Abstract
Background
Acute ischemic stroke (AIS) more frequently develops in patients with intracranial vertebral artery dissection (VAD) than extracranial VAD, and is associated with possible poor clinical outcomes. The aim of this study is to compare high-resolution magnetic resonance imaging (HR-MRI) findings and clinical features of VAD with and without AIS.
Methods
Twenty-nine lesions from 27 patients (15 male and 12 female patients; age range = 28–73 years) who underwent diffusion MRI and 3T HR-MRI within seven days were included. We classified VAD according to the presence of AIS lesions on diffusion MRI. Clinical features and HR-MRI findings (angiographic patterns, presence of double lumen sign, dissecting flap, posterior inferior cerebellar artery involvement, remodeling index, length of affected vessels, T1-signal intensity, area of intramural hematoma, and grades and patterns of vessel wall enhancement) were evaluated.
Results
Thirteen VADs with AIS and 16 without AIS were included. There were no significant differences in the clinical parameters (sex, age, risk factors, symptoms). More VADs with AIS presented as a steno-occlusive pattern than VADs without AIS. More VADs without AIS presented with aneurysmal dilation, larger mean remodeling index and longer mean length than VADs with AIS. Presence of intramural hematoma, T1-iso-signal intensity of intramural hematoma and contrast enhancement were significantly more common in VADs with AIS than without AIS.
Conclusions
Our study showed some differences in HR-MRI comparing intracranial VAD patients with and without AIS. Differing findings may facilitate a better understanding of intracranial VAD and risk assessment of AIS in these patients.
Keywords: Acute ischemic stroke (AIS), high-resolution magnetic resonance imaging (HR-MRI), vertebral artery dissection (VAD), intramural hematoma
Background
Spontaneous vertebral artery dissection (VAD) is a frequent cause of stroke in young patients, and has an approximate annual incidence rate between 1.0 and 1.5 per 100,000 individuals.1–4 VADs can be classified as extracranial dissection with/without intracranial dissection and intracranial dissection.5 Intracranial VAD is the most common type of intracranial artery dissection in Asian people.5,6 It is more frequently associated with the development of ischemic events than extracranial VAD.7 Stroke due to VAD is associated with a poor outcome, and the risk of stroke is evidently high in the first few days after dissection.7,8 Thus, early detection of VAD and timely antithrombotic therapy are important because VAD is a potentially treatable cause of stroke and transient ischemic attack.9,10
Digital subtraction angiography (DSA) has been the gold standard for the evaluation of intracranial artery disease, and it is advantageous with regard to the accuracy of the information pertaining to various geometric changes, hemodynamic information, and collateral blood supply that it provides. However, diagnosing VAD via DSA can sometimes be challenging because it cannot evaluate arterial wall pathology. Recently, VAD has been detected more frequently thanks to advances in neurovascular imaging.3,11 High-resolution vessel wall magnetic resonance imaging (HR-MRI) is now widely used for the evaluation of patients with clinically suspected VAD. It can be used to determine detailed wall features of VAD because it can provide high spatial resolution, and thus diagnoses based on it can be made with more confidence.
In this study, we investigate the morphologic features of VAD with acute ischemic stroke (AIS) via HR-MRI. We expected that characterization of these radiologic features may facilitate a better understanding of intracranial VAD and enhance the accuracy of estimation of the risk of AIS associated with VAD.
Methods
Study population
This retrospective study was approved by our institutional review board, and the requirement for informed consent was waived. Among the 398 patients who underwent 3.0T HR-MRI at our hospital between May 2013 and August 2017, a total of 27 patients (15 males and 12 females, mean age = 51.9 years, age range = 28–73 years) with 29 intracranial VADs were identified, including two patients with bilateral VAD lesions. Patients were retrospectively enrolled in accordance with the following criteria: (a) diagnosed with VAD based on clinical and radiological findings, (b) more than two imaging modalities (other than HR-MRI, such as DSA, computed tomography or magnetic resonance angiography (MRA)) were used to diagnose VAD, (c) no history of head trauma, (d) underwent HR-MRI within seven days of diffusion MRI being performed, (e) did not present with subarachnoid hemorrhage. Demographic data including sex, age, symptoms, and risk factors associated with acute stroke (i.e. hypertension, diabetes, hyperlipidemia, coronary artery disease, smoking, body mass index) were obtained from electronic medical records.
MRI protocol
HR-MRI was performed with either a 3T Philips scanner with an eight-channel head coil (Achieva, Philips Healthcare, Best, Netherlands) or a 3T Siemens scanner with a 64-channel head coil (Magnetom Skyra TIM TX TrueShape, Siemens Healthcare, Erlangen, Germany). The HR-MRI protocol consisted of three-dimensional (3D) time-of-flight (TOF) MRA, 3D proton density (PD) imaging, two-dimensional (2D) or 3D T1-weighted imaging (T1WI), and contrast-enhanced T1W1 (CE-T1WI). The PD imaging used turbo spin-echo sequences with parameters within the following ranges: repetition time (TR) 1200–2000 ms, echo time (TE) 30–33 ms, flip angle (FA) 90–120 degrees, matrix 300–320 × 264–300, field of view (FOV) 149–180 × 180 mm, section thickness 0.3–0.6 mm, and number of excitations (NEX) 1–2. For T1WI the parameters were within the following ranges: TR 670–1000 ms, TE 7.6–8.7 ms, FA 90–120 degrees, matrix 200–256 × 200–256, FOV 100–170 × 100–170 mm, section thickness 0.6–2.0 mm, and NEX 1–3. CE-T1WI was conducted following intravenous administration of gadoterate meglumine (Dotarem®, Guerbet, France) at a dose of 0.1 mmol/kg body weight.
Image analysis
Image analysis was performed on a picture archiving and communication system workstation, and quantitative analysis was performed using a Medical Imaging Interaction Toolkit (MITK). The VADs were classified as being either with AIS or without AIS. The diagnoses of AIS were made according to the presence of diffusion-restricted lesions on diffusion MRI. Radiologic diagnoses of VADs were made according to the Strategies Against Stroke Study for Young Adults in Japan criteria12 using more than two imaging modalities.
Vessel lumen analysis
The morphology of dissected vessels was classified as steno-occlusive, aneurysmal dilation, and string of pearls sign, according to the TOF MRA findings. Aneurysmal dilation and increased vessel diameter were visually defined as an increase in vessel diameter compared with normal-appearing arteries.13,14 Presence of a dissecting flap, double lumen sign at the dissected vessel, involvement of the posterior inferior cerebellar artery (PICA), length, and remodeling index were also assessed on TOF-MRA, PD, and pre-contrast and post-contrast T1-weighted magnetic resonance (MR) images. Remodeling index was calculated using a modified remodeling index: outer diameter of dissected vessel/([proximal normal diameter + distal normal diameter]/2).15
Vessel wall analysis
An intramural hematoma was defined as a crescent or oval-shaped area, and T1-signal intensity of intramural hematoma was observed. T1-signal intensity occupying more than half of the intramural hematomas was recorded as low-, iso-, or high-signal intensity compared with adjacent muscle. The relative signal intensity was calculated based on mean signal intensity of an intramural hematoma/mean signal intensity of adjacent muscle.16 The area of intramural hematoma was measured on short axial T1-weighted images using an MITK by manual drawing.
Contrast enhancement analysis
Abnormal wall enhancement of a dissected vessel wall was defined as definite enhancement compared with normal vessels or contralateral vertebral artery (VA), and classified as one of three grades according to the degree of enhancement (grade 0: similar to that of normal walls, grade 1: greater than that of grade 0 but less than that of venous enhancement, and grade 2: similar to or greater than that of venous enhancement).17 Patterns of contrast enhancement were classified as perivascular, intramural hematoma, luminal vessel wall enhancement, and intraluminal thrombosis.1
Statistical analysis
Categorical values were summarized as frequencies with percentages, and quantitative data were tabulated as mean ± standard deviation. All statistical analyses were performed using SAS® version 9.4 (SAS Institute Inc, Cary, NC, USA). Clinical data derived from VAD patients with and without AIS were compared via Student’s t-test, Fisher’s exact test, the chi-square test, and the Wilcoxon rank sum test. Fisher’s exact test was used for comparisons of frequency of intramural hematoma, T1-signal intensity of intramural hematoma, double lumen sign, dissecting flap, PICA involvement, and vessel wall enhancement of dissected vessels in VAD patients with and without AIS. Area of intramural hematoma, remodeling index, and length of dissected vessel were compared using the chi-square test. The Pearson correlation coefficient was used to evaluate the relationship between the interval between symptom onset and HR-MRI and intramural hematoma T1-signal intensity. Values of p < 0.05 were considered statistically significant.
Results
Clinical characteristics
Eight VAD patients with AIS exhibited acute infarction involving the cerebellum, and five patients presented with medullary infarction. Risk factors for atherosclerosis (hypertension, diabetes, hyperlipidemia, coronary artery disease, smoking, body mass index) did not differ significantly in VAD patients with and without AIS (p > 0.05). The demographic data of these patients are shown in Table 1. Most of the patients presented with symptoms at the time of admission, and only one patient without AIS did not present with any clinical symptoms, but it was detected incidentally via MRA. Dizziness was the most common symptom (69.2%) in VAD patients with AIS, and headache was the most common symptom (62.5%) in those without AIS. None of the patients had a history of trauma. The mean time interval between HR-MRI and diffusion MRI did not differ significantly in VAD patients with and without AIS. The mean time interval from symptom onset to diffusion MRI and mean time interval from symptom onset to HR-MRI in VAD patients with AIS were significantly shorter than were those in VAD patients without AIS.
Table 1.
Comparisons between VAD patients with and without AIS.
| With AIS (n = 13) | Without AIS (n = 14) | p value | |
|---|---|---|---|
| Male, n (%) | 8 (61.5) | 7 (50.0) | |
| Age, yearsa | 52.4 ± 15.1 | 52.6 ± 10.29 | 0.9614 |
| Risk factors | |||
| Hypertension, n (%) | 3 (23.1) | 3 (18.8) | 1.0000 |
| Diabetes, n (%) | 2 (15.4) | 0 (0) | 0.1921 |
| Hyperlipidemia, n (%) | 1 (7.7) | 3 (18.8) | 0.6059 |
| Coronary artery disease, n (%) | 2 (15.4) | 3 (18.8) | 1.0000 |
| Smoking, n (%) | 3 (23.1) | 2 (12.5) | 0.6322 |
| Body mass index (mean, kg/m2)a,c | 22.5 ± 7.2 | 20.6 ± 8.35 | 0.5026 |
| Symptoms | 13 (100) | 15 (93.8) | 0.5329 |
| Dizziness (%) | 9 (69.2) | 3 (18.8) | |
| Dysarthria (%) | 2 (15.4) | 0 (0) | |
| Dysphagia (%) | 0 (0) | 2 (12.5) | |
| Headache (%) | 0 (0) | 10 (62.5) | |
| Hemiparesis (%) | 1 (7.7) | 0 (0) | |
| Mental change (%) | 1 (7.7) | 0 (0) | |
| Incidental (%) | 0 (0) | 1 (6.3) | |
| HR-MRI to diffusion MRI time interval (days)b | 2 (0–6) | 2 (0–7) | 0.8230 |
| Symptom onset to diffusion MRI time interval (days)b | 1 (0–4) | 1 (0–56) | 0.0170 |
| Symptom onset to HR-MRI time interval (days)b | 4 (0–7) | 3 (1–62) | 0.0289 |
Data are presented as number (%) or mean ± standard deviation.
Data are presented as median (range).
Wilcoxon rank sum test.
VAD: vertebral artery dissection; AIS: acute ischemic stroke; HR-MRI: high-resolution magnetic resonance imaging; MRI: magnetic resonance imaging.
Vessel lumen analysis
Morphologic patterns on TOF MRA differed significantly in VAD with and without AIS. Twelve of 13 VAD (92.3%) patients with AIS exhibited steno-occlusive patterns (nine (75%), occlusion; three (25%), severe stenosis), and one (7.7%) exhibited aneurysmal dilation (Table 2). However, in VAD without AIS, aneurysmal dilation (56.3%), string of pearls sign (25.0%) and steno-occlusive pattern (18.8%) were present in that order of frequency. Increased vessel diameter was significantly more frequent in VAD without AIS than those with AIS (13 (81.3%) vs. 1 (7.7%)) (p = 0.0001). Double lumen sign and dissecting flap were also more frequent in VAD without AIS than VAD with AIS. Mean modified remodeling index was larger and mean length was shorter in VAD with AIS than VAD without AIS, but these differences were not statistically significant.
Table 2.
HR-MRI findings in VAD with and without AIS.
| With AIS (n = 13) | Without AIS (n = 16) | p value | |
|---|---|---|---|
| Vessel lumen analysis | |||
| Angiographic pattern | 0.0004 | ||
| Steno-occlusive, n (%) | 12 (92.3) | 3 (18.8) | |
| Severe stenosis, n (%) | 3 (25.0) | 3 (100) | |
| Occlusion, n (%) | 9 (75.0) | 0 (0) | |
| Aneurysmal dilation, n (%) | 1 (7.7) | 9 (56.3) | |
| Size of aneurysma | 3.04 | 2.79 ± 0.80 | |
| String of pearls sign, n (%) | 0 (0) | 4 (25) | |
| Increased vessel diameter, n (%) | 1 (7.7) | 13 (81.3) | 0.0001 |
| Double lumen sign, n (%) | 2 (15.4) | 4 (25) | 0.6628 |
| Dissecting flap, n (%) | 5 (38.5) | 9 (56.3) | 0.3404 |
| PICA involvement, n (%) | 5 (38.5) | 1 (6.3) | 0.0638 |
| Remodeling indexa | 1.2 ± 0.45 | 1.3 ± 0.44 | 0.4650 |
| Lengtha | 25.4 ± 17.93 | 17.1 ± 11.99 | 0.1490 |
| Vessel wall analysis | |||
| Intramural hematoma, n (%) | 12 (92.3) | 7(43.8) | 0.0084 |
| T1 SI of intramural hematoma | |||
| Iso SI, n (%) | 10 (76.9) | 2 (12.5) | 0.0449 |
| High SI, n (%) | 2 (15.4) | 5 (31.3) | |
| Low SI, n (%) | 0 (0.0) | 0 (0.0) | |
| Relative T1 signal intensitya | 1.2 ± 0.49 | 0.9 ± 1.15 | 0.4905 |
| Area (mm2)a | 294.7 ± 260.85 | 133 ± 84.74 | 0.1336 |
| Contrast enhancement analysis | |||
| Vessel wall enhancement, n (%) | 11 (84.6) | 9 (56.3) | 0.1296 |
| Grade 0, n (%) | 1 (7.7) | 7 (43.8) | 0.0410 |
| Grade 1, n (%) | 6 (46.2) | 2 (12.5) | |
| Grade 2, n (%) | 5 (38.5) | 7 (43.8) | |
| Vessel enhancement pattern | |||
| Perivascular, n (%) | 8 (61.5) | 7 (43.8) | 0.9039 |
| Intramural hematoma, n (%) | 0 (0.0) | 0 (0.0) | |
| Luminal vessel wall enhancement, n (%) | 2 (15.4) | 1 (6.3) | |
| Intraluminal thrombosis, n (%) | 1 (7.7) | 1 (6.3) | |
Data are presented as mean ± standard deviation.
HR-MRI: high-resolution magnetic resonance imaging; VAD: vertebral artery dissection; AIS: acute ischemic stroke; PICA: posterior inferior cerebellar artery; SI: signal intensity.
Vessel wall analysis
Intramural hematomas were more frequently observed in VAD with AIS (12; 92.3%) than VAD without AIS (seven; 43.8%) (p = 0.0057) (Table 2). T1 iso-signal intensity was more common in VAD with AIS than in VAD without AIS (p < 0.05) (Figures 1 and 2). In addition, there was a significant correlation between the time from symptom onset to HR-MRI and T1-signal intensity of intramural hematomas (r = 0.817, p = 0.012). The mean area of intramural hematomas in VAD with AIS (294.7 mm2 ± 260.85 mm2) was larger than it was in VAD without AIS (133 mm2 ± 84.74 mm2), but this difference was not statistically significant.
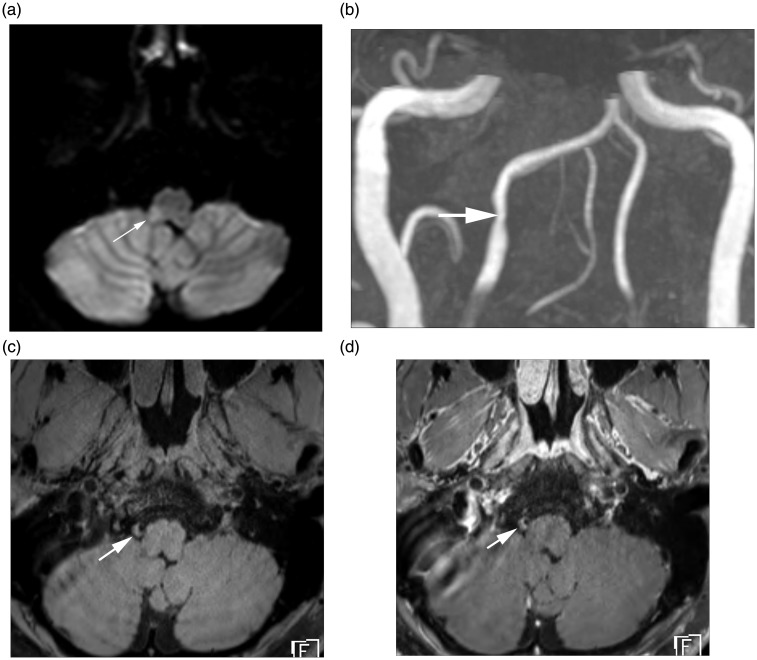
Figure 1.
HR-MRI findings of intracranial VAD with AIS. (a) Diffusion MRI demonstrates a focal acute infarction (arrow) involving right sided medulla. (b) TOF MR angiography reveals focal stenosis (arrow) at V4 segment of the right vertebral artery. (c) Axial pre-contrast T1-weighted MR image demonstrates crescentic T1 hyperintensity (arrow) at the stenotic segment, suggesting intramural hematoma. (d) Axial post-contrast T1-weighted image demonstrates perivascular enhancement (arrow) along the affected vessel.
HR-MRI: high-resolution magnetic resonance imaging; VAD: vertebral artery dissection; AIS: acute ischemic stroke; TOF: time of flight; MR: magnetic resonance.
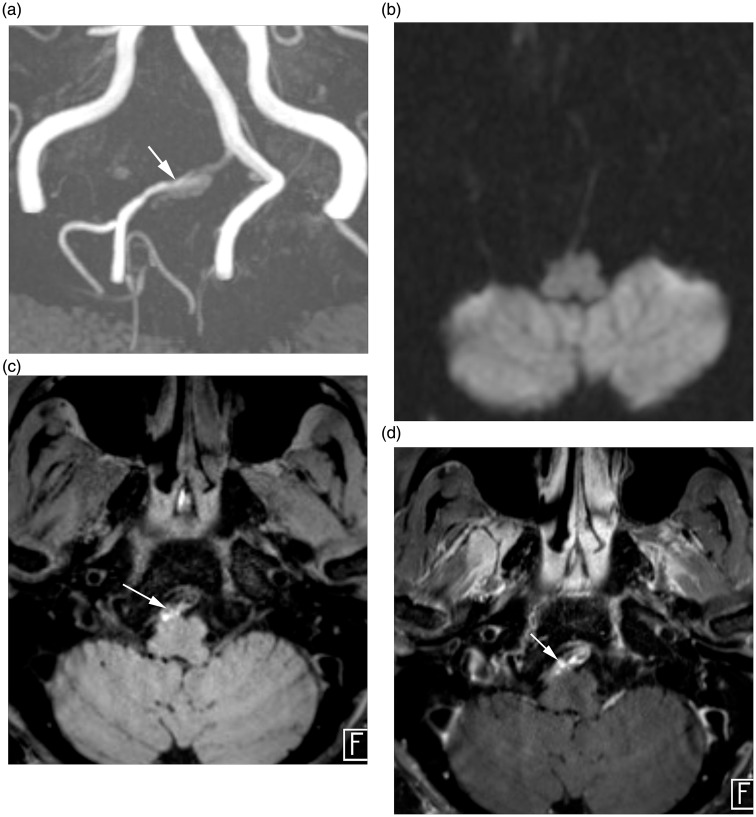
Figure 2.
HR-MRI findings of intracranial VAD without AIS. (a) TOF MR angiography demonstrate aneurysmal dilation (arrow) at V4 segment of the right vertebral artery. (b) Diffusion MRI reveals no evidence of diffusion restricted lesion involving brain parenchyma. (c) Axial pre-contrast T1-weighted image demonstrates intimal flap and hyperintense intramural hematoma (arrow). (d) Axial post-contrast T1-weighted image demonstrates perivascular enhancement (arrow) along the affected vessel.
HR-MRI: high-resolution magnetic resonance imaging; VAD: vertebral artery dissection; AIS: acute ischemic stroke; TOF: time of flight; MR: magnetic resonance.
Contrast enhancement analysis
Twenty VADs showed contrast enhancement along the luminal boundary or outer margin of dissected vessels. Contrast enhancement was more frequently observed in VAD with AIS (n = 11; 84.6%) than those without (n = 9; 56.3%). There was a significant correlation between the grading of contrast enhancement and the presence of AIS (p < 0.05). Grade 1 enhancements were more frequently observed in VAD with AIS than VAD without AIS (n = 6, 46.2% vs. n = 2, 12.5%) and grade 2 enhancements were more frequently observed in VAD without AIS than in VAD with AIS (n = 7; 43.8% vs. n = 5; 38.5%). The pattern of vessel wall enhancement did not significantly differ between VAD with and without AIS.
Discussion
Clinical presentations associated with intracranial VAD include ischemia, hemorrhage, and mass effect, and imaging findings vary according to clinical presentation.18 Subintimal dissection is likely to appear as irregular stenosis or occlusion of the VA by intramural hematoma, and is associated with posterior fossa infarction.18–20 However, subadventitial dissection frequently appears as aneurysmal dilation and tends to result in subarachnoid hemorrhage.18,21 The dissection reduces blood supply to the cerebellum and brainstem, which may cause infarction. In the current study, steno-occlusive lesions were more frequent in intracranial VADs with AIS and were more likely to be associated with intramural hematoma. In contrast, aneurysmal dilations were more frequent in intracranial VADs without AIS and were less likely to be associated with intramural hematoma. These results are consistent with a previous study in which intramural hematomas were more frequently associated with a steno-occlusive pattern than an aneurysmal dilation pattern.22 It is difficult to distinguish VAD from other lesions such as atherosclerosis or VA hypoplasia solely via a steno-occlusive pattern as determined by MRA. However, the pathology of the vessel wall as determined by HR-MRI can be used to distinguish VAD from other lesions, and thus derive a more confident diagnosis.
Hematoma exhibits a typical evolution of signal intensity related to the paramagnetic effects of the products of hemoglobin breakdown.9,21 Previous studies16,18,20,23,24 have reported that subacute-stage intramural hematomas exhibit high signal intensities on T1-weighted MRI. Hirai et al.23 also reported that most VADs showed higher T1-signal intensity at acute to subacute stages than at other stages. However, at early and chronic stages hematomas usually exhibit signals that are isointense compared to surrounding structures on T1-weighted images, thus T1 hyperintensity may not be apparent in vessel walls within a week of symptom onset.20 Therefore, acute-stage intramural hematoma may be obscured by surrounding isointense tissues.9 It is reported that T1- and T2-signal intensities of intramural hematomas significantly increased after one week of symptom onset.25 In the current study, more T1 iso-signal intensities of intramural hematomas were observed in VADs with AIS than those without. This suggested that the timing of VADs with AIS was earlier than that of VADs without AIS. In addition, more frequent T1 high-signal intensity in intramural hematomas in VAD patients without AIS may be explained by the passage of time. Changes of T1-signal intensity of intramural hematoma over time can make it possible to estimate the onset of the VAD. Finally, intramural hematoma is resorbed by inflammatory cell infiltrates and macrophage activity, and is replaced with granulation tissue.24
The presence of contrast enhancement has been used to evaluate plaque components and the degree of neovascularization.16,26 Perivascular enhancement may be another indicator of the acuteness of the dissection, and suggests an inflammatory component of the disease.1,10 Park et al.16 showed that strong enhancement was observed in the subacute stage and that vessel wall enhancement resolved over a few weeks. Interestingly, more VADs associated with AIS showed contrast enhancement than VADs that were not associated with AIS; however, strong enhancement was more frequent in VAD in patients without AIS. We suggest that the reason may be that there is a longer time from when the dissection occurs in VAD patients without AIS than in those with AIS. In addition, two of the intramural thromboses presented intense enhancement that facilitated differentiation from intramural hematoma.
With regard to the treatment of unruptured intracranial VADs, whether conservative management or treatments should be used remains controversial. Unruptured intracranial VAD has been known to follow a benign course with a high probability of spontaneous improvement.19,27 However, the risk of transient ischemic attack or acute infarction is highest in the first two weeks after VAD.19 Ischemic stroke in the posterior circulation is associated with an unfavorable outcome due to hemodynamic compromise or new embolization.7 Thus, VAD patients with more recent hematomas may require closer observation with acute management than those with chronic hematomas to prevent ischemic events.28 The risk associated with anticoagulation is high in intracranial VAD because of the possibility of subarachnoid hemorrhage.12,29 However, a recent study suggests that anticoagulation is safe in cases of intracranial VAD associated with ischemia if there is an absence of subarachnoid hemorrhage and dissecting aneurysm.21
To our knowledge, the current study is the first to focus solely on clinical and radiologic features of intracranial VAD associated with AIS. In particular, the interval between the diagnosis of acute infarctions and the performance of HR-MRI was shorter than it was in previous studies,1,19 and we believe that our study reflects the initial parameters of acute-stage VAD. Most of the cases were considered to be acute-stage lesions, and the findings of the study may be helpful with regard to deciding on the management of intracranial VADs.
The current study had several limitations. First, it was a retrospective study with a relatively small population. It is difficult to perform during the unstable stage of acute infarction, because the image acquisition time of HR-MRI is relatively long. Future studies with larger populations would be needed to support the findings of the current study. Second, T2-weighted images, which have been used in previous studies, were not included in our HR-MRI protocol.1 Instead, we used 2D and 3D PD images. Third, only T1-weighted images were used for the evaluation of intramural hematomas. Additional studies including T2-signal intensity of intramural hematomas are needed. Lastly, we did not perform fat-saturated T1/PD imaging, so the diagnosis of VAD may have been missed in some patients. Additional studies performed with fat saturation are needed for accurate evaluation of intramural hematomas.
Conclusion
Our study found some differences in HR-MR when comparing patients with and without AIS. We suggest the need for similar studies with larger sample sizes in order to confirm our findings. Differing findings between intracranial VAD patients with and without AIS may facilitate a better understanding of intracranial VAD and risk assessment of AIS in these patients.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bachmann R, Nassenstein I, Kooijman H, et al. Spontaneous acute dissection of the internal carotid artery: high-resolution magnetic resonance imaging at 3.0 Tesla with a dedicated surface coil. Invest Radiol 2006; 41: 105–111. [DOI] [PubMed] [Google Scholar]
- 2.Bogousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age: cause and prognosis. Arch Neurol 1987; 44: 479–482. [DOI] [PubMed] [Google Scholar]
- 3.Takano K, Yamashita S, Takemoto K, et al. MRI of intracranial vertebral artery dissection: evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology 2013; 55: 845–851. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen B, Malm J, Carlberg B, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in Northern Sweden. Stroke 1997; 28: 1702–1709. [DOI] [PubMed] [Google Scholar]
- 5.Kim B, Kim S, Kim D, et al. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology 2011; 76: 1735–1741. [DOI] [PubMed] [Google Scholar]
- 6.Huang YC, Chen YF, Wang YH, et al. Cervicocranial arterial dissection: experience of 73 patients in a single center. Surg Neurol 2009; 72(Suppl 2): S20–S27. discussion S27. [DOI] [PubMed] [Google Scholar]
- 7.Chang FC, Yong CS, Huang HC, et al. Posterior circulation ischemic stroke caused by arterial dissection: Characteristics and predictors of poor outcomes. Cerebrovasc Dis 2015; 40: 144–150. [DOI] [PubMed] [Google Scholar]
- 8.Czechowsky D, Hill MD. Neurological outcome and quality of life after stroke due to vertebral artery dissection. Cerebrovasc Dis 2002; 13: 192–197. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection. A systematic review. Neurologist 2012; 18: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann R, Nassenstein I, Kooijman H, et al. High–resolution magnetic resonance imaging (MRI) at 3.0 Tesla in the short-term follow-up of patients with proven cervical artery dissection. Invest Radiol 2007; 42: 460–466. [DOI] [PubMed] [Google Scholar]
- 11.Kim TW, Choi HS, Koo J, et al. Intramural hematoma detection by susceptibility-weighted imaging in intracranial vertebral artery dissection. Cerebrovasc Dis 2013; 36: 292–298. [DOI] [PubMed] [Google Scholar]
- 12.Minematsu K, Matsuoka H, Kasuya J. Cervicocepharic [sic] arterial dissections in Japan: analysis of 454 patients in the spontaneous cervicocephalic arterial dissections study I (SCADS-I). Stroke 2008; 39: 566. [Google Scholar]
- 13.Schoenhagen P, Ziada KM, Kapadia SR, et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000; 101: 598–603. [DOI] [PubMed] [Google Scholar]
- 14.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002; 105: 939–943. [DOI] [PubMed] [Google Scholar]
- 15.Jung SC, Kim HS, Choi CG, et al. Quantitative analysis using high-resolution 3T MRI in acute intracranial artery dissection. J Neuroimaging 2016; 26: 612–617. [DOI] [PubMed] [Google Scholar]
- 16.Park KJ, Jung SC, Kim HS, et al. Multi-contrast high-resolution magnetic resonance findings of spontaneous and unruptured intracranial vertebral artery dissection: qualitative and quantitative analysis according to stages. Cerebrovasc Dis 2016; 42: 23–31. [DOI] [PubMed] [Google Scholar]
- 17.Jung SC, Kim HS, Choi CG, et al. Spontaneous and unruptured chronic intracranial artery dissection. Clin Neuroradiol. Epub ahead of print 27 September 2016. DOI: 10.1007/s00062-016-0544-x. [DOI] [PubMed] [Google Scholar]
- 18.Yoon W, Seo JJ, Kim TS, et al. Dissection of the V4 segment of the vertebral artery: clinicoradiologic manifestations and endovascular treatment. Eur Radiol 2007; 17: 983–993. [DOI] [PubMed] [Google Scholar]
- 19.Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg 2011; 114: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 20.Hosoya T, Adachi M, Yamaguchi K, et al. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke 1999; 30: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 21.Metso TM, Metso AJ, Helenius J, et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke 2007; 38: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Lou X, Li Y, et al. Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: from the interventional neuroradiologists’ view. Acta Neurochir (Wien) 2014; 156: 515–525. [DOI] [PubMed] [Google Scholar]
- 23.Hirai T, Korogi Y, Murata Y, et al. Intracranial artery dissections: serial evaluation with MR imaging, MR angiography, and source images of MR angiography. Radiat Med 2003; 21: 86–93. [PubMed] [Google Scholar]
- 24.Habs M, Pfefferkorn T, Cyran CC, et al. Age determination of vessel wall hematoma in spontaneous cervical artery dissection: a multi-sequence 3T cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2011; 13: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heldner MR, Nedelcheva M, Yan X, et al. Dynamic changes of intramural hematoma in patients with acute spontaneous internal carotid artery dissection. Int J Stroke 2015; 10: 887–892. [DOI] [PubMed] [Google Scholar]
- 26.Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 2003; 107: 851–856. [DOI] [PubMed] [Google Scholar]
- 27.Mokri B, Houser OW, Sandok BA, et al. Spontaneous dissections of the vertebral arteries. Neurology 1988; 38: 880–885. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz NE, Vertinsky AT, Hirsch KG, et al. Clinical and radiographic natural history of cervical artery dissections. J Stroke Cerebrovasc Dis 2009; 18: 416–423. [DOI] [PubMed] [Google Scholar]
- 29.Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol 2000; 15: 316–321. [DOI] [PubMed] [Google Scholar]