Abstract
A core function of epithelia is to form barriers that separate tissue compartments within complex organisms. These barriers also separate the internal milieu from the external environment and are, therefore, an essential component of host defense. However, in many cases, a perfect barrier would be improbable with life itself. Examples include the air spaces within the lungs, the renal tubules, and the intestines. Here, we focus on the mechanisms by which barriers are assembled, maintained, and regulated in the context of health and disease. Because of its unique challenges and extensive study, we focus on the gastrointestinal tract as an organ-specific example of the essential contributions of the paracellular barrier to life.
1. Introduction
Epithelia separate distinct compartments and, in many cases, form barriers that limit exposure to the external environment. Barrier function develops during embryogenesis, even before distinct germ layers develop. Epithelia ultimately develop from all three germ layers, including skin, renal tubules, and gut from ectoderm, mesoderm, and endoderm, respectively [1]. Architectural differences in the organization of these diverse epithelia reflect their functional roles, but all rely on cell-cell adhesion and the intercellular junctions that direct morphogenesis and maintain tissue integrity.
2. Epithelial barriers and transepithelial transport
The structures responsible for epithelial intercellular adhesion were first demonstrated morphologically by transmission electron micrographs of rodent organs [2]. From apical to basolateral, these junctions, referred to collectively as the apical junction complex, are the tight junctions, adherens junctions, and desmosomes (Fig. 1A). Although organized differently, these molecularly-related structures serve similar functions in organisms from sea sponges to Drosophila.
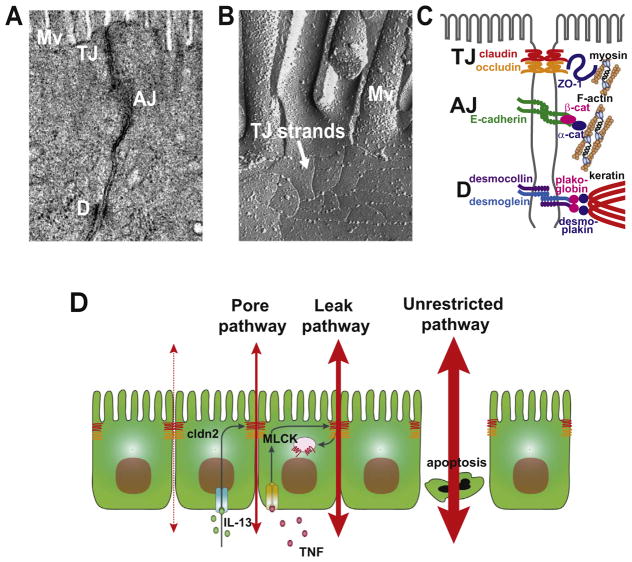
Fig. 1. Cell-cell adhesion in the intestinal epithelia.
(A) Transmission electron micrograph (TEM) image of intestinal epithelial cells. Microvilli (Mv), tight junction (TJ), adherens junction (AJ), and desmosomes (D). (B) Freeze-fracture electron micrograph image of intestinal epithelial cells. Noted the thin tight junction strands below the microvilli. (C) Localization and molecular components of tight junction, adherens junction, and desomosomes. (D) Mechanism by which cytokines increase pore and leak pathway permeability. Apoptosis is shown as a cause of increased unrestricted pathway flux.
Some tissues, such as the skin and bladder, can be nearly absolute barriers. Other epithelia, including those within the lungs, kidneys, and intestines, must balance barrier functions with vectorial transport in order to support water and salt homeostasis, nutrient absorption, and waste elimination. A significant proportion of this transepithelial transport occurs via the transcellular pathway and relies on specific proteins within apical and basolateral membranes. Such proteins include those that allow materials to cross the plasma membrane, e.g. ion channels, aquaporins, etc., as well as proteins that regulate endocytosis, exocytosis, and transcytosis. These pathways can, in some instances, be saturated. For example, high blood glucose concentrations, as can occur in dia3betes, translate to high glucose concentrations within renal tubular lumen that exceed the transport capacity of renal epithelial glucose transport proteins. This results in excess, un-reabsorbed glucose being present in the urine, which does not occur in a healthy state.
In contrast to the renal tubules, the gut lumen can be exposed to nutrient concentrations that exceed and saturate the transcellular transport systems in healthy individuals. This likely occurred regularly throughout evolution, when meals were unpredictable in frequency and content. The massive intestinal surface area can help to maximize absorption through increased paracellular flux that amplifies transcellular transport and ensures absorption of all nutrients from the lumen [3]. For example, Na+-coupled nutrient transport, as occurs with glucose and most amino acids, activates myosin light chain kinase (MLCK) within the ring of actin and myosin that encircles each cell at the level of the tight and adherens junctions [4]. Phosphorylation of myosin II regulatory light chain (MLC) within the perijunctional actomyosin ring results in size-selective increases in paracellular, i.e. tight junction, permeability. Along with the gradients established by transcellular transport, this increased permeability enhances paracellular water and nutrient transport by a mechanism known as solvent drag [5,6]. It is important, however, to recall that the paracellular pathway mediates passive transport. As a result, similar tight junction regulation can also enhance flux in the opposite direction, i.e. into the lumen, resulting in diarrhea.
3. Tight junction structure and molecular composition
When viewed by transmission electron microscopy, the tight junction is a nondescript confluence of adjacent plasma membranes at the intersection of apical and basolateral domains (Fig. 1A). The freeze fracture electron microscopy appearance of the tight junction is more striking, and is composed of an anastamosing network of strands (Fig. 1B). In molecular terms, the tight junction is made up of proteins arranged within specialized, raft-like, membrane domains. Transmembrane proteins include the claudin family of tetraspanning proteins (Fig. 1C) that direct assembly of tight junction strands [7]. In addition to membrane spanning domains, all claudins include a short, cytoplasmic N-terminal domain, a slightly longer cytoplasmic C-terminal tail, one intracellular loop, and two extracellular loops (ECLs). ECL1 contains the signature WGLWCC motif that defines the claudin family as well as several residues that determine charge-selectivity of paracellular pores [8]. ECL2 is less well studied, but does appear to contribute to trans interactions that stabilize claudin oligomers which form across the paracellular space. The C-terminal tails of most claudins end with a PDZ binding motif that facilitates trafficking to the tight junction. Other than the above exception, claudin C-tails vary in length and are the only region where there is substantial sequence diversity, explaining the location of epitopes recognized by claudin isoform-specific antibodies within the C-tail.
A second family of tetraspanning transmembrane proteins, the tight junction-associated Marvel proteins (TAMPs) occludin, tricellulin, and MarvelD3 (Fig. 1C) do not form claudin-like pores [9], but are able to regulate claudin function and tight junction structure [10]. To date, direct interactions between claudins and TAMPs have not been defined, but several studies have shown that TAMPs and claudins both bind to cytosolic scaffolding proteins such as ZO-1 (zonula occludens-1), a founding member of ZO protein family, and cingulin [7]. ZO-1, ZO-2 and cingulin can also bind filamentous actin (F-actin) and myosin (Fig. 1C), thereby linking the tight junction to the perijunctional actomyosin ring [11,12].
4. Coordination of transcellular and paracellular transport
Paracellular transport is driven by transepithelial electrochemical gradients that are created by transcellular transport. While neither active nor vectorial, the paracellular pathway is selective. Permeability is precisely regulated in response to physiological and pathophysiological stimuli. As noted above, claudin proteins direct tight junction strand assembly. While the prevailing hypothesis is that claudins also form the barrier, it is not yet certain whether claudins directly act as a barrier or, alternatively, create the barrier by organizing membrane lipids into specialized structures. It is, however, clear that claudins form channels that limit paracellular flux to molecules with diameters less than ~6 Å [13,14]. The charge selectivity of these channels is defined by specific residues within ECL1 [15]. While they can dictate relative selectivity for cations or anions, ion selectivity is far less stringent than that of most transmembrane ion channels. Nevertheless, like transmembrane ion channels, claudin channels are actively gated [16]. It is not known if, or how, this gating is regulated. For example, whether the increased paracellular flux activated by Na+-coupled nutrient transport is mediated through an increase in the number of paracellular pores [6] or, alternatively, an increase in the open probability of each pore has not been determined.
As noted above, paracellular absorption via solvent drag can amplify transcellular absorption to ensure that the intestine captures all available nutrients. Recent studies have, however, identified an additional mechanism by which paracellular ion flux is essential to ongoing nutrient absorption. These studies assessed knockout mice lacking claudin-2 and claudin-15, both of which form paracellular pores that accommodate monovalent cations, i.e. Na+. Mice lacking claudin-2 do not have an obvious phenotype. Nevertheless, an elegant study has shown that Na+ reabsorption within the renal tubule is inefficient, in that extra ATP is expended during this process in claudin-2 knockout mice [17]. Thus, as in the case of intestinal nutrient absorption, paracellular transport can amplify transcellular ion reabsorption within the renal tubule.
In contrast to the limited phenotype of claudin-2 knockout mice, claudin-15 knockout mice display dramatic intestinal hypertrophy [18]. Remarkably however, mice lacking both claudin-2 and claudin-15 die within the first few weeks of life due to nutrient malabsorption [19,20]. This is not due to anomalies of intestinal differentiation or reduced expression of Na+-coupled nutrient transporters. Rather, the defective absorption is the result of insufficient Na+ within the gut lumen. In general, normal diets do not contain sufficient amounts of Na+ to support all of the Na+-dependent absorption that occurs within the intestines. Thus, Na+ that is absorbed transcellularly must be recycled to the lumen to power additional rounds of transcellular, Na+-dependent absorption. This occurs across the tight junction via channels created by claudin-2 or claudin-15. Paracellular ion channels, therefore, allow Na+ recycling that is necessary for ongoing intestinal transcellular transport.
Transcellular and paracellular transport are also coordinated in the context of disease. For example, tumor necrosis factor-α (TNF) is well known to cause tight junction barrier loss by mechanisms that will be detailed below. The TNF related cytokine LIGHT (TNFSF14) causes tight junction barrier loss by the same mechanisms [21]. However, TNF, but not LIGHT, causes diarrhea. In fact, LIGHT actually enhances water absorption. This profound difference reflects the ability of TNF, but not LIGHT, to inhibit apical Na+-H+ exchange [21]. This exchange, mediated by NHE3, initiates transcellular Na+ absorption that generates the transepithelial ion gradient driving water absorption. When transcellular Na+ absorption continues, increased permeability results in greater water absorption than if tight junction permeability was not increased. In contrast, inhibition of transcellular Na+ absorption, and the resulting dissipation of the transcellular Na+ gradient, synergizes with increased paracellular permeability to allow fluid efflux, i.e. diarrhea.
5. Barrier function is regulated by modulating distinct paracellular pathways
Epithelial barriers reflect the presence of epithelial cells as well as sealing of the paracellular space by tight junctions. Epithelial damage, therefore, results in massive, non-selective barrier loss by a route that has been referred to as the unrestricted pathway (Fig. 1D). As described above, tight junction permeability also affects epithelial barrier properties more selectively. One mechanism that does not require new protein synthesis depends on the cytoskeleton. This includes the MLCK-dependent tight junction regulation that occurs in response to Na+-nutrient cotransport. Although this physiological barrier regulation is size-selective and limited to small, nutrient-sized molecules, myosin light chain kinase activation also underlies the pathophysiological barrier regulation induced by TNF and LIGHT. However, despite the shared central role of myosin light chain kinase, these examples of pathophysiological and physiological barrier regulation are strikingly different.
Na+-nutrient cotransport has only mild effects on tight junction ultrastructure, although condensation of the perijunctional actomyosin ring is detectable [22]. At the light microscopic level, tight junction proteins do not appear to be reorganized in response to physiological-range myosin light chain kinase activation or inhibition. In contrast, pathophysiological myosin light chain kinase-dependent barrier regulation involves caveolar endocytosis of occludin and increased permeability to much larger molecules, e.g. albumin [21,23]. It remains to be determined whether this difference simply reflects the magnitude of myosin light chain kinase activation or, alternatively, the contribution of a yet-to-be-defined second signal in the case of pathophysiological barrier regulation. Regardless, these observations indicate that there are at least two distinct routes across the tight junction. The first, which is size-and charge-selective and accommodates solutes with diameters up to ~6 Å has been termed the pore pathway [8,24,25]. This is a high-capacity paracellular route. In contrast, the leak pathway allows flux of much larger molecules, with diameters up to ~100 Å, is not charge-selective, and, even when maximally activated, is a relatively low-capacity paracellular route [8,24,25]. The bulk of available data indicate that the pore pathway reflects flux through claudin-based paracellular channels.
The molecular mechanisms of leak pathway flux have not yet been defined. Current hypotheses include transient disruptions of tight junction strands, and flux through the specialized areas of the tight junction such as those found at tricellular contacts sites, i.e. tricellular tight junctions [26]. These are not mutually exclusive. However, the proteins involved may differ depending on the site. For example, tricellulin, a TAMP that is concentrated at tricellular tight junctions, appears to seal the leak pathway at that site [26]. In contrast, the first TAMP identified, occludin, likely plays a similar role at bicellular tight junctions. The increased leak pathway flux resulting from TNF-induced occludin endocytosis can therefore be understood as a failure of leak pathway sealing due to occludin depletion [23]. Consistent with this, occludin overexpression prevents TNF-induced diarrhea in vivo [23]. Conversely, occludin knockdown cell lines displayed increased leak pathway permeability, and TNF does not cause further leak pathway flux increases in these cells. The mechanism by which occludin depletion increases leak pathway flux remains an active area of investigation. However, the observation that occludin knockout mice do not have serious intestinal barrier defects has led some to question the role of this protein. Further refinement of occludin’s contribution to in vivo function needs to be made through examination of occludin-deficient mice with response to stress, as well as more careful analyses of any compensatory mechanisms that may be activated in these mice due to occludin deficiency [27].
6. Barrier regulation along the crypt-villus axis
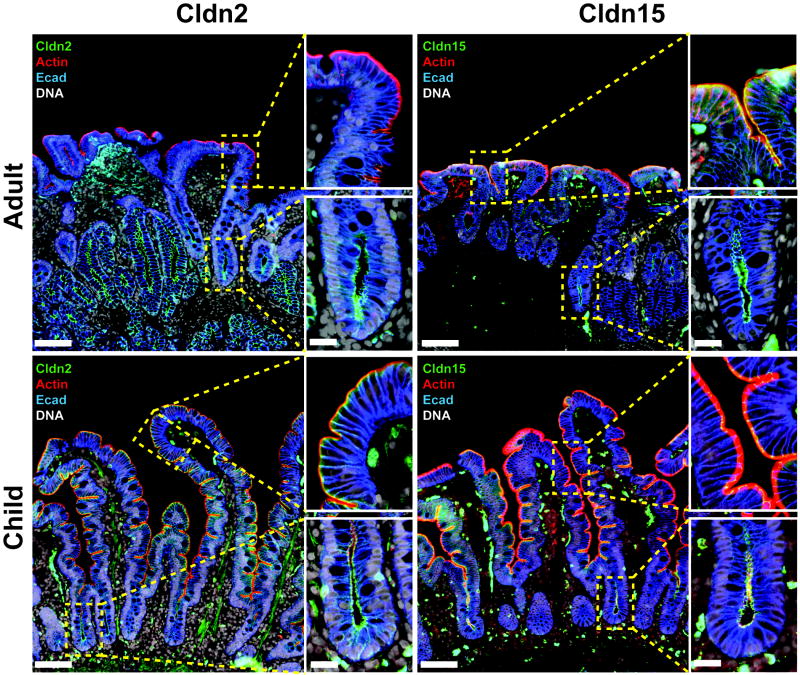
The small intestine provides a self-contained, spatially-resolved example of epithelial differentiation. The crypt is home to rapidly-cycling stem cells and some specialized cells, e.g. Paneth cells and endocrine cells [28,29]. However, the bulk of new cells generated within the crypt migrate upwards to the villus. These migrating cells first traverse the transit-amplifying zone, which is an area of the crypt in which proliferation continues during migration. These relatively-undifferentiated epithelial cells express CFTR, an apical Cl− channel, and other proteins that drive transcellular ion secretion. As migration continues, these epithelial cells shift their transcriptional programming to downregulate proteins involved in ion secretion in favor of those responsible for ion and nutrient absorption. As a result, the crypt is a net secretory compartment while the villus is primarily absorptive. Tight junctions play a critical role in defining functions that are thought to allow the crypt lumen to remain relatively sterile. A significant proportion of occludin is localized within cytoplasmic vesicles in crypt epithelia, whereas occludin is primarily concentrated at the tight junction in villus epithelia absent pathophysiology. In addition, clau-din-2 and claudin-15 are preferentially expressed within the crypt (Fig. 2). These expression and localization patterns allow water and Na+ to efflux through the paracellular pathway and follow transcellularly secreted Cl−. The relatively low expression of claudin-2 and claudin-15 within villus epithelium may also contribute to maintenance of the osmotic gradient generated by the intestinal villus counter-current exchanger. Similar to the countercurrent exchange mechanism that creates hypertonicity within the renal medulla, this process allows the interstitium within the villus to achieve Na+ concentrations greater than 500 mM [30]. This massive osmotic gradient across the epithelium greatly enhances villus fluid absorption. If claudin-2 and claudin-15 were expressed in the villus, the gradient would rapidly dissipate and villus fluid absorption would decrease. Interestingly, claudin-2 and claudin-15 expression is subject to developmental regulation, perhaps reflecting different nutrient sources during postnatal life. In rodents, claudin-2 is expressed at very high levels in intestinal crypts and villi at birth. Expression within villus epithelium decreases markedly as mice are weaned and shift from nursing to solid food, which contains lower concentrations of Na+-cotransported nutrients [31]. Claudin-15 expression is reciprocally regulated in rodents, likely explaining the normal growth of claudin-2 knockout mice. Our analyses of duodenal biopsies in humans show a similar pattern of claudin-2 and claudin-15 regulation during development, although some claudin-2 expression persists within crypt and villus epithelium in adults (Fig. 2).
Fig. 2. Differential expression of claudins during development in human intestine.
Expression pattern of human claudin-2 and -15 in the villus and crypt of duodenum biopsy from a female child (3-yr old) or female adult (80-yr old). Note that claudin-15 is a dominant Na+ channel tight junction protein in the villus of child. Scale bar=0.1 mm (low magnification images), 20 μm (insets).
7. Dynamic regulation of tight junction molecular architecture
Barrier function can be affected by expression and localization of tight junction proteins. A good example is regulation of claudin-2. In addition to its downregulation during postnatal development, claudin-2 expression within the intestines can increase in response to a variety of inflammatory stimuli, including interleukin- (IL-)1β [32], IL-6 [33], IL-9 [34], and IL-13 [35,36]. Where studied, this results in increased intestinal permeability to cations, but not anions or larger molecules [36]. Beyond this, claudin-2 activity can be regulated by intermolecular interactions and post-translational modifications. For example, phosphorylation at serine 208, near the C-tail, is required for claudin-2 retention within the plasma membrane [37]. Conversely, SUMOylation reduces claudin-2 membrane expression [38].
Under normal conditions, the bulk of claudin-2 is stably anchored at the tight junction, as demonstrated by fluorescence recovery after photobleaching (FRAP) analyses [39,40]. The claudin-2 mobile pool can, however, be increased by inhibition of casein kinase 2 (CK2). This effect of CK2 inhibition can be linked directly to dephosphorylation of a specific residue, serine 408, within the C-tail of occludin. A variety of in vitro and in vivo data indicate that, when dephosphorylated at serine 408, occludin binds to ZO-1 which, in turn, binds to claudin-2. These interactions do not occur when ZO-1 lacks either the PDZ1 domain necessary for binding claudin-2, or the U5GuK domain that mediates interactions with occludin. In contrast to its effect on claudin-2, CK2 inhibition reduces the mobile fraction of occludin, both in cultured intestinal epithelial monolayers and in enterocytes of transgenic mice expressing fluorescent occludin [40]. This is easily understood when one recognizes that, under typical conditions, the majority of tight junction-associated occludin is within the mobile fraction. CK2 inhibition triggers assembly of an occludin:ZO-1:claudin-2 complex that causes the mobile fractions of occludin and claudin-2 to converge. The effect between claudin-2 and occludin on one another’s mobility within the tight junction can be recapitulated within the tight junction-like strands that form in fibroblasts. In this setting, claudin-2 expression reduces the occludin mobile fraction.
In epithelial cells, assembly of the occludin:ZO-1:claudin-2 complex disrupts claudin-2 channels, thereby reducing paracellular cation and water flux. In vitro studies of cultured intestinal epithelial cells have shown the potential therapeutic effectiveness of this observation, as CK2 inhibition can reverse IL-13-induced barrier loss [40].
ZO-1 anchoring also appears to be involved in cytoskeletal regulation of tight junction permeability. Myosin light chain kinase inhibition in cells with active Na+-glucose cotransport reduces ZO-1 exchange at the tight junction, both in cultured intestinal epithelial monolayers and enterocytes within transgenic mice expressing fluorescent ZO-1 [41]. In contrast to the claudin-2 regulation discussed above, this regulation requires the ZO-1 actin binding region (ABR). ZO-1 interactions with occludin also appear to be required for TNF-induced occludin endocytosis, although the details have not yet been elucidated.
Further insights into potential mechanisms by which molecular interactions regulate tight junctions come from recent studies that identified TOCA-1 (transducer of cdc42-dependent actin activity) as a ZO-1 binding protein [42,43]. TOCA-1 trafficking to the tight junction requires interactions with the ZO-1 PDZ1 domain. TOCA-1 recruits the actin-nucleating factor N-WASP (neural Wiskott–Aldrich syndrome protein) to the tight junction. While loss of TOCA-1 might be expected to reduce F-actin content in the perijunctional actomyosin ring, it instead results in reorganization of perijunctional actin into dense, sarcomere-like, periodic actin arrays. This is associated with increased leak pathway flux and, more strikingly, reduced plasticity of cell shape.
Finally, recent work has shown that ZO-1 tethers the tight junction-like strands that form when claudin proteins are expressed in fibroblasts [44]. When the C-terminal three amino acids of claudin-2, which are required for association with ZO-1, are deleted, strand networks tend to expand over time. This expansion is constrained when ZO-1 is expressed.
8. Clinical implications of intestinal epithelial barrier loss
Increased intestinal permeability, or barrier dysfunction, has been linked to a number of gastrointestinal and systemic diseases, including inflammatory bowel disease, celiac disease, graft-versus-host-disease, diabetes, multiple sclerosis, and even autism [24]. While genetic polymorphisms have been linked to some human diseases (Table 1), barrier loss in most cases is not related to gene mutations. In most other cases the evidence in favor of a causative role of intestinal barrier loss in these disorders is limited [45]. Further, significant data from human subjects and genetically-modified mice suggest that, in the absence of other abnormalities, intestinal barrier loss of the magnitude associated with tight junction dysregulation is insufficient to cause disease. For example, although increased intestinal permeability is well-documented in Crohn’s disease, one of two forms of inflammatory bowel disease, it can also be present in a subset of healthy, first-degree relatives of Crohn’s disease patients [46]. This suggests that genetic or environmental factors shared with Crohn’s disease patients may contribute to barrier loss in their healthy relatives. Studies of spouses and other genetically unrelated individuals living in the same household indicate that environmental factors are not responsible for increased intestinal permeability in these otherwise healthy relatives. However, genetic analysis has shown that healthy relatives with increased permeability tend to carry specific polymorphisms of NOD2 that are associated with Crohn’s disease [47]. Thus, while these relatives may have some abnormality in both immune regulation and intestinal barrier function, they are clinically well. Some have proposed that these individuals are more likely to go on to develop Crohn’s disease, but there is currently no available data to support the hypothesis. Thus, these healthy relatives demonstrate that intestinal barrier defects alone are insufficient to cause disease in humans.
Table 1.
Tight junction proteins and associated diseases.
| I-A. Genetic mutations associated with human diseases | ||||
|---|---|---|---|---|
|
| ||||
| Tight junction proteins | Gene name | Mutations | Protein defect | Disease |
| Claudin-1 | CLDN1 | Frameshift/truncation | No expression | Neonatal ichithyosis and sclerosing cholangtitis |
| Claudin-5 | CLDN5/TMVCF | Deletion | No expression | Velo-cardio-facial syndrome |
| Claudin-14 | CLDN14 | Frameshift/truncation, substitution | Truncation, misfolding | Nonsyndromic deafness (DFNB29) |
| Claudin-16/paracelin-1 | CLDN16 | Truncation, splice site, missence | Trafficking defects | Familial hypomagnesemia, hypercalciuria, nephrocalcinosis |
| Claudin-19 | CLDN19 | Missence | Trafficking defects | Hypomagnesemia, hypercalciuria, nephrocalcinosis, visual impairment |
| Occludin | OCLN | Variable | Band-like calcification with simplified gyration and polymicrogyria | |
| Tricellulin | MARVELD2 | Splice site, truncation | Truncation | Autosomal recessive Nonsyndromic deafness (DFNB49) |
| ZO-2 | TJP2 | Substitution | Reduced claudin-1 affinity | Familial hypercholamenia |
| Peripheral myelin protein 22 | PMP22 | Deletion, duplication, mutations | Various | Peripheral polyneuropathies |
|
| ||||
| I-B. Altered protein expression in human diseases | ||||
|
| ||||
| Tight junction proteins | Protein levels/localization | Disease | ||
|
| ||||
| Claudin-1 | Downregulation | Atopic dermatitis, psoriasis | ||
| Upregulation | Ulcerative colitis | |||
| Claudin-2 | Downregulation | Gallstones | ||
| Upregulation | Crohn’s disease, ulcerative colitis, celiac disease, irritable bowel syndrome | |||
| Claudin-3 | Downregulation | Crohn’s disease, ulcerative colitis, celiac disease | ||
| Claudin-4 | Downregulation | Ulcerative colitis | ||
| Claudin-5 | Downregulation | Celiac disease | ||
| Claudin-7 | Downregulation | Ulcerative colitis, celiac disease, psoriasis | ||
| Claudin-8 | Downregulation | Crohn’s disease | ||
| Claudin-15 | Upregulation | Celiac disease | ||
| Claudin-23 | Downregulation | Intestine-type gastric cancer | ||
| Occludin | Downregulation | Crohn’s disease, ulcerative colitis, ichthyosis vulgaris | ||
| Upregulation | Psoriasis | |||
| ZO-1 | Downregulation | Ichthyosis vulgaris | ||
| Upregulation | Psoriasis | |||
| MLCK | Upregulation | Irritable bowel syndrome, Crohn’s disease, ulcerative colitis | ||
Similar studies of genetically modified mice show that modest barrier deficiencies, as opposed to the massive barrier defects associated with epithelial damage, do not progress to spontaneous disease. Indeed, data suggest that some of these animals develop compensatory immune responses protective from any ill effects of barrier loss. These mice have been followed to advanced ages, and do not appear to develop spontaneous disease over time. However, when subjected to insults that cause experimental inflammatory bowel disease in wild type mice, several studies have shown that mice with increased intestinal permeability fare worse than their counterparts. While many of these mouse models have phenotypes that include, but are not limited to, disruption of intestinal epithelial tight junction regulation, one model does provide an example of specifically-targeted disruption of epithelial barrier function within the intestine [48]. These transgenic mice express a constitutively-active myosin light chain kinase under the control of an intestinal epithelial-specific promoter. As expected, the mice have increased intestinal permeability. While they do not develop spontaneous disease, induced immune-mediated experimental inflammatory bowel disease results in far more severe intestinal and systemic injury relative to mice without the constitutively-active transgene. Further, overall survival is reduced in the transgenic mice. One conclusion that can be made from these data is that, while increased intestinal permeability is insufficient to cause disease, it can accelerate disease progression and enhance its severity. Human data also support this conclusion (Table 1). For example, in Crohn’s disease, studies of patients in clinical remission have shown that those with increased intestinal permeability are at greater risk of disease relapse [49].
To date, no human studies have assessed the potential therapeutic benefit of restoring the intestinal epithelial barrier. Studies in mice lacking the intestinal epithelial myosin light chain kinase isoform have, however, shown that these mice are resistant to barrier defects and initially have more modest clinical presentation, e.g. weight loss, during immune-mediated experimental inflammatory bowel disease relative to controls [50]. The protection afforded by myosin light chain kinase deficiency is apparent early in the course of disease. However, myosin light chain kinase knockout mice eventually do develop disease with similar clinical severity to mice that express myosin light chain kinase. Interestingly, the acceleration of disease in the knockout mice corresponds with the development of increased intestinal permeability in these mice. This increased intestinal permeability in later stages of disease occurred as a result of epithelial damage, rather than tight junction regulation. Thus, the myosin light chain kinase-deficient mice eventually develop barrier loss through tight junction-independent mechanisms and that barrier loss, in turn, contributes to disease progression. These results in mouse models might prompt some to pursue myosin light chain kinase inhibition as a therapeutic intervention in human patients. One cautionary note, however, is that the same myosin light chain kinase knockout mice fared worse than wild type mice when exposed to dextran sulfate sodium in a chemically-induced epithelial injury model of intestinal damage [50]. This likely reflects the important role of myosin light chain kinase in epithelial migration and wound healing. Thus, while myosin light chain kinase inhibition and tight junction preservation could be protective early in the disease process, consideration of myosin light chain kinase as a therapeutic target requires improved targeting of its specific functions.
9. Concluding remarks
The epithelial barrier is formed by molecular interactions that establish selectively-permeable intercellular junctions. Flux across these can be dynamically controlled by physiological and pathophysiological stimuli. Precise regulation of permeability of distinct routes passing through the tight junction, e.g. pore and leak pathways, is therefore essential for normal tissue homeostasis and overall health. This article is focused on the intestine as a model, but many of the principles presented are applicable to other epithelial tissues as well. Definition of fundamental mechanisms of barrier regulation and identification of molecular targets for therapeutic intervention hold promise for the development of treatments in epithelial barrier disorders.
Acknowledgments
Support
National Institutes of Health (R01DK61631, R01DK68271, R24DK099803) and the Crohn’s and Colitis Foundation of America.
References
- 1.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar M, Palade G. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin Sci. 1989;76:403–413. doi: 10.1042/cs0760403. [DOI] [PubMed] [Google Scholar]
- 4.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 5.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- 6.Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 7.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Gunzel D, Wolburg H, Piontek J, Huber O, Blasig IE. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–564. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhuo M, Pei L, Rajagopal M, Yu AS. Comprehensive cysteine-scanning mutagenesis reveals Claudin-2 pore-lining residues with different intrapore locations. J Biol Chem. 2014;289:6475–6484. doi: 10.1074/jbc.M113.536888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 15.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol – Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 16.Weber CR, Liang GH, Wang Y, Das S, Shen L, Yu AS, Nelson DJ, Turner JR. Claudin-2-dependent paracellular channels are dynamically gated. eLife. 2015;4:e09906. doi: 10.7554/eLife.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei L, Solis G, Nguyen MT, Kamat N, Magenheimer L, Zhuo M, Li J, Curry J, McDonough AA, Fields TA, Welch WJ, Yu AS. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J Clin Investig. 2016;126:2509–2518. doi: 10.1172/JCI83942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura A, Kitano Y, Hata M, Katsuno T, Moriwaki K, Sasaki H, Hayashi H, Suzuki Y, Noda T, Furuse M, Tsukita S. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523–534. doi: 10.1053/j.gastro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology. 2013;144:369–380. doi: 10.1053/j.gastro.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 21.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Investig. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 23.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 25.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 29.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Jodal M, Lundgren O. Countercurrent mechanisms in the mammalian gastrointestinal tract. Gastroenterology. 1986;91:225–241. doi: 10.1016/0016-5085(86)90463-4. [DOI] [PubMed] [Google Scholar]
- 31.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Kojima T, Murata M, Takano K, Go M, Chiba H, Sawada N. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp Cell Res. 2004;299:427–441. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr HA, Wirtz S, Vieth M, Waisman A, Rosenbauer F, McKenzie AN, Weigmann B, Neurath MF. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676–686. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 35.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Itallie CM, Tietgens AJ, Logrande K, Aponte A, Gucek M, Anderson JM. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J Cell Sci. 2012 doi: 10.1242/jcs.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Itallie CM, Mitic LL, Anderson JM. SUMOylation of claudin-2. Ann NY Acad Sci. 2012;1258:60–64. doi: 10.1111/j.1749-6632.2012.06541.x. [DOI] [PubMed] [Google Scholar]
- 39.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, Turner JR. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Itallie CM, Aponte A, Tietgens AJ, Gucek M, Fredriksson K, Anderson JM. The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks. J Biol Chem. 2013;288:13775–13788. doi: 10.1074/jbc.M113.466193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Itallie CM, Tietgens AJ, Krystofiak E, Kachar B, Anderson JM. A complex of ZO-1 and the bar-domain protein TOCA-1 regulates actin assembly at the tight junction. Mol Biol Cell. 2015;26:2769–2787. doi: 10.1091/mbc.E15-04-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol Biol Cell. 2016 doi: 10.1091/mbc.E16-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 47.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 50.Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, Abraham C, Turner JR. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]