Abstract
Background:
Bacteria and their by-products are etiological factors for the failure of endodontic treatment. Reduction of root canal bacterial contamination is one of the chief aims of root canal therapy. The aim of this study was to compare the effects of different rotary file tapers and two irrigation fluids on Enterococcus faecalis counts.
Materials and Methods:
In this ex vivo study Root canals of 72 human upper lateral incisors were enlarged to ISO #20 K-file. Then, the samples were sterilized and inoculated with E. faecalis for 72 h, divided into six experimental groups and prepared with #30 Flexmaster files with 0.02, 0.04, and 0.06 tapers and two different irrigation solutions such as normal saline and sodium hypochlorite. The control group (n = 10) was subdivided into two groups with or without bacterial inoculation and no mechanical instrumentation. Cleaning efficacy was evaluated in terms of the reduction of colony forming units (CFUs). T-test, ANOVA, Duncan, and Tukey tests were applied to the groups. A significant level of α = 0.05 was set for comparison between the groups.
Results:
The canals instrumented with 0.06 taper exhibited greater significant reduction in CFUs compared to canals instrumented with 0.04 and 0.02 taper (P < 0.05); 0.04 taper also resulted in greater significant reduction in CFUs than 0.02 taper (P < 0.05). In addition, no significant differences were observed in E. faecalis counts between the two irrigation fluids (P > 0.05).
Conclusion:
Under the conditions of this study, root canal preparation with greater taper resulted in canal cleanliness and better debridement.
Key Words: Bacterial load, dental instruments, Enterococcus faecalis, root canal preparation
INTRODUCTION
Bacteria and their by-products have a major role in pulpal and periapical pathogenesis.[1,2,3,4,5] It seems reasonable that elimination or reduction of pathogens as a prime objective in the successful treatment of apical periodontitis leads to successful treatment of this condition. In fact, when previously infected canals are rendered negative in bacterial sampling, an improved prognosis has been achieved.[6,7]
Among bacteria, Enterococcus faecalis is the most consistently reported organism from former cases. This organism is resistant to most intracanal medicaments and can survive up to a pH value of 11.5.[8] E. Faecalis can also survive prolonged starvation and grow as a monoinfection in treated canals in the absence of synergistic support from other bacteria.[9] Moreover, endodontic infections with E. faecalis are usually difficult to eliminate, even with intracanal calcium hydroxide dressing.[10]
Reduction in bacterial counts is accomplished by a triad of mechanical instrumentation such as cleaning and shaping with various irrigating solutions, and disinfection with intracanal medicaments.[4,11,12,13] When endodontic instruments were manufactured by the ISO, it was believed that the only way to reach the irrigation fluids to the critical apical 3-mm of the root canal, was apical preparation as wide as possible to reduce the microbial population and increase cleanliness.[14,15,16,17] Currently, after the introduction of nickel-titanium rotary systems, it is suggested that increasing the root canal taper should be implemented with apical preparation as narrow as possible.
Buchanan refers to advantages of “variable taper” as tapering with easy and simple use, enhanced and predictable cleaning and obturation outcomes even in inexperienced hands, adequate coronal enlargement, full deep shape, and apical resistance form in a simple instrument sequence.[18] In a recent study, de Gregorio et al. assessed only the effect of apical size and taper on irrigation solution volume delivered without analyzing the effect of irrigation solution on cleanliness and suggest that apical preparation of 40# taper 0.06 significantly increase the volume of irrigant at the working length.[19]
In a study rather similar to ours, Arvaniti and Khabbaz, Mohammadzadeh Akhlaghi et al., Cohenca et al., and Moshari et al. investigated the effects of taper, size and irrigation on root canal cleanliness. They stated greater size and taper with positive pressure irrigation could reduce the count of root canal bacteria. Our detailed methodology, however, is different from theirs.[2,20,21,22]
The aim of the present study, therefore, was to investigate the effects of different irrigation fluids and taper of the rotary system on root canal cleanliness in terms of decreases in colony forming units (CFUs) in the 3-mm apical area of the powdered root. The null hypothesis stated that the increase in taper does not affect E. faecalis counts.
MATERIALS AND METHODS
In this ex vivo study atotal of 82 human upper lateral incisors with one root canal, extracted due to orthodontic or periodontal reasons, were collected. Before preparation, all the teeth were radiographed in buccolingual and mesiodistal directions to exclude teeth with any aberrant canal morphology and to confirm a single canal. Teeth with severe root curvature, cracks or fractured roots, calcified root canals, immature apices, and decayed and filled teeth were excluded from the study. Ethics approval was granted by the Ethics Committee of Islamic Azad University, Isfahan (Khorasgan) branch (UREC 23810201902002).
The teeth were cleaned and debrided gently by the use of periodontal curette right after extraction. Then, they were immersed in 5.25% sodium hypochlorite (NaOCl) solution (Shamin chemical Co. Tehran, Iran) for 30 min for surface disinfection and stored in 0.9% sterile normal saline solution (Daroopakhsh, Tehran, Iran). In the first place, the teeth were cut from cementoenamel junction perpendicular to their long axis with a diamond disk (Horico H557F220) 12 mm from the root tip [Figure 1]. Patency of the root canals and presence of one root canal were ensured by using #10 and #15 K-files (Densply/Maillefer, Ballaigues, Switzerland). To reduce confounding variables, all the samples were primarily instrumented up to #20 K-file (Densply/Maillefer, Ballaigues, Switzerland) under copious irrigation with distilled water up to 11 mm working length. Then, roots were soaked in 17% ethylenediaminetetraacetic acid (Vericom, Korea) for 10 min, followed by 5.25% NaOCl for 10 min; finally, the samples were rinsed with sterile water. After preparation, the roots were randomly divided into six experimental groups (n = 12) and two control groups (n = 5).
Figure 1.
Standard cutting from cementoenamel junction.
All the samples were placed in brain-heart infusion (BHI) broth and autoclaved for 20 min 121°C and 15 psi. All the samples were autoclaved again in the same way as the first time for better accuracy. To ensure sterilization, all the samples were incubated separately in a micro-tube containing BHI for 24 h under aerobic and aseptic conditions at 37°C. If turbidity was observed in the micro-tube, it meant incomplete sterilization and the process of sterilization was repeated.
In this study, to create standard and controlled infection in all the cases, we used E. faecalis as a resistant bacterial species. This Gram-positive anaerobic bacterial species were obtained from the Department of Microbiology, Faculty of Medicine, Tehran University of Medical Sciences with an ID code of ATCC-29212. Furthermore, we used bile esculin agar since it allows the growth of enterococci and Streptococci, including E. faecalis.
A suspension of bacteria was prepared by adding 1 mL of a pure culture of E. faecalis (ATCC2912) grown in BHI broth; 0.05 mL of suspension was injected by volume sampler into each canal of the experimental group and positive control group [Figure 2]. The access cavity was sealed with intermediate restorative material Cavit (Premier Dental Products Co., Philadelphia, PA, USA). The roots were incubated at 37°C for 72 h separately.
Figure 2.
Contamination of samples.
The samples were kept sterile and divided into six groups (n = 12) after the incubation period. Preparation steps were performed as follows:
In all the groups, recapitulation with #15 K-file (Densply/Maillefer, Ballaigues, Switzerland) between different instruments was carried out, and after each file, 2 mL of irrigation solution was flushed. Canal preparation was carried out by single-length technique with Flexmaster rotary instruments.
Group I –Preparation was carried out with this sequence: Flexmaster 0.02 taper, #30; Flexmaster 0.04 taper, #30; Flexmaster 0.06 taper, #30, with 2 mL of 2.5% NaOCl irrigation after each file
Group II – Preparation was carried out with this sequence: Flexmaster 0.02 taper, #30; Flexmaster 0.04 taper, #30, with 2 mL of 2.5% NaOCl irrigation after each file
Group III – Preparation was carried out with this sequence: Flexmaster 0.02 taper, #30, with 2 mL of 2.5% NaOCl irrigation after each file
Group IV – Preparation was carried out with this sequence: Flexmaster 0.02 taper, #30; Flexmaster 0.04 taper, #30; Flexmaster 0.06 taper, #30, with 2 mL of normal saline irrigation after each file
Group V – Preparation was carried out with this sequence: Flexmaster 0.02 taper, #30; Flexmaster 0.04 taper, #30, with 2 mL of normal saline irrigation after each file
Group VI – Preparation was carried out with this sequence: Flexmaster 0.02 taper, #30, with 2 mL of normal saline irrigation after each file
Group VIIa – No instrumentation was performed after bacterial inoculation
Group VIIb – Neither bacterial inoculation nor mechanical instrumentation was performed.
After canal preparation and final rinsing with 10 mL of distilled water, 3-mm apical area of the root was powdered with a carbide bur driven by a Micromotor (NSK, Japan), low speed of 500 rpm by rinse of sterile distilled water and wait for drying. Then, transferred powders into tubes containing sterile BHI in the same condition for all the groups. After 10-fold serial dilutions in saline solution, aliquots of 0.1 mL were plated onto nutrient agar plates and incubated at 37°C for 48 h [Figure 3]. The CFUs were counted manually using a pen and click-counter after 24 h of growth.
Figure 3.
Microbiologic evaluation.
RESULTS
The comparison between the groups, according to two-way ANOVA with regard to the reaction, showed no interplay between 2.5% NaOCl and normal saline groups. However, one-way ANOVA showed significant differences between the groups.
Duncan test was used to assimilate E. faecalis counts in prepared teeth with 0.02, 0.04, 0.06 taper and 2.5% NaOCl as an irrigation solution. The test showed significant differences, suggesting that high tapering reduced bacteria more than low tapering. Different tapers in normal saline groups yielded the same results as the NaOCl groups.
E. Faecalis counts in prepared teeth with 0.02 taper and NaOCl and normal saline as irrigation solutions were assessed using t-test which showed no significant differences (P = 0.27); 0.04 and 0.06 taper with different irrigation solutions also showed no significant differences with P = 0.42 and P = 0.33, respectively.
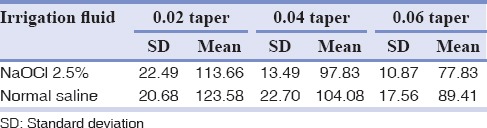
Canals instrumented with 0.06 taper exhibited greater reduction in CFUs compared to canals instrumented with 0.04 and 0.02 tapers (P < 0.001). Instrumentation with 0.04 taper also yielded a greater reduction in CFUs compared to canals instrumented with 0.02 taper (P < 0.001). Thus, canals instrumented with greater taper were cleaner, with no significant difference between the two irrigation solutions. CFU counts in each specimen in different groups are presented in Table 1.
Table 1.
Colony-forming unit counts in each of the specimens in different groups
DISCUSSION
The aim of the present study was to evaluate the effect of different irrigation solutions and taper of rotary systems on E. faecalis counts.
E. faecalis is a resistant bacterial species that believed to be the cause of endodontic treatment failure.
Based on the results of the present study, 2.5% NaOCl and normal saline did not exhibit significant differences. Selection of 2.5% NaOCl solution was based on the fact that lower or higher NaOCl concentrations have shown no significant differences in their antibacterial effects.[23]
This is consistent with Shabahang and Torabinejad who suggested the ineffectiveness of NaOCl to consistently disinfect root canals. They demonstrated that 50% of the root canals remained contaminated with E. faecalis despite irrigation with 1.3% or 5.25% NaOCl.[24] Another study used 1.25% NaOCl and found that about 38.1% of root canals remained contaminated with bacteria.[25] also Sjögren et al. in their clinical study stated that after the use of 0.5% NaOCl in debridement process, 40% of root canals remained infected.[26] Siqueira et al. studied on human extracted teeth were infected with E. faecalis and supposed after NaOCl 4% irrigation, 30%–40% of the root canals still contained viable bacteria.[27]
Gonçalves et al. and Rôças andSiqueira. reported reductions in bacterial counts with both NaOCl and chlorhexidine irrigants, with no significant differences between them.[28,29]
A substantial reduction in the bacterial counts was observed after chemomechanical preparation using either irrigant. This finding is consistent with many other studies,[11,30,31] confirming the essential role of chemomechanical procedures in eliminating intraradicular bacteria.[29]
Although there are some studies in contrary to our results,[32] it may be attributed to different methodologies.
However, antibacterial effects of NaOCl are recognized, the exact mechanism of microbial killing is not well clarified.[26] Therefore, we concluded that 2.5% NaOCl can reduce bacterial contamination, with no significant effect on E. faecalis. This can shows that elimination of E. faecalis might be more attributed to the mechanical action of instruments.
Another finding of the present study on different tapers is that root canal taper affected its cleanliness. This result can be compared with some previous studies, carried out by considering both size and taper. Mohammadzadeh Akhlaghi et al., Albrecht et al., Usman et al. and Lumley suggested that the greater size and taper could reduce the bacterial load.[2,33,34,35] Our results about greater taper are consistent with these studies.
To achieve the main aim of this study, we evaluated studies that prepared root canals with the same size but with different tapers. Consistent with our study results, Lee et al. suggested that an increase in taper leads to better debridement.[36] Singla et al. reported a significant decrease in bacterial counts with progressively larger tapers.[37] Lumley also reported that canals shaped with hand files of greater taper were significantly cleaner.[35] These findings are consistent with those of Dalton et al., Byström and Sundqvist.[38,39,40]
The results of Siqueira et al. were in contrast to the results of the present study. They suggested that canal preparation with different tapering had no effect on further reduction in bacterial counts in root canals.[41] The use of mandibular premolars in their research might be one of the reasons for the absence of significant differences in the results of their study. The cross-section of mandibular premolars is oval and wider in buccolingual direction. Despite the two instrumentation techniques that were performed in their study, the oval walls might remain unfilled. To solve this problem, anterior teeth with one root canal and circular cross-section anatomy were used in our study.
Arvaniti andKhabbaz also suggested that the cleanest part of the canal is in the middle third, with a statistically significant difference from the apical third. In their study, debris removal was almost complete with all the tapers, whereas the smear layer was not removed because of inadequate irrigation fluids.[20] According to this study, we designed our study to analyze the 3-mm apical area of the root canals.
None of the previous studies considered the penetration of bacteria into dentinal tubules. Hence, we were encouraged to plan a study with a different and accurate methodology. The study was therefore carried out by powdering 3-mm root end with a carbide bur, instead of paper points, piezo drills, or optical microscope observation.[37,38,42,43,44]
It is noteworthy that the strength of teeth with root canal treatment directly depends on the remaining intact tooth structure. Endodontic treatment processes result in the loss of tooth structure and weak root canal walls.[45,46]
CONCLUSION
The results of the present study showed that high tapering is more effective in root canal cleanliness, reducing E. faecalis counts to almost zero level. However, other clinical effects of larger instrumentation, including compromised restorability, fracture susceptibility, and canal path alterations should also be considered when using any instrumentation technique. Thus, root canals should be flared as needed and prepared in a conical shape with a gentle taper. Further studies are suggested to evaluate the effects of different tapers on VRF strength of the root.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadzadeh Akhlaghi N, Rahimifard N, Moshari A, Vatanpour M, Darmiani S. The effect of size and taper of apical preparation in reducing intra-canal bacteria: A Quantitative SEM study. Iran Endod J. 2014;9:61–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner JC, Falkler WA., Jr Bacteria in the apical 5 mm of infected root canals. J Endod. 1991;17:380–3. doi: 10.1016/s0099-2399(06)81989-8. [DOI] [PubMed] [Google Scholar]
- 4.Block RM, Bushell A, Rodrigues H, Langeland K. A histopathologic, histobacteriologic, and radiographic study of periapical endodontic surgical specimens. Oral Surg Oral Med Oral Pathol. 1976;42:656–78. doi: 10.1016/0030-4220(76)90217-6. [DOI] [PubMed] [Google Scholar]
- 5.Barbizam JV, Fariniuk LF, Marchesan MA, Pecora JD, Sousa-Neto MD. Effectiveness of manual and rotary instrumentation techniques for cleaning flattened root canals. J Endod. 2002;28:365–6. doi: 10.1097/00004770-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Engström B, Hård AF, Segerstad L, Ramström G, Frostell G. Correlation of positive cultures with the prognosis for root canal treatment. Odontol Revy. 1964;15:257–70. [Google Scholar]
- 7.Liet S, Sorin SM. Evaluation of clinical results based upon culturing root canals. J Br Endod Soc. 1969;3:3–6. doi: 10.1111/j.1365-2591.1969.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 8.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–5. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 9.Fabricius L, Dahlén G, Holm SE, Möller AJ. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res. 1982;90:200–6. doi: 10.1111/j.1600-0722.1982.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 10.Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15:308–20. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
- 11.Siqueira JF, Jr, Rôças IN, Paiva SS, Guimarães-Pinto T, Magalhães KM, Lima KC, et al. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:122–30. doi: 10.1016/j.tripleo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Rôças IN, Provenzano JC, Neves MA, Siqueira JF., Jr Disinfecting effects of rotary instrumentation with either 2.5% sodium hypochlorite or 2% Chlorhexidine as the main irrigant: A randomized clinical study. J Endod. 2016;42:943–7. doi: 10.1016/j.joen.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Munson MA, Pitt-Ford T, Chong B, Weightman A, Wade WG. Molecular and cultural analysis of the microflora associated with endodontic infections. J Dent Res. 2002;81:761–6. doi: 10.1177/0810761. [DOI] [PubMed] [Google Scholar]
- 14.Nair PN, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: A long-term light and electron microscopic follow-up study. J Endod. 1990;16:580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 15.Orstavik D, Kerekes K, Molven O. Effects of extensive apical reaming and calcium hydroxide dressing on bacterial infection during treatment of apical periodontitis: A pilot study. Int Endod J. 1991;24:1–7. doi: 10.1111/j.1365-2591.1991.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu MK, Wesselink PR. Efficacy of three techniques in cleaning the apical portion of curved root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:492–6. doi: 10.1016/s1079-2104(05)80134-9. [DOI] [PubMed] [Google Scholar]
- 17.Siqueira JF, Jr, Araújo MC, Garcia PF, Fraga RC, Dantas CJ. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod. 1997;23:499–502. doi: 10.1016/S0099-2399(97)80309-3. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan LS. The standardized-taper root canal preparation-part 1. Concepts for variably tapered shaping instruments. Int Endod J. 2000;33:516–29. doi: 10.1046/j.1365-2591.2000.00384.x. [DOI] [PubMed] [Google Scholar]
- 19.de Gregorio C, Arias A, Navarrete N, Del Rio V, Oltra E, Cohenca N, et al. Effect of apical size and taper on volume of irrigant delivered at working length with apical negative pressure at different root curvatures. J Endod. 2013;39:119–24. doi: 10.1016/j.joen.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Arvaniti IS, Khabbaz MG. Influence of root canal taper on its cleanliness: A scanning electron microscopic study. J Endod. 2011;37:871–4. doi: 10.1016/j.joen.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Cohenca N, Paranjpe A, Heilborn C, Johnson JD. Antimicrobial efficacy of two irrigation techniques in tapered and non-tapered canal preparations. A randomized controlled clinical trial. Quintessence Int. 2013;44:217–28. doi: 10.3290/j.qi.a29055. [DOI] [PubMed] [Google Scholar]
- 22.Moshari AA, Akhlaghi NM, Rahimifard N, Darmiani S. Reduction of Enterococcus faecalis in curved root canals after various sizes and tapers of canal preparation. J Conserv Dent. 2015;18:306–9. doi: 10.4103/0972-0707.159733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siqueira JF, Jr, Rôças IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26:331–4. doi: 10.1097/00004770-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29:576–9. doi: 10.1097/00004770-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Shuping GB, Orstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751–5. doi: 10.1097/00004770-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 27.Siqueira JF, Jr, Machado AG, Silveira RM, Lopes HO, de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J. 1997;30:279–82. doi: 10.1046/j.1365-2591.1997.00096.x. [DOI] [PubMed] [Google Scholar]
- 28.Gonçalves LS, Rodrigues RC, Andrade Junior CV, Soares RG, Vettore MV. The effect of sodium hypochlorite and chlorhexidine as irrigant solutions for root canal disinfection: A systematic review of clinical trials. J Endod. 2016;42:527–32. doi: 10.1016/j.joen.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Rôças IN, Siqueira JF., Jr Comparison of the in vivo antimicrobial effectiveness of sodium hypochlorite and chlorhexidine used as root canal irrigants: A molecular microbiology study. J Endod. 2011;37:143–50. doi: 10.1016/j.joen.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39:484–92. doi: 10.1111/j.1365-2591.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- 31.Zandi H, Rodrigues RC, Kristoffersen AK, Enersen M, Mdala I, Ørstavik D, et al. Antibacterial effectiveness of 2 root canal irrigants in root-filled teeth with infection: A Randomized clinical trial. J Endod. 2016;42:1307–13. doi: 10.1016/j.joen.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Berber VB, Gomes BP, Sena NT, Vianna ME, Ferraz CC, Zaia AA, et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int Endod J. 2000;39:10–7. doi: 10.1111/j.1365-2591.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht LJ, Baumgartner JC, Marshall JG. Evaluation of apical debris removal using various sizes and tapers of ProFile GT files. J Endod. 2004;30:425–8. doi: 10.1097/00004770-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. J Endod. 2004;30:110–2. doi: 10.1097/00004770-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Lumley PJ. Cleaning efficacy of two apical preparation regimens following shaping with hand files of greater taper. Int Endod J. 2000;33:262–5. doi: 10.1046/j.1365-2591.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Wu MK, Wesselink PR. The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from different-sized simulated plastic root canals. Int Endod J. 2004;37:607–12. doi: 10.1111/j.1365-2591.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 37.Singla M, Aggarwal V, Logani A, Shah N. Comparative evaluation of rotary protaper, profile and conventional step back technique on reduction in Enterococcus faecalis colony-forming units and vertical root fracture resistance of root canal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:105–10. doi: 10.1016/j.tripleo.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Dalton BC, Orstavik D, Phillips C, Pettiette M, Trope M. Bacterial reduction with nickel-titanium rotary instrumentation. J Endod. 1998;24:763–7. doi: 10.1016/S0099-2399(98)80170-2. [DOI] [PubMed] [Google Scholar]
- 39.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 40.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 41.Siqueira JF, Jr, Lima KC, Magalhães FA, Lopes HP, de Uzeda M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J Endod. 1999;25:332–5. doi: 10.1016/S0099-2399(06)81166-0. [DOI] [PubMed] [Google Scholar]
- 42.Singh M, Kasat VO. Efficacy of various combinations of irrigants and medicaments on Candida albican: An in vitro study. J Int Soc Prev Community Dent. 2015;5:157–62. doi: 10.4103/2231-0762.159947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paranjpe A, de Gregorio C, Gonzalez AM, Gomez A, Silva Herzog D, Piña AA, et al. Efficacy of the self-adjusting file system on cleaning and shaping oval canals: A microbiological and microscopic evaluation. J Endod. 2012;38:226–31. doi: 10.1016/j.joen.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Boutsioukis C, Gogos C, Verhaagen B, Versluis M, Kastrinakis E, Van der Sluis LW, et al. The effect of root canal taper on the irrigant flow: Evaluation using an unsteady computational fluid dynamics model. Int Endod J. 2010;43:909–16. doi: 10.1111/j.1365-2591.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 45.Guppy DR, Curtis RV, Ford TR. Dentine chips produced by nickel-titanium rotary instruments. Endod Dent Traumatol. 2000;16:258–64. doi: 10.1034/j.1600-9657.2000.016006258.x. [DOI] [PubMed] [Google Scholar]
- 46.Rundquist BD, Versluis A. How does canal taper affect root stresses? Int Endod J. 2006;39:226–37. doi: 10.1111/j.1365-2591.2006.01078.x. [DOI] [PubMed] [Google Scholar]


