Abstract
The past decade has witnessed a consolidation and refinement of the extraordinary progress made in taste research. This Review describes recent advances in our understanding of taste receptors, taste buds, and the connections between taste buds and sensory afferent fibres. The article discusses new findings regarding the cellular mechanisms for detecting tastes, new data on the transmitters involved in taste processing and new studies that address longstanding arguments about taste coding.
Taste buds are the peripheral organs of gustation and are located mainly in the tongue epithelium, although they are also present elsewhere in the oral cavity. They sample the chemical makeup of foods and beverages for nutrient content, palatability and potential toxicity. The substantial diversity and redundancy of the molecular receptors for these compounds may reflect the importance of identifying nutrients and avoiding chemical threats from the environment. The molecular recognition of tastants, which occurs at the apical tips of taste bud cells, ultimately results in sensory perceptions (for example, sweet, salty, and so on) that guide appetite and trigger physiological processes for absorbing nutrients and adjusting metabolism. This Review discusses the proteins and pathways that taste buds use to detect stimuli, the communication and modulation that occur between their cells, and the nerve fibres that innervate taste buds, as well as the principles of the coding by which information is conveyed from the periphery to neurons in the CNS. Each of these areas has seen many new developments, controversies and clarifications in the past decade.
Chemosensory transduction
Taste buds are sensory end organs that are located in the oral epithelium (BOX 1). The receptors on the chemosensitive apical tips of taste bud cells confer specificity to gustatory stimuli. Taste receptors come in many types, including several classes of G protein-coupled receptors (GPCRs) and ion channels (FIG. 1). Some stimuli interact with receptors to generate second messengers, whereas in other instances, the taste stimulus itself is transported into the cytoplasm of taste bud cells and activates downstream events.
Box 1. Taste buds and their distinct cell types.
Taste buds are clusters of columnar sensory cells that are embedded in the stratified epithelium of the tongue, palate and epiglottis. In mammals, each taste bud is a compact cluster of cells that resembles a garlic bulb, with 50–100 elongated cells extending from the base of the cluster to its apex and a few undifferentiated postmitotic cells at the base of the cluster. A classification scheme that integrates ultrastructural features and patterns of gene expression with cell function generally recognizes three cell types (in order of their relative abundance): type I, type II and type III cells (see the figure, part a; note that taste buds include many more cells than those that are depicted in the figure).
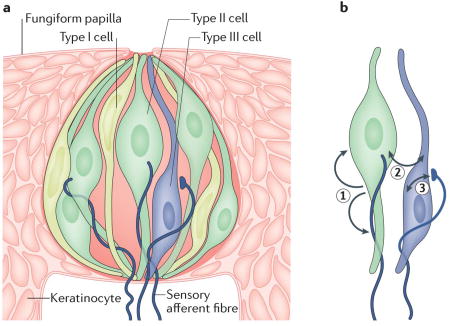
Type I cells comprise approximately half the total number of cells in a taste bud. They have narrow, irregularly shaped nuclei, are electron-dense and have wing-like cytoplasmic extensions that ensheath other taste bud cells162, 163. Type I cells seem to have glia-like functions. They express enzymes and transporters that are required to eliminate extracellular neurotransmitters112, 113, 164 and ion channels that are associated with the redistribution and spatial buffering of K+ (REF.165). Few other details are known about their function. Indeed, type I cells may be quite heterogeneous in terms of their gene expression patterns and their functions.
Approximately one-third of the cells in a taste bud are type II cells. These cells are larger in diameter than type I cells, have sizable spherical nuclei, and function as chemosensory receptors for sugars, amino acids and/or bitter stimuli as they express taste G protein-coupled receptors (GPCRs) and their downstream effectors. Most type II cells express one class of taste GPCR — namely, taste receptor type 1 (T1R) or T2R — and correspondingly respond only to one taste quality (for example, sweet or bitter, but not both)131, 132. It should be noted that T1R1, T1R2 and T1R3 are often co-expressed in taste bud cells, and, accordingly, responses to both sweet and umami stimuli can be detected in the same cells41, 114. The type II cells in a taste bud can differ in their expression of taste GPCRs such that each taste bud can respond to multiple taste stimuli.
Taste buds respond to more than one taste stimulus because they contain multiple type II cells of different specificities. Type III taste cells are the least numerous; they represent 2–20% of the cells in a taste bud, and their incidence varies regionally in the oral epithelium. For instance, taste buds on the anterior tongue (fungiform taste buds) often contain no more than a single type III cell, whereas taste buds in the posterior tongue may contain as many as ten. These cells display slender profiles and oblong nuclei. They do not express taste GPCRs but do contain the machinery required to detect sour taste4, 5, 68. Type III cells have ultrastructurally recognizable synapses162 with features such as clear and dense-cored vesicles, SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins and densities in which the membranes of the taste cell oppose nerve fibres166, 167. By contrast, type II cells lack synaptic vesicles and communicate with closely apposed afferent fibres via non-vesicular transmitter release (see the main text).
In addition to the taste receptors, there are also receptors for ATP that mediate transmission from type II cells to nerves and autocrine feedback onto type II cells (1); receptors for ATP, serotonin and GABA that are responsible for cell–cell communication between type II and type III cells (2); and finally, receptors for serotonergic (feedforward) transmission and glutamatergic (feedback) transmission between type III cells and gustatory afferent fibres (3) (see the figure, part b).
Taste cells within taste buds are separated from the mucosal surface, blood supply and surrounding epithelium by a selective barrier that includes enzymatic components (extracellular ATPases), physical components (the zonula occludens) and molecular components (claudins, chondroitin sulfate and other glycosaminoglycans)112, 168–171. The barrier probably regulates which small molecules (for example, drugs, trophic factors, hormones and neuropeptides) can penetrate into the taste bud from the mucosal, serosal or vascular environments to influence the function of its constituent cells.
Figure 1. Membrane proteins that transduce taste.
Type 2 taste receptors (T2Rs; bitter-taste receptors) are G protein-coupled receptors (GPCRs) that have short amino termini and may function as monomers (not shown) or dimers. T1Rs (sweet-taste and umami receptors) are also GPCRs, but they have long N termini that contain bilobed (venus flytrap) domains and function as dimers that use T1R3 as an obligate subunit. T1R1–T1R3 is an umami receptor, and T1R2–T1R3 is a sweet-taste receptor. All these taste GPCRs use a common transduction pathway that includes a Gβγ-activated phospholipase C (PLCβ2) and transient receptor potential cation channel subfamily M member 5 (TRPM5). The epithelial Na channel (ENaC) has three subunits and is thought to transduce salty taste in rodents. Glucose transporter type 4 (GLUT4) — which has 12 membrane-spanning segments — transports glucose by facilitative diffusion, whereas sodium/glucose cotransporter 1 (SGLT1) is Na dependent. One or both of these transporters are hypothesized to be part of an alternative glucose-sensing pathway that is similar to the one used in pancreatic β cells.
Taste bud cells can be organized into three main types, in part according to their function. In general, bitter, sweet and umami stimuli are detected by type II cells1–3, sour stimuli are detected by type III cells4–6, and salty (NaCl) stimuli are detected by as-yet-undefined taste bud cells7. Below, we describe the mechanisms by which gustatory stimuli are transduced by taste buds. We remind readers that sweet and bitter, for example, are sensory perceptions; the compounds that elicit them are labelled with these same names as a shorthand in this Review, to which purists may object.
Sweet
Taste buds detect sugars (probably as an indication of carbohydrates) and other sweet stimuli using diverse mechanisms. The best-studied receptor for sweet stimuli is the heterodimer formed of two GPCRs: namely, taste receptor type 1 member 2 (T1R2) and T1R3. These subunits were identified by screening for mRNAs that are preferentially expressed in mouse taste buds8 or by genetic linkage to Sac, which is a locus known to dictate sweet-taste sensitivity in mice9–11. When cultured cells are co-transfected with T1R2 and T1R3, they respond to sucrose, fructose, artificial sweeteners and some d-amino acids that elicit a sweet taste12, 13.
T1R2 and T1R3 belong to family C of the GPCRs14. They each possess a long extracellular amino terminus that forms a venus flytrap module (VFM). T1R2 and T1R3 function as a heterodimer, and have multiple ligand-binding sites. Nevertheless, the purified extracellular domain alone of either T1R2 or T1R3 is capable of binding many sugars and sugar alcohols15. Modelling and experiments with chimeric and point-mutated T1R2 and T1R3 have demonstrated that sugars and dipeptide sweeteners (for example, aspartame) bind in the cleft of the VFM, albeit at slightly different positions16–18. Intensely sweet proteins (for example, monellin and brazzein) bind in the VFM and in a cysteine-rich domain that links the VFM to the transmembrane region19, 20, and small-molecule sweeteners (for example, cyclamate) bind at residues in or near the transmembrane domains16.
Mice that lack T1R2 or T1R3 have been reported to lose all behavioural sensitivity and neural responses to sugars and artificial sweeteners21. However, another group has reported that knocking out the gene that encodes T1R3 (Tas1r3) variably affects responses to sugars while eliminating the detection of artificial sweeteners22. This finding suggests that T1R3-independent mechanisms probably exist for the detection of sugars and other sweeteners22, 23. One postulated T1R3-independent mechanism involves glucose transporter type 4 (GLUT4) and sodium/glucose cotransporter 1 (SGLT1), which have been shown to transport glucose into sweet-sensing taste cells, leading to a transient elevation of intracellular ATP24. ATP that is generated by this pathway blocks ATP-inhibited K+ channels (KATP channels) to depolarize the membrane24. Disaccharides such as sucrose are hydrolysed to hexoses and can then activate this pathway25. The involvement of a Na+-dependent transporter — that is, SGLT1 — in transducing sugars offers a plausible explanation for the potentiation of sweet taste by Na+ salts26.
T1R3-independent pathways for transducing sugars may also trigger physiological reflexes independently of sweet-taste perception. Orally applied sugar has long been known to produce a small but marked elevation in plasma insulin levels within minutes, long before the sugar is absorbed in the gut. This cephalic-phase insulin release (CPIR) is documented in rodents and humans, and requires intact taste nerves27–29. CPIR requires vagal stimulation of the pancreas29. Insulin release from the pancreas may also be stimulated by glucagon-like peptide 1 (GLP1; also known as incretin), which is secreted by sweet-sensing taste bud cells30, 31. Sugar-induced CPIR persists in Tas1r3-knockout mice32 and is mediated through the action of KATP channels33. Thus, at least two distinct and parallel sugar-sensing mechanisms seem to be initiated in taste buds: one that signals the perception of carbohydrate-rich foods (that is, sweet tastes, via T1R2–T1R3) and one that deploys a physiological reflex of insulin secretion (via a transporter).
Starch — which is a high-molecular-weight polymer of glucose — has long been considered to be tasteless to humans (but see REF.34). However, rodents show a strong appetitive preference for solutions containing smaller polymers such as Polycose35. Intriguingly, mice in which Tas1r3 and Tas1r2 have been knocked out can still detect Polycose36, 37. Given the nutritional importance of carbohydrates in most animals, the detection of sugars by glucose transporters and KATP channels may reflect a parallel mechanism for ensuring caloric sufficiency.
Umami
Some amino acids, notably glutamate and aspartate, have a savoury taste named umami. The prototypic stimulus for umami is monosodium glutamate (MSG). Glutamate is abundantly present in meat, fish, cheese and many vegetables. When 5ʹ nucleotides, such as 5ʹ inosine monophosphate (IMP), are present in small amounts alongside glutamate, there is a synergistic augmentation of umami taste38.
Taste cells detect umami stimuli through multiple receptors. A sizeable amount of literature documents a role for T1R1–T1R3 heterodimers in transducing the umami taste13, 21, 39. An initial report using Tas1r3-knockout mice, mice that lack Tas1r1 (which encodes T1R1) and double Tas1r3;Tas1r1-knockout mice claimed that T1R1–T1R3 heteromers were fully responsible for all umami taste detection21. However, studies using an independently generated Tas1r3-knockout line (see above) showed that the behavioural and neural responses of these mice to umami compounds (MSG and IMP) were nearly normal22, 40. Furthermore, a second line of mice in which Tas1r1 was knocked out also demonstrated near-normal responses to umami compounds in taste bud cells and nerves; the only major change was that the nucleotide-mediated augmentation of umami taste was lost41. Thus, it seems that T1R1–T1R3 responds primarily to mixtures of MSG and nucleotides.
Umami taste receptors other than T1R1–T1R3 are also present in taste bud cells; these include N-terminal-truncated, taste-specific variants of the two metabotropic glutamate receptors mGluR4 (REFS42, 43) and mGluR1 (REF.44). Both of these receptors are activated by glutamate at concentrations that are found in foods42, 44. Nerve recordings from mice that lack Grm4 (which encodes mGluR4) revealed decreased responses to glutamate and IMP, confirming that a fraction of the afferent nerve response to MSG in wild-type mice is attributable to mGluR4 (REF.45).
Bitter
Bitter taste is stimulated by an enormous variety of compounds that have diverse chemical structures, from simple salts to large complex molecules, many of which are toxic. Bitter-taste receptors (T2Rs) are class A GPCRs, and have short N termini and ligand-binding sites in their transmembrane segments46. Unlike T1Rs, the T2Rs are generally considered to function as monomers, although recent evidence suggests that they may also form homodimers and heterodimers47. Many mammalian genomes (including the human genome) have 20 or more genes that encode T2Rs; by contrast, only three T1R-encoding genes have been identified in mammalian genomes. Subsets of T2Rs are co-expressed in any given bitter-sensing taste bud cell48, 49. T1Rs (which detect sweet and umami tastes) and T2Rs are expressed in a non-overlapping pattern50, suggesting a separation of receptor cells that detect appetitive versus aversive stimuli.
Individual T2Rs in humans and rodents can be narrowly tuned to one or a very few bitter compounds, whereas others are broadly responsive to several bitter chemicals. The breadth of the receptive ranges of human T2Rs has been encyclopaedically documented in a thorough study of heterologously expressed taste receptors51. This study showed, for instance, that T2R3 responds to only a single compound (out of 94 different natural and synthetic compounds tested), whereas T2R14 responds to at least 33 compounds. Conversely, a single bitter compound often can activate multiple different T2Rs51–54. For example, quinine activates as many as nine different human T2Rs, whereas acetaminophen — an analgesic — stimulates just one human T2R51. This broad and overlapping range of ligand sensitivities of the T2Rs assures that this family of receptors responds to an enormous range of bitter-tasting chemicals (BOX 2). Presumably, this redundancy evolved to ensure the detection of potentially toxic (bitter-tasting) chemicals and thus prevent the consumption of harmful foods. An evolutionary note in this context is that orthologous receptors in mice and humans often are responsive to very different bitter tastants54, which suggests that receptors have been reassigned to ecologically relevant compounds and that the gene family has been subjected to selective pressures55. Many T2Rs exhibit functional polymorphisms that result in varying abilities to taste particular compounds56, and these polymorphisms may underlie differences in food preference57 (BOX 3).
Box 2. The discrimination of bitter tastes.
Taste receptor type 2 (T2R) family members comprise a large group of taste G protein-coupled receptors (GPCRs) that detect bitter compounds; there are more than 40 T2R family members in rodents and 25 functional genes in humans. When the family was first described, T2Rs were reported to be expressed in an all-or-none manner in individual cells50 or in a more combinatorial pattern48. If all T2Rs are expressed together in every bitter-sensing cell, the implication is that bitter is a homogeneous, singular taste quality50. Alternatively, if bitter-sensing cells express various combinations of T2Rs, it might be possible to discriminate bitter compounds48.
Evidence has accumulated for both of these possibilities. Ca imaging of mouse taste buds and single-unit recordings from peripheral axons in rats have revealed that taste bud cells and neurons do in fact discriminate among several bitter-tasting compounds, with some responding to denatonium, others responding to quinine or cycloheximide, and yet others responding to two or more of these compounds172, 173.
Recent studies that have systematically used numerous probe combinations on human taste buds have shown that chemosensory taste bud cells co-express overlapping subsets of 4–11 T2Rs and not the entire family49. In principle, this variation in co-expression could form the basis for the neural and perceptual discrimination of various bitter compounds. However, it is currently not known whether each T2R is expressed in a predictable combination with others or is expressed randomly. The former may be a prerequisite for discrimination. Although central neurons have been shown in some instances to discriminate among bitter compounds, this may involve additional receptor mechanisms and pathways157, 174, 175. Whether animals and humans are capable of behaviourally distinguishing bitter-tasting compounds is unresolved, and there is evidence both for175 and against176, 177 the discrimination of such compounds.
Box 3. Brussels sprouts and broccoli: the genetics of taste preference.
Variation in the genes that encode taste receptors probably generates different taste sensitivities among individuals. A well-known example involves the taste of phenylthiocarbamide (PTC) or related compounds. Individuals find PTC extremely bitter, moderately bitter or nearly tasteless (that is, they cannot detect the presence of PTC). These phenotypes were first reported nearly a century ago as an example of simple Mendelian inheritance with a dominant taster allele and a recessive non-taster allele178. The locus (TAS2R38, which encodes taste receptor type 2 member 38 (T2R38)) and underlying alleles were only recently identified179. Genetic analyses of human populations in Africa, Asia and Europe suggest that PTC-taster and non-taster alleles of TAS2R38 have been maintained by natural selection across more than 100,000 years of human evolution180. Similarly, an allele of the human gene TAS2R16 — which confers sensitivity to several β-glucopyranosides found in nature (for example, those in bitter almonds, bearberry and manioc) — has been subjected to positive selection pressure across human evolution181, 182.
The PTC-taster allele of TAS2R38 encodes a receptor that detects a range of natural bitter compounds, some of which are toxic and others of which are beneficial. This finding supports the interpretation that both alleles are preserved in human populations on the basis of varied human diets and environments. For instance, heterozygous individuals find anthocyanin-containing cruciferous vegetables (such as Brussels sprouts and broccoli) less bitter than do those who inherited two PTC-taster alleles. Consequently, homozygous ‘tasters’ tend to reject these vegetables and may thus avoid the hypothyroidism that results from consuming large quantities of anthocyanin compounds when dietary iodine is deficient. Curiously, polymorphisms in some human T2R-encoding genes are associated with variation in the perceived bitterness of ethanol and other stimuli that are usually considered to activate trigeminal fibres rather than taste buds183.
The anticancer benefit of phytochemicals present in cruciferous vegetables has motivated researchers to investigate whether there might be a relationship between certain bitter-taste receptor-encoding alleles, diet selection, and the incidence of colorectal cancer and other cancers. Most studies have failed to find an association between cancer incidence and polymorphisms in TAS2R14 (REF.184), TAS2R16 and TAS2R50 (REF.185). However, for TAS2R38, one laboratory has reported an association between a particular TAS2R38 haplotype and colorectal cancer risk186, although another group failed to detect such an association185.
The human T1R-encoding genes (which encode sweet or umami receptors) show great variability across human populations187. However, there are relatively few reports on taste phenotypes that are directly associated with these genetic alterations. A polymorphism in a non-coding region upstream of TAS1R3 was associated with a greater perceived intensity of sweetness that apparently resulted from increased transcription188. At least one polymorphism of TAS1R2 was reported to be associated with the increased intake of carbohydrates and elevated blood triglyceride levels in certain populations189, 190. Polymorphisms in TAS1R2 and TAS1R3 have also been associated with the risk of dental caries in children191. Furthermore, the ability of individuals to detect umami varies across populations and was shown to be associated with variation in TAS1R3 (REF.192).
Although genotype–phenotype associations are becoming apparent, functional studies on the receptors expressed by identified variants have yet to provide a molecular basis for the many observed variations in human taste and diet. Indeed, although taste receptor genetics may play a part in food selection, additional factors influence the complex human behaviours involved in this process.
Effector pathways for sweet, umami and bitter taste receptors
In spite of their diversity, T1Rs and T2Rs converge on a common intracellular signalling pathway. These GPCRs all couple to heterotrimeric G proteins that include Gβ3 and Gγ13, as well as Gαgus (also known as gustducin), Gα14 and Gαi (REFS58–60). Gα subunits were originally proposed to activate cAMP signalling61, but the current view is that they primarily function to regulate Gβγ subunits. cAMP also seems to have a longer-term role by maintaining signalling proteins in a responsive state through protein kinase A activation62. When T1Rs and T2Rs are activated by tastants, Gβγ dimers are released, which stimulates phospholipase Cβ2 to mobilize intracellular Ca2+ (REFS1, 58). Elevated cytosolic Ca2+ levels lead to the opening of transient receptor potential cation channel subfamily M member 5 (TRPM5), which is a cation-permeable channel that effectively depolarizes taste cells63–65. Interestingly, these same receptors and components of the same signalling pathways are also found in specialized cells in other tissues, where they may detect chemical stimuli without eliciting ‘taste’ per se (BOX 4).
Box 4. Extraoral taste receptors.
Taste G protein-coupled receptors (GPCRs) are not limited to the oral cavity or to sensing ingested foods and beverages. Instead, members of the large taste receptor type 2 (T2R) family, and the smaller T1R family, are found in several tissues and organs throughout the body95, 193, 194 (see the table). The function of many of these extraoral taste GPCRs remains incompletely explored.
Extraoral taste receptors that have been studied the most extensively and for which we have a better understanding of function include those in the gut and airways. Elegant studies have described a functional role for T2Rs (bitter-taste receptors) and their downstream signalling partners in the ciliated cells of the airway epithelium195, 196. These studies showed that bitter compounds elicit the secretion of bactericidal nitric oxide and stimulate ciliary beating, promoting the clearance of bacteria and noxious compounds from the airways. One particular bitter-taste receptor that is localized to airway epithelium, T2R38 (which is encoded by TAS2R38), is activated by acyl-homoserine lactone, which is a quorum-sensing molecule that is secreted by Gram-negative bacteria. Polymorphisms in TAS2R38 are linked to susceptibility to respiratory and chronic rhinosinusitis in humans, which emphasizes the importance of this bitter-taste receptor in the upper airways197, 198.
The nasal respiratory epithelium also includes another type of cell that expresses T2R: solitary chemosensory cells (SCCs). These chemosensory cells make synaptic contact with and release acetylcholine onto trigeminal sensory afferent fibres, thereby initiating a protective apnoea reflex199, 200. T2Rs on SCCs respond to quorum-sensing molecules and other bacterial secretions196, 201. The resulting Ca2+ signal seems to spread via gap junctions to surrounding respiratory epithelial cells, triggering an innate immune response that includes the secretion of antibacterial peptides198.
| Tissue | Taste GPCRs | Refs |
|---|---|---|
| Airway ciliated cells | T2Rs | 195 |
| Airway smooth muscle | T2Rs | 207 |
| Airway SCCs | T1Rs and T2Rs | 194, 197 |
| Brain: multiple regions | T1Rs and T2Rs | 208, 209 |
| Choroid plexus | T1Rs and T2Rs | 210 |
| Heart | T1Rs and T2Rs | 211 |
| Intestine | T1Rs and T2Rs | 202, 204, 212 |
| Keratinocytes | T2Rs | 213 |
| Kidney | T1Rs | 214 |
| Liver: bile ducts | T1Rs | 215 |
| Leukocytes | T1Rs and T2Rs | 214, 216, 217 |
| Pancreatic islets of Langerhans | T1Rs | 218 |
| Stomach | T2Rs | 202 |
| Testis | T1Rs and T2Rs | 214, 219, 220 |
| Thyroid gland | T2Rs | 221 |
It is intriguing that the same SCCs that express bitter-taste receptors that sense microbial secretions also have T1R sweet-taste receptors198. Glucose in the mucus is thought to be the stimulus for these receptors. Downstream effectors of the T1Rs tonically inhibit the T2R-mediated pathway in the same SCC. A high titre of bacteria in the airway mucus lowers glucose levels, releasing the T2R pathway from inhibition and promoting the antibacterial response. High glucose levels in the airway mucus of patients with diabetes may keep this protective pathway chronically suppressed198.
T1Rs and T2Rs are also expressed in the gastric a quantitative analysis of expression levels and cell types using a knock-in fluorescent reporter demonstrated that only a very limited number of T2Rs may be expressed. Moreover, the T2Rs are not expressed in enteroendocrine cells, as was originally suggested202, 203, but in goblet cells mechanisms204. Individual cells express some but not all components of the canonical taste signalling cascade205. The implication of this is that ingested nutrients and non-nutrient chemicals (such as glucose, peptides, amino acids, bitter compounds, fats and, possibly, the fermentation products of gut microbiota) stimulate taste receptors on gut cells to elicit various defence mechanisms and/or the secretion of appetite-regulating hormones98, 203, 204. Chemicals in the gastrointestinal lumen may also provoke SCCs to release transmitters — possibly acetylcholine206 — that act in a paracrine manner on neighbouring enteroendocrine cells.
Sour
The proximate stimulus for sour taste is intracellular acidification rather than extracellular protons66. Organic (‘weak’) acids, such as citric acid and acetic acid, are more potent stimuli of sour taste than are mineral (‘strong’) acids such as HCl. This is attributed to the greater membrane permeability of the undissociated organic acid molecule and the subsequent generation of protons in the cytoplasm. By contrast, mineral acids readily dissociate in the extracellular solution, but most cell membranes are relatively impermeable to protons. Thus, citric acid and acetic acid are more potent stimuli of sour taste than is HCl when tested at a similar pH. The taste bud cells that depolarize and produce Ca2+ responses to acids are the neuron-like type III cells5, 67, 68.
Throughout the past two decades, numerous plasma membrane ion channels have been proposed as transducers for sour taste, including epithelial Na+ channels (ENaCs)69, hyperpolarization-activated cyclic nucleotide-gated channels70 and acid-sensing ion channels (ASICs)71. More recently, two members of the TRP superfamily of ion channels — polycystic kidney disease protein 1-like 3 (PKD1L3) and PKD2L1 — were proposed as the major transducers of sour taste4, 72. However, all of these candidates have been ruled out as sour taste transducers either because they lacked biophysical characteristics that were consistent with sour-evoked responses in taste cells, or because mice in which the candidate receptor-encoding genes were knocked out retained all or most of their sensitivity to sour tastants73, 74.
Confocal imaging of pH and Ca2+ has shown that organic acids permeate type III cells, acidify the cytoplasm and block leak K+ channels to depolarize the cell membrane67, 75. These leak channels have recently been identified; specifically, type III cells express an inwardly rectifying K+ channel, KIR2.1, which is inhibited by intracellular protons6. Extracellular protons, such as those contributed from mineral acids or from dissociated organic acids, pass through a proton conductance that seems to be concentrated to the apical tips of type III cells68. The molecular identity of the proton channel that is responsible for this proton movement remains to be established. The influx of protons through the channel generates a small depolarizing current, and furthermore, the accumulation of protons inside the cell contributes to the inhibition of KIR2.1 channels. The net result in both cases is depolarization of the type III sour-sensing cells such that they reach the threshold for action potential initiation6.
Salty
The taste of NaCl is still somewhat of an enigma. Animals and humans readily consume NaCl below isotonic concentrations (that is, concentrations below approximately 150 mM). This appetite is presumably to ensure the adequate ingestion of an essential mineral. By contrast, higher concentrations of NaCl are normally aversive, which presumably reflects a survival mechanism that protects individuals against hypernatraemia and dehydration.
It remains unclear exactly which taste bud cells transduce NaCl and what the transduction mechanisms are. Some of the key observations in this context are that, in rodents, amiloride, a diuretic, decreases the amplitude of responses to NaCl in afferent nerve recordings76, 77 and reduces the behavioural response (licking) to NaCl solutions77. Yet, amiloride does not seem to change the perception of the saltiness of NaCl in humans77, 78. In rodents, amiloride affects neural responses mainly to NaCl; the effect on other salts is less consistent77. The dampening of NaCl taste by amiloride has long been interpreted as evidence for the essential role of the amiloride-sensitive Na+ channel ENaC in salty taste76, 79.
Fungiform taste bud cells loaded with a Ca2+ indicator have been reported to respond to apically applied NaCl7. When the gene encoding the obligatory α-subunit of ENaC was conditionally knocked out in taste cells, neural responses and the ability of mice to respond behaviourally to NaCl were lost7. These data confirmed earlier interpretations that ENaC is necessary for salty taste detection in rodents. However, no evidence has yet directly demonstrated that ENaC is the principal salt receptor in humans.
In a subsequent study, the authors reported that the signals for low (preferred) concentrations of NaCl originated in amiloride-sensitive taste bud cells, whereas high (aversive) NaCl concentrations were detected by completely separate amiloride-insensitive taste bud cells80. They further inferred that the two cell types transmitted their signals along separate afferent neurons. However, Ca2+ imaging subsequently showed that the amiloride sensitivity of afferent neurons was similar regardless of their NaCl sensitivity81; that is, high-NaCl versus low-NaCl sensitivity did not covary with amiloride sensitivity81.
To date, the identity of the taste bud cells that sense NaCl is not known. Amiloride-sensitive Na+ currents have been reported in taste bud cells that are neither type II or type III cells, based on their lack of voltage-gated currents82. By contrast, a report from another group demonstrated that the amiloride-blocked resting conductance was most prominent in cells that had the characteristics of type II cells83. Neither of these studies pinpointed the ENaC-mediated currents to cells that could be identified by type-specific molecular markers. Yet other reports assign amiloride-insensitive responses to NaCl to acid-sensing type III cells80, 84. This is an area ripe for clarification.
Fat
Dietary fats consist largely of triglycerides. Solid fats and oils differ in the length of the fatty acid chains of the triglyceride and the number of unsaturated positions. Most animals have a well-developed preference for fats. The sensations evoked by dietary fats most certainly include somatosensory components such as texture and viscosity. Whether fats stimulate a gustatory component remains debated. Rats lose their ability to detect and identify certain fats if the innervation of their taste buds is interrupted85 which supports a role for taste buds in sensing fats. The principle argument against fat as a taste quality is that triglycerides have not been shown to stimulate taste cells. However, oral lipase activity in rodents rapidly and effectively digests fats into free fatty acids in the immediate environment of taste buds86. Fatty acids themselves are effective taste stimuli (see below). In humans also, oral lipase activity is detectable but only at low levels87, 88, leaving its role in fat detection unresolved. There may not be a singular fat taste quality, as dietary free fatty acids evoke multiple orosensations — including ‘fatty’ and ‘irritating’ — depending on chain length and concentration89.
One of the earliest studies on fat taste reported that certain polyunsaturated fatty acids act directly on K+ channels, resulting in the prolonged depolarization of taste bud cells90. More recently, receptor-mediated mechanisms have been documented, including roles for a transporter for fats, CD36 (also known as platelet glycoprotein 4)91, 92, and two GPCRs, GPR40 and GPR120 (also known as free fatty acid receptors 1 and 4, respectively)93, 94. CD36 was reported to be localized to the apical tips of some taste bud cells92 and to elicit elevations in intracellular Ca2+ levels when stimulated by fatty acids96. How the transporter couples to Ca2+ signalling is not yet known. Intriguingly, oral stimulation of CD36 by certain fatty acids elevates pancreatic secretions92 — which is suggestive of a ‘cephalic phase response’, preparing the gut for digesting lipids. GPR120 is expressed in a subset of rodent type II cells and, when activated, mobilizes Ca2+, as is the case for T1R and T2R activation97. GPR120 seems to be distributed and function similarly in human taste buds89. In mice, knocking out the gene that encodes CD36, GPR40 or GPR120 results in partial deficits in fat taste92, 94.
In short, fat taste is complex, and no particular transduction mechanism has been unambiguously identified. Indeed, several transducer proteins may interact to generate the taste of fat.
Aside from the basic taste qualities discussed above — sweet, bitter, salty, sour and perhaps fatty — additional tastes have been described (BOX 5).
Box 5. Less-studied tastes.
Aside from the well-studied taste qualities (sweet, bitter, umami, sour and salty), food evokes additional sensory perceptions. One such sensation is chemesthesis. The pungency of chilli peppers, ginger, horseradish and black peppers is elicited by several unrelated compounds, including the vanilloids capsaicin and zingerone, the organosulfur compound allyl isothiocyanate, and the alkaloid piperine, respectively. These compounds are known to directly activate transient receptor potential cation channels (TRPs) — including TRPV1 and TRPA1 (REFS222–225) — that are expressed in nociceptors, causing irritation and/or pain. Hence, the pungency of peppers has been assumed to represent the activation of trigeminal nociceptors in the oral epithelium, which makes pungency not a taste modality per se (for example, see REF.226). However, subsets of neurons in the gustatory sensory ganglia have also been reported to express TRPA1 and TRPV1 (REF.227), and to display capsaicin-activated cation currents228. Thus, it remains to be established whether pungency is a specialized perception that originates solely from oral somatosensory (trigeminal) neurons or whether there is a genuine taste component as well. Trigeminal afferents — many of which terminate in close proximity to, if not actually within, taste buds — might release peptides that influence taste cell sensitivity, such as substance P or calcitonin gene-related peptide (CGRP)229–233. Another intriguing possibility is that capsaicin and other pungent stimuli activate keratinocytes, which then secrete neuroactive compounds to stimulate trigeminal or gustatory sensory afferent fibres, or both234, 235.
A less tangible food-related sensation that was reported more recently is ‘kokumi’, which is described as thickness, longevity and mouthfeel (REF.236). Glutathione, and related dipeptides and tripeptides, are the principal kokumi stimuli. On their own they are nearly tasteless, but, when they are combined with sucrose, NaCl, monosodium glutamate or more complex solutions (for example, broth), these compounds elicit kokumi sensations without altering the primary taste (for example, sweet and umami)237, 238. Extracellular Ca2+-sensing receptor (CaSR) — a G protein-coupled receptor that was originally identified in the parathyroid glands and is a key player in the body’s Ca2+ homeostasis — is expressed in subsets of taste bud cells and is a possible transducer for kokumi stimuli239. CaSR is reported to be allosterically modulated by sucrose and several other natural and synthetic sweeteners240, and this modulation may account for the interaction between kokumi compounds and other taste stimuli.
Another little-studied aspect of taste is the ability to detect certain nutrients that are deficient in the diet. For instance, humans normally report that Ca2+ salts have an unpleasant taste that includes bitterness and sourness241, and rodents show little taste preference for Ca2+ salts242. However, rodents greatly increase their intake of solutions containing Ca2+ salts if their diet is deficient in Ca2+ (REF.242). Curiously, Ca2+ appetite in mice243 and humans244 is associated with taste receptor type 1 member 3 (T1R3), which underlies sweet and umami tastes, rather than with CaSR. It remains unclear whether Ca2+ produces a consciously perceived taste that drives appetite.
Neurotransmitters and modulators
At least five neurotransmitters have been identified in taste buds. Their release and possible roles in taste have been examined in detail, and are discussed below. In addition, several peptides that function as hormones or neuromodulators interact with the taste system by influencing the sensitivity or the output of taste buds. As the topic of the role of peptides in taste has been reviewed recently98, we focus here on small-molecule transmitters.
ATP
Early suggestions that ATP is a transmitter for taste buds were based on immunostaining for P2X purinoceptors in nerves associated with taste buds in rat tongues99. A role for this transmitter in taste signalling was validated in a study showing that sheets of lingual epithelium containing taste buds secreted ATP when stimulated with bitter tastants100. Furthermore, when the genes that encode the purinoceptors P2X2 and P2X3 were knocked out, mice became taste-blind to sweet, salty, bitter and umami100.
Later studies showed, however, that in these knockout mice, the evoked release of ATP also was markedly diminished, somewhat weakening the argument that these receptors are the exclusive postsynaptic targets on sensory afferent fibres101. Nevertheless, most evidence supports the essential role of P2X2 and P2X3 as principal players in taste transmission. Experiments that used cellular biosensor cells to detect transmitter release have demonstrated the tastant-evoked release of ATP from individual type II cells102, 103. ATP release is greatly reinforced and amplified by autocrine feedback via the P2X and P2Y receptors expressed by type II cells101, 104 (see below), which accounts for the loss of ATP secretion when the genes that encode P2X2 and P2X3 are knocked out.
Type II taste receptor cells secrete transmitters onto afferent fibres through an atypical, non-vesicular mechanism that involves large-pore membrane channels, which were initially proposed to include pannexin 1 (which is encoded by Panx1)102 or connexins103. The identity of the ATP-release channel was subsequently revised to calcium homeostasis modulator protein 1 (CALHM1)105. A feature that made pannexin 1 channels particularly attractive as potential ATP-release channels was their gating by the combined action of depolarization and intracellular Ca2+ (REFS102, 106), both of which occur simultaneously in taste cells during tastant-induced stimulation. Conversely, a confounding feature of CALHM1 channels as the conduit for ATP release is their requirement for an unphysiologically low level of extracellular Ca2+ for gating105, 107. Nevertheless, the finding that Panx1-knockout mice showed continued secretion of ATP and taste afferent transmission108, 109, combined with the observation that Calhm1-knockout mice exhibit taste deficits105, 110, firmly swayed the balance towards CALHM1 channels as the ATP-release channels that function in taste cells.
The high concentration of intracellular ATP (in the millimolar range) drives efflux through CALHM1 channels when they are opened by taste-evoked action potentials105. Even in the absence of synaptic clefts between type II cells and afferent fibres, ATP secreted into the confined extracellular spaces reaches sufficient concentrations to stimulate P2X receptors on nearby afferent fibres100. Interestingly, large ‘atypical’ mitochondria are located directly below the plasma membrane of type II cells near sites where innervating afferent fibres pass in close apposition111. These mitochondria are optimally positioned as potential sources of presynaptic ATP for afferent transmission.
Mechanisms for removing extracellular ATP and thus terminating taste-evoked synaptic transmission include the degradation of ATP by the ectoATPase on the surface of type I cells112, 113 and by an ectonucleotidase on type III cells114. The by-products of ATP breakdown, ADP and adenosine, contribute positive autocrine feedback by activating P2Y and A2 adenosine receptors, respectively, on type II cells104, 114. ATP also amplifies its own release by activating P2X receptors on type II cells101, 104. When this autocrine feedback is disrupted, an insufficient amount of ATP is secreted to activate nerves. ATP secreted from type II cells also acts as a paracrine stimulus for type III cells, leading to 5-hydroxytryptamine (5-HT; also known as serotonin) release from the latter cells102 (see below).
5-HT
The first taste neurotransmitter for which taste-evoked release was demonstrated experimentally was 5-HT115. Studies using cellular biosensors identified type III cells as the source of stimulus-secreted 5-HT. This was consistent with the long-established finding that these cells synthesize 5-HT and store it in synaptic vesicles116–118. Several distinct stimuli, including KCl-induced depolarization and sour tastants, elicit 5-HT release from type III cells in a depolarization-dependent and Ca2+-dependent manner119. Patch-clamp recordings from type III cells have demonstrated depolarization-induced vesicular exocytosis, presumably of the 5-HT-containing vesicles contained in these cells120.
Type III taste bud cells also secrete 5-HT in response to the paracrine release of ATP from type II cells (see below). Curiously, 5-HT released from type III cells is mediated by two separate mechanisms115 that involve either the mobilization of intracellular Ca2+ stores following ATP stimulation104, or Ca2+ influx triggered by sour taste stimuli or KCl-induced depolarization119, 120.
One function of 5-HT in taste buds seems to be to inhibit ATP secretion from type II cells104 (but see REF.121), providing negative feedback to taste excitation. Synaptically released 5-HT also excites sensory afferent fibres at well-defined synapses122, 123.
GABA
The inhibitory transmitter GABA is synthesized by, stored in and released from type III cells119, 124, 125. Correspondingly, type II taste cells express GABA type A receptors (GABAARs) and GABABRs receptors125. GABA seems to act within the taste bud to inhibit ATP release from type II cells119. Furthermore, sensory afferent neurons and their peripheral, taste bud-innervating processes express GABAARs125. Indeed, nearly every gustatory sensory neuron expresses GABAARs in addition to P2X2 and P2X3 receptors for the aforementioned purinergic signalling from taste bud cells126. Intriguingly, GABAARs seem to be limited to the neuronal soma and peripheral processes; the central processes of these neurons lack these receptors126. GABA inhibits the activation of gustatory ganglion neurons in vitro127. However, although it is inferred that GABA secreted in the periphery modulates afferent responses, this has not yet been demonstrated.
Acetylcholine and noradrenaline
In addition to secreting ATP, type II cells also secrete acetylcholine (ACh), as suggested by early findings that taste buds are rich in acetylcholinesterase128, 129. ACh release seems to function as an autocrine mechanism for increasing type II cell release of ATP, either by the selfsame type II cell or by the spread of ACh released from adjacent type II cells. Biosensor cell assays have also established that type III cells can uptake and re-release noradrenaline117, 130; the function of noradrenaline in taste remains unclear.
Cell–cell communication
A view of cell–cell interactions within taste buds has developed that suggests that signal processing in the peripheral organs of taste is more complex than was previously thought.
Sweet, bitter and umami compounds directly stimulate type II taste bud cells, and this is consistent with the expression patterns of the taste GPCRs for these compounds. The ATP that is released from type II cells during tastant-induced stimulation activates sensory afferent fibres, as described above, but ATP also excites adjacent type III cells via their P2Y receptors102, 104. Thus, in addition to being directly activated by acidic (sour) taste stimuli, type III cells also respond — albeit indirectly — to sweet, bitter and umami tastants131, 132.
The stimulation of type III cells by ATP induces them to secrete 5-HT and GABA, which in turn inhibit ATP release from type II cells. 5-HT and GABA provide negative feedback onto type II cells during tastant-induced activation104, 119, 121, 133. Details of how the release of 5-HT and GABA is balanced, what specific conditions stimulate 5-HT and/or GABA release, and the eventual impact of inhibitory transmitters on the taste afferent signal have yet to be explored.
Another form of cell–cell communication may exist between taste buds. The activation of one taste bud has been reported to inhibit surrounding taste buds, although the mechanism of inhibition was not determined in these studies134–136. Glutamate, presumably released from the collateral branches of afferent axons during tastant-induced stimulation, was recently shown to selectively activate type III cells137, 138. As the activation of type III cells induces the release of 5-HT and GABA, the glutamate-evoked release of inhibitory transmitters may explain the inhibition by axon collaterals137. However, species differences in axon branching and patterns of innervation139 limit a clear elucidation of this inhibitory mechanism.
The autocrine, paracrine and synaptic circuits that are present in taste buds are detailed in REF.140 and summarized in BOX 1.
Taste coding
How information from taste buds is transmitted to the CNS (specifically to neurons in the nucleus of the solitary tract) and, in particular, how signals discriminating sweet, sour, salty, bitter, umami and possibly other tastes are encoded are thorny questions. There has been more than 75 years of heated debate over these issues. At one extreme is the labelled-line coding model, which states that individual taste bud cells exclusively identify a unique taste quality (for example, sweet taste) and synapse with afferent fibres that are dedicated to that quality141, 142. Moreover, the central projections of the afferent neurons are ‘labelled’ by that same taste quality and synapse with dedicated hindbrain neurons that relay the information for that one quality to higher brain centres, thereby establishing a ‘labelled line’ of transmitted information.
An early suggestion of labelled-line coding in the periphery came from single-unit recordings in rodents, which showed that individual fibres respond best to one taste quality (for example, “NaCl-best”), although activity was also elicited by tastants of other qualities143. Studies on a number of other types of mammal have shown that sweet-responsive fibres are particularly well-tuned to sugars and artificial sweeteners144.
The expression patterns of taste receptors in taste buds lend strong evidence for taste quality distinction at the level of taste cells. T2Rs, which detect bitter stimuli, are not found in cells that express T1Rs, which detect sweet or umami stimuli12. Moreover, sour-sensing cells are a separate cell type from both T1R-expressing and TR2-expressing cells4, 5. Furthermore, the genetic ablation of only type III cells results in the selective loss of sour taste, as observed both at the behavioural level and in afferent recordings4. In addition, the expression of a modified opioid receptor — receptor activated solely by a synthetic ligand (RASSL) — in T1R-expressing cells drives behavioural preference for a compound that normally has no taste: namely, the synthetic opioid spiradoline21. Conversely, the expression of RASSL in T2R-expressing cells resulted in an aversion to spiradoline145. The implication is that sweet and bitter ‘labels’ are resident in particular cells; activating the afferent fibres that innervate these cells would thus result in stereotypical responses, consistent with labelled-line coding.
An alternative model for how taste is encoded in the periphery states that information is transmitted by combinatorial activity in multiple peripheral afferent fibres81, 146, 147 (FIG. 2). According to this model, ensembles of dissimilar afferent fibres are activated by a taste stimulus. The overall combination of fibres activated encodes the taste quality, such as sweet. Indeed, such combinatorial coding has long been established for colour vision and characterizes odour recognition in the olfactory system148.
Figure 2. The combinatorial model of taste coding.
Individual type II taste bud cells are mostly tuned to one taste quality (for example, bitter, sweet or salty): that is, they are ‘specialists’ (umami has been omitted for clarity). The type III cells sense sour tastes and also respond secondarily to other taste stimuli via cell-to-cell (paracrine) communication within the taste bud (represented by the arrows between the taste bud cells). Thus, type III cells can be termed ‘generalists’. Some afferent ganglion neurons receive input from taste cells that respond to a single taste quality and hence would be specialist neurons. Other afferent ganglion neurons receive input from many taste cells or from type III cells and thus are multiply sensitive ‘generalist’ neurons. Moving to the CNS, sensory ganglion cells converge on hindbrain neurons in the nucleus of the solitary tract.
Strong evidence for the combinatorial coding of peripheral taste comes from several lines of experimentation. First, taste buds contain both narrowly tuned cells and more broadly responsive ones. Specifically, separate populations of type II cells respond mainly to a single taste quality (for example, sweet or bitter). By contrast, type III taste bud cells respond directly to sour stimuli and indirectly (via cell–cell communication) to multiple taste stimuli131, 132. Second, there are decades of electrophysiological recordings from individual sensory neurons that innervate taste buds in animals ranging from rodents to primates. Many of these recordings have revealed the existence of highly tuned neurons — which are also referred to as ‘specialists’ — that respond overwhelmingly only to stimuli of one taste quality143, 144, 149–153. However, these same recordings also reveal neurons — called ‘generalists’ — that respond to two or more different taste qualities. The relative proportions of such specialist and generalist neurons vary substantially depending on the species, the choice of stimuli presented and the concentration of the stimulus. Often, one taste stimulus is most efficacious for a given neuron (for example, “sucrose-best” (REF.143)), but even that property is not always fixed. Specifically, increasing the concentration of tastant-containing solutions (that is, increasing the stimulus strength) converts seemingly narrowly tuned neurons into broadly responsive ones, and neurons with one best stimulus at low concentration acquire a different ‘best stimulus’ at higher concentrations81. These observations are inconsistent with the fixed, labelled-line model of taste coding.
A third possibility is that taste qualities are encoded by different temporal patterns of activity in gustatory neurons. Although temporal coding has been implicated in brainstem and cortical gustatory centres154–157, there is no evidence that information in peripheral sensory neurons is encoded by spike timing. Indeed, an early study showed that stimulating human taste buds with a train of electrical pulses evoked a taste sensation that did not change when the stimulus rate was altered158. However, this study did not test different patterns of electrical stimulation.
Taste coding in central taste pathways is beyond the scope of this Review and has been comprehensively reviewed elsewhere159–161. It suffices to say, however, that the three models — labelled-line coding, combinatorial processing and temporal patterning — continue to be raised as explanations of how taste is encoded in higher brain centres, including the primary gustatory cortex.
Conclusions
Until recently, many researchers thought that questions underlying the cellular and molecular mechanisms of the basic taste qualities had been settled. This Review explains that although we indeed understand many of the receptors and transmitters that are involved in detecting sweet, sour, salty, bitter and umami tastes, major gaps in our knowledge remain. Issues awaiting resolution include the molecular identity of additional signalling pathways for detecting umami and sweet tastes; the cells involved in detecting salty tastes; whether ‘fatty’ is a basic taste; the role of cell–cell communication in taste buds; and, more broadly, how distinct taste qualities are encoded in sensory afferent fibres and beyond following their initial detection in the taste bud.
Acknowledgments
The authors acknowledge support from the US National Institutes of Health (grants R01DC000374 and R01DC007630 (to S.D.R.), R01DC006308 (to N.C.), and R21DC012746 and R01DC014420 (to S.D.R. and N.C.), and Ajinomoto Co., Inc.
Glossary
- Intracellular acidification
The increased concentration of cytosolic hydrogen ions, which corresponds to a decrease in cytoplasmic pH. Intracellular acidification can result from metabolic processes, the dissociation of organic (weak) acids, or the influx of protons through channels or transporters.
- Taste coding
The computational system by which trains of action potentials in sensory cells convey information about the quality, concentration and other features of a sensory stimulus.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. This study demonstrates that sweet-taste and bitter-taste receptors signal via a common pathway that includes PLCβ2 and TRPM5. Mice lacking the genes that encode these signalling proteins are shown to lose taste sensitivity for sweet, bitter and umami. [DOI] [PubMed] [Google Scholar]
- 2.Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J. Comp. Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 3.DeFazio RA, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J. Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. This study uses Ca2+ imaging and single-cell reverse transcription PCR to show that cells with taste GPCRs (T1Rs and T2Rs) and their downstream effectors are distinct from taste cells that express proteins for vesicular neurotransmitter release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye W, et al. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc. Natl Acad. Sci. USA. 2016;113:E229–E238. doi: 10.1073/pnas.1514282112. This patch-clamp study shows that cytoplasmic acidification excites sour-sensing taste bud cells by blocking the inwardly rectifying K+ channel KIR2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoon MA, et al. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 9.Max M, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat. Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 10.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat. Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 11.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J. Neurochem. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 12.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. This study shows that the heterologous expression of T1R2 and T1R3 confers sensitivity to sugars and synthetic sweeteners. [DOI] [PubMed] [Google Scholar]
- 13.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. This study shows that the heterologous expression of taste GPCRs T1R1 and T1R3 confers sensitivity to many amino acids, including glutamate. [DOI] [PubMed] [Google Scholar]
- 14.Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 15.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr. Biol. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, et al. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl Acad. Sci. USA. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui M, et al. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr. Pharm. Des. 2006;12:4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 18.Masuda K, et al. Characterization of the modes of binding between human sweet taste receptor and low molecular- weight sweet compounds. PLoS ONE. 2012;7:e35380. doi: 10.1371/journal.pone.0035380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assadi-Porter FM, et al. Key amino acid residues involved in multi-point binding interactions between brazzein, a sweet protein, and the T1R2–T1R3 human sweet receptor. J. Mol. Biol. 2010;398:584–599. doi: 10.1016/j.jmb.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang P, et al. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 2004;279:45068–45075. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- 21.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. This study involves the genetic ablation of the taste receptors T1R1, T1R2 or T1R3, and the results suggested that these receptors are necessary and sufficient for behavioural responses to sweet and umami tastes in mice (but see reference 22) [DOI] [PubMed] [Google Scholar]
- 22.Damak S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. This study shows that knockout of the gene that encodes T1R3 results in a selective loss of taste sensitivity to artificial sweeteners but does not abolish responses to sugars and umami (but see reference 21) [DOI] [PubMed] [Google Scholar]
- 23.Treesukosol Y, Smith KR, Spector AC. The functional role of the T1R family of receptors in sweet taste and feeding. Physiol. Behav. 2011;105:14–26. doi: 10.1016/j.physbeh.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc. Natl Acad. Sci. USA. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukumaran SK, et al. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc. Natl Acad. Sci. USA. 2016;113:6035–6040. doi: 10.1073/pnas.1520843113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumazawa T, Kurihara K. Large enhancement of canine taste responses to sugars by salts. J. Gen. Physiol. 1990;95:1007–1018. doi: 10.1085/jgp.95.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am. J. Physiol. 1984;246:R88–R95. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- 28.Tonosaki K, Hori Y, Shimizu Y, Tonosaki K. Relationships between insulin release and taste. Biomed. Res. 2007;28:79–83. doi: 10.2220/biomedres.28.79. [DOI] [PubMed] [Google Scholar]
- 29.Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol. Behav. 2011;103:44–50. doi: 10.1016/j.physbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokrashvili Z, et al. Endocrine taste cells. Br. J. Nutr. 2014;111(Suppl. 1):S23–S29. doi: 10.1017/S0007114513002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takai S, et al. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29:2268–2280. doi: 10.1096/fj.14-265355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glendinning JI, et al. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309:R552–R560. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glendinning JI, et al. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;312:R597–R610. doi: 10.1152/ajpregu.00433.2016. References 32 and 33 demonstrate the involvement of taste buds in stimulating insulin release immediately following sugar ingestion. The mechanism is independent of the sweet-taste receptors T1R2 and T1R3, and instead uses a transduction pathway similar to that used in pancreatic islet β cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapis TJ, Penner MH, Lim J. Humans can taste glucose oligomers independent of the hT1R2/hT1R3 sweet taste receptor. Chem. Senses. 2016;41:755–762. doi: 10.1093/chemse/bjw088. [DOI] [PubMed] [Google Scholar]
- 35.Sclafani A. Carbohydrate taste, appetite, and obesity: an overview. Neurosci. Biobehav. Rev. 1987;11:131–153. [PubMed] [Google Scholar]
- 36.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R218–R235. doi: 10.1152/ajpregu.00089.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi S. Basic properties of umami and effects on humans. Physiol. Behav. 1991;49:833–841. doi: 10.1016/0031-9384(91)90192-q. [DOI] [PubMed] [Google Scholar]
- 39.Li X, et al. Human receptors for sweet and umami taste. Proc. Natl Acad. Sci. USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem. Senses. 2006;31:351–357. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- 41.Kusuhara Y, et al. Taste responses in mice lacking taste receptor subunit T1R1. J. Physiol. 2013;591:1967–1985. doi: 10.1113/jphysiol.2012.236604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat. Neurosci. 2000;3:113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am. J. Clin. Nutr. 2009;90:738S–742S. doi: 10.3945/ajcn.2009.27462H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am. J. Clin. Nutr. 2009;90:743S–746S. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- 45.Yasumatsu K, et al. Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. J. Physiol. 2015;593:1021–1034. doi: 10.1113/jphysiol.2014.284703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behrens M, Meyerhof W. Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Semin. Cell Dev. Biol. 2013;24:215–221. doi: 10.1016/j.semcdb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Kuhn C, Bufe B, Batram C, Meyerhof W. Oligomerization of TAS2R bitter taste receptors. Chem. Senses. 2010;35:395–406. doi: 10.1093/chemse/bjq027. [DOI] [PubMed] [Google Scholar]
- 48.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 49.Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J. Neurosci. 2007;27:12630–12640. doi: 10.1523/JNEUROSCI.1168-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adler E, et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 51.Meyerhof W, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. This study comprehensively expresses human bitter-taste receptors in heterologous cells to de-orphan them and catalogue the compounds that active them. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn C, et al. Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sainz E, et al. Functional characterization of human bitter taste receptors. Biochem. J. 2007;403:537–543. doi: 10.1042/BJ20061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lossow K, et al. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J. Biol. Chem. 2016;291:15358–15377. doi: 10.1074/jbc.M116.718544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Risso D, Tofanelli S, Morini G, Luiselli D, Drayna D. Genetic variation in taste receptor pseudogenes provides evidence for a dynamic role in human evolution. BMC Evol. Biol. 2014;14:198. doi: 10.1186/s12862-014-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bufe B, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dotson CD, Shaw HL, Mitchell BD, Munger SD, Steinle NI. Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite. 2010;54:93–99. doi: 10.1016/j.appet.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang L, et al. Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat. Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 59.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 60.Tizzano M, et al. Expression of Gα14 in sweettransducing taste cells of the posterior tongue. BMC Neurosci. 2008;9:110. doi: 10.1186/1471-2202-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 62.Clapp TR, et al. Tonic activity of Gα-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–3787. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez CA, et al. A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J. Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl Acad. Sci. USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyall V, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am. J. Physiol. Cell Physiol. 2001;281:C1005–C1013. doi: 10.1152/ajpcell.2001.281.3.C1005. This study shows that cytosolic acidification is a prerequisite for the sour taste-induced stimulation of taste bud cells. [DOI] [PubMed] [Google Scholar]
- 67.Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J. Physiol. 2003;547:475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl Acad. Sci. USA. 2010;107:22320–22325. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. Proton currents through amiloridesensitive Na channels in hamster taste cells. Role in acid transduction. J. Gen. Physiol. 1992;100:803–824. doi: 10.1085/jgp.100.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens DR, et al. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- 71.Ugawa S, et al. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J. Neurosci. 2003;23:3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishimaru Y, et al. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl Acad. Sci. USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richter TA, Dvoryanchikov GA, Roper SD, Chaudhari N. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J. Neurosci. 2004;24:4088–4091. doi: 10.1523/JNEUROSCI.0653-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horio N, et al. Sour taste responses in mice lacking PKD channels. PLoS ONE. 2011;6:e20007. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J. Neurophysiol. 2004;92:1928–1936. doi: 10.1152/jn.00273.2004. [DOI] [PubMed] [Google Scholar]
- 76.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 77.Halpern BP. Amiloride and vertebrate gustatory responses to NaCl. Neurosci. Biobehav. Rev. 1998;23:5–47. doi: 10.1016/s0149-7634(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 78.Ossebaard CA, Smith DV. Amiloride suppresses the sourness of NaCl and LiCl. Physiol. Behav. 1996;60:1317–1322. doi: 10.1016/s0031-9384(96)00258-2. [DOI] [PubMed] [Google Scholar]
- 79.Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494:472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kretz O, Barbry P, Bock R. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J. Histochem. Cytochem. 1999;47:51–64. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- 81.Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Breadth of tuning in taste afferent neurons varies with stimulus strength. Nat. Commun. 2015;6:8171. doi: 10.1038/ncomms9171. This study uses Ca2+ imaging in anaesthetized mice to show that gustatory afferent neurons respond to single or multiple taste quality stimuli depending on their concentration; this finding provides support for combinatorial taste coding (but see reference 141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bigiani A, Cuoghi V. Localization of amiloridesensitive sodium current and voltage-gated calcium currents in rat fungiform taste cells. J. Neurophysiol. 2007;98:2483–2487. doi: 10.1152/jn.00716.2007. [DOI] [PubMed] [Google Scholar]
- 84.Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA. Amilorideinsensitive salt taste is mediated by two populations of type III taste cells with distinct transduction mechanisms. J. Neurosci. 2016;36:1942–1953. doi: 10.1523/JNEUROSCI.2947-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stratford JM, Curtis KS, Contreras RJ. Chorda tympani nerve transection alters linoleic acid taste discrimination by male and female rats. Physiol. Behav. 2006;89:311–319. doi: 10.1016/j.physbeh.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 86.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R447–R454. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 87.Voigt N, et al. The role of lipolysis in human orosensory fat perception. J. Lipid Res. 2014;55:870–882. doi: 10.1194/jlr.M046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kulkarni BV, Mattes RD. Lingual lipase activity in the orosensory detection of fat by humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R879–R885. doi: 10.1152/ajpregu.00352.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galindo MM, et al. G protein-coupled receptors in human fat taste perception. Chem. Senses. 2012;37:123–139. doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 90.Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am. J. Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 91.Fukuwatari T, et al. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 92.Laugerette F, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsumura S, et al. Colocalization of GPR120 with phospholipase-Cβ2 and α-gustducin in the taste bud cells in mice. Neurosci. Lett. 2009;450:186–190. doi: 10.1016/j.neulet.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 94.Cartoni C, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avau B, Depoortere I. The bitter truth about bitter taste receptors: beyond sensing bitter in the oral cavity. Acta Physiol. (Oxf.) 2016;216:407–420. doi: 10.1111/apha.12621. [DOI] [PubMed] [Google Scholar]
- 96.Gaillard D, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 97.Adachi S, et al. Behavioral palatability of dietary fatty acids correlates with the intracellular calcium ion levels induced by the fatty acids in GPR120-expressing cells. Biomed. Res. 2014;35:357–367. doi: 10.2220/biomedres.35.357. [DOI] [PubMed] [Google Scholar]
- 98.Calvo SS, Egan JM. The endocrinology of taste receptors. Nat. Rev. Endocrinol. 2015;11:213–227. doi: 10.1038/nrendo.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bo X, et al. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–1111. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- 100.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. The authors of this study identify ATP as a taste neurotransmitter that acts on P2X2 and P2X3 receptors expressed by sensory afferent fibres that innervate taste buds. [DOI] [PubMed] [Google Scholar]
- 101.Huang YA, et al. Knocking out P2X receptors reduces transmitter secretion in taste buds. J. Neurosci. 2011;31:13654–13661. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang YJ, et al. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc. Natl Acad. Sci. USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romanov RA, et al. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J. Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taruno A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. In this paper, patch clamp, molecular biological and genetic knockout studies demonstrate that ATP is released from taste bud cells during gustatory stimulation through CALHM1 channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 107.Ma Z, Tanis JE, Taruno A, Foskett JK. Calcium homeostasis modulator (CALHM) ion channels. Pflugers Arch. 2016;468:395–403. doi: 10.1007/s00424-015-1757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tordoff MG, et al. Normal taste acceptance and preference of PANX1 knockout mice. Chem. Senses. 2015;40:453–459. doi: 10.1093/chemse/bjv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vandenbeuch A, Anderson CB, Kinnamon SC. Mice lacking pannexin 1 release ATP and respond normally to all taste qualities. Chem. Senses. 2015;40:461–467. doi: 10.1093/chemse/bjv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tordoff MG, et al. Salty taste deficits in CALHM1 knockout mice. Chem. Senses. 2014;39:515–528. doi: 10.1093/chemse/bju020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang R, Montoya A, Bond A, Walton J, Kinnamon JC. Immunocytochemical analysis of P2X2 in rat circumvallate taste buds. BMC Neurosci. 2012;13:51. doi: 10.1186/1471-2202-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J. Comp. Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vandenbeuch A, et al. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc. Natl Acad. Sci. USA. 2013;110:14789–14794. doi: 10.1073/pnas.1309468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Adenosine enhances sweet taste through A2B receptors in the taste bud. J. Neurosci. 2012;32:322–330. doi: 10.1523/JNEUROSCI.4070-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang YJ, et al. Mouse taste buds use serotonin as a neurotransmitter. J. Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nada O, Hirata K. The monoamine-containing cell in the gustatory epithelium of some vertebrates. Arch. Histol. Jpn. 1977;40:197–206. doi: 10.1679/aohc1950.40.supplement_197. [DOI] [PubMed] [Google Scholar]
- 117.Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J. Comp. Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- 118.Kim DJ, Roper SD. Localization of serotonin in taste buds: a comparative study in four vertebrates. J. Comp. Neurol. 1995;353:364–370. doi: 10.1002/cne.903530304. [DOI] [PubMed] [Google Scholar]
- 119.Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS ONE. 2011;6:e25471. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. J. Neurosci. 2010;30:14695–14701. doi: 10.1523/JNEUROSCI.1570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jaber L, Zhao FL, Kolli T, Herness S. A physiologic role for serotonergic transmission in adult rat taste buds. PLoS ONE. 2014;9:e112152. doi: 10.1371/journal.pone.0112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fujita T, Kanno T, Kobayashi S. The Paraneuron. Springer Science & Business Media; 2012. [Google Scholar]
- 123.Larson ED, et al. The role of 5-HT3 receptors in signaling from taste buds to nerves. J. Neurosci. 2015;35:15984–15995. doi: 10.1523/JNEUROSCI.1868-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Obata H, Shimada K, Sakai N, Saito N. GABAergic neurotransmission in rat taste buds: immunocytochemical study for GABA and GABA transporter subtypes. Brain Res. Mol. Brain Res. 1997;49:29–36. doi: 10.1016/s0169-328x(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 125.Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J. Neurosci. 2011;31:5782–5791. doi: 10.1523/JNEUROSCI.5559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dvoryanchikov G, Pereira E, Williams C, Roper SD, Chaudhari N. GABA as afferent taste neurotransmitter. Chem. Senses. 2013;38:643. [Google Scholar]
- 127.Koga T, Bradley RM. Biophysical properties and responses to neurotransmitters of petrosal and geniculate ganglion neurons innervating the tongue. J. Neurophysiol. 2000;84:1404–1413. doi: 10.1152/jn.2000.84.3.1404. [DOI] [PubMed] [Google Scholar]
- 128.Paran N, Mattern CF. The distribution of acetylcholinesterase in buds of the rat vallate papilla as determined by electron microscope histochemistry. J. Comp. Neurol. 1975;159:29–44. doi: 10.1002/cne.901590104. [DOI] [PubMed] [Google Scholar]
- 129.Dando R, Roper SD. Acetylcholine is released from taste cells, enhancing taste signalling. J. Physiol. 2012;590:3009–3017. doi: 10.1113/jphysiol.2012.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J. Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J. Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. This study uses Ca2+ imaging in lingual slice preparations to show that GPCR-expressing type II taste bud cells are tuned to single taste qualities, whereas type III cells respond to and integrate signals from multiple cells in the taste bud. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshida R, et al. Discrimination of taste qualities among mouse fungiform taste bud cells. J. Physiol. 2009;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chaudhari N. Synaptic communication and signal processing among sensory cells in taste buds. J. Physiol. 2014;592:3387–3392. doi: 10.1113/jphysiol.2013.269837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murayama N. Interaction among different sensory units within a single fungiform papilla in the frog tongue. J. Gen. Physiol. 1988;91:685–701. doi: 10.1085/jgp.91.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Riddle DR, Hughes SE, Belczynski CR, DeSibour CL, Oakley B. Inhibitory interactions among rodent taste axons. Brain Res. 1990;533:113–124. doi: 10.1016/0006-8993(90)91803-o. [DOI] [PubMed] [Google Scholar]
- 136.Vandenbeuch A, Pillias AM, Faurion A. Modulation of taste peripheral signal through interpapillar inhibition in hamsters. Neurosci. Lett. 2004;358:137–141. doi: 10.1016/j.neulet.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 137.Huang YA, Grant J, Roper S. Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS ONE. 2012;7:e30662. doi: 10.1371/journal.pone.0030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vandenbeuch A, et al. Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neurosci. 2010;11:77. doi: 10.1186/1471-2202-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zaidi FN, Whitehead MC. Discrete innervation of murine taste buds by peripheral taste neurons. J. Neurosci. 2006;26:8243–8253. doi: 10.1523/JNEUROSCI.5142-05.2006. This study uses retrograde labelling to show that mouse taste buds are innervated by only three, four or five sensory afferent neurons. Conversely, the authors estimate that a sensory afferent neuron in mice innervates only a single taste bud. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roper SD, Chaudhari N. In: Handbook of Brain Microcircuits. 2. Shepherd G, Grillner S, editors. Ch. 26. Oxford Univ. Press; 2017. pp. 277–283. [Google Scholar]
- 141.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barretto RP, et al. The neural representation of taste quality at the periphery. Nature. 2014;517:373–376. doi: 10.1038/nature13873. This study uses Ca2+ imaging in anaesthetized mice to show that taste-detecting afferent neurons respond predominantly to single taste quality stimuli, which supports the idea of labelled-line taste coding (but see reference 80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frank M. An analysis of hamster afferent taste nerve response functions. J. Gen. Physiol. 1973;61:588–618. doi: 10.1085/jgp.61.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees II. single chorda tympani fibers. Physiol. Behav. 1997;61:829–841. doi: 10.1016/s0031-9384(96)00562-8. [DOI] [PubMed] [Google Scholar]
- 145.Mueller KL, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 146.Erickson RP. Olfaction and Taste. In: Zotterman Y, editor. Proceedings of the First International Symposium. Pergamon Press; 1963. pp. 205–213. [Google Scholar]
- 147.Erickson RP. A study of the science of taste: on the origins and influence of the core ideas. Behav. Brain Sci. 2008;31:59–75. doi: 10.1017/S0140525X08003348. [DOI] [PubMed] [Google Scholar]
- 148.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 149.Pfaffmann C. Gustatory afferent impulses. J. Cell. Comp. Physiol. 1941;17:243–258. [Google Scholar]
- 150.Lundy RF, Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J. Neurophysiol. 1999;82:2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- 151.Sollars SI, Hill DL. In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones. J. Physiol. 2005;564:877–893. doi: 10.1113/jphysiol.2005.083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J. Neurophysiol. 2006;95:674–685. doi: 10.1152/jn.00793.2005. [DOI] [PubMed] [Google Scholar]
- 153.Yoshida R, et al. Taste responsiveness of fungiform taste cells with action potentials. J. Neurophysiol. 2006;96:3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- 154.Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J. Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J. Neurophysiol. 2003;90:1418–1431. doi: 10.1152/jn.00177.2003. [DOI] [PubMed] [Google Scholar]
- 156.Geran L, Travers S. Temporal characteristics of gustatory responses in rat parabrachial neurons vary by stimulus and chemosensitive neuron type. PLoS ONE. 2013;8:e76828. doi: 10.1371/journal.pone.0076828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilson DM, Boughter JD, Jr, Lemon CH. Bitter taste stimuli induce differential neural codes in mouse brain. PLoS ONE. 2012;7:e41597. doi: 10.1371/journal.pone.0041597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Von Bekesy G. Sweetness produced electrically on the tongue and its relation to taste theories. J. Appl. Physiol. 1964;19:1105–1113. doi: 10.1152/jappl.1964.19.6.1105. [DOI] [PubMed] [Google Scholar]
- 159.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav. Cogn. Neuroci. Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 160.Carleton A, Accolla R, Simon SA. Coding in the mammalian gustatory system. Trends Neurosci. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat. Rev. Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 162.Murray RG. Cellular relations in mouse circumvallate taste buds. Microsc. Res. Tech. 1993;26:209–224. doi: 10.1002/jemt.1070260304. This is an early compilation of high-resolution electron micrographs that support current interpretations of the structure and function of the different types of taste cell that are described in this Review. [DOI] [PubMed] [Google Scholar]
- 163.Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J. Comp. Neurol. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 164.Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate–aspartate transporter, GLAST, in rat taste buds. Eur. J. Neurosci. 2000;12:3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 165.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J. Comp. Neurol. 2009;517:1–14. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J. Comp. Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 167.Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J. Comp. Neurol. 2004;471:59–71. doi: 10.1002/cne.20021. [DOI] [PubMed] [Google Scholar]
- 168.Holland VF, Zampighi GA, Simon SA. Morphology of fungiform papillae in canine lingual epithelium: location of intercellular junctions in the epithelium. J. Comp. Neurol. 1989;279:13–27. doi: 10.1002/cne.902790103. [DOI] [PubMed] [Google Scholar]
- 169.Witt M, Kasper M. Immunohistochemical distribution of CD44 and some of its isoforms during human taste bud development. Histochem. Cell Biol. 1998;110:95–103. doi: 10.1007/s004180050270. [DOI] [PubMed] [Google Scholar]
- 170.Michlig S, Damak S, Le Coutre J. Claudin-based permeability barriers in taste buds. J. Comp. Neurol. 2007;502:1003–1011. doi: 10.1002/cne.21354. [DOI] [PubMed] [Google Scholar]
- 171.Dando R, et al. A permeability barrier surrounds taste buds in lingual epithelia. Am. J. Physiol. Cell Physiol. 2015;308:C21–C32. doi: 10.1152/ajpcell.00157.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 173.Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J. Neurophysiol. 2009;101:1598–1612. doi: 10.1152/jn.91168.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oliveira-Maia AJ, et al. Nicotine activates TRPM5-dependent and independent taste pathways. Proc. Natl Acad. Sci. USA. 2009;106:1596–1601. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Delwiche JF, Buletic Z, Breslin PA. Covariation in individuals’ sensitivities to bitter compounds: evidence supporting multiple receptor/transduction mechanisms. Percept. Psychophys. 2001;63:761–776. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- 177.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J. Neurosci. 2002;22:1937–1941. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Blakeslee AF. Genetics of sensory thresholds: taste for phenyl thio carbamide. Proc. Natl Acad. Sci. USA. 1932;18:120–130. doi: 10.1073/pnas.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim UK, et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. This genetic study identifies the human locus responsible for the inheritance of the taster and non-taster phenotypes for the bitter compound PTC. [DOI] [PubMed] [Google Scholar]
- 180.Wooding S, et al. Natural selection and molecular evolution in PTC, a bitter-taste receptor gene. Am. J. Hum. Genet. 2004;74:637–646. doi: 10.1086/383092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Soranzo N, et al. Positive selection on a high sensitivity allele of the human bitter-taste receptor TAS2R16. Curr. Biol. 2005;15:1257–1265. doi: 10.1016/j.cub.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 182.Campbell MC, et al. Limited evidence for adaptive evolution and functional effect of allelic variation at rs702424 in the promoter of the TAS2R16 bitter taste receptor gene in Africa. J. Hum. Genet. 2014;59:349–352. doi: 10.1038/jhg.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Campa D, et al. A gene-wide investigation on polymorphisms in the taste receptor 2R14 (TAS2R14) and susceptibility to colorectal cancer. BMC Med. Genet. 2010;11:88. doi: 10.1186/1471-2350-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nolden AA, McGeary JE, Hayes JE. Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiol Behav. 2016;156:117–127. doi: 10.1016/j.physbeh.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schembre SM, Cheng I, Wilkens LR, Albright CL, Marchand le L. Variations in bittertaste receptor genes, dietary intake, and colorectal adenoma risk. Nutr. Cancer. 2013;65:982–990. doi: 10.1080/01635581.2013.807934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Carrai M, et al. Association between TAS2R38 gene polymorphisms and colorectal cancer risk: a casecontrol study in two independent populations of Caucasian origin. PLoS ONE. 2011;6:e20464. doi: 10.1371/journal.pone.0020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim UK, Wooding S, Riaz N, Jorde LB, Drayna D. Variation in the human TAS1R taste receptor genes. Chem. Senses. 2006;31:599–611. doi: 10.1093/chemse/bjj065. [DOI] [PubMed] [Google Scholar]
- 188.Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr. Biol. 2009;19:1288–1293. doi: 10.1016/j.cub.2009.06.015. The human psychophysics and signal detection analyses carried out in this study indicate that a TAS1R3 allele is associated with an increased sensitivity to sucrose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eny KM, Wolever TM, Corey PN, El-Sohemy A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am. J. Clin. Nutr. 2010;92:1501–1510. doi: 10.3945/ajcn.2010.29836. [DOI] [PubMed] [Google Scholar]
- 190.Ramos-Lopez O, Panduro A, Martinez-Lopez E, Roman S. Sweet taste receptor TAS1R2 polymorphism (Val191Val) is associated with a higher carbohydrate intake and hypertriglyceridemia among the population of West Mexico. Nutrients. 2016;8:101. doi: 10.3390/nu8020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Haznedaroglu E, et al. Association of sweet taste receptor gene polymorphisms with dental caries experience in school children. Caries Res. 2015;49:275–281. doi: 10.1159/000381426. [DOI] [PubMed] [Google Scholar]
- 192.Chen QY, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am. J. Clin. Nutr. 2009;90:770S–779S. doi: 10.3945/ajcn.2009.27462N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Finger TE, Kinnamon SC. Taste isn’t just for taste buds anymore. F1000 Biol. Rep. 2011;3:20. doi: 10.3410/B3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol. Metab. 2013;24:92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 195.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lee RJ, Chen B, Redding KM, Margolskee RF, Cohen NA. Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorumsensing molecules require taste signaling components. Innate Immun. 2014;20:606–617. doi: 10.1177/1753425913503386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee RJ, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee RJ, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. This study shows that bitter-taste receptors expressed in upper airway cells sense bacteria that cause sinusitis and trigger an innate immunity. It also shows that sweet-taste receptors expressed in the same cells regulate this pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Finger TE, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc. Natl Acad. Sci. USA. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Saunders CJ, Christensen M, Finger TE, Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc. Natl Acad. Sci. USA. 2014;111:6075–6080. doi: 10.1073/pnas.1402251111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tizzano M, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl Acad. Sci. USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wu SV, et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl Acad. Sci. USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol. 2007;7:557–562. doi: 10.1016/j.coph.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Prandi S, et al. A subset of mouse colonic goblet cells expresses the bitter taste receptor Tas2r131. PLoS ONE. 2013;8:e82820. doi: 10.1371/journal.pone.0082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 206.Schutz B, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front. Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deshpande DA, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010;16:1299–1234. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun. 2011;406:146–151. doi: 10.1016/j.bbrc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 210.Tomas J, Santos CR, Quintela T, Goncalves I. “Tasting” the cerebrospinal fluid: another function of the choroid plexus? Neuroscience. 2016;320:160–171. doi: 10.1016/j.neuroscience.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 211.Foster SR, et al. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS ONE. 2013;8:e64579. doi: 10.1371/journal.pone.0064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem. Soc. Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 213.Wolfle U, et al. Expression and functional activity of the bitter taste receptors TAS2R1 and TAS2R38 in human keratinocytes. Skin Pharmacol. Physiol. 2015;28:137–146. doi: 10.1159/000367631. [DOI] [PubMed] [Google Scholar]
- 214.Kiuchi S, et al. Genomic structure of swine taste receptor family 1 member 3, TAS1R3, and its expression in tissues. Cytogenet. Genome Res. 2006;115:51–61. doi: 10.1159/000094801. [DOI] [PubMed] [Google Scholar]
- 215.Taniguchi K. Expression of the sweet receptor protein, T1R3, in the human liver and pancreas. J. Vet. Med. Sci. 2004;66:1311–1314. doi: 10.1292/jvms.66.1311. [DOI] [PubMed] [Google Scholar]
- 216.Lee N, et al. Mouse neutrophils express functional umami taste receptor T1R1/T1R3. BMB Rep. 2014;47:649–654. doi: 10.5483/BMBRep.2014.47.11.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maurer S, et al. Tasting Pseudomonas aeruginosa biofilms: human neutrophils express the bitter receptor T2R38 as sensor for the quorum sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Front. Immunol. 2015;6:369. doi: 10.3389/fimmu.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Reimann F, et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mosinger B, et al. Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc. Natl Acad. Sci. USA. 2013;110:12319–12324. doi: 10.1073/pnas.1302827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xu J, Cao J, Iguchi N, Riethmacher D, Huang L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2013;19:17–28. doi: 10.1093/molehr/gas040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Clark AA, et al. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015;29:164–172. doi: 10.1096/fj.14-262246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caterina MJ, et al. The capsaicin receptor: a heatactivated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 223.Everaerts W, et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr. Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 224.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl Acad. Sci. USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Roper SD. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 2014;223:827–871. doi: 10.1007/978-3-319-05161-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu L, Simon SA. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol. Behav. 2000;69:363–378. doi: 10.1016/s0031-9384(00)00209-2. [DOI] [PubMed] [Google Scholar]
- 227.Katsura H, Tsuzuki K, Noguchi K, Sakagami M. Differential expression of capsaicin-, menthol-, and mustard oil-sensitive receptors in naive rat geniculate ganglion neurons. Chem. Senses. 2006;31:681–688. doi: 10.1093/chemse/bjl009. [DOI] [PubMed] [Google Scholar]
- 228.Nakamura S, Bradley RM. Characteristics of sodium currents in rat geniculate ganglion neurons. J. Neurophysiol. 2011;106:2982–2991. doi: 10.1152/jn.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Finger TE. Peptide immunohistochemistry demonstrates multiple classes of perigemmal nerve fibers in the circumvallate papilla of the rat. Chem. Senses. 1986;11:135–144. [Google Scholar]
- 230.Wang Y, Erickson RP, Simon SA. Modulation of rat chorda tympani nerve activity by lingual nerve stimulation. J. Neurophysiol. 1995;73:1468–1483. doi: 10.1152/jn.1995.73.4.1468. [DOI] [PubMed] [Google Scholar]
- 231.Simon SA, Liu L, Erickson RP. Neuropeptides modulate rat chorda tympani responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1494–R1505. doi: 10.1152/ajpregu.00544.2002. [DOI] [PubMed] [Google Scholar]
- 232.Grant J. Tachykinins stimulate a subset of mouse taste cells. PLoS ONE. 2012;7:e31697. doi: 10.1371/journal.pone.0031697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huang AY, Wu SY. Calcitonin gene-related peptide reduces taste-evoked ATP secretion from mouse taste buds. J. Neurosci. 2015;35:12714–12724. doi: 10.1523/JNEUROSCI.0100-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 235.Baumbauer KM, et al. Keratinocytes can modulate and directly initiate nociceptive responses. eLife. 2015;4:e09674. doi: 10.7554/eLife.09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ueda Y, Sakaguchi M, Hirayama K, Miyajima R, Kimizuka A. Characteristic flavor constituents in water extract of garlic. Agric. Biol. Chem. 1990;54:163–169. [Google Scholar]
- 237.Dunkel A, Köster J, Hofmann T. Molecular and sensory characterization of γ-glutamyl peptides as key contributors to the kokumi taste of edible beans (Phaseolus vulgaris L.) J. Agric. Food Chem. 2007;55:6712–6719. doi: 10.1021/jf071276u. [DOI] [PubMed] [Google Scholar]
- 238.Ohsu T, et al. Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 2010;285:1016–1022. doi: 10.1074/jbc.M109.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maruyama Y, Yasuda R, Kuroda M, Eto Y. Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells. PLoS ONE. 2012;7:e34489. doi: 10.1371/journal.pone.0034489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Medina J, et al. Positive allosteric modulation of the calcium-sensing receptor by physiological concentrations of glucose. J. Biol. Chem. 2016;291:23126–23135. doi: 10.1074/jbc.M116.729863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tordoff MG. Some basic psychophysics of calcium salt solutions. Chem. Senses. 1996;21:417–424. doi: 10.1093/chemse/21.4.417. [DOI] [PubMed] [Google Scholar]
- 242.McCaughey SA, Forestell CA, Tordoff MG. Calcium deprivation increases the palatability of calcium solutions in rats. Physiol. Behav. 2005;84:335–342. doi: 10.1016/j.physbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 243.Tordoff MG, et al. Involvement of T1R3 in calciummagnesium taste. Physiol. Genom. 2008;34:338–348. doi: 10.1152/physiolgenomics.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tordoff MG, Alarcon LK, Valmeki S, Jiang P. T1R3: a human calcium taste receptor. Sci. Rep. 2012;2:496. doi: 10.1038/srep00496. [DOI] [PMC free article] [PubMed] [Google Scholar]




