Abstract
Natural killer (NK) cells maintain immune homeostasis by detecting and eliminating damaged cells. Simultaneous activating and inhibitory input are integrated by NK cells, with the net signal prompting cytotoxicity and cytokine production, or inhibition. Chief among the inhibitory signals for NK cells are “self” human leukocyte antigen (HLA) molecules, which are sensed by killer immunoglobulin-like receptors (KIR). Through a process called “education”, the functional capabilities of each NK cell are counterbalanced by their sensitivity for inhibition by co-inherited “self” HLA. Since genes for HLA and the killer immunoglobulin-like receptors (KIR) that bind them are polymorphic, polygenic and independently segregate, NK education and function differ even between related individuals. In this review, we describe how variances in NK education, reactivity and sensitivity for inhibition impact reproductive success, infection, cancer, inflammatory and autoimmune diseases.
Education for NK potential – activation, inhibition, and regulation
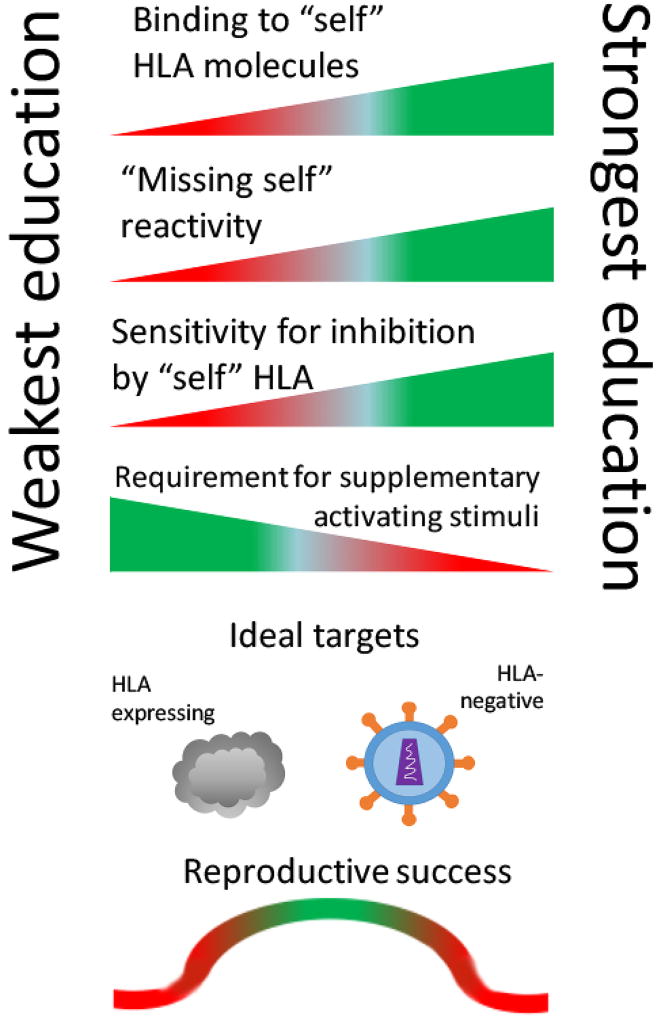
Natural killer (NK) cells were initially characterized for their ability to lyse target cells lacking “self” major histocompatibility class I expression (MHC) [1]. Recognition of “missing self” in humans enables distinction of self from non-self tissues, and detection of diseased cells with downregulated human leukocyte antigen (HLA) expression, which occurs in some viral infections and cancers [2–4]. This “missing self” reactivity of NK cells is not, however, absolute. Rather, the avidity of interactions between NK receptors and HLA creates a spectrum of functionality among the NK cells both within and between individuals, where the reactive potential of each NK cell, endowed by a process of “NK education,” is counterbalanced by its dominant sensitivity for inhibition by co-inherited “self” HLA [5–7]. This process is called NK “education” (Figure 1).
Figure 1. NK education and impacts on human health.
NK cell education represents a continuum of reactivity and inhibition, determined by interactions with co-inherited HLA. Cells capable of high avidity binding exhibit the most potent “missing self” reactivity and sensitivity for inhibition by “self” HLA class I molecules. Uneducated NK cells do not bind “self” HLA molecules, leading to weak “missing self” capabilities, but also insensitivity for inhibition. NK education determines the threshold for NK reactivity, with the strength of NK education inversely correlated with the requirement for additional activating input (i.e. pro-inflammatory signals or bound antibodies). NK education equips populations to detect damaged cells exhibiting a variety of HLA phenotypes, with uneducated NK cells as the best effectors against HLA-expressing targets (i.e. most cancers) and educated NK cells as the best effectors against HLA-negative targets (i.e. certain viral infections). Successful pregnancy is fostered by specialized uterine NK cells (uNK), educated to enable trophoblast invasion and placentation, while managing fetal growth.
In humans, NK education occurs most through interactions between HLA class I molecules and inhibitory receptors, such as the CD94/NKG2A heterodimer and, more prominently, the killer immunoglobulin-like receptors (KIR), as extensively reviewed (Table 1) [3,8–10]. While several models have been advanced to describe how NK education occurs, they generally agree that cumulative interaction between inhibitory receptors and HLA ligands dictates the level of response of an NK cell to activating signals, such as stress ligands, inflammatory cytokines, and Fc receptor engagement [6,11,12]. The HLA and KIR gene families represent the most polymorphic and polygenic receptor-ligand pair in the human genome, having co-evolved to enable diverse NK cell responsiveness [13,14]. Genes for KIR and HLA segregate independently, yielding diverse compound genotoypes [15]. Functional interactions between co-inherited KIR and HLA drive NK education, which enables a unique form of immunologic diversity with a minimum number of germline-encoded genes [15]. Finally, immunologic experiences can further modulate NK cell functions, through establishment of memory-like or adaptive NK cell populations via epigenetic remodelling [16,17].
Table 1.
Receptor-ligand partnerships contributing to NK cell education
| HLA epitope | Cognate receptor | ||
|---|---|---|---|
| HLA-C1 (Asn77) | KIR2DL2 KIR2DS2 | (major), | KIR2DL3, |
| HLA-C2 (Lys80) | KIR2DL1, KIR2DS1 | KIR2DL2 | (minor), |
| HLA-Bw4 | KIR3DL1 | ||
| HLA-F (open conformation) | KIR3DS1 | ||
| HLA-A11 | KIR3DL2 | ||
| HLA-E, presenting HLA leader sequences | NKG2A | ||
| pan HLA | LIR-1 | ||
Allelic variation influences the overall avidity of receptor-ligand interactions by controlling surface expression densities and binding affinities [18–20]. We and others have investigated the impact of allelic variation on human health for KIR3DL1 and its ligand HLA-Bw4, the most diverse and evolutionarily most ancient KIR-HLA partnership [21–23]. HLA-B alleles can be divided based on the Bw6 or Bw4 epitope encoded at positions 77–83, with further division of Bw4 into Bw4-80I or Bw4-80T subtypes based on a dimorphism (isoleucine vs threonine) at position 80. KIR3DL1 alleles encompass the activating KIR3DS1 alleles, and inhibitory alleles expressed at high, low and null cell surface densities [24,25]. Interestingly, the null KIR3DL1 allele is expressed exclusively intracellularly, which prevents inhibition by Bw4-expressing target cells, but permits education [26,27]. The remaining subtypes of Bw4 and KIR3DL1 mediate education and inhibition, with the strongest outcomes occurring between the densely expressed and strong binding KIR3DL1-high and Bw4-80I allotypes [18].
Binding to “self” HLA is not required for survival and maintenance of NK cells, which exhibit a spectrum of HLA binding from none to weak to strong, as a result of cumulative contribution from multiple inhibitory receptor types, their expression levels, and affinities to HLA [18–20]. “Uneducated” NK cells, non-binding and weakly-binding NK cells are beneficial in disease states where HLA expression persists, but set with a higher threshold for reactivity to avoid auto-aggression [10,26]. Nevertheless, in the presence of activating stimuli, such as antibodies bound to target cells for antibody-dependent cellular cytotoxicity (ADCC), or a cytokine-conditioned pro-inflammatory microenvironment, uneducated NK cells can readily be recruited for target cell killing [28,29].
Though considerations of NK education most often concern inhibitory KIR, it is now understood that other receptor-ligand interactions titrate the NK reactivity/inhibition balance. The inhibitory CD94/NKG2A and LIR-1 both bind conserved regions of HLA and increase NK response potential by providing additional binding to “self” HLA [30,31]. Conversely, NK education can also be reduced by activating receptors in the context of high ligand exposure: KIR2DS1+ NK cells, normally responsive to HLA-C2+ targets, are refractory to stimulation when they are collected from HLA-C2 homozygous individuals, having been rendered tolerant to self [32]. KIR2DS2 and KIR3DS1 have likewise been reported to bind HLA-C1 and HLA-F, respectively [33–35]; whether these interactions contribute to education and tolerance has not been described.
Altogether, educated and uneducated NK cells establish a complementary system of responsiveness to enable reactivity against HLA-sufficient and HLA-deficient target cells, with important implications in reproduction, autoimmune, infectious and malignant disease (Table 2). In this review, we focus on interactions between KIR and HLA focusing on their combined influence on human health.
Table 2.
Associations of NK education in human health and disease
| KIR-HLA combination |
Education status |
Health/Disease state |
Impact |
|---|---|---|---|
| KIR2DL1 + HLA-C2 | Educated | Pregnancy | Increased risk of pre-eclampsia, stillbirth and low birthweight (especially with fetal HLA-C2) [50,93,94] |
|
|
|||
| Infectious disease | Enriched among individuals acquiring Dengue virus infection [95] | ||
|
|
|||
| Infectious disease | Enriched among HCMV seropositive NKG2C+ cells in persons with HCMV [74] | ||
|
|
|||
| KIR2DL1 without HLA-C2 | Uneducated | Infectious disease | Lower reactivation of HCMV after kidney transplant [96] |
|
|
|||
| Cancer | Protective against AML relapse following allogeneic HCT in patients with AML (missing ligand) [83,97] | ||
|
| |||
| KIR2DL2 + HLA-C1 | Strongly educated | Autoimmune disease | Protection against ulcerative colitis [98] |
|
|
|||
| Inflammatory disease | Enriched in patients with Kawasaki disease [99] | ||
|
|
|||
| Cancer/infectious disease | Associated with younger age of onset for hepatocellular carcinoma in persons infected with hepatitis B [100] | ||
|
|
|||
| Infectious disease/cancer | Enriched among persons infected with high-risk human papillomavirus and patients developing invasive cervical cancer [101] | ||
|
|
|||
| Cancer | Protection against relapse of hematological malignancy in patients receiving umbilical cord blood transplantation [102] | ||
|
| |||
| KIR2DL2 + HLA-C2 | Uneducated – weakly educated | Infectious disease | Lower reactivation of HCMV after kidney transplant [96] |
|
|
|||
| Autoimmune disease | Increased susceptibility to type I diabetes [103] | ||
|
| |||
| KIR2DS1 + HLA-C2 | Educated (tolerogenic) | Pregnancy | Enriched in patients with recurrent miscarriage [49] |
|
|
|||
| Autoimmune disease | Protective against multiple sclerosis [67] | ||
|
| |||
| KIR2DS1, without HLA-C2 | Uneducated (reactive) | Pregnancy | Enriched in patients with failed pregnancy after in vitro fertilization [105] |
|
|
|||
| Cancer | Protection against AML relapse after hematopoietic cell transplantation [104] | ||
|
|
|||
| Cancer | Protection against relapse of hematological malignancy in patients receiving umbilical cord blood transplantation [102] | ||
|
| |||
| KIR2DL3 + HLA-C1 | Educated | Infectious disease | increased resolution of HCV infection compared with 2DL2+C1 or 2DL1+C2; possibly due to weaker inhibitory signals [106] |
|
|
|||
| Infectious disease | Resistance to Ebola infection [107] | ||
|
|
|||
| Infectious disease | Higher incidence of cerebral malaria in infected persons [108] | ||
|
|
|||
| Infectious disease | Underrepresented among individuals acquiring Dengue virus infection [95] | ||
|
|
|||
| Infectious disease | Enriched among patients resolving hepatitis B infection; underrepresented in patients with chronic hepatitis B infection [109] | ||
|
|
|||
| Infectious disease | Enriched among HCMV seropositive NKG2C+ cells in persons with HCMV [74] | ||
|
|
|||
| Infectious disease/cancer | Enriched among persons infected with high-risk human papillomavirus and patients developing invasive cervical cancer [101] | ||
|
|
|||
| Cancer | Protection against relapse of hematological malignancy in patients receiving umbilical cord blood transplantation [102] | ||
|
| |||
| KIR2DS2 + HLA-C1 | Infectious disease | Protective against HCV and Dengue virus [110] | |
|
| |||
| KIR2DL3 without HLA-C1 | Uneducated | Autoimmune disease | Protection against ulcerative colitis [98] |
|
|
|||
| Cancer | Protection against AML relapse in patients receiving hematopoietic cell transplantation [102,104,111] | ||
|
| |||
| KIR3DL1 + HLA-Bw4 | Educated | Infectious disease | High affinity pairs (KIR3DL1-high + HLA-Bw4-80I) protect against HIV progression to AIDS [71] |
|
|
|||
| Infectious disease | Enriched among individuals acquiring Dengue virus infection [95] | ||
|
|
|||
| Cancer | low affinity pairs are associated with the protection against relapse patients with AML after hematopoietic cell transplantation [26] | ||
|
|
|||
| Cancer | Enriched in patients with kidney cancer and non small cell lung carcinoma compared with healthy controls [112] | ||
|
| |||
| KIR3DL1 without HLA-Bw4 | Uneducated | Cancer | Protection against AML relapse after hematopoietic cell transplantation [26,83,97] |
|
|
|||
| Cancer | Improved overall survival and cancer control in patients treated with 3F8 antibody (Bw4-negative and weak binding pairs of KIR3DL1 + HLA-Bw4) [29,84,85] | ||
|
|
|||
| Infectious disease/cancer | Higher likelihood of hepatitis C-associated hepatocellular carcinoma [113] | ||
|
|
|||
NK education in pregnancy
Successful pregnancy requires invasion of the fetal trophoblast and formation of spiral arteries, facilitated by NK cells. These processes must be carefully balanced: extensive invasion is associated with high birth weight and high-risk delivery; poor invasion is associated with preeclampsia, low birth weight and recurrent miscarriage [36].
Uterine NK cells (uNK) are the most abundant lymphocytes in the decidua, and are key players in successful pregnancy [37–39]. Through interactions with HLA expressed on extravillous trophoblast cells (EVT), uNK cells facilitate trophoblast invasion and placentation [37]. EVT lack expression of HLA-B and HLA-A; but exhibit HLA-E and HLA-C [40,41], whose cognate NKG2A and KIR2D receptors are overexpressed on uNK cells [38,40,42,43]. Moreover, uNK cells are enriched for educated populations: HLA-C1+ and HLA-C2+ mothers demonstrate increased frequencies of uNK cells expressing cognate KIR2DL3 and KIR2DL1, respectively [43,44]. In this way, maternal NK cells are equipped to enable tissue remodeling while remaining sensitive to inhibition, creating an optimal molecular environment for trophoblast invasion.
Fetal HLA-C2 of paternal origin in KIR2DS1+, HLA-C2-negative mothers exhibit high birth weight and successful placentation [36,38,45]. In contrast, KIR2DS1 is underrepresented and HLA-C2 is enriched among women experiencing recurrent miscarriages [46]. Similarly, the more inhibitory KIR-A haplotype, which lacks KIR2DS1, and HLA-C2 are enriched in women experiencing recurrent miscarriage and in their partners [47–49]. KIR-A haplotypes and fetal HLA-C2 of paternal origin are similarly enriched among patients with pre-eclampsia, where placental development and fetal growth are compromised due to poor invasion of the trophoblast [36,50,51]. Together, these studies suggest that uNK education that favors maternal NK responsiveness over inhibition may enable successful placentation and spiral artery formation.
NK education in autoimmune and inflammatory disease
The etiopathology of autoimmune and inflammatory diseases, including the role(s) of NK cells, remains largely to be determined, but is associated with activation of T and B lymphocytes against self proteins and persistent inflammation mediated by innate and adaptive cells. The available evidence suggests that NK cells fulfill both beneficial and detrimental roles, implying that inter-patient variability, possibly in NK education, could underlie the pathology of inflammatory and autoimmune disease.
Across a spectrum of inflammatory disorders, including Kawasaki disease, rheumatoid arthritis, multiple sclerosis (MS), systemic lupus erythematosus and type I diabetes, decreased numbers and function of circulating NK cells precede disease flares [52–58], suggesting that NK cells are important for controlling inflammation. In apparent contrast, NK cells are indispensable in the priming phase for subsequent development of experimental myasthenia gravis, and IFN-γ produced by NK cells exacerbates experimental autoimmune encephalopathy and diabetes in mouse models [59], suggesting that the role for NK cells could differ between diseases and/or patients.
In patients responding to modern treatments for MS including mitoxantrone, daclizumab or natalizumab – therapies that control lymphocyte expansion – selective enrichment and maturation of the NK population and its cytotoxic capacities are observed in responding patients [58,60–62]. In patients treated for MS and systemic lupus erythematosus, antibodies aimed at depleting auto-aggressive B cells, including alemtuzumab and rituximab, function by recruiting NK cells for antibody-dependent cellular cytotoxicity (ADCC) [63,64]. Their success is associated with enrichment of NK cells, and depletion of the NK population eliminates the efficacy of treatment in experimental models [63,64]. Several studies have now reported a protective role of KIR2DS1 against MS, enhanced by the presence ofo its HLA-C2 ligand [65–67], a configuration that supports tolerance of NK cells bearing this activating receptor[32]. Altogether, these observations support a regulatory role for NK cells in preventing autoimmune and inflammatory disease, but the mechanism(s) through which this occurs and the contribution of NK education remains largely to be investigated.
NK education in infectious disease
Given the prominent role for T cells in detecting viral antigens loaded onto HLA molecules, it is not surprising that many viruses have evolved to reduce surface expression of HLA. Although this creates a target for educated NK cells, viral adaptations, including encoding HLA mimics or selective HLA downregulation facilitate virus spread throughout populations.
In HIV infection, downregulation of HLA is limited to the HLA-A and HLA-B loci, with HLA-C expression persisting or even upregulated [68–70]. To NK cells educated by KIR3DL1 and HLA-B, HIV infected cells therefore present themselves as missing-self targets, and killing proceeds in a manner reflective of education strength [18,68]. As a result, HIV-infected individuals harbouring compound genotypes of KIR3DL1 and HLA-Bw4, particularly combinations of high density and high-affinity receptor-ligand, proceed more slowly to AIDS than those lacking the HLA-Bw4 epitope [71].
Graded control of HIV infection occurs, correlated to the extent of NK education and the sensitivity of KIR3DL1+ NK cells to missing self targets [71]. We recently demonstrated that the high-avidity combination of HLA-Bw4 molecules exhibiting isoleucine at position 80 (Bw4-80I) and KIR3DL1 receptors expressed at high surface densities show greater degranulation and IFN-γ production in response to HLA-negative K562 target cells compared with all other combinations of KIR3DL1 and HLA-Bw4 [18]. The latter still exhibited greater reactivity that that of KIR3DL1+ NK cells collected from individuals lacking HLA-Bw4 epitopes, however. Importantly, this hierarchy predicted direct cytotoxicity of NK cells against HIV-infected autologous CD4+ T cells, a finding consistent with hierarchical control of HIV infection based on a patient’s compound KIR3DL1 and HLA-B genotypes.
An alternate viral strategy to evade detection by education is to trigger inhibition of NK cells by maintaining normal HLA expression. Both Zika virus and HIV induce upregulation of HLA-C expression on infected cells [70,72]. Since HLA-C interacts primarily with NK cells and not with T cells, and the vast majority of HLA-C molecules exhibit at least one HLA-C1 or HLA-C2 epitope [73], this strategy enables escape from immune surveillance through HLA-C-mediated inhibition of KIR2DL-expressing NK cells. Finally, adaptive NKG2C+ NK cells expanding in response to human cytomegalovirus (HCMV) infection exhibit self-specific KIR[74]. Whether education of this population is beneficial to controlling the virus is not known, but the expression of an activating receptor on an educated NK cell population may help to overcome HLA-driven inhibition.
Viruses may also employ molecular mimicry to avoid detection, whereby expression of virally-encoded HLA-like proteins by infected cells trigger inhibitory signaling in NK cells. For example, HCMV encodes an HLA-like protein, UL-18, that triggers inhibition of NK cells through the conserved leukocyte inhibitory receptor-1, but does not present antigen to T cells [75,76]. In this way, the HCMV has evolved to evade recognition by both major cytolytic lymphocyte subsets.
NK education in cancer
Although HLA expression is often assumed to be low on tumour cells, recent findings reveal that HLA expression persists at some detectable level and/or can easily be induced on the majority of tumours, a conclusion empirically supported by success observed with immune checkpoint inhibitor therapy for multiple different cancers [77,78]. We and others have demonstrated that HLA expression is upregulated in the presence of inflammation, including that driven by cancer therapy itself [29,79]. It is the exceptional tumour that exhibits loss of HLA expression at the genetic level and usually under severe immunological pressure [80], and the majority of studies support a prominent role for uneducated NK cells in cancer control.
A potent role of NK cells in cancer control is evident in patients with acute myelogenous leukemia (AML) receiving hematopoietic cell transplantations (HCT). A significant “graft-versusleukemia” (GVL) effect occurs during HCT in some patients, where donor-derived NK cells insensitive to inhibition by the HLA expressed on a recipient’s leukemia cells mediate potent cancer control [81–83]. Initially, this was thought to reflect killing of “missing self” targets by educated NK cells following HLA-mismatched HCT in which the recipient lacked HLA ligands present in the donor, but surprisingly, a GVL impact has also been observed in patients undergoing HLA-matched HCT. Mechanistically, in the pro-inflammatory pro-stimulatory post-HCT environment, lack of HLA binding of inhibitory KIR expressed on uneducated cells permits NK reactivity against leukemia [28]. As a result, patients lacking one or more of the inhibitory KIR-binding HLA ligands, a so-called “missing ligand” benefit, exhibit lower relapse and improved overall survival compared with those expressing all three KIR ligands.
We recently demonstrated that subtype variability for KIR3DL1 and HLA-B can approximate a “missing ligand” benefit in AML recipients of HLA-matched HCT even if all KIR ligands are present in the patient. Specifically, donor KIR3DL1 and HLA-B subtype combinations predictive of weak or no inhibition are associated with improved AML control after HCT in comparison to combinations predictive of strong inhibition [26]. This may provide a novel opportunity for the transplant physician to select among HLA-matched donors for those carrying KIR3DL1 subtypes unlikely to be inhibited by the tumour to prevent AML relapse after transplant. It is noteworthy that the combinations most beneficial in AML control are the opposite to those beneficial in HIV infection, highlighting the fundamental importance of HLA expression on the diseased cell in determining which NK subpopulation and education level will have the most clinical impact.
The same allele subtype variability has now been demonstrated to impact the success of treatment for other cancers, including those treated with monoclonal antibodies, including rituximab and anti-GD2 [84,85]. In patients bearing all three KIR ligands, these antibody therapies are less effective, as educated NK cells are inhibited from performing cytotoxicity against antibody-tagged tumour cells. As in patients with AML, non-engaging KIR-HLA combinations enable cytotoxicity to proceed, leading to better tumour control and overall survival in these patients.
Manipulating NK education to restore health
Recognizing the power of NK education in determining the outcomes of disease and treatment, many laboratories are now focused on developing NK-based immunotherapies and immunodiagnostics, where they have several advantages. Unlike T cells, NK cells do not generate a graft-versus-host response; therefore, HLA matching is not required for adoptive transfer. Good manufacturing protocols exist for expansion of NK cells ex vivo, and NK education is maintained during both expansion and following adoptive transfer [86–89]. Because NK cell education is defined by a relatively restricted number of receptor and ligand subtypes, it may be possible to establish off-the-shelf approaches using NK-modifying agents and/or NK cell banks to precisely control and redirect NK cell function. These approaches, however successful they may be at ex vivo activation and expansion, cannot fully control for the dominant effects of in vivo inhibition of the transferred NK cells by HLA expression on the recipient’s diseased cells.
Interrupting NK cell inhibition as a strategy to enhance NK cell function against HLA-expressing virus infected and malignant cells has therefore become a goal of many laboratories. To minimize KIR3DL1-mediated NK cell inhibition following HCT in patients with AML, we are undertaking a prospective trial to select among HLA-matched donors for those whose KIR3DL1 allele subtypes enable weak or no inhibition (clinicaltrials.gov NCT02450708). This approach is highly feasible: For 93% of patients in a pilot test, we identified at least one donor whose KIR3DL1 allele subtypes predicted for weak or no inhibition by the donor and recipient HLA-B [26].
An alternative approach is to block inhibition with antibodies targeted against inhibitory receptors. The anti-KIR antibody, 1-7F9/IPH2101, is being tested for this purpose. Though initial studies in mice demonstrated a promising improvement in lymphoma control [90], clinical trials of this same antibody as a single agent in the treatment of multiple myeloma and AML have not demonstrated a substantial benefit of anti-KIR antibodies [91,92]. Approaches to combine this antibody with others to increase NK cell activation, alterations in antibody dosing or schedule, or modifications to prevent binding to activating receptors may be required to unleash the clinical potential of this and other antibodies targeting inhibitory receptors. Whether these antibodies can be employed to promote NK aggression against virally-infected cells or support successful pregnancy; or antibodies against activating receptors may be beneficial in autoimmune diseases, has not been explored.
Conclusions
Variable interactions between KIR and HLA, driven by gene and allele-level diversity, strongly influence NK cell education with impacts on human health. No one pattern of NK education (or co-inheritance of particular KIR and HLA genes) can be universally classified as beneficial or detrimental, as their benefits are disease-specific. Understanding how NK education contributes to successful pregnancy, disease development, resolution and successful treatment may identify KIR and HLA genes as biomarkers for prognosis and their proteins as targets for precision therapy.
Highlights.
NK cell education is endowed by independently-segregating polygenic KIR and HLA which, combined, enable diverse effector functions
Education counterbalances each NK cell’s effector potential with sensitivity for inhibition
Uneducated and educated NK cells have roles in successful pregnancy, disease resistance and susceptibility
Acknowledgments
The authors apologize to those researchers whose work could not be included in this review due to space restrictions. This review was supported by NIH grants AI125651, HL129472, CA23766, AI069197, AI123658, and funding from The Leukemia and Lymphoma Society and Alex’s Lemonade Stand Foundation to KCH. This work was supported by a grant from the Banting Research Foundation, the Canadian Cancer Society ant the Canadian Institutes of Health Research - Institute of Cancer Research (grant #705275) and The Beatrice Hunter Cancer Research Institute to JEB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kung SK, Miller RG. The NK1.1 antigen in NK-mediated F1 antiparent killing in vitro. J Immunol. 1995;154:1624–1633. [PubMed] [Google Scholar]
- 3.Nash WT, Teoh J, Wei H, Gamache A, Brown MG. Know Thyself: NK-Cell Inhibitory Receptors Prompt Self-Tolerance, Education, and Viral Control. Frontiers in Immunology. 2014;5:175. doi: 10.3389/fimmu.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malmberg K-J, Carlsten M, Björklund A, Sohlberg E, Bryceson YT, Ljunggren H-G. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 5**.Boudreau JE, Liu X-R, Zhao Z, Zhang A, Shultz LD, Greiner DL, Dupont B, Hsu KC. Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class I. Immunity. 2016;45:280–291. doi: 10.1016/j.immuni.2016.07.005. Using a NOD-RAG1−/−IL2Rgc−/− mouse model that transgenically expresses human HLA, Boudreau et al., demonstrated that NK cell education is conveyed by HLA present from hematopoietic and stromal cell sources. Once programmed, NK cell education is maintained by HLA present in cis in the NK cell itself. This is potentially important in the context of adoptive NK cell therapies, where maintenance of NK cell function and change in education will predict the activity of NK cells after transfer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. The Journal of Immunology. 2009;182:4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Tian Z. NK cell education via nonclassical MHC and non-MHC ligands. 2016;14:321–330. doi: 10.1038/cmi.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Sunwoo JB, Yang L, Choi T, Song Y-J, French AR, Vlahiotis A, Piccirillo JF, Cella M, Colonna M, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK Cell Education by Inhibitory Receptors for MHC Class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Brodin P, Höglund P. Beyond licensing and disarming: A quantitative view on NK-cell education. Eur J Immunol. 2008;38:2934–2937. doi: 10.1002/eji.200838760. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song Y-J, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 13.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manser AR, Weinhold S, Uhrberg M. Human KIR repertoires: shaped by genetic diversity and evolution. Immunol Rev. 2015;267:178–196. doi: 10.1111/imr.12316. [DOI] [PubMed] [Google Scholar]
- 15.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 16.Tesi B, Schlums H, Cichocki F, Bryceson YT. Epigenetic Regulation of Adaptive NK Cell Diversification. Trends Immunol. 2016;37:451–461. doi: 10.1016/j.it.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 17**.Schlums H, Cichocki F, Tesi B, Theorell J, Béziat V, Holmes TD, Han H, Chiang SCC, Foley B, Mattsson K, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. Following exposure to certain stimuli, adaptive NK cells differentiate, marked by epigenetic alterations. Schlums, Cichocki et al., were the first to systematically characterize these changes in human NK cells collected from human cytomegalovirus seropositive donors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Boudreau JE, Mulrooney TJ, Le Luduec J-B, Barker E, Hsu KC. KIR3DL1 and HLA-B Density and Binding Calibrate NK Education and Response to HIV. The Journal of Immunology. 2016;196:3398–3410. doi: 10.4049/jimmunol.1502469. This study investigated variations in KIR3DL1 and HLA-B subtypes and their role in NK cell education in primary NK cells. In it, the authors demonstrated that avidity of receptor-ligand interactions, as dictated by receptor and ligand surface expression levels and by receptor-ligand affinity, determine the magnitude of NK cell responsiveness against HLA-negative target cells and autologous HIV-infected cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frazier WR, Steiner N, Hou L, Dakshanamurthy S, Hurley CK. Allelic variation in KIR2DL3 generates a KIR2DL2-like receptor with increased binding to its HLA-C ligand. The Journal of Immunology. 2013;190:6198–6208. doi: 10.4049/jimmunol.1300464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moesta AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008;180:3969–3979. doi: 10.4049/jimmunol.180.6.3969. [DOI] [PubMed] [Google Scholar]
- 21*.Saunders PM, Pymm P, Pietra G, Hughes VA, Hitchen C, O’Connor GM, Loiacono F, Widjaja J, Price DA, Falco M, et al. Killer cell immunoglobulin-like receptor 3DL1 polymorphism defines distinct hierarchies of HLA class I recognition. Journal of Experimental Medicine. 2016;213:791–807. doi: 10.1084/jem.20152023. KIR3DL1 and HLA-B form the most extensive receptor-ligand partnership in the human genome, with impacts on binding avidity and NK education. In this work, Saunders et al. refine the understanding of binding between KIR3DL1 and HLA-B subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor GM, McVicar D. The yin-yang of KIR3DL1/S1: molecular mechanisms and cellular function. Critical reviews in immunology. 2013;33:203–218. doi: 10.1615/critrevimmunol.2013007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parham P, Norman PJ, Abi-Rached L, Guethlein LA. Variable NK Cell Receptors Exemplified by Human KIR3DL1/S1. The Journal of Immunology. 2011;187:11–19. doi: 10.4049/jimmunol.0902332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreau JE, Le Luduec J-B, Hsu KC. Development of a novel multiplex PCR assay to detect functional subtypes of KIR3DL1 alleles. PLoS ONE. 2014;9:e99543. doi: 10.1371/journal.pone.0099543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardiner CM, Guethlein LA, Shilling HG, Pando M, Carr WH, Rajalingam R, Vilches C, Parham P. Different NK cell surface phenotypes defined by the DX9 antibody are due to KIR3DL1 gene polymorphism. J Immunol. 2001;166:2992–3001. doi: 10.4049/jimmunol.166.5.2992. [DOI] [PubMed] [Google Scholar]
- 26**.Boudreau JE, Giglio F, Gooley TA, Stevenson PA, Le Luduec J-B, Shaffer BC, Rajalingam R, Hou L, Hurley CK, Noreen H, et al. KIR3DL1/ HL A-B Subtypes Govern Acute Myelogenous Leukemia Relapse After Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35:2268–2278. doi: 10.1200/JCO.2016.70.7059. Following hematopoietic cell transplantation for treatment of hematologic malignancies, patients benefit from a "missing ligand" impact, where uneducated NK cells, refractory to inhibition by the HLA presented on tumor cells, mediate alloreactive control of cancer regrowth. Boudreau et al demonstrated that NK-mediated alloreactivity could proceed if donor KIR3DL1 subtypes were poorly sensitive or insensitive to inhibition by the recipient/donor HLA in HLA-matched HCT. This research, importantly, opens the possibility to select among HLA-matched donors to maximize post-transplant NK-mediated alloreactivity, even in patients encoding all KIR ligands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taner SB, Pando MJ, Roberts A, Schellekens J, Marsh SGE, Malmberg K-J, Parham P, Brodsky FM. Interactions of NK Cell Receptor KIR3DL1*004 with Chaperones and Conformation-Specific Antibody Reveal a Functional Folded State As Well As Predominant Intracellular Retention. The Journal of Immunology. 2010;186:62–72. doi: 10.4049/jimmunol.0903657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Venstrom JM, Liu X-R, Pring J, Hasan RS, O'Reilly RJ, Hsu KC. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113:3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarek N, Le Luduec J-B, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, Modak S, Heller G, Dupont B, Cheung N-KV, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122:3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Horowitz A, Djaoud Z, Nemat-Gorgani N, Blokhuis J, Hilton HG, Béziat V, Malmberg K-J, Norman PJ, Guethlein LA, Parham P. Class I HLA haplotypes form two schools that educate NK cells in different ways. Sci Immunol. 2016;1:eaag1672–eaag1672. doi: 10.1126/sciimmunol.aag1672. The invariant receptor, NKG2A, binds HLA-E presenting peptides derived from leader sequences of with methionine encoded at position −21 (−21M). Horowitz et al., demonstrated that −21M is rarely encoded on HLA haplotypes with ligands for KIR. The authors conclude that HLA haplotypes have co-evolved with NK cells to enable two modes for NK education: one which relies primarily on NKG2A and one which relies on KIR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yawata M, Yawata N, Draghi M, Partheniou F, Little A-M, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112:2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pittari G, Liu XR, Selvakumar A, Zhao Z, Merino E, Huse M, Chewning JH, Hsu KC, Dupont B. NK Cell Tolerance of Self-Specific Activating Receptor KIR2DS1 in Individuals with Cognate HLA-C2 Ligand. The Journal of Immunology. 2013;190:4650–4660. doi: 10.4049/jimmunol.1202120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Garcia-Beltran WF, Hölzemer A, Martrus G, Chung AW, Pacheco Y, Simoneau CR, Rucevic M, Lamothe-Molina PA, Pertel T, Kim T-E, et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. 2016;17:1067–1074. doi: 10.1038/ni.3513. Identification of ligands for activating KIR has been elusive. In this report, the authors demonstrate that HLA-F in its open conformation (i.e. without β2m or bound peptide) binds to KIR3DS1 to stimulate activation. Open conformation HLA-F is highly conserved, and expressed on the surface of activated CD4+ T cells, providing a mechanistic explanation for a known protective impact of KIR3DS1 in HIV. This work opens the possibility to directly and broadly explore the role of KIR3DS1 in NK education and disease control. See also reference 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.David G, Djaoud Z, Willem C, Legrand N, Rettman P, Gagne K, Cesbron A, Retiere C. Large Spectrum of HLA-C Recognition by Killer Ig-like Receptor (KIR)2DL2 and KIR2DL3 and Restricted C1 Specificity of KIR2DS2: Dominant Impact of KIR2DL2/KIR2DS2 on KIR2D NK Cell Repertoire Formation. The Journal of Immunology. 2013;191:4778–4788. doi: 10.4049/jimmunol.1301580. [DOI] [PubMed] [Google Scholar]
- 35**.Burian A, Wang KL, Finton KAK, Lee N, Ishitani A, Strong RK, Geraghty DE. HLA-F and MHC-I Open Conformers Bind Natural Killer Cell Ig-Like Receptor KIR3DS1. PLoS ONE. 2016;11:e0163297–12. doi: 10.1371/journal.pone.0163297. Identification of ligands for activating KIR has been elusive. In this report, the authors demonstrate that HLA-F in its open conformation (i.e. without β2m or bound peptide) binds to KIR3DS1 to stimulate activation. Open conformation HLA-F is highly conserved, and expressed on the surface of activated CD4+ T cells, providing a mechanistic explanation for a known protective impact of KIR3DS1 in HIV. This work opens the possibility to directly and broadly explore the role of KIR3DS1 in NK education and disease control. See also reference 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Redman CC, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colucci F. The role of KIR and HLA interactions in pregnancy complications. Immunogenetics. 2017;69:557–565. doi: 10.1007/s00251-017-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell–activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Li Y-P, Ding B, Zhao Y-R, Chen Z-J, Xu C-Y, Fu Y-B, Wang X-T. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J Obstet Gynaecol Res. 2014;40:1288–1295. doi: 10.1111/jog.12329. [DOI] [PubMed] [Google Scholar]
- 40.King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, Burrows TD, Loke YW. Recognition of Trophoblast HLA Class I Molecules by Decidual NK Cell Receptors—A Review. Placenta. 2000;21:S81–S85. doi: 10.1053/plac.1999.0520. [DOI] [PubMed] [Google Scholar]
- 41.King A, Hiby SE, Verma S, Burrows T, Gardner L, Loke YW. Uterine NK cells and trophoblast HLA class I molecules. Am J Reprod Immunol. 1997;37:459–462. doi: 10.1111/j.1600-0897.1997.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 42.Moffett A, Colucci F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev. 2015;267:283–297. doi: 10.1111/imr.12323. [DOI] [PubMed] [Google Scholar]
- 43.Male V, Sharkey A, Masters L, Kennedy PR, Farrell LE, Moffett A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur J Immunol. 2011;41:3017–3027. doi: 10.1002/eji.201141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Sharkey AM, Xiong S, Kennedy PR, Gardner L, Farrell LE, Chazara O, Ivarsson MA, Hiby SE, Colucci F, Moffett A. Tissue-Specific Education of Decidual NK Cells. The Journal of Immunology. 2015;195:3026–3032. doi: 10.4049/jimmunol.1501229. Successful pregnancy is facilitated by uterine NK cells, which facilitate trophoblast invasion and formation of the spiral arteries. In this work, Sharkey et al demonstrate that the education of NK cells in the decidua is markedly different from that of the circulating peripheral blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, Gjessing HK, Carrington M, Moffett A. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. The Journal of Immunology. 2014;192:5069–5073. doi: 10.4049/jimmunol.1400577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Zhao Y-R, Jiao Y-L, Wang L-C, Li J-F, Cui B, Xu C-Y, Shi Y-H, Chen Z-J. Increased activating killer immunoglobulin-like receptor genes and decreased specific HLA-C alleles in couples with recurrent spontaneous abortion. Biochem Biophys Res Commun. 2007;360:696–701. doi: 10.1016/j.bbrc.2007.06.125. [DOI] [PubMed] [Google Scholar]
- 47.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008;23:972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 48.Alecsandru D, Garrido N, Vicario JL, Barrio A, Aparicio P, Requena A, Garcia-Velasco JA. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum. Reprod. 2014;29:2637–2643. doi: 10.1093/humrep/deu251. [DOI] [PubMed] [Google Scholar]
- 49.Dambaeva SV, Lee DH, Sung N, Chen C-Y, Bao S, Gilman-Sachs A, Kwak-Kim J, Beaman KD. Recurrent Pregnancy Loss in Women with Killer Cell Immunoglobulin-Like Receptor KIR2DS1 is Associated with an Increased HLA-C2 Allelic Frequency. Am J Reprod Immunol. 2016;75:94–103. doi: 10.1111/aji.12453. [DOI] [PubMed] [Google Scholar]
- 50.Hiby SE, Walker JJ, O'shaughnessy KM, Redman CWG, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakimuli A, Chazara O, Byamugisha J, Elliott AM, Kaleebu P, Mirembe F, Moffett A. Pregnancy, parturition and preeclampsia in women of African ancestry. Am. J. Obstet. Gynecol. 2014;210:510–520.e1. doi: 10.1016/j.ajog.2013.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 53.Villanueva J, Lee S, Giannini EH, Graham TB, Passo MH, Filipovich A, Grom AA. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res. Ther. 2005;7:R30–7. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res. Ther. 2013;15:216. doi: 10.1186/ar4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge X, Li C-R, Yang J, Wang G-B. Aberrantly decreased levels of NKG2D expression in children with kawasaki disease. Scand J Immunol. 2013;77:389–397. doi: 10.1111/sji.12022. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen N, Ødum N, Ursø B, Lanier LL, Spee P. Cytotoxicity of CD56bright NK Cells towards Autologous Activated CD4+ T Cells Is Mediated through NKG2D, LFA-1 and TRAIL and Dampened via CD94/NKG2A. PLoS ONE. 2012;7:e31959. doi: 10.1371/journal.pone.0031959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunter PJ, Nistala K, Jina N, Eddaoudi A, Thomson W, Hubank M, Wedderburn LR. Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis & Rheumatology. 2010;62:896–907. doi: 10.1002/art.27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chanvillard C, Jacolik RF, Infante Duarte C, Nayak RC. The Role of Natural Killer Cells in Multiple Sclerosis and Their Therapeutic Implications. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balasa B, Deng C, Lee J, Bradley LM, Dalton DK, Christadoss P, Sarvetnick N. Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med. 1997;186:385–391. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bielekova B, Howard T, Packer AN, Richert N, Blevins G, Ohayon J, Waldmann TA, McFarland HF, Martin R. Effect of Anti-CD25 Antibody Daclizumab in the Inhibition of Inflammation and Stabilization of Disease Progression in Multiple Sclerosis. Arch Neurol. 2009;66:483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, O'Neill G, Neyer L, Sheridan J, Wang C, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. The Lancet Neurology. 2010;9:381–390. doi: 10.1016/S1474-4422(10)70033-8. [DOI] [PubMed] [Google Scholar]
- 62.Skarica M, Eckstein C, Whartenby KA, Calabresi PA. Novel mechanisms of immune modulation of natalizumab in multiple sclerosis patients. Journal of Neuroimmunology. 2011;235:70–76. doi: 10.1016/j.jneuroim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Anolik JH. B cell biology and dysfunction in SLE. Bulletin of the NYU hospital for joint diseases. 2007;65:182–186. [PubMed] [Google Scholar]
- 64.Kazkaz H, Isenberg D. Anti B cell therapy (rituximab) in the treatment of autoimmune diseases. Current Opinion in Pharmacology. 2004;4:398–402. doi: 10.1016/j.coph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Shahsavar F, Mapar S, Ahmadi SAY. Multiple sclerosis is accompanied by lack of KIR2DS1 gene: A meta-analysis. GDATA. 2016;10:75–78. doi: 10.1016/j.gdata.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bettencourt A, Silva AM, Carvalho C, Leal B, Santos E, Costa PP, Silva BM. The role of KIR2DS1 in multiple sclerosis - KIR in Portuguese MS patients. Journal of Neuroimmunology. 2014;269:52–55. doi: 10.1016/j.jneuroim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Fusco C, Guerini FR, Nocera G, Ventrella G, Caputo D, Valentino MA, Agliardi C, Gallotti J, Morra VB, Florio C, et al. KIRs and their HLA ligands in Remitting–Relapsing Multiple Sclerosis. Journal of Neuroimmunology. 2010;229:232–237. doi: 10.1016/j.jneuroim.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 69.Apps R, Meng Z, Del Prete GQ, Lifson JD, Zhou M, Carrington M. Relative expression levels of the HLA class-I proteins in normal and HIV-infected cells. The Journal of Immunology. 2015;194:3594–3600. doi: 10.4049/jimmunol.1403234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, et al. Influence of HLA-C Expression Level on HIV Control. Science. 2013;340:87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin MP, Gao X, Lee J-H, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 72*.Glasner A, Oiknine Djian E, Weisblum Y, Diab M, Panet A, Wolf DG, Mandelboim O. Zika virus escapes NK cell detection by upregulating MHC class I molecules. J Virol. 2017 doi: 10.1128/JVI.00785-17. In this work, Glasner et al., demonstrate, for the first time, that Zika virus infection broadly induces upregulation of HLA. Direct measurement of specific HLA alleles is challenging with the available reagents, but the authors demonstrate that recombinant KIR2DL2 proteins can bind Zika virus infected cells. These results imply that HLA-C, at a minimum, is among the ligands upregulated in response to infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parham P, Norman PJ, Abi-Rached L, Guethlein LA. Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:800–811. doi: 10.1098/rstb.2011.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Béziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debré P, et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 75*.Chen KC, Stanton RJ, Banat JJ, Wills MR. Leukocyte Immunoglobulin-Like Receptor 1-Expressing Human Natural Killer Cell Subsets Differentially Recognize Isolates of Human Cytomegalovirus through the Viral Major Histocompatibility Complex Class I Homolog UL18. J Virol. 2016;90:3123–3137. doi: 10.1128/JVI.02614-15. UL18 is a homolog of HLA encoded by human cytomegalovirus (HCMV) which, upon binding to the LIR-1 inhibitory receptor, triggers inhibition of NK cells. The work described by Chen at el demonstrates that the efficiency of this inhibition differs between viral strains. Moreover, they show that LIR-1, not NKG2C, is the dominant receptor predicting NK-mediated control of HCMV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cerboni C, Achour A, Wärnmark A, Mousavi-Jazi M, Sandalova T, Hsu M-L, Cosman D, Kärre K, Carbone E. Spontaneous mutations in the human CMV HLA class I homologue UL18 affect its binding to the inhibitory receptor LIR-1/ILT2/CD85j. Eur J Immunol. 2006;36:732–741. doi: 10.1002/eji.200425220. [DOI] [PubMed] [Google Scholar]
- 77**.Malmberg K-J, Sohlberg E, Goodridge JP, Ljunggren H-G. Immune selection during tumor checkpoint inhibition therapy paves way for NK-cell “missing self” recognition. Immunogenetics. 2017;69:547–556. doi: 10.1007/s00251-017-1011-9. The vast majority of cancers maintain HLA at the genetic level, which can be induced by inflammation. In this and reference 78, an emerging phenomenon, where HLA expression is lost at the genetic level following checkpoint inhibition therapy, is described. This work is important because it implies that educated NK cells will be important complementary mediators for successful checkpoint inhibition therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. The vast majority of cancers maintain HLA at the genetic level, which can be induced by inflammation. In this and reference 77, an emerging phenomenon, where HLA expression is lost at the genetic level following checkpoint inhibition therapy, is described. This work is important because it implies that educated NK cells will be important complementary mediators for successful checkpoint inhibition therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Propper DJ, Chao D, Braybrooke JP, Bahl P, Thavasu P, Balkwill F, Turley H, Dobbs N, Gatter K, Talbot DC, et al. Low-dose IFN-gamma induces tumor MHC expression in metastatic malignant melanoma. Clin Cancer Res. 2003;9:84–92. [PubMed] [Google Scholar]
- 80.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, Perrelli NF, Cosentino C, Torri F, Angius A, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 81.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O'Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, Guethlein LA, Trachtenberg EA, Haagenson M, Horowitz MM, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84**.Forlenza CJ, Boudreau JE, Zheng J, Le Luduec J-B, Chamberlain E, Heller G, Cheung N-KV, Hsu KC. KIR3DL1 Allelic Polymorphism and HLA-B Epitopes Modulate Response to Anti-GD2 Monoclonal Antibody in Patients With Neuroblastoma. J Clin Oncol. 2016;34:2443–2451. doi: 10.1200/JCO.2015.64.9558. This paper was the first to demonstrate that variations in KIR3DL1 and HLA-B allele subtypes predict the efficacy of antibody-mediated cellular cytotoxicity in patients with neuroblastoma treated with anti-GD2 antibody. This demonstrates that the efficacy of antibodies used for cancer immunotherapy may be variably effective and endorses KIR and HLA allele typing to enhance prognosis and deliver precise therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85**.Erbe AK, Wang W, Reville PK, Carmichael L, Kim K, Mendonca EA, Song Y, Hank JA, London WB, Naranjo A, et al. HLA-Bw4-I-80 Isoform Differentially Influences Clinical Outcome As Compared to HLA-Bw4-T-80 and HLA-A-Bw4 Isoforms in Rituximab or Dinutuximab-Based Cancer Immunotherapy. Frontiers in Immunology. 2017;8:675. doi: 10.3389/fimmu.2017.00675. Erbe et al. demonstrated that patients with neuroblastoma receiving anti-GD2 or rituximab were more likely to benefit from the immunotherapy if they exhibited KIR3DL1 and the Bw4-80T isoform. These findings endorse KIR and HLA genotyping for patients with cancer undergoing immunotherapy, where outcomes may differ as a function of their genotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of Highly Cytotoxic Human Natural Killer Cells for Cancer Cell Therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boudreau JE, Liu X-R, Zhao Z, Zhang A, Shultz LD, Greiner DL, Dupont B, Hsu KC. Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class I. Immunity. 2016;45:280–291. doi: 10.1016/j.immuni.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89**.Wang W, Erbe AK, Alderson KA, Phillips E, Gallenberger M, Gan J, Campana D, Hank JA, Sondel PM. Human NK cells maintain licensing status and are subject to killer immunoglobulin-like receptor (KIR) and KIR-ligand inhibition following ex vivo expansion. Cancer Immunol Immunother. 2016;65:1047–1059. doi: 10.1007/s00262-016-1864-z. NK cells expanded ex vivo are potent tools for cancer immunotherapy. This paper demonstrates that NK cells expanded ex vivo maintain their education and sensitivity to inhibition even after ex vivo expansion and activation, opening the possibility of selecting NK cell donors for expansion and precision immunotherapy based on their education. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuséri N, Bonnafous C, Czerwinski D, Rajapaksa A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benson DM, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, Bakan C, Andre P, Efebera Y, Tiollier J, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.InnatePharma. Innate pharma announces top-line results from effikir trial evaluating the efficacy of lirilumab as a single agent in elderly patients with acute myeloid leukemia [Internet] 2017 httpinnate-pharma.comennews-eventspress-releasesinnatepharma-announces-top-line-results-effikir-trial-evaluating-efficacy-lirilumab-singleagent-elderly-patients-acute-myeloid-leukemia, [no volume]
- 93.Nakimuli A, Chazara O, Hiby SE, Farrell L, Tukwasibwe S, Jayaraman J, Traherne JA, Trowsdale J, Colucci F, Lougee E, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci USA. 2015;112:845–850. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faridi RM, Agrawal S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Human Reproduction. 2011;26:491–497. doi: 10.1093/humrep/deq341. [DOI] [PubMed] [Google Scholar]
- 95.Beltrame LM, Sell AM, Moliterno RA, Clementino SL, Cardozo DM, Dalalio MM, Fonzar UJ, Visentainer JE. Influence of KIRgenes and their HLA ligands in susceptibility to dengue in a population from southern Brazil. Tissue Antigens. 2013;82:397–404. doi: 10.1111/tan.12256. [DOI] [PubMed] [Google Scholar]
- 96.Hadaya K, de Rham C, Bandelier C, Ferrari-Lacraz S, Jendly S, Berney T, Buhler L, Kaiser L, Seebach JD, Tiercy JM, et al. Natural killer cell receptor repertoire and their ligands, and the risk of CMV infection after kidney transplantation. Am. J. Transplant. 2008;8:2674–2683. doi: 10.1111/j.1600-6143.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 97.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O apos Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones DC, Edgar RS, Ahmad T, Cummings JRF, Jewell DP, Trowsdale J, Young NT. Killer Ig-like receptor (KIR) genotype and HLA ligand combinations in ulcerative colitis susceptibility. Genes Immun. 2006;7:576–582. doi: 10.1038/sj.gene.6364333. [DOI] [PubMed] [Google Scholar]
- 99*.Bossi G, Mannarino S, Pietrogrande MC, Salice P, Dellepiane RM, Cremaschi AL, Corana G, Tozzo A, Capittini C, De Silvestri A, et al. Genetic epistasis between killer immunoglobulin-like receptors and human leukocyte antigens in Kawasaki disease susceptibility. Genes Immun. 2015;16:481–487. doi: 10.1038/gene.2015.34. Although much research has been performed on the implications of NK education in pregnancy, infectious disease and cancer, less is understood of the role of NK cells in autoimmune and infectious disease. The paper by Bossi et al studied 100 children with Kawasaki disease, comparing their KIR and HLA genotypes with those of 270 healthy individuals. They found enrichment of KIR2DL2 and KIR2DS2 + HLA-C1 compound genotypes among patients, implicating NK cell education in inflammatory disease. [DOI] [PubMed] [Google Scholar]
- 100.Pan N, Qiu J, Sun H, Miao F, Shi Q, Xu J, Jiang W, Jin H, Xie W, He Y, et al. Combination of Human Leukocyte Antigen and Killer Cell Immunoglobulin-Like Receptor Genetic Background Influences the Onset Age of Hepatocellular Carcinoma in Male Patients with Hepatitis B Virus Infection. Clin Dev Immunol. 2013;2013:1–7. doi: 10.1155/2013/874514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rizzo R, Gentili V, Rotola A, Bortolotti D, Cassai E, Di Luca D. Implication of HLA-C and KIRAlleles in Human Papillomavirus Infection and Associated Cervical Lesions. Viral Immunol. 2014;27:468–470. doi: 10.1089/vim.2014.0017. [DOI] [PubMed] [Google Scholar]
- 102.Sekine T, Marin D, Cao K, Li L, Mehta P, Shaim H, Sobieski C, Jones R, Oran B, Hosing C, et al. Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood. 2016;128:297–312. doi: 10.1182/blood-2016-03-706317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shastry A, Sedimbi SK, Rajalingam R, Nikitina-Zake L, Rumba I, Wigzell H, Sanjeevi CB. Combination of KIR 2DL2 and HLA-C1 (Asn 80) confers susceptibility to type 1 diabetes in Latvians. Int J Immunogenet. 2008;35:439–446. doi: 10.1111/j.1744-313X.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 104.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105*.Morin SJ, Treff NR, Tao X, Scott RT, Franasiak JM, Juneau CR, Maguire M. Combination of uterine natural killer cell immunoglobulin receptor haplotype and trophoblastic HLA-C ligand influences the risk of pregnancy loss: a retrospective cohort analysis of direct embryo genotyping data from euploid transfers. Fertility and Sterility. 2017;107:677–683.e2. doi: 10.1016/j.fertnstert.2016.12.004. There are strong associations with NK cell education and successful pregnancy, but most have relied solely on the mothers’ genotypes or paternal antigens to infer what the fetus may carry. In this study, researchers used DNA from in vitro fertilized eggs to directly genotype embryos, correlating maternal and embryo KIR and HLA genotypes with the ultimate success or loss of pregnancy and found that fetal HLA-C1 was associated with protection from pregnancy loss in mothers carrying KIR-A haplotypes. [DOI] [PubMed] [Google Scholar]
- 106.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 107.Wauquier N, Padilla C, Becquart P, Leroy E, Vieillard V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics. 2010;62:767–771. doi: 10.1007/s00251-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hirayasu K, Ohashi J, Kashiwase K, Hananantachai H, Naka I, Ogawa A, Takanashi M, Satake M, Nakajima K, Parham P, et al. Significant Association of KIR2DL3-HLA-C1 Combination with Cerebral Malaria and Implications for Co-evolution of KIR and HLA. PLoS Pathog. 2012;8:e1002565–12. doi: 10.1371/journal.ppat.1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Bona D, Aiello A, Colomba C, Bilancia M, Accardi G, Rubino R, Giannitrapani L, Tuttolomondo A, Cascio A, Caiaffa MF, et al. KIR2DL3 and the KIR ligand groups HLA-A-Bw4 and HLA-C2 predict the outcome of hepatitis B virus infection. J Viral Hepat. 2017;24:768–775. doi: 10.1111/jvh.12698. [DOI] [PubMed] [Google Scholar]
- 110.Naiyer MM, Cassidy SA, Magri A, Cowton V, Chen K, Mansour S, Kranidioti H, Mbirbindi B, Rettman P, Harris S, et al. KIR2DS2 recognizes conserved peptides derived from viral helicases in the context of HLA-C. Sci Immunol. 2017;2:eaal5296. doi: 10.1126/sciimmunol.aal5296. [DOI] [PubMed] [Google Scholar]
- 111.Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, Moss PAH, Briggs DC. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103:1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 112.Omar Al S, Middleton D, Marshall E, Porter D, Xinarianos G, Raji O, Field JK, Christmas SE. Associations between genes for killer immunoglobulin-like receptors and their ligands in patients with solid tumors. HIM. 2010;71:976–981. doi: 10.1016/j.humimm.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 113.De Re V, Caggiari L, De Zorzi M, Repetto O, Zignego AL, Izzo F, Tornesello ML, Buonaguro FM, Mangia A, Sansonno D, et al. Genetic Diversity of the KIR/HLA System and Susceptibility to Hepatitis C Virus-Related Diseases. PLoS ONE. 2015;10:e0117420–21. doi: 10.1371/journal.pone.0117420. [DOI] [PMC free article] [PubMed] [Google Scholar]