Abstract
Background:
There has been increasing interest in the study of the association between Vitamin D receptor (VDR) gene polymorphisms and risk of chronic periodontitis. However, the results remain inconclusive. To better understand the roles of VDR polymorphisms (BsmI, TaqI, FokI, and ApaI) in chronic periodontitis susceptibility, we conducted this systematic review and meta-analysis.
Materials and Methods:
The PubMed, Google Scholar, and Web of Science database were systemically searched to determine all the eligible studies about VDR polymorphisms and risk of chronic periodontitis up to April 2017. Odds ratio (OR) and 95% confidence interval (CI) were used to evaluate the associations between VDR polymorphisms and chronic periodontitis risk. All the statistical analyses were performed by Comprehensive Meta-Analysis. All P values were two-tailed with a significant level at 0.05.
Results:
Finally, a total of 38 case–control studies in 19 publications were identified which met our inclusion criteria. There are ten studies with 866 chronic periodontitis cases and 786 controls for BsmI, 16 studies with 1570 chronic periodontitis cases and 1676 controls for TaqI, five studies with 374 chronic periodontitis cases and 382 controls for FokI, and seven studies with 632 chronic periodontitis cases and 604 controls for ApaI. Overall, no significant association was observed between VDR gene BsmI, TaqI, FokI, and ApaI polymorphisms and risk of chronic periodontitis in any genetic model. Subgroup analysis stratified by ethnicity suggested a significant association between BsmI polymorphism and chronic periodontitis risk in the Caucasian subgroup under allele model (A vs. G: OR = 1.747, 95% CI = 1.099–2.778, P = 0.018). Further, no significant associations were observed when stratified by Hardy–Weinberg equilibrium status for BsmI, TaqI, and ApaI.
Conclusion:
Our results suggest that BsmI, TaqI, FokI, and ApaI polymorphisms in the VDR gene might not be associated with risk of chronic periodontitis in overall population.
Key Words: Chronic periodontitis, meta-analysis, polymorphism, Vitamin D receptor
INTRODUCTION
Periodontal disease is the leading cause of tooth loss in the world, with chronic periodontitis the most common form representing the vast majority of all periodontal diseases.[1] Periodontal disease is highly prevalent and has multiple negative impacts on quality of life.[2] Chronic periodontitis is a reversible inflammation in response to intraoral plaque bacteria,[1,2] which affects the periodontal tissues resulting in irreversible apical migration of the junctional epithelium, loss of periodontal attachment, and ultimately tooth loss.[3,4] Periodontitis is a complex multifactorial disease that involves the interaction of genetic and environmental factors such as sex, age, smoking, and systemic diseases.[5,6] Among the genes suggested to be involved in periodontitis are genes that code for interleukin-1 (IL-1), IL-10, Vitamin D receptor (VDR), transforming growth factor beta (matrix metalloproteinase-3), IL-6, IL-1 β, tumor necrosis factor-alpha, etc.[7,8,9,10,11] The involvement of VDR gene polymorphisms have been suggested in the etiology of both aggressive and chronic periodontitis.[10] The gene-encoding VDR is localized to human chromosome 12q13-14 region and is spanned approximately 100 kb long, comprising at least five promoter regions, eight protein-coding exons, and six untranslated exons, which are alternatively spliced.[12,13,14,15] To date, several functional polymorphisms have been reported in the VDR genes, including FokI in exon 2 (C/T, rs10735810), BsmI in intron 8 (G/A, rs1544410), ApaI in intron 8 (A/C, rs7975232), Tru9 I in intron 8 (G/A, rs757343), and TaqI in exon 9 (T/C, rs731236).[16,17]
In 2001, Yoshihara et al. reported first association between the VDR gene BsmI G/A (rs1544410) polymorphism and aggressive periodontitis in a Japanese population.[18] Two years later, Tachi et al. reported associations between TaqI T/C (rs731236) and FokI C/T (rs2228570) polymorphisms and chronic periodontitis in the same population.[19] Later, the VDR polymorphisms susceptibility to chronic periodontitis have been investigated in different populations. Not surprisingly, the results obtained by different investigators vary. Therefore, to provide a more comprehensive assessment of the associations of the VDR polymorphisms (BsmI, TaqI, FokI, and ApaI) with chronic periodontitis, we carried out this systematic review and meta-analysis of all eligible studies published up to March 2017.
MATERIALS AND METHODS
Search Strategy
To identify eligible studies for this meta-analysis, we searched the MEDLINE (PubMed), Google Scholar, and Web of Science (Thomson-Reuters) for all eligible articles published up to March 20, 2017, that examined the association between the BsmI, TaqI, FokI, and ApaI polymorphisms of the VDR gene and chronic periodontitis risk. The following terms were included in the search: “periodontal disease,” “chronic periodontitis,” “Vitamin D receptor,” “VDR,” “BsmI (rs1544410, intron 8, +63980 G > A),” “TaqI (rs731236, exon 9, +65058 T > C),” “FokI (rs2228570, exon 2, +30920 C > T),” “ApaI (rs7975232, intron 8, +64978 C > A),” “polymorphism,” “mutation,” “variant,” “gene,” “genotype,” “SNP,” and “allele.” The search was not restricted by the publication year or language. To identify potentially relevant studies, we manually searched reference lists of eligible studies, reviews, and related meta-analyses. In addition, we also contacted the authors to get more data as possible as we can. If there were multiple reports of the same study or overlapping data, only the study with the largest sample sizes or the most recent one was selected in our meta-analysis and the others were excluded.
Inclusion and exclusion criteria
Studies were selected according to the following inclusion criteria: (1) full-text published studies; (2) epidemiological studies with case–control or cohort design; (3) investigating the association of VDR – BsmI, TaqI, FokI, and ApaI polymorphisms with chronic periodontitis risk; (4) providing sufficient genotype data or information that could help infer the results in the studies to calculate the odds ratios (ORs) with a 95% confidence interval (CI). The exclusion criteria were as follows: (1) studies with only case group (no control population), case reports, commentaries, and reviews; (2) studies on other polymorphisms of VDR gene; and (3) studies without detail genotype frequencies, which were unable to calculate OR.
Data extraction
Two investigators independently extracted data using a pre-designed form. For each study, the following information was extracted: name of first author, publication year, country where the study was conducted, ethnicity, polymorphisms, genotypic testing method, number of cases and controls, genotype frequency in cases and controls, minor allele frequencies (MAFs) in control subjects, and Hardy–Weinberg equilibrium (HWE) test in control subjects. Diverse ethnicities were categorized as Caucasian, Asian, African, and Mixed, which included more than one race. Disagreements were resolved in consultation with the third reviewer.
Statistical analyses
All the statistical analyses were performed by Comprehensive Meta-Analysis software version 2.0 (Biostat, Englewood Cliffs, I.N.J., USA). All P values were two-tailed with a significant level at 0.05. The strength of associations was assessed using ORs and 95% CIs and the significance of pooled ORs was examined by Ztest. The pooled ORs were determined for BsmI under the allele (A vs. G), homozygote (AA vs. GG), heterozygote (AG vs. GG), dominant (AA + AG vs. GG), and recessive (AA vs. AG + GG) models. The TaqI was evaluated using the allele (C vs. T), homozygote (CC vs. TT), heterozygote (CT vs. TT), dominant (CC + CT vs. TT), and recessive (CC vs. CT + TT) models. The FokI polymorphism was assessed using the allele (T vs. C), homozygote (TT vs. CC), heterozygote (TC vs. CC), dominant (TT + TC vs. CC), and recessive (TT vs. TC + CC) models. The ApaI polymorphism was assessed using the allele model (T vs. G), homozygote (TT vs. GG), heterozygote (TG vs. GG), dominant model (TT + TG vs. GG), and recessive (TT vs. TG + GG) models. Heterogeneity between studies was evaluated by Cochran's Q-test and I2 statistics. P < 0.10 or I2 > 50% indicated significant heterogeneity. If substantial heterogeneity was detected, the random effects model (the DerSimonian-Laird method) was used; otherwise, the fixed effects model (the Mantel-Haenszel method) was utilized. Furthermore, to explore the source of between-study heterogeneity, subgroup analyses were performed. The one-way sensitivity analyses were performed to survey the stability of the results, namely, a single study in the meta-analysis was omitted each time to reflect the influence of the individual data set to the pooled OR. Publication bias was examined using a Begg's funnel plot or Egger's plot, and the significance level was set at 0.05 for both. A HWE test of the VDR gene polymorphisms in healthy subjects was examined using Chi-square test and deviation was considered when P < 0.01.
RESULTS
Study characteristics
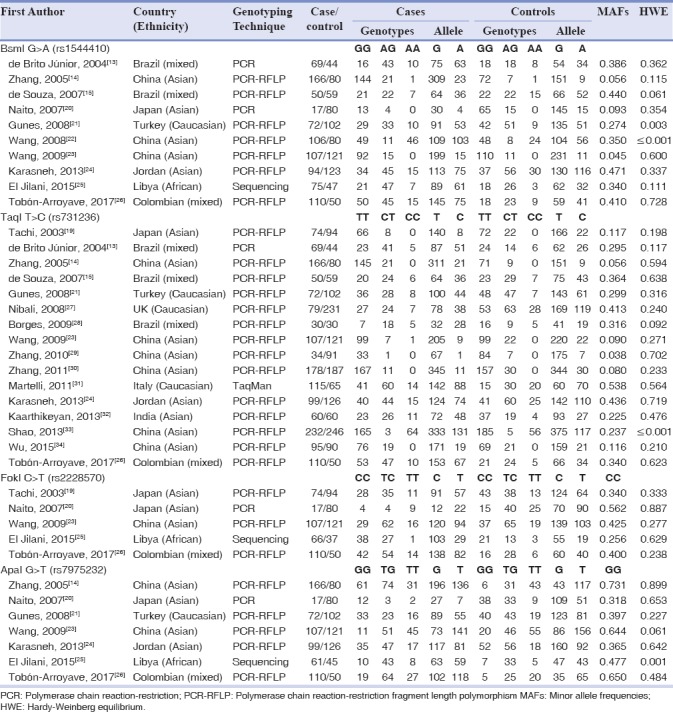
Initially, we have identified 52 publications through the database search. After reading the titles and abstracts, two publications with duplicate titles and four articles that were review articles or assessed unrelated diseases were excluded. Finally, a total of 38 case–control studies in 19 publications[13,14,15,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] were identified met our inclusion criteria. There are ten studies[13,14,15,20,21,22,23,24,25,26] with 866 chronic periodontitis cases and 786 concerning BsmI, 16 studies[13,14,15,19,21,23,26,27,28,29,30,31,32,33,34] with 1570 chronic periodontitis cases and 1676 controls concerning TaqI, five studies[19,20,23,25,26] with 374 chronic periodontitis cases and 382 controls concerning FokI, and seven studies[14,20,21,23,24,25,26] with 632 chronic periodontitis cases and 604 controls concerning ApaI. Main characteristics of the 38 included case–control studies were described in Table 1. Briefly, four main ethnic populations, including Caucasians, Asians, African, and Mixed, from eight countries were involved in this study. The countries of these studies included Brazil, China, Japan, Turkey, Jordan, Libya, Italy, India, and Colombia. The genotypes in control group for four case–control studies including two studies for BsmI,[21,22] one study for TaqI,[25] and one study for ApaI[33] were not consistent with HWE (P < 0.05). The characteristics of each study included in this meta-analysis are presented in Table 1.
Table 1.
Characteristics of studies included in Vitamin D receptor polymorphisms and chronic periodontitis
Quantitative synthesis
Vitamin D receptor-BsmI polymorphism
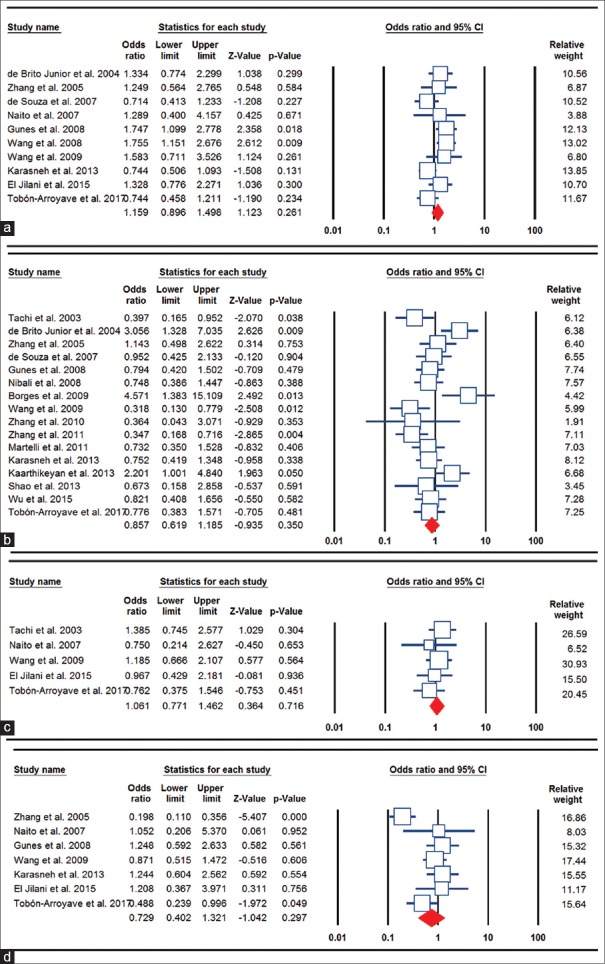
The main results of BsmI polymorphism meta-analysis are shown in Table 2. The pooled results based on all included studies did not show a significant association between BsmI polymorphism and chronic periodontitis risk under all genetic models (allele model: A vs. G, OR = 1.159, 95% CI = 0.896–1.498, P = 0.261, Figure 1a; homozygote model: AA vs. GG, OR = 1.043, 95% CI = 0.738–1.475, P = 0.810; heterozygote model: AG vs. GG, OR = 1.182, 95% CI = 0.918–1.522, P = 0.196; dominant model: AA + AG vs. GG, OR = 1.174, 95% CI = 0.933–1.476, P = 0.171; and recessive model: AA vs. AG + GG, OR = 1.968, 95% CI = 0.702–1.334, P = 0.842). In the subgroup analyses, there was a significant association between BsmI polymorphism and chronic periodontitis risk only under the allele model (A vs. G: OR = 1.747, 95% C = 1.099–2.778, P = 0.018) in the Caucasian population, but not in Asian, Mixed, and African populations. When stratifying the studies by HWE status, the association between BsmI polymorphism and chronic periodontitis risk was not significant in all genetic models.
Table 2.
The meta-analysis of BsmI A/G (rs1544410) polymorphism and chronic periodontitis risk
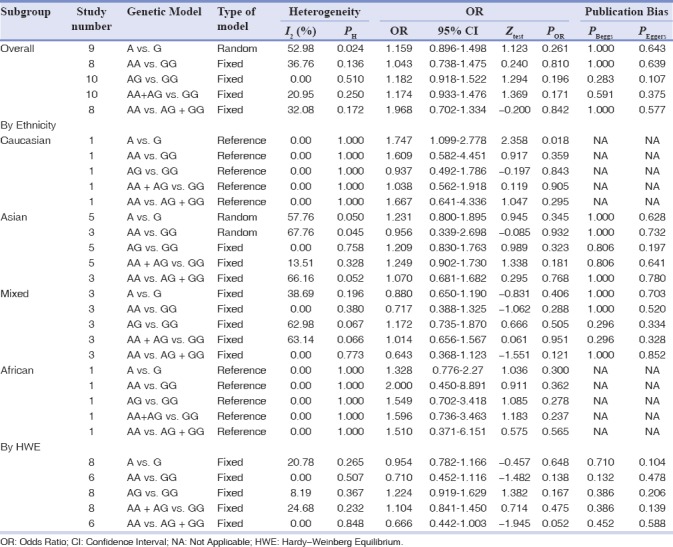
Figure 1.
Forest plots for the association of the Vitamin D receptor polymorphisms and chronic periodontitis susceptibility. (a) BsmI (allele model: A vs. G); (b) TaqI (heterozygote model: CT vs. TT); (c) FokI (dominant model: TT + TC vs. CC); and (d) ApaI (recessive model: TT vs. TG + GG).
Vitamin D receptor-TaqI polymorphism
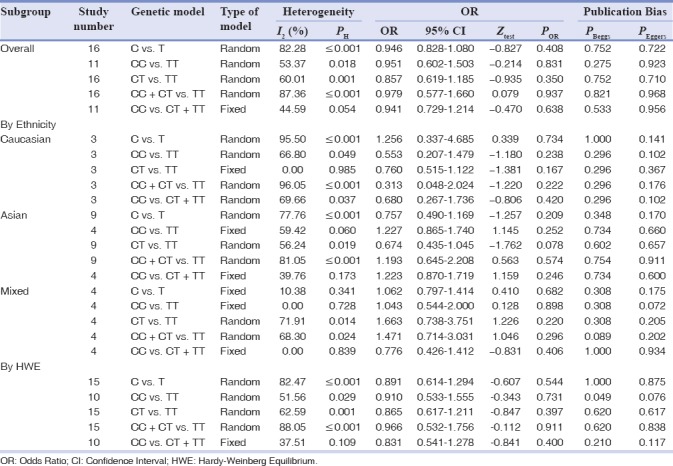
The main results of this meta-analysis are shown in Table 3. Overall, no significant associations were found between TaqI polymorphism and chronic periodontitis risk when all studies were pooled into the meta-analysis under all genetic models (allele model: C vs. T: OR = 0.946, 95% C = 0.828–1.080, P = 0.408; homozygote model: CC vs. TT: OR = 0.951, 95% CI = 0.602–1.503, P = 0.831, Figure 1b; heterozygote model: CT vs. TT: OR = 0.857, 95% CI = 0.619–1.185, P = 0.350; the dominant model: CC + CT vs. TT: OR = 0.979, 95% CI = 0.577–1.660, P = 0.937; recessive model: CC vs. CT + TT: OR = 0.941, 95% CI = 0.729–1.214, P = 0.638). In the subgroup analyses by ethnicity, there was no a significant association between TaqI polymorphism and chronic periodontitis risk in Caucasian, Asians, African, and Mixed populations. No significant associations were observed when stratified by HWE status.
Table 3.
The meta-analysis of TaqI T/C (rs731236) polymorphism and chronic periodontitis risk
Vitamin D receptor-FokI polymorphism
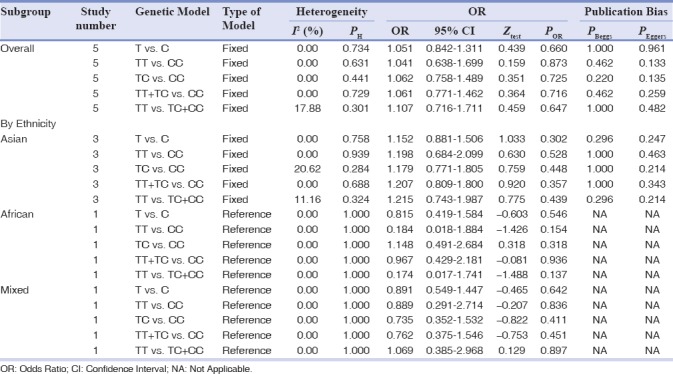
The main results of FokI polymorphism meta-analysis are shown in Table 4. The overall analyses suggested no significant association between the FokI and chronic periodontitis susceptibility in all genetic models (allele model: T vs. C: OR = 1.051, 95% CI = 0.842–1.311, P = 0.660; homozygote model: TT vs. CC: OR = 1.041, 95% CI = 0.638–1.699, P = 0.873; heterozygote model: TC vs. CC: OR = 1.062, 95% CI = 0.758–1.489, P = 0.725; the dominant model: TT + TC vs. CC: OR = 1.061, 95% CI = 0.771–1.462, Figure 1c, P = 0.716; recessive model: TT vs. TC + CC: OR = 1.107, 95% CI = 0.716–1.711, P = 0.647). In the subgroup analyses, there was no significant association between FokI polymorphism and chronic periodontitis risk in the Asians, African, and Mixed populations.
Table 4.
The meta-analysis of FokI C/T (rs2228570) polymorphism and chronic periodontitis risk
Vitamin D receptor-ApaI polymorphism
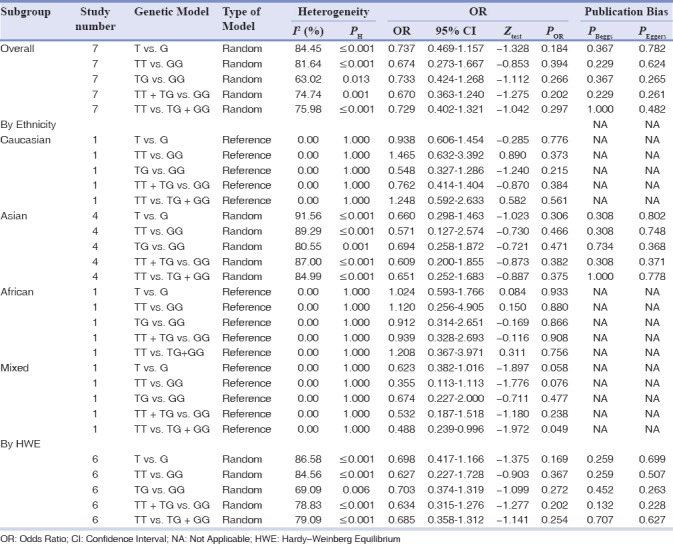
Table 5 gives the summary results for the association of the ApaI with the risk of chronic periodontitis based on all five genetic models. However, there was no significant association between the ApaI and chronic periodontitis susceptibility under all genetic models (allele model: T vs. G: OR = 0.737, 95% CI = 0.469–1.157, P = 0.184; homozygote model: TT vs. GG: OR = 0.674, 95% CI = 0.273–1.667, P = 0.394; heterozygote model: TG vs. GG: OR = 0.733, 95% CI = 0.424–1.268, P = 0.266; the dominant model: TT + TG vs. GG: OR = 0.670, 95% CI = 0.363–1.240, P = 0.202; recessive model: TT vs. TG + GG: OR = 0.729, 95% CI = 0.402–1.321, P = 0.297, Figure 1d. We then performed stratified analysis by ethnicity and found a significant association between the ApaI polymorphism and chronic periodontitis risk in the mixed population under recessive model (TT vs. TG + GG: OR = 0.488, 95% CI = 0.239–0.996, P = 0.049), but not in the Caucasian, Asian, and African populations. Moreover, no significant associations were observed when stratified by HWE status.
Table 5.
The meta-analysis of ApaI G/T (rs7975232) polymorphism and chronic periodontitis risk
Minor allele frequencies
We have calculated MAFs for controls from the corresponding genotype distribution. Frequencies of the BsmI G > A (rs1544410), TaqI T > C (rs731236), FokI C > T (rs2228570), and ApaI G > T (rs7975232) alleles were in range of 0.045–0.471, 0.038–0.436, 0.256–0.425, and 0.318–0.650, respectively. Moreover, the variant alleles had different representations among controls of different Asian descents [Table 1].
Sensitivity analysis
In addition, we have performed sensitivity analysis by omitting four studies in which the genotype distributions of BsmI, TaqI, FokI, and ApaI polymorphisms in the healthy controls significantly deviated from the HWE. However, the significance of pooled ORs not influenced by omitting those studies, indicating that the results was stable.
Publication bias
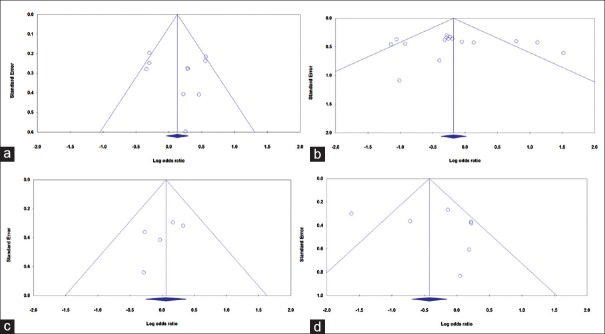
We have assessed the publication bias of the literature qualitatively by funnel plots and estimated quantitatively by Begg's and Egger's tests. The shape of the funnel plots no revealed evidence of obvious asymmetry. Moreover, the Egger's test was used to provide further statistical evidence of funnel plot symmetry. However, we did not find any evidence of publication bias of the literatures regarding the BsmI, TaqI, FokI, and ApaI polymorphisms and chronic periodontitis under the all genetic models [Figure 2a–d].
Figure 2.
Begg's funnel plots of the Vitamin D receptor polymorphisms and chronic periodontitis susceptibility for publication bias test. (a) BsmI (allele model: A vs. G); (b) TaqI (heterozygote model: CT vs. TT); (c) FokI (dominant model: TT + TC vs. CC); and (d) ApaI (recessive model: TT vs. TG + GG).
DISCUSSION
In this study, we examined the relationship between the polymorphism of widely investigated common polymorphisms in VDR gene and chronic periodontitis susceptibility. In 2010, Deng et al. performed a meta-analysis on VDR gene polymorphisms associations with periodontitis.[35] However, their results under cruise as their methodology in overall and subgroup analyses is not considerably correct. Thus, our main purpose of performing this meta-analysis was to improve statistical power and obtain more accurate quantitative results by increasing the sample size. Our meta-analysis indicates that BsmI polymorphism was not associated with increased chronic periodontitis risk when all eligible studies were pooled into the meta-analysis. In further stratified and sensitivity analyses, significantly increased chronic periodontitis risk was observed under the allele model in Caucasians for BsmI, but not in other ethnicities. Our results showed that carrying the BsmI minor allele may be a risk factor for chronic periodontitis in Caucasians. Similarly, some studies have shown that BsmI is associated with an increased risk of some autoimmune diseases including systemic lupus erythematosus[36] and diabetes type 1.[37] However, the functional significance of the BsmI polymorphism, which is located near the 3'-UTR region in intron 8, remains unclear. This polymorphism does not change the amino acid sequence of the encoded protein. However, it seems that BsmI may alter polyadenylation of the VDR mRNA transcript and thus affect gene expression through regulation of mRNA stability.[38]
In the present meta-analysis, we also failed to detect any association between TaqI and ApaI polymorphisms and chronic periodontitis susceptibility in all four genetic models, even by ethnicity. Our results were consistent with a previous meta-analysis on VDR gene polymorphisms with susceptibility to different conditions such as melanoma,[39] female reproductive cancers,[40] atopic dermatitis,[41] and polycystic ovary syndrome.[42] The reason for this lack of association could be explained with further haplotype studies. Some studies suggested that ApaI and TaqI polymorphisms are located at the 3'end of the VDR gene neighboring the 3'UTR region, which do not result in changes in the predicted amino acid sequence of the VDR.[43] In addition, our data suggest that FokI not significantly associated with chronic periodontitis susceptibility. The functional FokI polymorphism is the most important start codon polymorphism in VDR gene, which is located in exon 2 at the 5' coding region of the gene.[41,42,43]
Heterogeneity is an important problem when interpreting the results of a meta-analysis,[44,45,46] and identifying the sources of heterogeneity is one of the most important goals of meta-analysis.[47,48,49] In this study, significant between-study heterogeneity in the pooled analyses of all included studies was found for TaqI and ApaI polymorphisms. However, for these polymorphisms, the heterogeneity not disappeared fully after subgroup analysis by ethnicity and excluding the studies not in HWE.
We conducted the largest and most comprehensive quantitative meta-analysis of the relationship between VDR gene polymorphisms and chronic periodontitis risk. However, the results of the present meta-analysis should also be interpreted within the context of its limitations. First, we have performed this meta-analysis based mainly on the results from published studies. Future analyses may include data from more unpublished datasets. Second, due to limited individual data, we did not conduct a more precise analysis on other covariates such as age, gender, and environmental factors. Third, compared with many large-sample genetic association studies, the sample size of included studies in our meta-analysis is relatively small, especially for FokI and ApaI, which may limit the statistical power of this meta-analysis. Finally, the current data support the multifactorial nature of periodontitis, and both genetic and environmental factors are involved in the pathogenesis of this disease. Therefore, our results may be affected by additional confounding factors, such as gender, age, environmental factors, and we could not take this into account in this meta-analysis because studies did not report the data.
CONCLUSION
We failed to detect any association between BsmI, TaqI, FokI, and ApaI polymorphisms in the VDR gene and chronic periodontitis susceptibility in the overall population. Moreover, due to limited sample size, more large-scale, multi-racial association studies are required to further clarify the genetic association between various VDR gene polymorphisms and risk of chronic periodontitis.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgments
We would like to thank Professor Seyed Mehdi Kalantar and Professor Mohammad Hasan Sheikhha for their cooperation in this study.
REFERENCES
- 1.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghannad F, Nica D, Fulle MI, Grenier D, Putnins EE, Johnston S, et al. Absence of alphavbeta6 integrin is linked to initiation and progression of periodontal disease. Am J Pathol. 2008;172:1271–86. doi: 10.2353/ajpath.2008.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–51. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AlJehani YA. Risk factors of periodontal disease: Review of the literature. Int J Dent 2014. 2014:182513. doi: 10.1155/2014/182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Taba M, Jr, Souza SL, Mariguela VC. Periodontal disease: A genetic perspective. Braz Oral Res. 2012;26(Suppl 1):32–8. doi: 10.1590/s1806-83242012000700006. [DOI] [PubMed] [Google Scholar]
- 7.Jia XW, Yuan YD, Yao ZX, Wu CJ, Chen X, Chen XH, et al. Association between IL-4 and IL-4R polymorphisms and periodontitis: A meta-analysis. Dis Markers 2017. 2017:8021279. doi: 10.1155/2017/8021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding C, Ji X, Chen X, Xu Y, Zhong L. TNF-α gene promoter polymorphisms contribute to periodontitis susceptibility: Evidence from 46 studies. J Clin Periodontol. 2014;41:748–59. doi: 10.1111/jcpe.12279. [DOI] [PubMed] [Google Scholar]
- 9.Vijayalakshmi R, Geetha A, Ramakrishnan T, Emmadi P. Genetic polymorphisms in periodontal diseases: An overview. Indian J Dent Res. 2010;21:568–74. doi: 10.4103/0970-9290.74226. [DOI] [PubMed] [Google Scholar]
- 10.Brett PM, Zygogianni P, Griffiths GS, Tomaz M, Parkar M, D'Aiuto F, et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005;84(12):1149–53. doi: 10.1177/154405910508401211. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Zhu Y, Singh P, Ajmera DH, Song J, Ji P, et al. Association of common variants in MMPs with periodontitis risk. Dis Markers 2016. 2016:1545974. doi: 10.1155/2016/1545974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annamaneni S, Bindu CH, Reddy KP, Vishnupriya S. Association of Vitamin D receptor gene start codon (Fok1) polymorphism with high myopia. Oman J Ophthalmol. 2011;4:57–62. doi: 10.4103/0974-620X.83654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Brito Júnior RB, Scarel-Caminaga RM, Trevilatto PC, de Souza AP, Barros SP. Polymorphisms in the Vitamin D receptor gene are associated with periodontal disease. J Periodontol. 2004;75:1090–5. doi: 10.1902/jop.2004.75.8.1090. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JC, Geng HO, Ma WB, Huang P, Pang RY, Zhang YH, et al. Association of Vitamin D receptor gene polymorphisms with the susceptibility to chronic periodontitis of Han nationality. Zhonghua Kou Qiang Yi Xue Za Zhi. 2005;40:50–3. [PubMed] [Google Scholar]
- 15.de Souza CM, Braosi AP, Luczyszyn SM, Avila AR, de Brito RB, Jr, Ignácio SA, et al. Association between Vitamin D receptor gene polymorphisms and susceptibility to chronic kidney disease and periodontitis. Blood Purif. 2007;25:411–9. doi: 10.1159/000109235. [DOI] [PubMed] [Google Scholar]
- 16.Uitterlinden AG, Fang Y, van Meurs JB, van Leeuwen H, Pols HA. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol. 2004;89-90:187–93. doi: 10.1016/j.jsbmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 17.Colombini A, Brayda-Bruno M, Lombardi G, Croiset SJ, Ceriani C, Buligan C, et al. BsmI, ApaI and TaqI polymorphisms in the Vitamin D receptor gene (VDR) and association with lumbar spine pathologies: An Italian case-control study. PLoS One. 2016;11:e0155004. doi: 10.1371/journal.pone.0155004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshihara A, Sugita N, Yamamoto K, Kobayashi T, Miyazaki H, Yoshi H, et al. Analysis of Vitamin D and Fcgamma receptor polymorphisms in Japanese patients with generalized early-onset periodontitis. J Dent Res. 2001;80:2051–4. doi: 10.1177/00220345010800120501. [DOI] [PubMed] [Google Scholar]
- 19.Tachi Y, Shimpuku H, Nosaka Y, Kawamura T, Shinohara M, Ueda M, et al. Vitamin D receptor gene polymorphism is associated with chronic periodontitis. Life Sci. 2003;73:3313–21. doi: 10.1016/j.lfs.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Naito M, Miyaki K, Naito T, Zhang L, Hoshi K, Hara A, et al. Association between Vitamin D receptor gene haplotypes and chronic periodontitis among Japanese men. Int J Med Sci. 2007;4:216–22. doi: 10.7150/ijms.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunes S, Sumer AP, Keles GC, Kara N, Koprulu H, Bagci H, et al. Analysis of Vitamin D receptor gene polymorphisms in patients with chronic periodontitis. Indian J Med Res. 2008;127:58–64. [PubMed] [Google Scholar]
- 22.Wang HY, Pan YP, Teng D, Zhao J, Lin L. The relativity between chronic periodontitis and the genetic polymorphisms of Vitamin D receptor and estrogen receptor. Zhonghua Kou Qiang Yi Xue Za Zhi. 2008;43:236–9. [PubMed] [Google Scholar]
- 23.Wang C, Zhao H, Xiao L, Xie C, Fan W, Sun S, et al. Association between Vitamin D receptor gene polymorphisms and severe chronic periodontitis in a Chinese population. J Periodontol. 2009;80:603–8. doi: 10.1902/jop.2009.080465. [DOI] [PubMed] [Google Scholar]
- 24.Karasneh JA, Ababneh KT, Taha AH, Al-Abbadi MS, Marzouka NA, Jaradat SM, et al. Association of Vitamin D receptor gene polymorphisms with chronic and aggressive periodontitis in Jordanian patients. Eur J Oral Sci. 2013;121:551–8. doi: 10.1111/eos.12085. [DOI] [PubMed] [Google Scholar]
- 25.El Jilani MM, Mohamed AA, Zeglam HB, Alhudiri IM, Ramadan AM, Saleh SS, et al. Association between Vitamin D receptor gene polymorphisms and chronic periodontitis among Libyans. Libyan J Med. 2015;10:26771. doi: 10.3402/ljm.v10.26771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobón-Arroyave SI, Isaza-Guzmán DM, Pineda-Trujillo N. Association study of Vitamin D receptor (VDR)-related genetic polymorphisms and their haplotypes with chronic periodontitis in Colombian population. J Clin Diagn Res. 2017;11:ZC60–6. doi: 10.7860/JCDR/2017/23967.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nibali L, Parkar M, D'Aiuto F, Suvan JE, Brett PM, Griffiths GS, et al. Vitamin D receptor polymorphism (-1056 Taq-I) interacts with smoking for the presence and progression of periodontitis. J Clin Periodontol. 2008;35:561–7. doi: 10.1111/j.1600-051X.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- 28.Borges MA, Figueiredo LC, Brito RB, Jr, Faveri M, Feres M. Microbiological composition associated with Vitamin D receptor gene polymorphism in chronic periodontitis. Braz Oral Res. 2009;23:203–8. doi: 10.1590/s1806-83242009000200018. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Meng HX, Zhao HS, Li QY, Xu L, Chen ZB, et al. Correlation study on polymorphisms of Vitamin D receptor gene in patients with periodontitis. Beijing Da Xue Xue Bao Yi Xue Ban. 2010;42:37–40. [PubMed] [Google Scholar]
- 30.Zhang Q, Peng MY, Ma M. The association between VDR gene TaqI SNPs and moderate to severe chronic periodontitis in Ningxia population. Chin J Gerontol. 2011;31:3447–9. [Google Scholar]
- 31.Martelli FS, Mengoni A, Martelli M, Rosati C, Fanti E. VDR TaqI polymorphism is associated with chronic periodontitis in Italian population. Arch Oral Biol. 2011;56:1494–8. doi: 10.1016/j.archoralbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Kaarthikeyan G, Jayakumar ND, Padmalatha O, Varghese S, Anand B. Analysis of association of TaqI VDR gene polymorphism with the chronic periodontitis in Dravidian ethnicity. Indian J Hum Genet. 2013;19:465–8. doi: 10.4103/0971-6866.124377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao T. The Association of VDR Polymorphisms with Chronic Periodontitis. Master Thesis of Kunming Medical University; 2013 [Google Scholar]
- 34.Wu L, Lin J, Wang DL, Zhao J. Association between chronic periodontitis and Vitamin D receptor gene polymorphisms among Uygur adults in Moyu county of Xinjiang. J Oral Sci Res. 2015;31:995–9. [Google Scholar]
- 35.Deng H, Liu F, Pan Y, Jin X, Wang H, Cao J, et al. BsmI, TaqI, ApaI, and FokI polymorphisms in the Vitamin D receptor gene and periodontitis: A meta-analysis of 15 studies including 1338 cases and 1302 controls. J Clin Periodontol. 2011;38:199–207. doi: 10.1111/j.1600-051X.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- 36.Azab SF, Ali YF, Farghaly MA, Hamed ME, Allah MA, Emam AA, et al. Vitamin D receptor gene BsmI polymorphisms in Egyptian children and adolescents with systemic lupus erythematosus: A case-control study. Medicine (Baltimore) 2016;95:e5233. doi: 10.1097/MD.0000000000005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada A, Kanazawa Y, Motohashi Y, Yamada S, Maruyama T, Ikegami H, et al. Evidence for association between Vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. J Autoimmun. 2008;30:207–11. doi: 10.1016/j.jaut.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Feng M, Li H, Chen SF, Li WF, Zhang FB. Polymorphisms in the Vitamin D receptor gene and risk of autoimmune thyroid diseases: A meta-analysis. Endocrine. 2013;43:318–26. doi: 10.1007/s12020-012-9812-y. [DOI] [PubMed] [Google Scholar]
- 39.Zeljic K, Kandolf-Sekulovic L, Supic G, Pejovic J, Novakovic M, Mijuskovic Z, et al. Melanoma risk is associated with Vitamin D receptor gene polymorphisms. Melanoma Res. 2014;24:273–9. doi: 10.1097/CMR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 40.Mun MJ, Kim TH, Hwang JY, Jang WC. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas. 2015;81:256–65. doi: 10.1016/j.maturitas.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Kılıç S, Sılan F, Hız MM, Işık S, Ögretmen Z, Özdemir Ö, et al. Vitamin D receptor gene BSMI, FOKI, APAI, and TAQI polymorphisms and the risk of atopic dermatitis. J Investig Allergol Clin Immunol. 2016;26:106–10. doi: 10.18176/jiaci.0020. [DOI] [PubMed] [Google Scholar]
- 42.Bagheri M, Abdi Rad I, Hosseini Jazani N, Nanbakhsh F. Vitamin D receptor TaqI gene variant in exon 9 and polycystic ovary syndrome risk. Int J Fertil Steril. 2013;7:116–21. [PMC free article] [PubMed] [Google Scholar]
- 43.Niu MY, Wang L, Xie AM. ApaI, BsmI, FokI, and TaqI polymorphisms in the Vitamin D receptor gene and Parkinson's disease. Chin Med J (Engl) 2015;128:1809–14. doi: 10.4103/0366-6999.159358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sobhan MR, Forat-Yazdi M, Mazaheri M, Zare-Shehneh M, Neamatzadeh H. Association between the DNA repair gene XRCC3 rs861539 polymorphism and risk of osteosarcoma: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18:549–55. doi: 10.22034/APJCP.2017.18.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jafari-Nedooshan J, Forat Yazdi M, Neamatzadeh H, Zare Shehneh M, Kargar S, Seddighi N, et al. Genetic association of XRCC1 gene rs1799782, rs25487 and rs25489 polymorphisms with risk of thyroid cancer: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18:263–70. doi: 10.22034/APJCP.2017.18.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gohari M, Neámatzadeh H, Jafari MA, Mazaheri M, Zare-Shehneh M, Abbasi-Shavazi E, et al. Association between the p53 codon 72 polymorphism and primary open-angle glaucoma risk: Meta-analysis based on 11 case-control studies. Indian J Ophthalmol. 2016;64:756–61. doi: 10.4103/0301-4738.195002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehdinejad M, Sobhan MR, Mazaheri M, Zare-Shehneh M, Neamatzadeh H, Kalantar SM. Genetic Association between ERCC2, NBN, RAD51 Gene Variants and Osteosarcoma Risk: a Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2017;18(5):1315–1321. doi: 10.22034/APJCP.2017.18.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoram-Abadi KM, Forat-Yazdi M, Kheirandish S, Saeidi N, Zarezade Z, Mehrabi N, et al. DNMT3B-149 C>T and -579 G>T polymorphisms and risk of gastric and colorectal cancer: A meta-analysis. Asian Pac J Cancer Prev. 2016;17:3015–20. [PubMed] [Google Scholar]
- 49.Namazi A, Abedinzadeh M, Nourbaksh P, Neamatzadeh H. Association between the XRCC3 thr241Met polymorphism and risk of colorectal cancer: A meta analysis of 5,193 cases and 6,645 controls. Asian Pac J Cancer Prev. 2015;16:2263–8. doi: 10.7314/apjcp.2015.16.6.2263. [DOI] [PubMed] [Google Scholar]