Abstract
Background
Initial studies have provided a mixed perspective of the efficacy of d-cycloserine (DCS) for augmenting the efficacy of exposure-based cognitive behavioral therapy (CBT) for panic disorder. In this multicenter trial, we examine the magnitude of DCS augmentation effects for an ultra-brief program of CBT.
Methods
We conducted a double-blind, controlled trial at three treatment sites, randomizing 180 adults with a primary diagnosis of panic disorder to 5 sessions of treatment, with study pill (50 mg DCS or matching placebo) administered 1 hour prior to the final 3 sessions. Two booster sessions were subsequently provided, and outcome was assessed at post-treatment and 1-month, 2-month, and 6-month follow-up assessments. The primary outcome was the degree of reduction in the Panic Disorder Severity Scale. Additional analyses examined the role of severity and current antidepressant or benzodiazepine use as moderators of DCS augmentation effects.
Results
DCS augmentation resulted in significant benefit only early in the trial, with no beneficial effects of DCS augmentation evident at follow-up evaluations. We did not find that baseline severity or antidepressant or benzodiazepine use moderated DCS efficacy, but benzodiazepine use was associated with lower efficacy of CBT regardless of augmentation condition.
Conclusions
Consistent with other recent multicenter trials, the benefit of DCS was less than indicated by pilot study and reflected an acceleration of treatment response evident at treatment endpoint, but no advantage in response over follow-up evaluation. Our results did not support severity or concomitant medication moderators observed in previous trials of DCS augmentation.
Clinical Trials Registry Name
Exposure, D-Cycloserine Enhancement, and Genetic Modulators in Panic Disorder (DCSPanic)
Clinical Trials URL
Clinical Trials Registry Number
Keywords: anxiety, panic attacks/agoraphobia, CBT, clinical trials, pharmacotherapy
Introduction
Comparative trials1 and meta-analytic comparisons2 show that cognitive behavioral therapy (CBT) is at least as effective as pharmacotherapy for treatment of panic disorder; and also offers strong treatment acceptance and tolerability1; 3, improvement in comorbid conditions4, and maintenance of treatment gains and strong cost-efficacy5; 6. Despite these strengths, many patients fail to respond adequately to CBT7. The addition of benzodiazepines or antidepressants to CBT is a readily available augmentation strategy, but meta-analyses indicate that the benefits of these strategies are modest and quickly lost during follow-up intervals when medication use is not maintained8; 9. Further, animal studies, clinical observation, and randomized trial data suggest that traditional antidepressant and anxiolytic medications may interfere with extinction learning10–12, attenuating the magnitude of benefits that otherwise might be afforded by the combination of two active treatments. Accordingly, there is a clear need for alternative augmentation strategies to enhance outcomes for panic disorder.
The development of d-cycloserine (DCS) as an augmentation strategy for exposure-based CBT has been noted to be one of the particular achievements of translational research13. Since the first report of clinical application of DCS by Ressler and associates14, DCS augmentation of exposure-based CBT has been examined in over a dozen randomized trials in the anxiety and trauma-related disorders15; 16. For our pilot study in panic disorder, DCS was administered prior to the final three sessions of a five session protocol; significant advantages, reflecting large effect sizes, were seen relative to placebo augmentation at treatment endpoint and 1-month follow-up17. Subsequently, Siegmund and colleagues18 examined DCS augmentation of CBT for panic disorder in an 11-session protocol. They reported strong overall treatment effects, but no randomized treatment differences between DCS and placebo-augmented patients. Nonetheless, they found an interaction (reflecting a large effect size) indicating a faster treatment response among more severely ill patients receiving DCS. These results are consistent with studies of other anxiety disorders that have shown an acceleration of treatment response with DCS augmentation, observed in the absence of an endpoint advantage when longer protocols of treatment are used19; 20. In contrast, endpoint advantages for DCS are more consistently observed when especially brief treatments are used, in line with the hypothesis that endpoint advantages are primarily seen when the placebo-augmented group does not have additional exposure sessions to allow them to catch up, in terms of response, to the DCS-augmented group15.
The purpose of the present study is to examine DCS augmentation of exposure-based CBT for panic disorder in an adequately-powered, double-blind, clinical trial. Unlike other multicenter DCS trials that studied medication-free patients20, we allowed patients taking antidepressant or benzodiazepine medications, ensuring that we were evaluating DCS under the conditions by which it is likely to be applied clinically, including patients who have failed to respond to pharmacotherapy. We hypothesized that DCS-augmentation of brief exposure-based CBT would enhance treatment outcome relative to augmentation with pill placebo, and that these advantages would be maintained over follow-up. Given the findings suggesting moderation of DCS effects by disorder severity18 and antidepressant21 or benzodiazepine12 medication, emergent since the design of this trial, we also evaluated potential moderator effects for disorder severity and concomitant medication as additions to our planned analysis.
Materials and Methods
Participants
Participants were recruited and enrolled at Boston University (BU; n = 68), the Institute of Living in Hartford, Connecticut (IOL; n = 59), and a combined site of Massachusetts General Hospital/Rush (MGH/Rush; n = 53), as the second author (MHP) changed institutions during the study. All study procedures were approved by the Institutional Review Boards of each of these sites and was performed in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Interested participants were screened by phone and, if eligible, were invited for in-person informed consent and diagnostic and severity evaluations with masters- or doctoral-level clinicians. To be considered for the study patients had to be 18 years or older and have a primary (disorder of greatest distress/disability) DSM-IV diagnosis of panic disorder, with current Clinical Global Impression-Severity (CGI-S) score of greater than 4. Diagnostic exclusion criteria included a lifetime history of a psychotic disorder, bipolar disorder, or a developmental disorder; posttraumatic stress disorder, substance use disorder, eating disorder, or organic mental disorder within the past 6 months; agoraphobia sufficiently severe as to limit the patient’s ability to travel to and participate in weekly sessions; suicidal ideation or suicidal behaviors within the past 6 months; and personality dysfunction likely to interfere with study participation.
Medical exclusion factors included serious medical illness or instability for which hospitalization may be likely within the next year; current or past history of seizures (other than febrile seizures in childhood); pregnant women, lactating women, and women of childbearing potential who were not using medically accepted forms of contraception; history of head trauma causing loss of consciousness, seizure, or ongoing cognitive impairment; current use of isoniazid. Furthermore, patients participating in concurrent psychotherapy initiated within three months of baseline as well as those with past CBT for panic disorder that emphasized interoceptive exposure were ineligible for the study. Finally, eligible individuals were either free of concurrent psychotropic medication for at least two weeks prior to initiation of randomized treatment or were on eight weeks of stable dosages of medication.
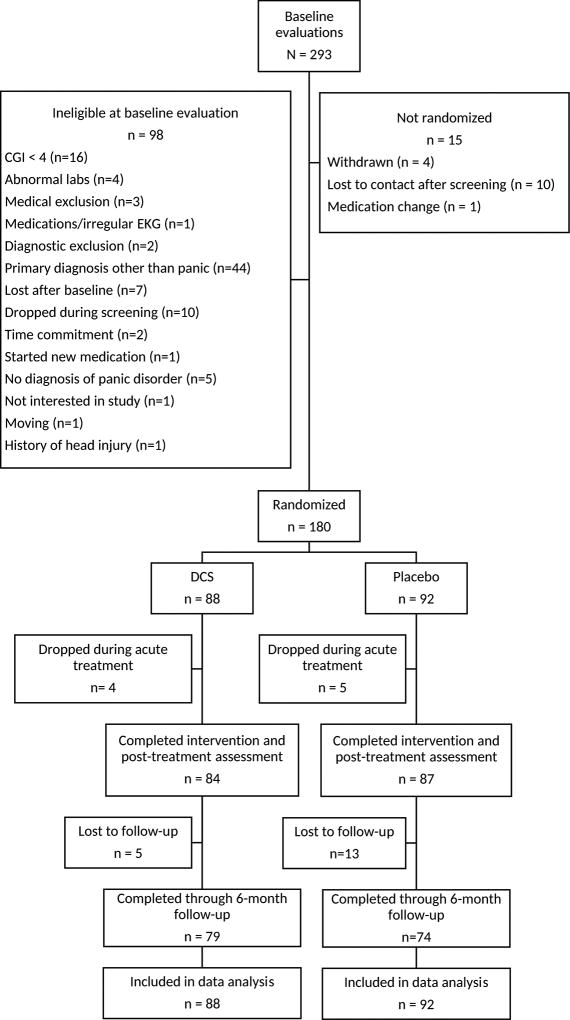
Participants were enrolled in the study between July 2008 and January 2013 with follow-up assessments completed by August 2013. Participant flow throughout the study is summarized in Figure 1. Participants were considered randomized when they took their first study pill; the study was considered completed when follow-up sample size targets were met. A total of 180 adults were randomized to DCS or placebo augmentation. A total of 171 completed treatment, and 18 of these participants were lost to follow-up prior to the final follow-up assessment.
Figure 1.
CONSORT diagram
Assessments
Core assessment windows were baseline, prior to session 4, one week following session 5 (treatment endpoint), and follow-up assessments at 1, 2, 3, and 6 months after completion of the acute treatment. Clinicians also completed the CGI prior to every treatment visit as further specified below. Panic disorder and psychiatric comorbidities were evaluated by the Structured Clinical Interview for DSM-IV22. Agreement for a between-clinician assessment of the primary diagnosis of panic disorder was 100% in a subsample of 52 participants re-evaluated at one site, using a different structured interview (the Anxiety Disorders Interview Schedule for DSM-IV;23).
The primary treatment outcome measure was the Panic Disorder Severity Scale (PDSS;24), a widely applied measure of panic disorder severity that has good inter-rater reliability24 and an alpha of 0.71 in the context of the present study. Raters completed training and ongoing calibration across sites using recorded severity evaluations. Other clinician-rated outcome measures included the Clinician Global Impression-Severity Scale (CGI-S;25) with use of specific rating anchor points, the Montgomery-Asberg Depression Rating Scale (MADRS;26), the Hamilton Anxiety Rating Scale- structured interview version (SIGH-A;27), and the Range of Impaired Functioning Tool (LIFE-RIFT;28). The CGI-S was used in determining whether patients met the “CGI-S of 1 or 2” component of the “remission status” criteria (i.e., zero panic attacks and CGI-S of 1 or 2 at endpoint). Self-report secondary outcome measures included the Anxiety Sensitivity Index (ASI;29) and the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q;30).
Randomized Intervention
Patients were randomized to receive either adjunctive DCS or placebo, which were administered as a 50-mg pill one-hour prior to the exposure procedures on three study visits (i.e., sessions 3–5 of exposure treatment). Randomization was stratified by site and symptom severity (i.e., CGI severity score of ≥ 5). Within each stratum, we used block randomization with varying block sizes. Randomization assignments were developed by the statistical team at Yale University, were generated prior to allocating participants, and were concealed until the end of the study. All study staff involved in patient care, evaluation, or supervision were blind to group assignment until the end of the study. Adverse events were monitored and elicited by open questioning of the study clinician, assessing adverse events occurring over the last hour as well as during the week following the last dose of DCS.
CBT
The core treatment for augmentation was a 5-session, manualized protocol of CBT with weekly sessions31. The first session (60 min) provided patients with a model of panic disorder and its treatment with CBT, and included initial monitoring assignments (cognitions around panic attacks). In the second session (60 min), patients were introduced to interoceptive exposure (exposure to somatic sensations of anxiety; e.g., hyperventilation to induce dizziness, paresthesias, flushes, etc.) and more active experiences evaluating and changing their thoughts associated with anxiety and panic (cognitive restructuring). The next three sessions were devoted to more intensive interoceptive exposure, delivered in a 90-min format, and preceded by use of the blinded study medication. Sessions 4 and 5 continued this program and also included interoceptive exposure practice outside the office (in order to provide patients with practice with sensations in situations that may motivate agoraphobic avoidance). Home practice assignments were assigned after each session. Participants were also provided with a booster session of CBT following the one- and two-month follow-up assessments. Study therapists were doctoral and graduate-student level providers who first viewed videotaped presentation of all core interventions, and then supervision by the first author in monthly cross-site phone calls in addition to weekly supervision provided on site.
Data Analysis
Power calculations were informed by pilot data indicating a large effect size for DCS augmentation17, but we used a more conservative estimate of d = 0.6 to select power for this multicenter trial. A 6-month follow-up sample size of 130 provided more than 90% power to detect an effect of that size assuming two-sided alpha level of 0.05.
Generalized linear mixed effects models (GLMM) were used to account for the longitudinal nature of the data and to use all available data for each subject. The primary outcome measure was percent change from baseline on the PDSS. Secondary outcomes measures included: (a) remission status and (b) percent change from baseline on anxiety sensitivity, quality of life satisfaction, degree of impairment, depression symptoms, and non-panic anxiety symptoms. We used likelihood-based estimation methods that provide valid results under missing-at-random assumptions. Comparison of dropout rates by treatment group was performed using a χ2 test. In sensitivity analysis of remission rates, dropouts were counted as non-remitted.
We conducted separate analyses for the primary outcome measure (continuous PDSS score) and for the secondary continuous outcome measures with treatment group as a between-subject factor, time as a within-subject factor and the interaction between treatment and time. We also controlled for site effects by including site (BU, IOL or MGH/Rush) and its interactions with treatment and time in the models. The best fitting variance-covariance structure for the linear mixed effects models was selected based on Bayesian Information Criterion32. For the binary outcome variable remission, we used a GLMM approach with logit link and random subject effects. Effects in the analysis of the primary outcome measure (PDSS) were considered significant at the p < 0.05 level; whereas, effects in the analysis of the secondary outcome measures were considered significant at the Bonferroni-adjusted p < 0.01 significance levels. In addition, we assessed whether baseline disorder severity, antidepressant use, or benzodiazepine use moderated augmentation effects from DCS. These variables were examined alone and in interaction with the randomized treatment conditions in the context of the GLMM models described above. Adverse events were examined separately using Fisher's Exact Tests when the number of events was greater than 0.
Results
Participants and Pre-treatment Comparability
The randomized sample consisted of 107 women (59%) and 73 men, with a mean age of 35.4 (SD = 12.1) years. The majority of the participants were white (83%). Almost half the sample (51%) was taking psychiatric medications at study entry, with 34 (18.9%) taking a combination of antidepressant and benzodiazepine medication, 30 (16.7%) taking an antidepressant alone, and 28 (15.6%) taking a benzodiazepine alone. Consistent with the inclusion criteria, all participants on concomitant medication were on a stable dosage for a minimum of eight weeks prior to study entry and agreed to maintain this stable dosage throughout the trial.
As shown in Table 1, the randomized treatment groups were comparable at baseline on all outcome measures (all p's > 0.05). Likewise, there were no significant differences between randomized treatment groups in benzodiazepine use (37.0% for placebo and 31.8% for DCS, χ2 (1)=0.53, p=0.47) and in antidepressant use (37.0% for placebo and 34.1% for DCS, χ2 (1)=0.16, p=0.69). There was a statistically significant association between antidepressant and benzodiazepine use at baseline (χ2 (1)=15.3, p<0.0001); participants who were taking one of these medications were more likely to be using the second medication as well.
Table 1.
Sample characteristics at the baseline assessment.
| DCS | PBO | p | |
|---|---|---|---|
| Age | 35.75 (11.59) | 35.18 (12.84) | 0.76 |
| Number (percent) female | 49 (55.7%) | 58 (63.0%) | 0.36 |
| Number (percent) nonwhite* | 12 (13.8%) | 18 (19.8%) | 0.32 |
| PDSS | 13.30 (4.50) | 13.37 (3.39) | 0.90 |
| MADRS | 11.43 (8.47) | 11.42 (8.78) | 1.00 |
| SIGH-A | 14.32 (9.49) | 13.63 (8.36) | 0.60 |
| LIFE-RIFT | 9.65 (3.49) | 9.78 (3.01) | 0.78 |
| Q-LES-Q | 47.24 (9.26) | 47.05 (9.87) | 0.89 |
| ASI | 32.88 (11.50) | 34.02 (12.23) | 0.51 |
Note: PDSS: Panic Disorder Severity Scale, MADRS: Montgomery-Asberg Depression Rating Scale, SIGH-A: Hamilton Anxiety Rating Scale-structured interview version, LIFE-RIFT: Range of Impaired Functioning Tool, Q-LES-Q Quality of Life Enjoyment and Satisfaction Questionnaire, and ASI: Anxiety Sensitivity Index
178 subjects provided complete ethnic and racial data.
Site Differences
Sites differed in participant severity at baseline. ANOVA with follow-up pairwise comparisons indicated that participants at IOL were less severe than MGH/Rush on the PDSS at baseline (p < .001), with severity scores for BU midway between the other two sites. Covariation of PDSS severity at baseline did not eliminate the site effects at posttreatment described below. Sites also differed on secondary severity variables at baseline. For example, BU differed from the other two sites by having participants with lower depression severity as assessed by the MADRS (p-values < .009), and younger participants with higher role functioning than the IOL site (p<.01). IOL participants also had lower quality of life and higher anxiety sensitivity than the other sites (p<.04). Consideration of these site differences in secondary severity variables did not eliminate site main effects on PDSS described below.
Dropout and Adverse Events
Eighteen subjects out of 92 (5 during treatment and 13 during follow-up, 20% total) in the control group compared to 9 out of 88 (4 during treatment and 5 during follow-up, 10% total) in the DCS group dropped out. These differences were not statistically significant (χ2 (1) = 3.08, p = 0.21).
As shown in Table 2, the rate of adverse events was low in both groups. Eleven DCS patients (12.5%) endorsed fatigue, compared to 3 (3.3%) placebo patients (p = 0.03). No other adverse events differed significantly between the groups.
Table 2.
Adverse events by treatment group.
| DCS | PBO | |
|---|---|---|
| Nausea/vomiting | 6.8% | 7.6% |
| Gastrointestinal distress | 2.3% | 3.3% |
| Headache | 4.6% | 3.3% |
| Fatigue | 12.5% | 3.3% * |
| Sedation | 5.7% | 3.3% |
| Jitteriness/tremor | 3.4% | 1.1% |
| Agitation/restlessness | 2.3% | 0.0% |
| Dizziness/lightheadedness | 5.7% | 10.9% |
| Anxiety/panic | 2.3% | 0.0% |
| Impaired concentration | 1.1% | 1.1% |
| Dry mouth | 4.5% | 1.1% |
| Blurred vision | 1.1% | 1.1% |
| Tachycardia | 2.3% | 0.0% |
| Paresthesias | 1.1% | 4.4% |
| Shortness of breath | 4.6% | 2.2% |
| Itching | 2.3% | 0.0% |
| Chills | 1.1% | 0.0% |
| Derealization | 1.1% | 1.1% |
| Menstrual irregularity | 1.1% | 1.1% |
p < .05
Additional Treatment during Follow-up
At the 3-month follow-up assessment, 3 participants reported additional treatment during the period since the post-treatment assessment: 1 started new psychotherapy, and 3 started a self-help program. Between the 3- and 6-month follow-up, 3 reported starting new psychotherapy and 2 reported starting a self-help program. The use of alternative treatment was not significantly different across the randomized treatment conditions (Fishers exact test, p = .41)
Primary Outcomes
Panic Disorder Severity Scale (PDSS)
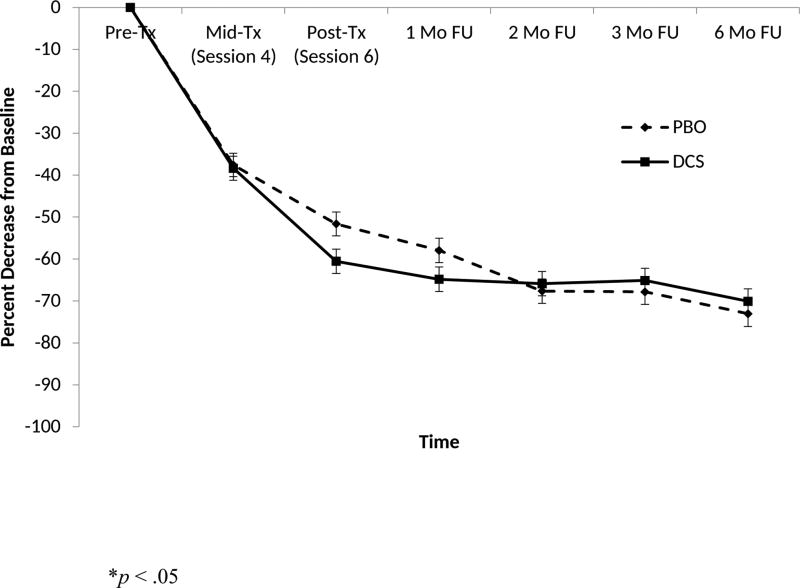
For the primary continuous outcome (percent change in PDSS score), treatment by time interaction was significant (F5,792 = 2.30, p = 0.04). The treatment main effect (F1,792 = 0.26, p = 0.61) was not significant, but the main effect of time was significant (F5,792 = 51.24, p < 0.0001). Both treatment groups improved significantly. The pre-specified comparison at the end of treatment was significant (F1,792 = 4.78, p = 0.03) and reflected a small-to-medium effect size (d = 0.3), with the DCS group showing more improvement on average (see Figure 2). The site main effect and the site by treatment interaction were significant (F2,792 = 6.52, p = 0.002 and F2,792 = 4.25, p = 0.01, respectively). Responsivity to DCS augmentation was significantly higher at the BU and MGH/Rush sites than at the IOL site, with mean scores reflecting greater benefit for DCS only at the former two sites. Additionally, greater overall reduction in PDSS scores, regardless of randomization condition, occurred at the IOL and MGH sites relative to the BU site (p < .01).
Figure 2.
Least squares means and standard errors of reductions on the Panic Disorder Severity Scale for patients receiving cognitive behavioral therapy plus d-cycloserine (DCS) or cognitive behavioral therapy plus placebo (PBO).
Remission Status
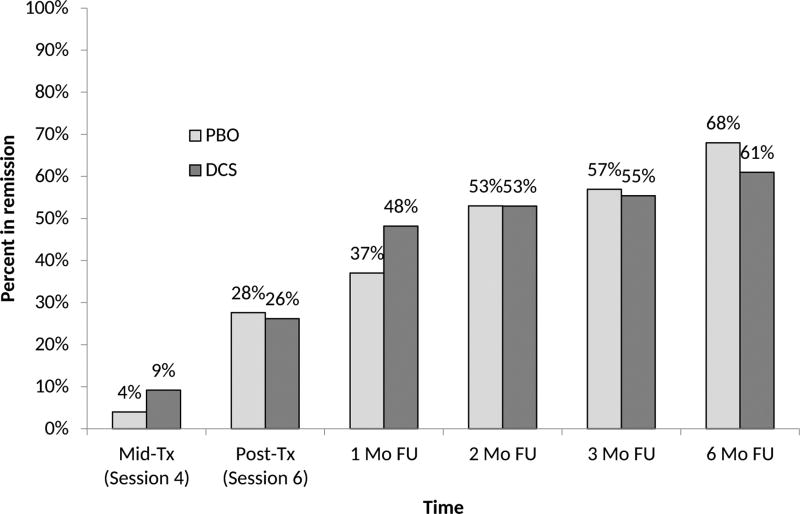
Examination of remission rates found no significant main effect of treatment and no treatment by time interaction. There was a significant main effect of time (F 5,792 = 29.16, p < 0.0001). The main effect for site was not significant at the Bonferroni-adjusted level of 0.01 (F2,792 = 3.66, p = 0.03). The percentage of remitted subjects (based on the completer sample) increased steadily over time (from 6.7% at session 4 to 26.9% at session 6, and then up to 64.3% at 6 month follow-up, see Figure 3). Sensitivity analysis when counting dropouts as non-remitted showed the same substantive results.
Figure 3.
Proportion of patients meeting remission criteria following cognitive behavioral therapy plus d-cycloserine (DCS) or cognitive behavioral therapy plus placebo (PBO).
Dimensional Secondary Outcomes
No significant differences between DCS versus placebo augmentation were evident, but there was consistent evidence of improvement for both treatment groups, with significant improvement over time for anxiety sensitivity (ASI: F4,611 =17.84, p < 0.0001), role functioning (LIFE-RIFT: F4,624 = 5.97, p = 0.0001), and quality of life (QLESQ: F4,622 = 3.45, p = 0.008). Improvement in non-panic anxiety was limited to the IOL and MGH/Rush sites, and was not seen for depression (MADRS: F4,575 = 0.55, p = 0.70). Other site differences include a greater percent improvement in ASI and Q-LES-Q at the IOL site than at the MGH/Rush site or at the BU site (p's < 0.01), and a greater change in LIFE-RIFT scores at the IOL and MGH/Rush sites than at the BU site (p = 0.002 and p < 0.0001, respectively).
Moderator Analyses
Severity
Consideration of baseline severity alone (F1,792 = 0.01, p = 0.993) and in interaction (F1,792 = 0.84, p = 0.36) with randomized treatment revealed no significant effects or trends.
Benzodiazepine and Antidepressant Use
Of the main and interactive effects of adjunctive pharmacotherapy, only the main effect of benzodiazepine use at baseline was significant. Those who used benzodiazepines at baseline had lower percent change in PDSS than those who did not take these medications (F1,792 = 5.33, p = 0.02). Likewise, benzodiazepine use at baseline was associated with lower odds for remission across time points and treatments (χ2 (1) = 8.44, p = 0.004). Among individuals using benzodiazepines on a daily basis (n=45), total daily dose (expressed in alprazolam equivalents; M(SD) = 4.63(13.90)) was not related to percent change in PDSS.
Discussion
There is increasing evidence that DCS augmentation has a primary action of speeding treatment response15. The current study fits this pattern. We found a benefit of DCS augmentation on our primary outcome measure (percent change in PDSS) at the conclusion of five sessions of treatment, but advantages for DCS were not maintained by the one-month follow-up assessment and beyond. Also, the magnitude of this endpoint benefit was well below the effect sizes observed in our pilot study17. Accordingly, the strong response observed for the treatment of panic disorder after just five sessions in both treatment arms, combined with the increasing response observed over booster sessions and the follow-up period (with the number of participants meeting the criteria for treatment response rising from 26.9% at posttreatment to 64.3% at 6-month follow-up), and the physician effort needed for a pharmacologic augmentation strategy, leads us away from routinely recommending DCS augmentation of panic disorder. Our data suggest that the brief, focused CBT provided across five sessions of treatment provided the essential therapeutic learning that could be consolidated across the booster session and follow-up period, and that DCS did not offer benefit beyond this process.
Other investigators have suggested that no antidepressant use21; 33 and baseline severity18 may help identify patients who are likely to respond better to DCS augmentation. However, we were not able to support either of these emergent findings in the current study. We did find that CBT may be less effective in individuals taking benzodiazepines, consistent with clinical trial and observation data11; 12; 34. As such, prior to considering augmentation of CBT with these patients, strategies for treatment in the context of benzodiazepine discontinuation should be considered35.
A number of limitations of our study deserve note. Our study targeted response in a brief trial, in a generally-selected sample of treatment-seeking adults with panic disorder, including patients failing to respond adequately to pharmacotherapy. It remains an open question whether DCS augmentation can rescue treatment response in individuals who have failed previous trials of exposure-based CBT, but there are initial indications that this might be the case36; 37. Also, in both animal models and clinical trials38; 39, the degree of low fear achieved at the conclusion of an exposure session appears to moderate DCS augmentation response. In the current study, we did not systematically collect fear estimates across exposure, and hence we are not able to examine this factor as a moderator. Additionally, unexpected site effects were observed for several of our outcome variables. Notably, patients at the IOL site demonstrated greater impairment and greater improvements in ASI and Q-LES-Q scores over time than those at the BU or MGH/Rush sites despite evidence of less augmentation offered by the study drug in the primary outcome measure (i.e., PDSS) at IOL. Hence, the greater improvement in both study conditions on primary and secondary variables of interest at the IOL site may have obscured potential augmentation effects. Statistically accounting for site differences in ASI and Q-LES-Q scores did not eliminate site main effects on the PDSS outcome measure.
Conclusion
This study adds to the growing literature on DCS suggesting that it accelerates response early in treatment, but that this relative advantage is attenuated over time. Our data further suggest that an ultra-brief CBT program for panic disorder demonstrates efficiency and efficacy31, and warrants consideration for further application in clinical practice.
Acknowledgments
This research was supported by NIMH grants R01-MH081116 to Dr. Otto, R01-MH081132 to Dr. Pollack, and R01-MH081130 to Dr. Tolin. Funding agencies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Financial Disclosures
Dr. Otto reports serving, in the last three years, as a paid consultant for MicroTransponder Inc., Concert Pharmaceuticals, and ProPhase, providing expert consensus opinion for Otsuka Pharmaceuticals, receiving royalty support for use of the SIGH-A from ProPhase, receiving book royalties from Oxford University Press, Routledge, and Springer, and receiving research support for other projects from NIH.
Dr. Pollack reports serving on the advisory board or consulting for Clintara, Concert Pharmaceuticals, Corcept Therapeutics, Edgemont Pharmaceuticals, Eli Lilly, Ironwood Pharmaceuticals, Medavante, Merck, Palo Alto Health Sciences, and Project Plus, receiving equity from Doyen Medical, Medavante, Mensante Corporation, Mindsite, and Targia Pharmaceuticals, and receiving royalties/patents for SIGH-A and SAFER interviews.
Dr Dowd reports receiving research funding from Pfizer, Janssen and Edgemont Pharmaceuticals and serves as a paid consultant with Clintara and Otsuka Pharmaceutical.
Dr. Hofmann receives support from NIH/NCCIH (R01AT007257), NIH/NIMH (R01MH099021, R34MH099311, R34MH086668, R21MH102646, R21MH101567, K23MH100259), and the Department of the Army for work unrelated to the studies reported in this article. He receives compensation for his work as an advisor from the Palo Alto Health Sciences and Otsuka America Pharmaceutical, Inc., and for his work as a Subject Matter Expert from John Wiley & Sons, Inc. and SilverCloud Health, Inc. He also receives royalties and payments for his editorial work from various publishers.
Dr. Gueorguieva reports consulting for Palo Alto Health Sciences.
Dr. Krystal JHK consults for AbbVie, AMGEN, Astellas Pharma Global Development, AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Co, Euthymics Bioscience and Neurovance (a subsidiary of Euthymic Bioscience), Forum Pharmaceuticals, Janssen Research and Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, and Takeda Industries. JHK is on the scientific advisory board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, and Pfizer Pharmaceuticals. JHK holds stock in Biohaven Medical Sciences, and stock options in Mnemosyne Pharmaceuticals. JHK holds three patents/inventions: (1) US Patent 5 447 948 (5 September 1995); (2) US Patent 8 778 979 B2 (15 July 2014); and (3) US application 14/197 767 filed on 5 March 2014; United States application or PCT international application 14/306 382 filed on 17 June 2014. In addition, Dr. Krystal was supported by the Yale Center for Clinical Investigation (UL1RR024139), US Department of Veterans Affairs via its support for the National Center for Post Traumatic Stress Disorder and Consortium to Alleviate PTSD, and the US National Institute on Alcohol Abuse and Alcoholism (P50AA012879).
Dr. Simon reports research grants from the American Foundation for Suicide Prevention, Department of Defense, Highland Street Foundation, and NIH; speaking/CME/consulting from the MGH Psychiatry Academy and Pfizer Pharmacuticals, and spousal equity from G Zero and Gatekeeper.
Dr. Tolin reports receiving research funding from Palo Alto Health Sciences, Inc.
Footnotes
Dr. Pearlson and Ms. Szuhany report no conflicts.
References
- 1.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283(19):2529–36. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 2.Mitte K. A meta-analysis of the efficacy of psycho- and pharmacotherapy in panic disorder with and without agoraphobia. J Affect Disord. 2005;88(1):27–45. doi: 10.1016/j.jad.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann SG, Barlow DH, Papp LA, et al. Pretreatment attrition in a comparative treatment outcome study on panic disorder. Am J Psychiatry. 1998;155(1):43–7. doi: 10.1176/ajp.155.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Tsao JC, Lewin MR, Craske MG. The effects of cognitive-behavior therapy for panic disorder on comorbid conditions. J Anxiety Disord. 1998;12(4):357–71. doi: 10.1016/s0887-6185(98)00020-6. [DOI] [PubMed] [Google Scholar]
- 5.McHugh RK, Otto MW, Barlow DH, et al. Cost-efficacy of individual and combined treatments for panic disorder. J Clin Psychiatry. 2007;68(7):1038–44. doi: 10.4088/jcp.v68n0710. [DOI] [PubMed] [Google Scholar]
- 6.Otto MW, Pollack MH, Maki KM. Empirically supported treatments for panic disorder: costs, benefits, and stepped care. J Consult Clin Psychol. 2000;68(4):556–63. [PubMed] [Google Scholar]
- 7.Pollack MH, Otto MW, Roy-Byrne PP, et al. Novel treatment approaches for refractory anxiety disorders. Depress Anxiety. 2008;25(6):467–76. doi: 10.1002/da.20329. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305–12. doi: 10.1192/bjp.188.4.305. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N, Churchill R, Furukawa TA. Combination of psychotherapy and benzodiazepines versus either therapy alone for panic disorder: a systematic review. BMC Psychiatry. 2007;7:18. doi: 10.1186/1471-244X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burghardt NS, Sigurdsson T, Gorman JM, et al. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73(11):1078–86. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otto MW, McHugh RK, Kantak KM. Combined Pharmacotherapy and Cognitive-Behavioral Therapy for Anxiety Disorders: Medication Effects, Glucocorticoids, and Attenuated Treatment Outcomes. Clin Psychol (New York) 2010;17(2):91–103. doi: 10.1111/j.1468-2850.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothbaum BO, Price M, Jovanovic T, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171(6):640–8. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biol Psychiatry. 2006;60(4):319–21. doi: 10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 15.Otto MW, Kredlow MA, Smits JA, et al. Enhancement of Psychosocial Treatment With d-Cycloserine: Models, Moderators, and Future Directions. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues H, Figueira I, Lopes A, et al. Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PLoS One. 2014;9(7):e93519. doi: 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto MW, Tolin DF, Simon NM, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67(4):365–70. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Siegmund A, Golfels F, Finck C, et al. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011;45(8):1042–7. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Chasson GS, Buhlmann U, Tolin DF, et al. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther. 2010;48(7):675–9. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann SG, Smits JA, Rosenfield D, et al. D-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170(7):751–8. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson E, Hedman E, Enander J, et al. D-Cycloserine vs Placebo as Adjunct to Cognitive Behavioral Therapy for Obsessive-Compulsive Disorder and Interaction With Antidepressants: A Randomized Clinical Trial. JAMA Psychiatry. 2015;72(7):659–67. doi: 10.1001/jamapsychiatry.2015.0546. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID I/P, version 2.0) New York: Biometrics Research Department; 1995. [Google Scholar]
- 23.Di Nardo PAB, T A, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) New York: Oxford University Press; 1994. [Google Scholar]
- 24.Shear MK, Brown TA, Barlow DH, et al. Multicenter collaborative panic disorder severity scale. Am J Psychiatry. 1997;154(11):1571–5. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- 25.Guy W. ECDEU assessment manual for psychopharmacology: publication ADM 76-338. Washington DC: US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13(4):166–78. [PubMed] [Google Scholar]
- 28.Leon AC, Solomon DA, Mueller TI, et al. A brief assessment of psychosocial functioning of subjects with bipolar I disorder: the LIFE-RIFT. Longitudinal Interval Follow-up Evaluation-Range Impaired Functioning Tool. J Nerv Ment Dis. 2000;188(12):805–12. doi: 10.1097/00005053-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Peterson RA, Reiss S. Anxiety Sensitivity Index manual. Worthington, OH: International Diagnostic Services, Inc.; 1992. [Google Scholar]
- 30.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 31.Otto MW, Tolin DF, Nations KR, et al. Five sessions and counting: considering ultra-brief treatment for panic disorder. Depress Anxiety. 2012;29(6):465–70. doi: 10.1002/da.21910. [DOI] [PubMed] [Google Scholar]
- 32.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310–7. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 33.Paul IA, Nowak G, Layer RT, et al. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994;269(1):95–102. [PubMed] [Google Scholar]
- 34.Westra HA, Stewart SH, Conrad BE. Naturalistic manner of benzodiazepine use and cognitive behavioral therapy outcome in panic disorder with agoraphobia. J Anxiety Disord. 2002;16(3):233–46. doi: 10.1016/s0887-6185(02)00091-9. [DOI] [PubMed] [Google Scholar]
- 35.Otto MW, McHugh RK, Simon NM, et al. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behav Res Ther. 2010;48(8):720–7. doi: 10.1016/j.brat.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrell LJ, Waters AM, Boschen MJ, et al. Difficult-to-treat pediatric obsessive-compulsive disorder: feasibility and preliminary results of a randomized pilot trial of D-cycloserine-augmented behavior therapy. Depress Anxiety. 2013;30(8):723–31. doi: 10.1002/da.22132. [DOI] [PubMed] [Google Scholar]
- 37.Norberg MM, Gilliam CM, Villavicencio A, et al. D-cycloserine for treatment nonresponders with obsessive-compulsive disorder: A case report. Cognitive Behavioral Practice. 2012;19:338–345. [Google Scholar]
- 38.Smits JA, Rosenfield D, Otto MW, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013;47(10):1455–61. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smits JA, Rosenfield D, Otto MW, et al. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry. 2013;73(11):1054–8. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]