In a recent publication in Science Immunology, Carmona-Rivera et al. (1) report that HLA-DRB1*04:01 transgenic mice immunized with fibroblast-like synoviocytes (FLSs) loaded with neutrophil extracellular traps (NETs) developed antibodies specific to citrullinated rheumatoid arthritis (RA) autoantigens. This work adds support for the role of citrullination in NETs as a critical component of RA pathogenesis. In the context of these findings, this manuscript offers an opportunity to revisit the nuances of defining citrullination during NETosis and to reexamine the definition of antibodies specific for citrullinated RA autoantigens.
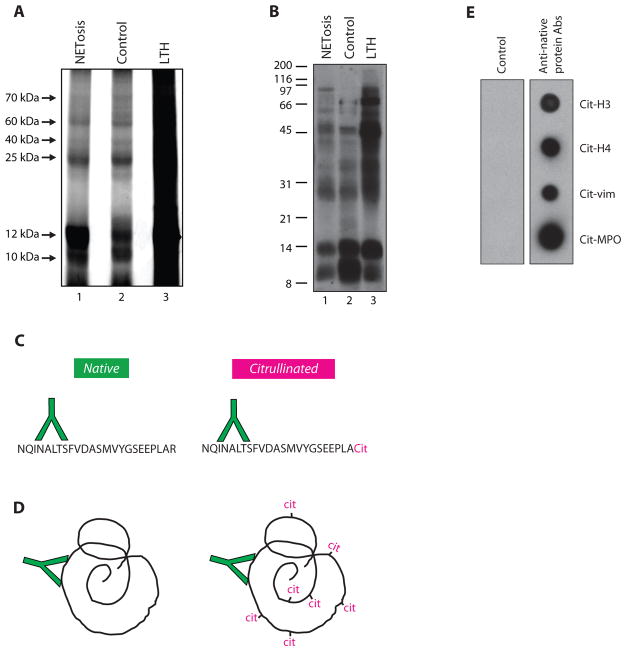
The major foundation for this work is supported by the idea that citrullination in NETs provides the antigens that initiate an anti-citrullinated protein antibody (ACPA) response (1, 2). To strengthen this critical component, Carmona-Rivera et al. showed in Fig. 1A of the manuscript that citrullinated proteins are generated during NETosis. To induce NETs, the authors used soluble immunoglobulin M (IgM) purified from non-RA patients with monoclonal IgM cryoglobulinemia with rheumatoid factor reactivity. Using a new method to detect citrullination (i.e., rhodamine-phenylglyoxal), Carmona-Rivera et al. showed patterns of citrullination that have not been described in NETs elsewhere (3, 4). Since we and others have questioned the extent of citrullination in NETs (except for some citrullination of histones) (4, 5), we addressed whether the findings by Carmona-Rivera et al. may result from the use of rhodamine-phenylglyoxal to detect citrullinated proteins in NETs. Using a canonical inducer of NETosis (phorbol 12-myristate 13-acetate, PMA) and rhodamine-phenylglyoxal, we replicated the patterns of citrullination depicted by Carmona-Rivera (Fig. 1A, lane 1). However, the inclusion of controls demonstrated that, except for one protein of ~12 kDa more prominent in NETs (likely a histone), citrullinated proteins were similarly detected in control (unstimulated) neutrophils (Fig. 1A, lane 2). The limited extent of citrullination found in NETs was highlighted when compared to mechanisms that induce robust hypercitrullination, such as ionomycin from Streptomyces conglobatus, which saturated the detection assay in the time required to detect citrullination in NETs and control cells (Fig. 1A, lane 3). These findings are consistent with patterns of hypercitrullination induced by bacterial and host pore-forming proteins (3, 4, 6), which result from a process termed leukotoxic hypercitrullination (LTH) (4). Since Carmona-Rivera et al. did not include controls in manuscript figure 1A, it is impossible to define the significance and magnitude of their results and whether citrullination detected in cryoglobulin-induced NETosis may only correspond to background found in control unstimulated neutrophils.
Fig. 1.
NETosis is not a source of cellular hypercitrullination. (A and B) Healthy control peripheral blood neutrophils were incubated alone (lane 2, unstimulated control) or co-incubated with 100 nM PMA to induce NETosis (lane 1), or with 1 μM ionomycin to induce leukotoxic hypercitrullination (LTH) (lane 3). Reactions were stopped after 4 hrs at 37°C. Total protein citrullination was detected using rhodamine-phenylglyoxal (A). RA autoantigens were detected by immunoblotting using high titer ACPA+ RA serum (B). Samples were electrophoresed on 15% (A) or 12.5% (B) SDS-PAGE. The data shown in B is representative of sera from 5 individuals with RA (C and D) Schematic representation of a small peptide (C) and a protein (D) in their native and citrullinated forms. By targeting any sequence not affected by citrullination, antibodies to native proteins can similarly recognize both native and citrullinated peptides and proteins. The peptide sequence in C is from MPO and was obtained from figure 1F in Carmona-Rivera et al. (1). (E) Purified histone H3 (H3), histone H4 (H4) (New England Biolabs), vimentin (vim) (Peprotech) and MPO (Millipore) were citrullinated in vitro with purified recombinant human PAD4. The citrullinated (cit) proteins were detected by dot blot using irrelevant mouse IgG (Sigma Cat# M5409) (control) or commercial antibodies (Abs) to native histone H3, histone H4, vimentin (Cell Signaling, clone # 96C10, L64C1 and 5G3F10, respectively) and MPO (R&D Systems, clone # 392105).
The importance of including informative controls is similarly underscored in additional figures in the manuscript by Carmona-Rivera et al. In manuscript figure 1B, Carmona-Rivera et al. demonstrated that ACPAs target several proteins in NETs. While the detection of antigens in NETs by RA autoantibodies is reproducible using PMA (Fig. 1B, lane 1), the inclusion of unstimulated neutrophils demonstrates that the same bands are also found (some even more prominently) in control cells (Fig. 1B, lane 2). Importantly, this is far less than the prominent detection of autoantigens in hypercitrullinated neutrophils (Fig. 1B, lane 3). Nevertheless, the absence of controls in manuscript figure 1B makes it impossible to confirm whether citrullinated autoantigens are indeed generated during NETosis. Since dying cells redistribute their intracellular proteins, it is not surprising that citrullinated proteins found in control neutrophils may be redistributed during NETosis (or any other form of cell death) and detected with ACPAs by immunofluorescence, as observed in Fig. 1C of the manuscript. ACPA binding is also observed in cells that are not NETting (Carmona-Rivera et al., fig. S1), supporting the idea that NETosis is not a generator of hypercitrullination but rather a redistributor of an existent steady-state citrullinome in neutrophils.
The identification of citrullinated proteins in unstimulated neutrophils is a finding that we have confirmed by mass spectrometry (MS) [see table S2 in reference (3)]. Stimuli with the potential to enhance citrullination therefore require inclusion of proper controls to determine if citrullination is generated (both qualitatively and quantitatively) above the background of non-stimulated cells. Otherwise, hundreds of stimuli can be proposed to induce citrullination (which can be confirmed by rhodamine-phenylglyoxal and MS) simply by detecting the background citrullinome that is found in neutrophils. Indeed, the absence of controls in Fig. 1E and table S1 of the manuscript questions whether the limited number of citrullinated proteins detected in NETs [which are not representative of the entire known RA citrullinome (6, 7)] were generated during NETosis or merely represent redistribution of the steady state citrullinome found in neutrophils.
Last, although it is certain that antibodies targeting the same antigen both as native and citrullinated can be found in RA (8, 9), this finding has been overexploited to justify caveats in the study of ACPAs in experimental models of arthritis and in NETs. Since antibodies to native sequences can bind any region of the antigen that is not affected by citrullination, as illustrated in Fig. 1C and D, these antibodies can target both native and citrullinated small peptides and proteins (Fig. 1C and D, respectively). However, that does not mean that they should be defined as ACPAs. To prove this simple idea, the experiment showed in manuscript figure 6C (used to demonstrate ACPAs in mice immunized with FLS loaded with NETs) was reproduced using anti-native protein antibodies from commercial sources (Fig. 1E). As expected, antibodies to native proteins, but not irrelevant mouse IgG, strongly reacted with the citrullinated proteins. Since ACPAs are not necessary in mice to develop arthritis (10), the production of antibodies to native proteins can explain the induction of arthritis by FLS loaded with NETs. Indeed, the epitope chip analysis showed in manuscript figures 6H and S13 demonstrates that the antibody response in these mice is predominant to native sequences. Nevertheless, in the absence of controls, these antibodies can be erroneously defined as specific to citrullinated antigens. In this context, antibodies to native and citrullinated myeloperoxidase and neutrophil elastase in manuscript Fig. 1G and 1I were determined by comparing titers in RA vs. osteoarthritis. Although this analysis can define whether these antibodies are significantly associated with RA, it provides no evidence that the antibodies are specific to citrullinated sequences. Defining an antibody as an ACPA requires demonstrating its preferential binding to the citrullinated vs. native version of an antigen.
In summary, although differences in experimental conditions may be used to justify discrepancies regarding the role of NETosis in generating citrullinated autoantigens, the purpose of this letter is to underscore the importance of including strict controls to study citrullination in NETs. The data from Carmona-Rivera et al., in combination with our work, suggests that NETosis is not a generator of citrullinated autoantigens, except for some citrullination of histones that is not exclusive to NETosis (3). Therefore, the role of NETosis as a trigger of the ACPA response in mice and in RA should be taken with caution.
Acknowledgments
Funding: The study was supported by The Jerome L. Greene Foundation and National Institute of Arthritis and Musculoskeletal and Skin (NIAMS)/National Institutes of Health (NIH) grant R01 AR069569. The content is solely the responsibility of the author and does not necessarily represent the official views of NIAMS or the NIH.
Footnotes
Competing interests: F.A. is an inventor on issued patent no. 8,975,033 held by The Johns Hopkins University that covers “Human autoantibodies specific for PAD3 which are cross-reactive with PAD4 and their use in the diagnosis and treatment of rheumatoid arthritis and related diseases.” F.A. received a grant from MedImmune and has served as consultant for Bristol-Myers Squibb Company and Pfizer.
References and notes
- 1.Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, Liu Y, Bicker KL, Wahamaa H, Hoffmann V, Catrina AI, Thompson P, Buckner JH, Robinson WH, Fox DA, Kaplan MJ. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2:eaag3358. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, van EJ, Rosen A, Andrade F. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci Transl Med. 2013;5:209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konig MF, Andrade F. A critical reappraisal of neutrophil extracellular traps (NETs) and NETosis mimics based on differential requirements for protein citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth HV, Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6 doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, Rosen A, Nigrovic PA, Sokolove J, Giles JT, Moutsopoulos NM, Andrade F. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tutturen AE, Fleckenstein B, de Souza GA. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J Proteome Res. 2014;13:2867–2873. doi: 10.1021/pr500030x. [DOI] [PubMed] [Google Scholar]
- 8.Brink M, Hansson M, Ronnelid J, Klareskog L, Rantapaa DS. The autoantibody repertoire in periodontitis: a role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Antibodies against uncitrullinated peptides seem to occur prior to the antibodies to the corresponding citrullinated peptides. Ann Rheum Dis. 2014;73:e46. doi: 10.1136/annrheumdis-2014-205498. [DOI] [PubMed] [Google Scholar]
- 9.Konig MF, Giles JT, Nigrovic PA, Andrade F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann Rheum Dis. 2016;75:2022–2028. doi: 10.1136/annrheumdis-2015-208529. [DOI] [PubMed] [Google Scholar]
- 10.Konig MF, Darrah E, Andrade F. Insights into the significance of peptidylarginine deiminase 4 and antibodies against citrullinated antigens in the absence of “true ACPAs” in an experimental model of arthritis: comment on the article by Shelef et al. Arthritis Rheumatol. 2014;66:2642–2644. doi: 10.1002/art.38719. [DOI] [PubMed] [Google Scholar]