| Control | 2 | 2 | 0 | 10 | 10 | 0 |
| Control | 1 | 1 | 0 | 10 | 10 | 0 |
| Median (IQR) | 0.5 (0–1.25) | 2 (1–2) | 40 (10–60) | 45 (27.5–52.5) |
Parent vessel completely occluded.
LEA: liquid embolic agent; IQR: interquartile range.
Injection of liquid embolic between two layers of FD mesh proved technically challenging, and one injection was aborted due to microcatheter occlusion. In two other aneurysms, injection of LEA led to parent artery occlusion while injecting between the layers of FD mesh, but aneurysms were still visible, filling retrogradely through distal anastomoses. This re-treatment strategy also improved angiographic scores at six months in three out of four (median angiographic score changed from 0.5 to 2). The three additional months of follow-up did not lead to progression towards occlusion for the two control aneurysms, which remained widely patent at six months with unchanged angiographic scores.
Magnified stereoscopic photography and pathological study of the re-treated aneurysms with improved angiographic scores (Figures 3 and 4) showed partial occlusion of the aneurysmal fundi, filled with thrombus with variable degrees of organisation and covered by a layer of endothelial cells. The aneurysms that were angiographically unchanged after re-treatment were characterised by empty aneurysm cavities (two out of six), and intra-aneurysmal thrombus that was not organised (one out of six). The relationship between the degree of neointimal coverage of devices, size of the residual aneurysm, and the content and degree of organisation of the intra-aneurysmal contents of the residual aneurysms was unclear.
In cases treated with repeat flow diversion and liquid embolics, the liquid embolic was found to have successfully crossed the mesh of the initially deployed FD, as PHIL was located inside the aneurysmal sac in all cases, as well as between the two layers of flow diverting mesh (Figure 4). The two cases in which the parent vessel had become occluded also showed clear evidence of LEA in the carotid artery.
At pathology, the control aneurysms that were not re-treated at three months showed empty aneurysm cavities, connected to small leaks in the neointima that covered the FDs.
Discussion
It was possible using a canine bifurcation aneurysm model to reproduce the clinical problem of interest: treatment failures after flow diversion. Residual aneurysms at three months remained unchanged at six months when not re-treated, because of small leaks (pores) in the neointima covering the devices. All animals that were re-treated with more flow diversion, with or without adjunctive liquid embolics, failed to go on to complete or near-complete aneurysm occlusion. The use of a liquid embolic was neither successful nor safe, at least in this preliminary experience, in contrast to a previous experiment in dogs.15
Residual aneurysms after flow diversion are not rare.2 In some patients with mass effect, symptoms may progress in spite of follow-up angiograms showing only small residua.16 Clinically, it is not clear at which point in time and how large a residuum shown by angiography signifies that flow diversion has failed, or that re-treatment should be considered.17 Some flow-diverted aneurysms may become progressively occluded over time, but watchful waiting can also result in aneurysm rupture.18,19
To conduct this experiment properly requires the selection of an animal model known to be prone to failure after flow diversion. A previous systematic review has shown that canine aneurysms are most likely to fail when the aneurysms are large, the necks are wide, when aneurysms are located at a bifurcation, and when the construct includes a collateral branch.8–10,12,20 These four factors were all considered in selecting this model for this work. This experiment suggests, at least in this model, that once the leaks from the parent vessel into the aneurysm are lined by mature neointima, they are unlikely to become occluded spontaneously with time, and that re-treatment with another layer of FD does not lead to aneurysm occlusion.
The heterogeneous pathological findings we observed are difficult to interpret. We are tempted to speculate that the endothelialised crescents that were found between the thrombus that failed to organise and the aneurysm wall, also shown to be connected to the neointimal leaks at the neck, are a sign that treatment has failed. Similar crescents have been described to explain failures of simple coiling in some models21 and have prompted a hypothesis that the endothelium may play an important role in failures and recurrences, and that endothelial denudation could improve long-term results of endovascular treatment of aneurysms.22
Some aneurysms that appeared completely or near-completely occluded on angiography were filled with unorganised thrombus covered with an endothelial layer, leaving crescentic circulating spaces connected to leaks in the neointima that covered the FD devices. Similar findings have been associated with delayed aneurysm rupture after FD, in spite of apparently successful occlusion or near occlusion.23,24
LEAs have previously been used to treat human aneurysms.25–27 In animal models, LEAs have been shown to be more effective but more difficult to control than coils.28,29 Intra-aneurysmal injection of a LEA under the protection of a partially deployed FD (to prevent migration of embolic material in the parent vessel) has recently been described in experimental aneurysms with good results.15 Our exploratory experience with telescoping FDs and the injection of PHIL between the FDs was found to be more hazardous than we anticipated. The technique will need further development before consideration as a future potential option to treat failures of flow diversion in patients.
There are several limitations to this study: the number of animals was small, and the follow-up periods were short. However, a previous study has shown that failures at three months lead to no further improvement with time, at least in canine models.30 Other limitations include that cervical carotid experimental aneurysm models differ from spontaneous intracranial aneurysms, and canine and human biology is different; any extrapolation of these results to the clinical realm must be with caution.
Conclusion
Failure of flow diversion can be reproduced in canine models. Re-treatment of residual flow-diverted experimental aneurysms with additional flow diversion, even when supplemented with LEAs, did not lead to aneurysm occlusion.
Funding
Robert Fahed received a research grant from La Société Française Neuro-Vasculaire (SFNV).
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 3.Kan P, Srinivasan VM, Mbabuike N, et al. Aneurysms with persistent patency after treatment with the pipeline embolization device. J Neurosurg 2016; 126: 1–5. [DOI] [PubMed] [Google Scholar]
- 4.Farzin B, Brosseau L, Jamali S, et al. Flow diverters: inter and intra-rater reliability of porosity and pore density measurements. J Neurointerv Surg 2015; 7: 734–739. [DOI] [PubMed] [Google Scholar]
- 5.Chalouhi N, Tjoumakaris S, Starke RM, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013; 44: 2150–2154. [DOI] [PubMed] [Google Scholar]
- 6.Mattingly T, Van Adel B, Dyer E, et al. Failure of aneurysm occlusion by flow diverter: a role for surgical bypass and parent artery occlusion. J Neurointerv Surg 2015; 7: e13. [DOI] [PubMed] [Google Scholar]
- 7.Darsaut TE, Salazkin I, Gentric JC, et al. Temporary surgical clipping of flow-diverted arteries in an experimental aneurysm model. J Neurosurg 2016; 125: 283–288. [DOI] [PubMed] [Google Scholar]
- 8.Darsaut TE, Bing F, Salazkin I, et al. Testing flow diverters in giant fusiform aneurysms: a new experimental model can show leaks responsible for failures. AJNR Am J Neuroradiol 2011; 32: 2175–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahed R, Gentric JC, Salazkin I, et al. Flow diversion of bifurcation aneurysms is more effective when the jailed branch is occluded: an experimental study in a novel canine model. J Neurointerv Surg 2017; 9: 311–315. [DOI] [PubMed] [Google Scholar]
- 10.Fahed R, Raymond J, Ducroux C, et al. Testing flow diversion in animal models: a systematic review. Neuroradiology 2016; 58: 375–382. [DOI] [PubMed] [Google Scholar]
- 11.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cereb Blood Flow Metab 2011; 31: 991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darsaut TE, Bing F, Salazkin I, et al. Flow diverters can occlude aneurysms and preserve arterial branches: a new experimental model. AJNR Am J Neuroradiol 2012; 33: 2004–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamran M, Yarnold J, Grunwald IQ, et al. Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology 2011; 53: 501–508. [DOI] [PubMed] [Google Scholar]
- 14.Gentric JC, Salazkin I, Gevry G, et al. Compaction of flow diverters improves occlusion of experimental wide-necked aneurysms. J Neurointerv Surg 2016; 8: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 15.Berenstein A. Treatment of experimental aneurysms with a new liquid embolic agent and a retrievable stent: proof of concept and feasibility study. J Neurointerv Surg 2016; 8: 934–939. [DOI] [PubMed] [Google Scholar]
- 16.Abla AA, Zaidi HA, Crowley RW, et al. Optic chiasm compression from mass effect and thrombus formation following unsuccessful treatment of a giant supraclinoid ICA aneurysm with the Pipeline device: open surgical bailout with STA-MCA bypass and parent vessel occlusion. J Neurosurg Pediatr 2014; 14: 31–37. [DOI] [PubMed] [Google Scholar]
- 17.Raymond J, Darsaut TE, Guilbert F, et al. Flow diversion in aneurysms trial: the design of the FIAT study. Interv Neuroradiol 2011; 17: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szikora I, Marosfoi M, Salomvary B, et al. Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2013; 34: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estrade L, Makoyeva A, Darsaut TE, et al. In vitro reproduction of device deformation leading to thrombotic complications and failure of flow diversion. Interv Neuroradiol 2013; 19: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahed R, Darsaut TE, Gentric JC, et al. Flow diversion: what can clinicians learn from animal models? Neuroradiology 2017; 59: 255–261. [DOI] [PubMed] [Google Scholar]
- 21.Raymond J, Darsaut T, Salazkin I, et al. Mechanisms of occlusion and recanalization in canine carotid bifurcation aneurysms embolized with platinum coils: an alternative concept. AJNR Am J Neuroradiol 2008; 29: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond J, Guilbert F, Metcalfe A, et al. Role of the endothelial lining in recurrences after coil embolization: prevention of recanalization by endothelial denudation. Stroke 2004; 35: 1471–1475. [DOI] [PubMed] [Google Scholar]
- 23.Hampton T, Walsh D, Tolias C, et al. Mural destabilization after aneurysm treatment with a flow-diverting device: a report of two cases. J Neurointerv Surg 2011; 3: 167–171. [DOI] [PubMed] [Google Scholar]
- 24.Chow M, McDougall C, O’Kelly C, et al. Delayed spontaneous rupture of a posterior inferior cerebellar artery aneurysm following treatment with flow diversion: a clinicopathologic study. AJNR Am J Neuroradiol 2012; 33: E46–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon S, Archer K, Mericle R. Multicenter registry of liquid embolic treatment of cerebral aneurysms. World Neurosurg 2014; 82: e731–e738. [DOI] [PubMed] [Google Scholar]
- 26.Molyneux AJ, Cekirge S, Saatci I, et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004; 25: 39–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002; 96: 474–482. [DOI] [PubMed] [Google Scholar]
- 28.Struffert T, Roth C, Romeike B, et al. Onyx in an experimental aneurysm model: histological and angiographic results. J Neurosurg 2008; 109: 77–82. [DOI] [PubMed] [Google Scholar]
- 29.Murayama Y, Vinuela F, Tateshima S, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. AJNR Am J Neuroradiol 2000; 21: 1726–1735. [PMC free article] [PubMed] [Google Scholar]
- 30.Darsaut TE, Bing F, Salazkin I, et al. Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: an in vivo study in canines. J Neurosurg 2012; 117: 37–44. [DOI] [PubMed] [Google Scholar]