Abstract
In 2011, the National Institute on Aging and Alzheimer’s Association created separate diagnostic recommendations for the preclinical, mild cognitive impairment, and dementia stages of Alzheimer’s disease. Scientific progress in the interim led to an initiative by the National Institute on Aging and Alzheimer’s Association to update and unify the 2011 guidelines. This unifying update is labeled a “research framework” because its intended use is for observational and interventional research, not routine clinical care. In the National Institute on Aging and Alzheimer’s Association Research Framework, Alzheimer’s disease (AD) is defined by its underlying pathologic processes that can be documented by postmortem examination or in vivo by biomarkers. The diagnosis is not based on the clinical consequences of the disease (i.e., symptoms/signs) in this research framework, which shifts the definition of AD in living people from a syndromal to a biological construct. The research framework focuses on the diagnosis of AD with biomarkers in living persons. Biomarkers are grouped into those of β amyloid deposition, pathologic tau, and neurodegeneration [AT(N)]. This ATN classification system groups different biomarkers (imaging and biofluids) by the pathologic process each measures. The AT(N) system is flexible in that new biomarkers can be added to the three existing AT(N) groups, and new biomarker groups beyond AT(N) can be added when they become available. We focus on AD as a continuum, and cognitive staging may be accomplished using continuous measures. However, we also outline two different categorical cognitive schemes for staging the severity of cognitive impairment: a scheme using three traditional syndromal categories and a six-stage numeric scheme. It is important to stress that this framework seeks to create a common language with which investigators can generate and test hypotheses about the interactions among different pathologic processes (denoted by biomarkers) and cognitive symptoms. We appreciate the concern that this biomarker-based research framework has the potential to be misused. Therefore, we emphasize, first, it is premature and inappropriate to use this research framework in general medical practice. Second, this research framework should not be used to restrict alternative approaches to hypothesis testing that do not use biomarkers. There will be situations where biomarkers are not available or requiring them would be counterproductive to the specific research goals (discussed in more detail later in the document). Thus, biomarker-based research should not be considered a template for all research into age-related cognitive impairment and dementia; rather, it should be applied when it is fit for the purpose of the specific research goals of a study. Importantly, this framework should be examined in diverse populations. Although it is possible that β-amyloid plaques and neurofibrillary tau deposits are not causal in AD pathogenesis, it is these abnormal protein deposits that define AD as a unique neurodegenerative disease among different disorders that can lead to dementia. We envision that defining AD as a biological construct will enable a more accurate characterization and understanding of the sequence of events that lead to cognitive impairment that is associated with AD, as well as the multifactorial etiology of dementia. This approach also will enable a more precise approach to interventional trials where specific pathways can be targeted in the disease process and in the appropriate people.
Keywords: Alzheimer’s disease diagnosis, Preclinical Alzheimer’s disease, Biomarkers Alzheimer’s disease, CSF biomarkers Alzheimer’s disease, Alzheimer’s disease imaging, Amyloid PET, Tau PET
1. Preamble
Alzheimer’s disease (AD) was initially defined as a clinical-pathologic entity, which was diagnosed definitely at autopsy and in life as possible or probable AD [1]. Over time, however, the distinction between neuropathologic change (which implies change from normal) and clinical symptoms became blurred. Consequently, the term AD is often used to describe two very different entities: prototypical clinical syndromes without neuropathologic verification and AD neuropathologic changes. However, a syndrome is not an etiology but rather a clinical consequence of one or more diseases. A biological rather than a syndromal definition of AD is a logical step toward greater understanding of the mechanisms underlying its clinical expression. Disease-modifying interventions must engage biologically defined targets, and the dementia syndrome does not denote a specific biological target(s). Furthermore, in order to discover interventions that prevent or delay the initial onset of symptoms a biologically based definition of the disease that includes the preclinical phase is needed. Thus, a framework suitable for interventional trials should be founded on a biologically based definition of AD; and, it is only rational that the framework is harmonized across interventional and observational research.
Neuropathologic examination is the standard for defining AD—plaques and tangles define AD as a unique disease among several that can lead to dementia. Validated, widely used biomarkers exist that are proxies for AD neuropathologic changes. We propose a research framework grounded on a biomarker-based definition of AD in living people. In many situations, however, biomarker characterization of research participants is not possible. Research without biomarkers has and will continue to constitute a vital part of the effort to evaluate the dementia and mild cognitive impairment (MCI) syndromes. Also, this framework does not limit but rather enhances research into broadly defined dementia by providing a biologically based definition of one cause of dementia, AD.
The AD field is fortunate that biomarkers of important categories of neuropathologic change, that is, β-amyloid (Aβ) deposition, pathologic tau, and neurodegeneration, have been and are being developed. This framework is focused on characterizing research participants with these biomarkers. AD biomarker characterization will identify some research participants who have no AD biomarker abnormalities and some who likely have diseases other than AD. This research framework does not ignore these individuals but rather provides a system for characterizing them alongside individuals who are in the Alzheimer’s continuum.
2. Background: Rationale for updating 2011 NIA-AA guidelines for AD
In 2011, the National Institute on Aging and Alzheimer’s Association (NIA-AA) created separate sets of diagnostic guidelines for the symptomatic or “clinical” stages of AD, that is, MCI and dementia [2,3]. Recommendations were also created for a stage of AD in individuals without overt symptoms, called “preclinical AD” [4]. The criteria for the symptomatic stages were intended, in part, to aid routine clinical diagnostic decision-making and to provide researchers a common framework to define these clinical stages [2,3,5]. The recommendations for preclinical AD were not designed for routine clinical care but rather to provide researchers a common language to identify and stage research participants who were not cognitively impaired but had abnormal AD biomarkers [4,5]. The framework described in this document also has this latter intention—to provide researchers a common language with which to communicate observations.
Since the publication of the 2011 guidelines, data have continued to accumulate indicating that the cognitive decline in AD occurs continuously over a long period [6–8], and that progression of biomarker measures is also a continuous process that begins before symptoms [9–14]. Thus, the disease is now regarded as a continuum rather than three distinct clinically defined entities [15]. This concept was recognized but was not formalized in the 2011 NIA-AA guidelines [4,5].
A common theme in the 2011 recommendations was the use of imaging and cerebrospinal fluid (CSF) biomarkers. In symptomatic individuals, biomarkers were used to refine confidence that AD pathologic changes contributed to a person’s cognitive impairments [2,3,5]. In the case of preclinical AD, biomarkers were used to define the construct [4]. In the 2011 recommendations, amyloid biomarkers were placed at the apex of the biomarker hierarchy preclinically [4], whereas in contrast, all AD biomarkers, including those reflecting neurodegeneration, were placed on equal footing in the MCI and dementia guidelines [2,3]. Although this discrepancy was noted at the time [5], there is now a growing consensus that application of biomarkers should be harmonized conceptually across the disease continuum and that biomarkers of neurodegeneration are not equivalent to those reflecting amyloid and pathologic tau accumulation [16].
A major motivation for updating the 2011 guidelines has been the evolution in thinking about biomarkers. Studies published since 2011 have reinforced the idea that certain imaging and CSF biomarkers are valid proxies for neuropathologic changes of AD. Imaging-to-autopsy comparison studies have established that amyloid positron emission tomography (PET) is a valid in vivo surrogate for Aβ deposits (in brain parenchyma/vessel walls) [17–24]. It is also now widely accepted that CSF Aβ42 (or the Aβ42/ Aβ40 ratio) is a valid indicator of the abnormal pathologic state associated with cerebral Aβ [25]. An additional development has been the introduction of PET ligands for pathologic tau [26–28]. By contrast, additional research has highlighted the fact that measures of neurodegeneration or neuronal injury that are commonly used in AD research—magnetic resonance imaging (MRI), fluoro-deoxyglucose (FDG) PET, and CSF total tau (T-tau)—are not specific for AD but rather are nonspecific indicators of damage that may derive from a variety of etiologies, for example, cerebrovascular injury [29].
Based on this background, NIA-AA leadership commissioned a work group whose charge was to examine the 2011 guidelines in the context of current scientific knowledge and if appropriate update them. Members of the work group were selected by NIA-AA leadership with the goals of providing a range of scientific expertise, broad representation of different stakeholders and professional organizations involved with AD research, and gender and geographic diversity (including both within the United States and international scientists).
3. Guiding principles for updating NIA-AA guidelines for AD
The charge to the 2018 NIA-AA work group was to unify and update the 2011 recommendations in a manner that is consistent with current understanding of the AD continuum. The work group approached this mandate with several guiding principles.
First, the overall objective was to create a scheme for defining and staging the disease across its entire spectrum. Experience with the 2011 NIA-AA recommendations has shown that a common framework for defining and staging the disease facilitates standardized reporting of research findings across the field [30–45].
Second, we determined that these recommendations should be cast as a “research framework,” not as diagnostic criteria or guidelines. Unlike the 2011 NIA-AA criteria for MCI or AD dementia based on clinical criteria (i.e., without biomarkers) [2,3], the 2018 research framework is not intended for general clinical practice. It is called a “research framework” because it needs to be thoroughly examined and modified if needed before being adopted into general clinical practice. There are two categories of studies that will achieve this ultimate goal: longitudinal cohort studies and randomized placebo controlled trials. Cohort studies, particularly community- and population-based cohorts, will examine the extent to which temporal relationships and patterns of signs, symptoms, and biomarkers expected by this framework align with what is observed. These results will support convergent and divergent validity. Trials showing that an intervention modifies both biomarkers and signs and symptoms will establish criterion validity (i.e., a disease-modifying effect). Other areas of medicine have used this approach to define pathologic processes using biomarkers, for example, bone mineral density, hypertension, hyperlipidemia, and diabetes are defined by biomarkers. Interventions modulating these biomarkers have been shown to reduce the likelihood of developing fractures and myocardial and cerebral infarctions [46,47].
Third, the committee recognized the research framework must function in two major contexts—observational cohort studies and interventional trials.
The committee took a stepwise approach to creating the 2018 research framework by posing a series of questions where each incremental step built on earlier conclusions.
4. The term “Alzheimer’s disease” refers to an aggregate of neuropathologic changes and thus is defined in vivo by biomarkers and by postmortem examination, not by clinical symptoms
We approached the definition of AD with the distinction between a syndrome and a disease in mind. Some will argue that a specific syndrome, that is, a multidomain amnestic dementia (after other potential etiologies have been excluded), should define AD in living people. Our position, however, is that dementia is not a “disease” but rather is a syndrome composed of signs and symptoms that can be caused by multiple diseases, one of which is AD. As we elaborate in the following paragraph, there are two major problems with using a syndrome to define AD; it is neither sensitive nor specific for the neuropathologic changes that define the disease, and it cannot identify individuals who have biological evidence of the disease but do not (yet) manifest signs or symptoms [48,49].
It is now well established that the prototypical multidomain amnestic dementia phenotype historically used to define probable AD [1] does not “rule in” AD pathologic change (which implies change from normal) at autopsy [50–52] and the absence of the syndrome does not “rule out” AD pathologic change. From 10% to 30% of individuals clinically diagnosed as AD dementia by experts do not display AD neuropathologic changes at autopsy [50], and a similar proportion has normal amyloid PET or CSF Aβ42 studies [53–62]. Thus, the multidomain amnestic dementia phenotype is not specific; it can be the product of other diseases as well as AD [51]. Nonamnestic clinical presentations, that is, language, visuospatial, and executive disorders, may also be due to AD [63–66]. In addition, AD neuropathologic changes are often present without signs or symptoms, especially in older persons. Thirty to forty percent of cognitively unimpaired (CU) elderly persons have AD neuropathologic changes at autopsy [67–69], and a similar proportion has abnormal amyloid biomarkers [33,53–55,60,70–73]. The fact that an amnestic multidomain dementia is neither sensitive nor specific for AD neuropathologic change suggests that cognitive symptoms are not an ideal way to define AD.
The traditional approach to incorporating biomarkers into models of AD began with patients’ clinical symptoms, which appear relatively late in the disease, and worked backward to relate symptoms to biomarker findings. The committee recommends a different approach where the neuropathologic changes detected by biomarkers define the disease. Defining AD by biomarkers indicative of neuropathologic change independent from clinical symptoms represents a profound shift in thinking. For many years, AD was conceived as a clinical-pathological construct [1]; it was assumed that if an individual had typical amnestic multidomain symptoms, they would have AD neuropathologic changes at autopsy and if symptoms were absent, they would not have AD at autopsy. Symptoms/signs defined the presence of the disease in living persons, and therefore, the concepts of symptoms and disease became interchangeable. AD later became a clinical-biomarker construct with the International Work Group (IWG) [64,74,75] and 2011 NIA-AA guidelines where biomarkers were used to support a diagnosis of AD in symptomatic individuals, but the definition of AD was not divorced from clinical symptoms (with the exceptions of the 2011 NIA-AA recommendations on preclinical AD and IWG criteria in autosomal dominant mutation carriers, and NIA-AA neuropathologic guidelines).
5. AD biomarkers
Various imaging and CSF biomarkers are widely used in AD and brain aging research, and an organized approach is needed for a generalizable research framework. The committee addressed this by following the recommendations from a recent position paper that outlined an unbiased descriptive classification scheme for biomarkers used in AD and brain aging research [16]. The scheme [which is labeled AT(N)] recognizes three general groups of biomarkers based on the nature of the pathologic process that each measures (Table 1) [16]. See section 9.4 for explanation of (N) notation. Biomarkers of Aβ plaques (labeled “A”) are cortical amyloid PET ligand binding [76,77] or low CSF Aβ42 [78–80]. Biomarkers of fibrillar tau (labeled “T”) are elevated CSF phosphorylated tau (P-tau) and cortical tau PET ligand binding [79,81–83]. Biomarkers of neurodegeneration or neuronal injury [labeled “(N)”] are CSF T-tau [84], FDG PET hypometabolism, and atrophy on MRI [85–91].
Table 1.
AT(N) biomarker grouping
| A: Aggregated Aβ or associated pathologic state |
| CSF Aβ42, or Aβ42/Aβ40 ratio |
| Amyloid PET |
| T: Aggregated tau (neurofibrillary tangles) or associated pathologic state |
| CSF phosphorylated tau |
| Tau PET |
| (N): Neurodegeneration or neuronal injury |
| Anatomic MRI |
| FDG PET |
| CSF total tau |
Abbreviations: Aβ, β amyloid; CSF, cerebrospinal fluid.
NOTE. See section 9.4 for explanation of (N) notation.
A limitation of the 2011 NIA-AA recommendations was that biomarkers were grouped into just two categories— amyloid and tau-related neurodegeneration. Tauopathy and neurodegeneration were placed into the same biomarker category. In persons with only AD, it is reasonable to assume that neurodegeneration is closely associated with pathologic tau. However, it is increasingly recognized that neurodegeneration/injury, even in classic AD brain regions, also occurs in non-AD conditions. This is particularly so in elderly individuals where comorbidities are common [92]. AT(N) classification provides a solution to this problem, which is to separate biomarkers that are specific for pathologic tau from those that are nonspecific measures of neurodegeneration/neuronal injury.
The AT(N) system was designed with both a CSF and an imaging biomarker in each of the three biomarker groups (Table 1) [16]. Thus, complete AT(N) biomarker characterization of research participants is possible using either imaging or CSF biomarkers alone. However, some research groups may prefer a mixture of imaging and CSF biomarkers for AT(N) characterization. For example, when lumbar puncture and MRI are accessible but PET is not, investigators may choose to use CSF Aβ42 and P-tau as the A and T biomarkers and MRI as the (N) biomarker.
6. Definition of AD
Once the committee agreed that AD should be defined as a biologic construct that is identified by biomarkers in living people, the next logical question was “what biomarker signature or profile(s) defines AD?” The committee agreed that only biomarkers that are specific for hallmark AD proteinopathies (i.e., Aβ and pathologic tau) should be considered as potential biomarker definitions of the disease. Different possible biomarker profiles were considered.
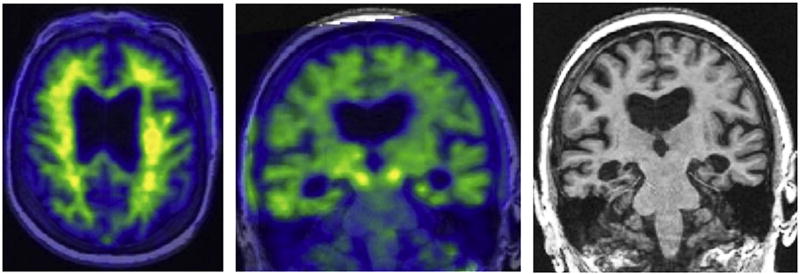
Numerous studies have shown that CU individuals with abnormal amyloid biomarkers have more rapid progression of atrophy, hypometabolism, and clinical/cognitive decline than individuals without biomarker evidence of Aβ deposition [13,33,80,93–99] The proportion of amyloid PET-positive clinically normal individuals by age nearly perfectly parallels the (increasing) age-specific prevalence of individuals clinically diagnosed as AD dementia 15–20 years later [53]. The first biomarkers to become abnormal in carriers of deterministic AD mutations are those of Aβ [9–11,14]. These human data and animal model data [100] suggest a causal upstream role for Aβ in the pathogenesis of AD; and although β-amyloidosis alone is insufficient to cause cognitive deterioration directly, it may be sufficient to cause downstream pathologic changes (i.e., tauopathy and neurodegeneration) that ultimately lead to cognitive deterioration. These findings are supported by clinicopathologic studies as well [101,102]. Consequently, a widely held view is that amyloid biomarkers represent the earliest evidence of AD neuropathologic change currently detectable in living persons [9,11,72,103,104]. This suggests that abnormal β-amyloidosis biomarkers alone could serve as the defining signature of AD. However, both Aβ and paired helical filament (PHF) tau deposits are required to fulfill neuropathologic criteria for AD [105,106], which suggests that evidence of abnormalities in both Aβ and pathologic tau biomarkers should be present to apply the label “Alzheimer’s disease” in a living person (Fig. 1). With these considerations in mind, the committee agreed on the following definitions.
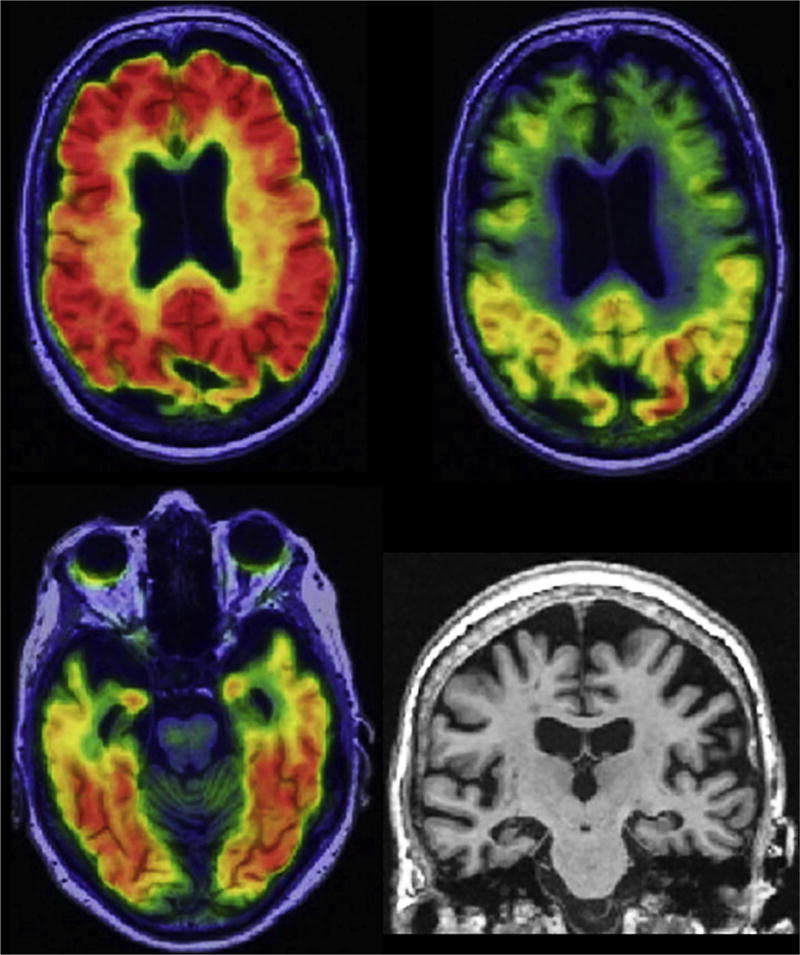
Fig. 1.
Alzheimer’s disease with dementia. A 75-year-old woman with amnestic multidomain dementia. Participant in the Mayo Alzheimer’s Disease Research Center. Abnormal amyloid PET with Pittsburgh compound B (top left), tau PET with flortaucipir (top right and bottom left), and atrophy on MRI (bottom right). Biomarker profile A+T+(N)+.
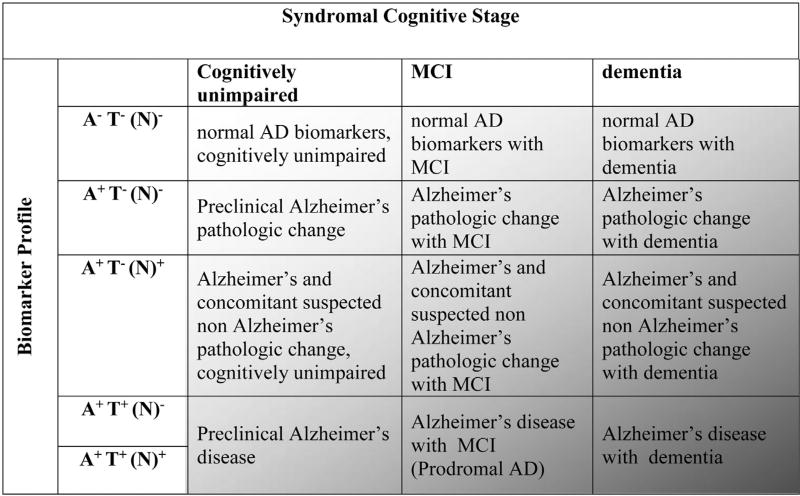
An individual with biomarker evidence of Aβ deposition alone (abnormal amyloid PET scan or low CSF Aβ42 or Aβ42/Aβ40 ratio) with a normal pathologic tau biomarker would be assigned the label “Alzheimer’s pathologic change” (Table 2, Fig. 2, Text Box 1). The term “Alzheimer’s disease” would be applied if biomarker evidence of both Aβ and pathologic tau was present (Table 2, Fig. 1, Text Box 1). Alzheimer’s pathologic change and AD are not regarded as separate entities but earlier and later phases of the “Alzheimer’s continuum” (an umbrella term that includes both). These definitions are applied independently from clinical symptoms. They also meet our specifications to function equally well across the disease spectrum: from early-through late-life onset, from presymptomatic through symptomatic phases, and for typical and atypical clinical presentations.
Table 2.
Biomarker profiles and categories
| AT(N) profiles | Biomarker category | |
|---|---|---|
| A-T-(N)- | Normal AD biomarkers | |
| A+T-(N)- | Alzheimer’s pathologic change | Alzheimer’s continuum |
| A+T+(N> | Alzheimer’s disease | |
| A+T+(N)+ | Alzheimer’s disease | |
| A+T-(N)+ | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change | |
| A-T+(N)- | Non-AD pathologic change | |
| A-T-(N)+ | Non-AD pathologic change | |
| A-T+(N)+ | Non-AD pathologic change | |
Abbreviation: AD, Alzheimer’s disease.
NOTE. See text for explanation of (N) notation.
NOTE. Binarizing the three AT(N) biomarker types leads to eight different biomarker “profiles”. Every individual can be placed into one of the three general biomarker “categories” based on biomarker profiles: those with normal AD biomarkers (no color), those with non-AD pathologic change (dark grey), and those who are in the Alzheimer’s continuum (light grey). The term “Alzheimer’s continuum” is an umbrella term that denotes either Alzheimer’s pathologic change or AD.
NOTE. If an individual has an abnormal amyloid biomarker study, but a biomarker for tau is not available, then the individual is placed into the “Alzheimer’s continuum”. A missing biomarker group can be labeled with an asterisk (*). For example, A+(N)+ without a T biomarker would be A+T*(N)+.
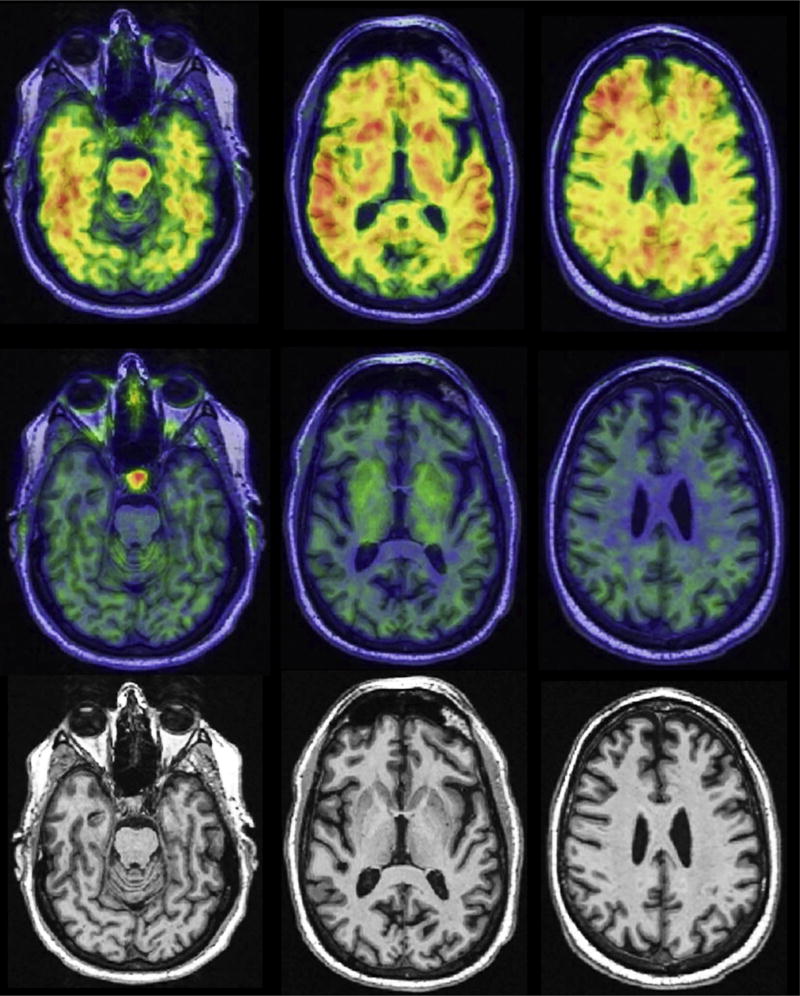
Fig. 2.
Preclinical Alzheimer’s pathologic change. A cognitively unimpaired 67-year-old man. Participant in the Mayo Clinic Study of Aging. Abnormal amyloid PET (Pittsburgh compound B, top row), no uptake on tau PET (with flortaucipir, middle row), no atrophy on MRI (bottom row). Biomarker profile A+T−(N)−.
Text Box 1 Glossary.
Alzheimer disease (AD) – refers to Aβ plaques and pathologic tau deposits, defined in vivo by abnormal biomarkers of Aβ and pathologic tau (both are required)
Alzheimer’s pathologic change – early stage of Alzheimer’s continuum, defined in vivo by an abnormal Aβ biomarker with normal pathologic tau biomarker
Alzheimer’s continuum – refers to individuals with biomarker designation of either AD or Alzheimer’s pathologic change
Alzheimer’s clinical syndrome – recommended terminology for clinically ascertained multi- (or single-) domain amnestic syndrome or a classic syndromal variant (i.e., what has historically been labeled “possible or probable AD”). It applies to both mildly impaired and demented individuals. The term “Alzheimer’s disease” is reserved for situations where neuropathologic or biomarker evidence of the disease (i.e. Aβ plaques and pathologic tau deposits) is present
Biomarker group – refers to three different pathologic processes of AD that a biomarker can measure: Aβ (A), pathologic tau (T), and neurodegeneration/neuronal injury (N)
Biomarker profile – binarizing each of the three biomarker groups into normal/abnormal (+/−) results in eight possible biomarker profiles: A+T−(N) −, A+T+(N) −, etc
Biomarker category – biomarker profiles are grouped into three possible biomarker categories: normal AD biomarkers, A−T−(N)−; Alzheimer’s continuum any A+ combination, and non-Alzheimer’s pathologic change (i.e., suspected non-Alzheimer’s pathophysiology or SNAP), A−T+(N)−, A−T−(N)+ or A−T+(N) +
Cognitively unimpaired – cognitive performance in the nonimpaired range for that individual, defined as not mild cognitive impairment or demented
Neurobehavioral symptoms – symptoms attributable to mood or behavioral disorders, for example, anxiety, depression, and apathy
Transitional cognitive decline – cognitive performance in the nonimpaired range but with a subjective complaint of cognitive decline, or a subtle decline measured on longitudinal cognitive testing, or neurobehavioral symptoms, or combinations of these
7. Staging
We next developed a system for staging severity. Our guiding principles were the following. Two types of information about the research participant are staged independently from each other: (1) grading disease severity using biomarkers and (2) grading the severity of cognitive impairment. Measures used to define AD must be specific for the disease, whereas measures used to stage severity need not be. Thus, different measures have different roles (Text Box 2). Aβ biomarkers determine whether or not an individual is in the Alzheimer’s continuum. Pathologic tau biomarkers determine if someone who is in the Alzheimer’s continuum has AD because both Aβ and tau are required for a neuropathologic diagnosis of the disease. Neurodegenerative/neuronal injury biomarkers and cognitive symptoms, neither of which is specific for AD, are used only to stage severity not to define the presence of the Alzheimer’s continuum.
Text Box 2 AT(N)(C) measures have different roles for definition and staging.
Definition
A: Aβ biomarkers determine whether or not an individual is in the Alzheimer’s continuum.
T: Pathologic tau biomarkers determine if someone who is in the Alzheimer’s continuum has Alzheimer’s disease.
Staging severity
(N): Neurodegenerative/neuronal injury biomarkers
(C): Cognitive symptoms
A and T indicate specific neuropathologic changes that define Alzheimer’s disease, whereas (N) and (C) are not specific to Alzheimer’s disease and are therefore placed in parentheses.
8. Biomarker profiles and categories
In many research studies, it will be most appropriate to treat biomarkers of amyloid, pathologic tau, and neurodegeneration/neuronal injury as continuous measures without using normal/abnormal cut points. However, biomarkers used in medicine often use a cut point denoting normal versus abnormal values to support management decisions for an individual patient. The need for discrete categorization of biomarker continua is also obvious for AD clinical trials, where explicit cut points serve as inclusion/exclusion criteria.
The addition of a normal/abnormal cut point for each AT(N) biomarker group results in eight different AT(N) “biomarker profiles” (Table 2, Text Box 1): A+T−(N)−, A+T + (N) + , etc. Based on the definitions of Alzheimer’s pathologic change and AD outlined earlier, the ATN biomarker system assigns every individual to one of three “biomarker categories” (Table 2, Text Box 1): (1) individuals with normal AD biomarkers; (2) those in the Alzheimer’s continuum (subdivided into Alzheimer’s pathologic change and AD); and (3) those with a normal amyloid biomarker but with abnormal T or (N), or both. This latter biomarker profile implies evidence of one or more neuropathologic processes other than AD [35,40,107] and has been labeled “suspected non-Alzheimer’s pathophysiology” (or SNAP) [38].
It is worthwhile emphasizing that, like the 2012 NIA-AA classification system for AD neuropathic change [105,106], AT(N) scoring of biomarkers is independent from clinical symptoms.
Although the term “stage” is more familiar, we use the term “biomarker profile” (Table 2) because the term “stage” implies a sequence, that is, stage 1 always precedes stage 2, etc. The AT(N) biomarker system does not imply a specific order of events nor does it imply causality. It is a system for grouping biomarkers and classifying research participants on the basis of biomarker profiles. A−T−(N)− represents a state without evidence of pathologic change that is detectable by AT(N) biomarkers, whereas A+T+(N)+ represents an advanced pathologic state. Staging can be accomplished by combining information from each of the three biomarker groups; the more biomarker groups that are abnormal, the more advanced the pathologic stage. The rate of cognitive decline is significantly greater for cognitively impaired and CU individuals who have abnormalities in both an amyloid biomarker and a second biomarker type (which could be CSF T-tau or P-tau, atrophy, or hypometabolism) in comparison to individuals who have neither or only one of these biomarker abnormalities [30–35,39,40,42–45]. These data firmly establish that more advanced disease defined by biomarkers predicts greater likelihood of and more rapid cognitive decline. Thus, a solid evidence base exists proving that combinations of biomarker abnormalities are useful for staging the Alzheimer’s continuum.
8.1. Alternatives to binary biomarker groups
Given that Alzheimer’s pathologic change and AD are defined by biomarkers, a single cut point is needed in many situations. However, as pointed out in the AT(N) position paper [16], other options are possible. In many research situations, biomarkers are best treated as continuous variables. For example, the risk of short-term cognitive decline increases continuously with worsening (N) biomarkers, and this may be true of T biomarkers as well [108,109].
A three-range approach might also be useful where the three ranges are defined by two cut points, one lenient and the other more conservative [16,110,111]. If these three ranges were labeled—clearly normal (0), intermediate range (1), and clearly abnormal (2)—then a two–cut point biomarker profile might look like A2T1(N)0, etc. Designating an intermediate range using two cut points has evolved in other diseases for clinical care, for example, pre-hypertension (a stage now called “elevated”) and prediabetes have proved to be useful constructs in medicine. Numeric severity grading within different pathologic categories is also analogous to the tumor, nodes, metastasis (TNM) system used for staging all non–central nervous system solid tumors [112,113]. Characteristics of the primary tumor (T) are graded from 0–1; nodal (N) involvement from 0–3; and distant metastases (M) are graded 0–1.
8.2. Personalized medicine
The AT(N) system moves AD research in the direction of personalized medicine by coding pathologic change in three categories for each research participant and allows for future flexibility by adding other biomarkers as they are discovered and validated. This level of granularity in biomarker classification, combined with genetic and clinical information, will presumably be useful in tailoring treatment to the individual when appropriate specific treatments become available.
9. Characteristics and limitations of biomarkers
9.1. CSF versus imaging biomarkers
While we place imaging and CSF biomarkers into common groups, a fundamental difference between the two should be recognized. CSF biomarkers are measures of the concentrations of proteins in CSF from the lumbar sac that reflect the rates of both production (protein expression or release/secretion from neurons or other brain cells) and clearance (degradation or removal) at a given point in time [114,115]. Imaging measures, on the other hand, represent the magnitude of the neuropathologic load or damage accumulated over time. Low CSF Aβ42 is therefore best considered a biomarker of a pathologic state that is associated with amyloid plaque formation and not a measure of amyloid plaque load as amyloid PET is. Similarly, CSF P-tau is best considered a biomarker of a pathologic state that is associated with PHF tau formation and not a measure of pathologic tau deposits as tau PET is.
Discordances between imaging and CSF biomarkers may occur [36,41,116–119]. In some situations, discordance in normal/abnormal labels between an imaging and CSF biomarker within a study is simply a product of how cut points were established that can be rectified by adjusting them. The continuous relationship between CSF Aβ42 and amyloid PET, however, is “L-shaped” rather than linear [116,117,120]. This may be due to a temporal offset between these two measures [121–123]. In the limited data currently available, tau PET ligand binding is linearly correlated with elevated CSF P-tau [82,83,115]; however, the correlation is imperfect. This may be in part because P-tau seems to plateau later in the disease [14] while the tau PET signal continues to increase [124]. Given these observations, one might ask “how could a CSF and an imaging measure be used as biomarkers of a common pathologic process?” The answer lies in the chronic nature of AD, which spans years to decades. Thus, an ongoing active pathologic state, denoted by CSF, and the accumulation of neuropathologic load, denoted by imaging, will be concordant over the long term.
9.2. Tau PET
Tau PET is a new modality, and the ligands that have been evaluated to date are considered first-generation compounds. These compounds suffer from limitations, the most common being off-target binding [125]. However, at least one first-generation ligand has emerged as a reliable biomarker of 3R/4R PHF tau deposits [28]. Autoradiographic studies have shown that the most widely studied ligand, flortaucipir, does not bind to amyloid plaques, TAR DNA Binding Protein 43 (TDP43), argyrophilic grains, or α-synuclein. Flortaucipir binds weakly or not at all to sole 4R or sole 3R tau deposits in primary tauopathies [126–128]. In vivo imaging to autopsy comparisons also indicates specific binding of flortaucipir to PHF tangles [23] and correlation with the Braak neurofibrillary tangles stage [129]. Elevated tau PET binding in both medial temporal lobe structures and the neocortex is strongly associated with positive amyloid PET scans and with clinical impairment across the normal aging to dementia clinical spectrum [82,130–141]. New tau PET ligands are in the early stages of development and evaluation [142], and there is optimism that some of the limitations of the first-generation compounds will be addressed in the next generation of tau PET ligands.
9.3. CSF T-tau and P-tau
The most thoroughly examined P-tau epitope as a CSF biomarker for AD is threonine 181 (P-tau181) [143], but assays for the concentration of P-tau231 and P-tau199 correlate tightly with P-tau181 and show very similar diagnostic accuracy [144]. CSF levels of T-tau and P-tau are tightly correlated within cohorts of AD patients and controls [145], and the correlation between CSF T-tau and P-tau is typically much higher than between CSF T-tau and MRI measures of atrophy or FDG PET [36,115]. Therefore, it is reasonable to ask why not place both CSF T-tau and P-tau in the pathologic tau biomarker group. The answer lies in the divergent behavior of these two measures in other diseases. There is a marked temporary increase in T-tau, with no change in P-tau, in traumatic brain injury and stroke that correlates with the severity of neuronal damage [146,147]. It is difficult to rationalize how changes in T-tau in such patients could be attributed to brain PHF tau deposition. Furthermore, in Creutzfeldt-Jakob disease, a disorder characterized by very rapid neurodegeneration but not PHF tau accumulation, there is a very marked increase in CSF T-tau (10–20 times more than in AD), whereas P-tau shows no or minor change [148,149]. The only disorder that consistently shows an increase in CSF P-tau is AD [143], whereas this biomarker is normal in other neurodegenerative disorders. The level of CSF P-tau also does correlate with severity of PHF tau accumulation after death [81,150]. Taken together, these data indicate that CSF T-tau reflects the intensity of neuronal damage at a specific point [114], whereas elevated CSF P-tau reflects an abnormal pathologic state associated with PHF tau formation.
9.4. Biomarkers of neurodegeneration or neuronal injury
Biomarkers in the (N) group (Table 1) are indicators of neurodegeneration or neuronal injury resulting from many causes; they are not specific for neurodegeneration due to AD. In any individual, the proportion of observed neurodegeneration/injury that can be attributed to AD versus other possible comorbid conditions (most of which have no extant biomarker) is unknown. These are recognized limitations of the (N) category of biomarkers. In addition, unlike A and T, (N) biomarkers do not map onto neuropathologic findings used to diagnose AD. For these reasons, we have placed (N) in parenthesis, indicating the fundamental differences between (N) and AT.
For purposes of simplification, it might be tempting to eliminate the (N) biomarker group from the research framework. However, the combination of an abnormal MRI, CSF T-tau, or FDG PET study with an abnormal amyloid biomarker provides much more powerful prediction of future cognitive decline [30–35,39,40,42–45] than an abnormal amyloid study alone. This is logical given that neurodegeneration, particularly synapse loss, is the aspect of AD neuropathologic change that correlates most closely with symptoms [151]. Thus, the (N) biomarker group provides important pathologic staging information; and for this reason, it seems inadvisable to eliminate this group of biomarkers from the AD research framework. Also, without the (N) group, the difference between A+T−(N)− (see Fig. 2) and A+T−(N)+ (see Fig. 3) would not be formally captured, that is, both would be placed into the same A+T− biomarker group. Comparison of the images in Fig. 2 and Fig. 3 shows that these two individuals obviously belong in different biomarker groups. We believe that A+T−(N)+ represents evidence of comorbidity, that is, A+T− represents Alzheimer’s pathologic change while in the A+T− context, (N) + represents evidence of non-AD neurodegeneration/neuronal injury [152] and thus A+T−(N)− and A+T−(N)+ indicate meaningfully different pathologic states [153].
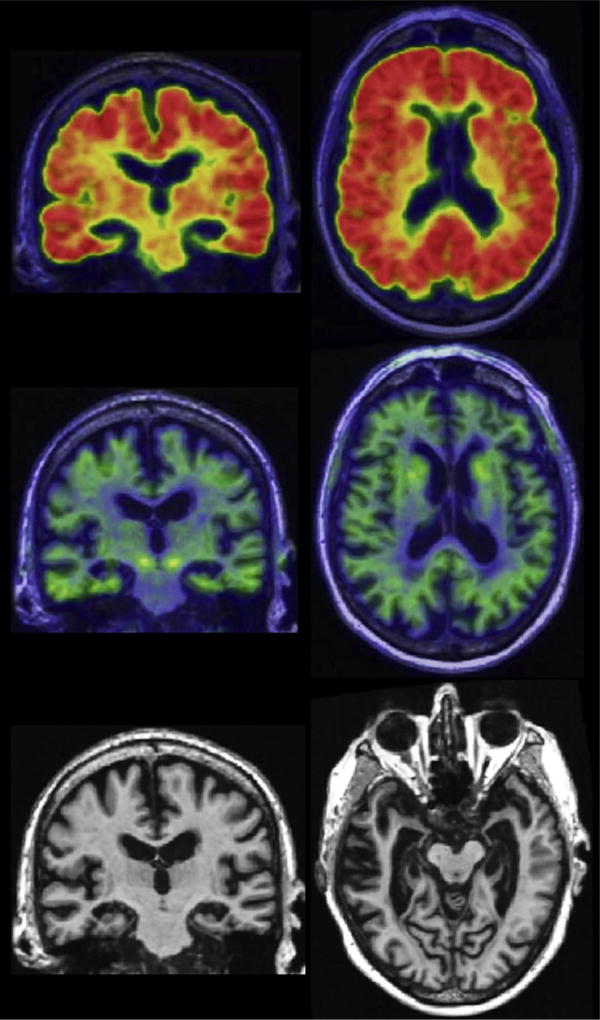
Fig. 3.
Alzheimer’s and concomitant suspected non-Alzheimer’s pathologic change with dementia. A 91-year-old male with severe amnestic dementia. Participant in the Mayo Alzheimer’s Disease Research Center. Abnormal amyloid PET with Pittsburgh compound B (top row), normal tau PET (flortaucipir, middle row), and severe medial temporal atrophy on MRI (bottom row). The biomarker profile (A +T−(N)+) suggests the patient has Alzheimer’s pathologic change (A+T−) plus an additional degenerative condition [(N)+], likely hippocampal sclerosis.
It is important to note some differences among biomarkers in the (N) group [114]. Atrophy on MRI likely reflects cumulative loss and shrinkage of the neuropil [154–156]. CSF T-tau likely indicates the intensity of neuronal injury at a given point in time [108,114,157,158]. FDG PET likely indicates both cumulative loss of the neuropil and functional impairment of neurons. These differences may result in discordances [36,43,115,119,159].
9.5. Limitations
None of the biomarkers are as sensitive as direct examination of tissue at autopsy. Absolute sensitivity of amyloid PET relative to an autopsy gold standard has been assessed [160]. Typical cut points used for 18F amyloid PET ligands roughly label individuals with none to sparse neuritic plaques normal and individuals with moderate to high neuritic plaque load abnormal [18,22]. A typical cut point used for 11C Pittsburgh compound B approximately labels individuals with Thal phase 0–1 normal and individuals with Thal phase 2–5 abnormal [21]. Thus, a negative amyloid PET scan should not be equated with the complete absence of Aβ in the brain or even with absent or sparse neuritic plaques. Clinicopathologic studies suggest that low levels of pathologic changes are associated with subtle cognitive deficits among CU persons [8,161]. The amount of pathologic tau that can be present in the brain below the in vivo tau PET detectable threshold is unknown at this time. This limitation is important to bear in mind when considering the distinction between Alzheimer’s pathologic change and AD, which hinges on in vivo detection of pathologic tau deposits; however, neither CSF P-tau nor tau PET is expected to identify minimal neurofibrillary changes that are detectable by neuropathologic examination. Similarly, the number of neurons or neuronal processes that must be lost to detect atrophy on MRI or hypometabolism on FDG PET is not known. For every biomarker, there must be an in vivo limit of detection, which is true for any biomarker not just those discussed here.
9.6. Flexibility to incorporate new biomarkers
The current form of the NIA-AA research framework is designed around biomarker technology that is presently available. TDP43 and α-synuclein proteinopathies, micro infarcts, hippocampal sclerosis, and argyrophilic grains can occur alone, or more frequently, along with AD pathologic changes [162,163]; however, validated biomarkers are not presently available for them. The AT(N) biomarker scheme is expandable to incorporate new biomarkers (Text Box 3). For example, a vascular biomarker group could be added, that is, ATV(N), when a clear definition of what constitutes V+ is developed. And, when biomarkers for TDP43 and α-synuclein are developed, AT(N) can be expanded to incorporate these as well. An important pathologic process in AD is activation of the innate immune system, with both astrocytosis and microgliosis [164]. Biomarkers of these changes are not yet widely accepted though some are emerging [165–169] and when developed could likewise be added to the biomarker scheme. CSF neurogranin is presumed to measure synaptic degeneration and loss [170,171], and neurofilament light chain [172] is presumed to measure axonal injury. When they have been more thoroughly studied, these measures should serve as biomarkers of damage to the neuropil in the (N) group of biomarkers. In fact, these may ultimately be preferable to T-tau as a CSF-based (N) biomarker. Because CSF P-tau and T-tau are highly correlated in AD and are equally correlated with tau PET [124], they do not seem to provide independent information in AD.
Text Box 3 Flexibility of the AT(N) system.
The AT(N) system is designed to incorporate new biomarkers within existing AT(N) groups. For example, neurofilament light chain (cerebrospinal fluid or plasma) or neurogranin will likely be added to the (N) group.
The AT(N) system is also designed to incorporate new biomarkers in categories beyond AT(N). The notation ATX(N) might be useful when conceptualizing the incorporation of new biomarker groups, where X represents an array of biomarkers that may become available in the future. For example, when a measure that incorporates and appropriately weights the many sources of information about cerebrovascular disease has been developed and standardized, AT(N) will be expanded to ATV(N). When biomarkers for both V and synuclein have been developed, AT(N) will be expanded to ATVS(N), and so on for biomarkers of inflammation (I), TDP43, etc.
Cut points: Cut points should be selected to fit the specific research question(s) of interest. The framework is outlined using a single cut point approach, which labels each biomarker group normal (−) or abnormal (+). This approach is conceptually straightforward and will always be needed in some use cases, for example, as an inclusion criterion in clinical trials. However, a two–cut point approach (lenient and conservative) might have great appeal. If the research question centered on the earliest detectable evidence of Alzheimer’s pathologic change, then a lenient cut point would be appropriate. If the research questions required high diagnostic certainty, then more conservative cut points would be appropriate.
Conceptually, it might be useful to think of ATX(N), where X is an array of biomarkers of specific pathologic processes, which hopefully will become available in the future (TDP43, synuclein, etc.), and (N) represents cumulative brain injury from all etiologies. Even if biomarkers of all known brain pathologic processes became available, a sensitive but nonspecific (N) biomarker would still be useful because it seems certain that some proportion of cumulative brain injury would remain unexplained by all available disease biomarkers.
9.7. Biomarkers other than AT(N)
While we focus on biomarkers of AD, we emphasize that other currently available biomarkers have a valuable role to play. Several different MRI measures provide information about cerebrovascular disease. Although a biomarker for α-synuclein does not yet exist, decreased striatal dopamine transporter uptake of 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane single-photon emission computed tomography (dopamine transporter, DaTscan) is thought to reflect nigrostriatal degeneration in Lewy body disease [173]. Likewise, the FDG PET cingulate island sign is often present in Lewy body disease [174]. These tests may provide useful information about non-AD pathologic processes and may be used alone or concordantly with AT(N) biomarkers to provide a more complete picture of the heterogeneous etiologic nature of dementia. For example, in an individual with an A+T−(N)+ biomarker profile and cerebral infarction(s), atrophy is attributable at least in part to vascular brain injury.
The fact that most dementia is multifactorial presents a challenge both for diagnosis and treatment. In individuals with multiple brain neuropathologic processes, each makes some contribution to the individual’s cognitive impairment. In an individual with multiple neuropathologic processes, treating one of them (i.e., AD) should have a beneficial effect. Therefore, using biomarkers to aid in discovery of treatments for AD should not be delayed until biomarkers of all possible etiologies for dementia have been developed.
Finally, while many neuropathologic processes are known to contribute to cognitive impairment, it seems likely that new pathologic entities will be discovered in the future. And, biomarkers of these new diseases, when developed, will enhance the ability of investigators to more fully characterize the dementia spectrum.
10. Cognitive staging
Like biomarkers, cognitive performance exists on a continuum. An obvious approach to cognitive staging therefore is to use continuous cognitive instruments, which may be the preferred outcome measure in many modern clinical trials [175]. While recognizing that cognition does exist on a continuum, the committee felt it was also appropriate to outline categorical cognitive staging schemes. In the 2011 NIA-AA guidelines, cognitive staging was implicit rather than explicit. Three different documents were published describing preclinical AD, MCI, and dementia; however, these categories have at times been interpreted to indicate three distinct entities. In the research framework, we avoid the notion of separate entities and instead refer to the “cognitive continuum”.
One of the specifications of the NIA-AA research framework was that it be applicable in two distinct research contexts—interventional trials and observational research. In many if not most modern AD interventional trials, individuals are selected for inclusion with the aid of biomarkers. The studies are concerned only with a defined portion of the population—those in the Alzheimer’s continuum. For observational research, on the other hand, the research questions often require that all members of a recruited sample are included (those with non-AD pathologic changes, normal AD biomarkers, and those in the Alzheimer’s continuum). In these studies, research questions often hinge on the presence of heterogeneity within the cohort, which is substantially screened out of AD trial cohorts. We therefore outline two types of categorical clinical staging schemes. The first is syndromal categorical cognitive staging that uses traditional syndromal categories and is applicable to all members of a recruited cohort (i.e., includes all biomarker profiles). The second is a numeric clinical staging scheme that is applicable only to those in the Alzheimer’s continuum, which the committee felt might be particularly useful in clinical trials.
The committee also recognized that cognitive staging has to function both when prior longitudinal clinical or cognitive testing evaluations are available for participants and when prior information is unavailable and the participant is being evaluated for the first time.
10.1. Syndromal categorical cognitive staging
The syndromal cognitive staging scheme divides the cognitive continuum into three traditional categories—CU, MCI, and dementia, with dementia further subdivided into mild, moderate, and severe stages (Table 3). This three-category division serves as the basis for cognitive categorization in many large ongoing studies [53,176–178]. Numerous researchers feel that it has been and continues to be effective for clinical research and that abandoning it would unnecessarily disrupt ongoing studies. Dividing the cognitive continuum into these three syndromal categories also has been adopted by many medical practitioners [179]. It has also been codified for clinical practice in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria [180] by the terms “mild neurocognitive disorder” (essentially MCI) and “major neurocognitive disorder” (essentially dementia).
Table 3.
Syndromal staging of cognitive continuum: Applicable to all members of a research cohort independent from biomarker profiles
| Cognitively unimpaired |
| Cognitive performance within expected range for that individual based on all available information. This may be based on clinical judgment and/or on cognitive test performance (which may or may not be based on comparison to normative data, with or without adjustments for age, education, occupation, sex, etc.). |
| Cognitive performance may be in the impaired/abnormal range based on population norms, but performance is within the range expected for that individual. |
| A subset of cognitively unimpaired individuals may report subjective cognitive decline and/or demonstrate subtle decline on serial cognitive testing. |
| Mild cognitive impairment |
| Cognitive performance below expected range for that individual based on all available information. This may be based on clinical judgment and/ or on cognitive test performance (which may or may not be based on comparison to normative data with or without adjustments for age, education, occupation, sex, etc.). |
| Cognitive performance is usually in the impaired/abnormal range based on population norms, but this is not required as long as the performance is below the range expected for that individual. |
| In addition to evidence of cognitive impairment, evidence of decline in cognitive performance from baseline must also be present. This may be reported by the individual or by an observer (e.g., study partner) or observed by change on longitudinal cognitive testing/behavioral assessments or by a combination of these. |
| May be characterized by cognitive presentations that are not primarily amnestic*. |
| Although cognitive impairment is the core clinical criteria, neurobehavioral disturbance may be a prominent feature of the clinical presentation†. |
| Performs daily life activities independently, but cognitive difficulty may result in detectable but mild functional impact on the more complex activities of daily life, either self-reported or corroborated by a study partner. |
| Dementia |
| Substantial progressive cognitive impairment that affects several domains and/or neurobehavioral symptoms. May be reported by the individual or by an observer (e.g., study partner) or observed by change on longitudinal cognitive testing. |
| Cognitive impairment and/or neurobehavioral symptoms result in clearly evident functional impact on daily life. No longer fully independent/requires assistance with daily life activities. This is the primary feature differentiating dementia from MCI. |
| May be subdivided into mild, moderate, and severe |
Abbreviation: MCI, mild cognitive impairment.
For MCI and dementia: Cognitive impairment may be characterized by presentations that are not primarily amnestic.
Tor MCI and dementia: Although cognition is the core feature, neurobehavioral changes—for example, changes in mood, anxiety, or motivation— commonly coexist and may be a prominent part of the presentation.
Although the definitions of CU, MCI, and dementia (Table 3) are largely the same as in the 2011 NIA-AA guidelines, there are differences (Text Box 4). For example, the 2011 guidelines included only those CU individuals who had an abnormal amyloid biomarker study (i.e., preclinical AD). In contrast, in the NIA-AA research framework, the definition of CU is independent from biomarker findings. In the 2011 guideline for MCI, the diagnosis was based on clinical judgment when all available information about the patient was considered. In the NIA-AA research framework, the diagnosis can be based on clinical judgment or on cognitive test performance alone. In the 2011 guidelines, an amnestic multidomain dementia was labeled “probable or possible AD by clinical criteria” without requiring biomarker documentation of AD. In the NIA-AA research framework, the labels CU, MCI, and dementia denote only severity of cognitive impairment and are not used to infer its etiology.
Text Box 4 Changes from National Institute on Aging and Alzheimer’s Association (NIA AA) 2011.
The NIA-A A research framework builds on but implements a number of changes from the 2011 NIA-A A guidelines. In this research framework, the term “Alzheimer disease (AD)” refers to pathologic processes and therefore in living persons is defined by biomarkers. In the 2011 NIA-AA guidelines, an individual with a classic dementia syndrome and in whom biomarkers were not available (or were conflicting) was labeled possible or probable AD. In contrast, in this research framework, such an individual is labeled Alzheimer’s clinical syndrome, which describes a syndrome not a probabilistic pathologic diagnosis. In this research framework, AD is defined as a continuous process in both cognitive and biomarker domains rather than as three separate clinical entities in the 2011 guidelines. Use of biomarkers is harmonized across the disease continuum in this research framework, which was not the case in 2011. Biomarkers are grouped into those of Aβ, pathologic tau, and neurodegeneration or neuronal injury, unlike 2011 where tau and neurodegeneration/neuronal injury biomarkers were placed into the same category. Unlike 2011, biomarker staging includes all members of the population, that is, individuals in the Alzheimer’s continuum, with non-AD pathologic changes, and with normal biomarker profiles. The research framework outlines two different systems for staging the severity of cognitive symptoms. A syndromal categorical scheme largely preserves the three clinical categories from 2011: cognitively unimpaired, mild cognitive impairment, and dementia. This is applicable to all members of the population regardless of biomarker profile. A numeric clinical staging scheme is defined only for individuals in the Alzheimer’s continuum.
10.2. Nomenclature
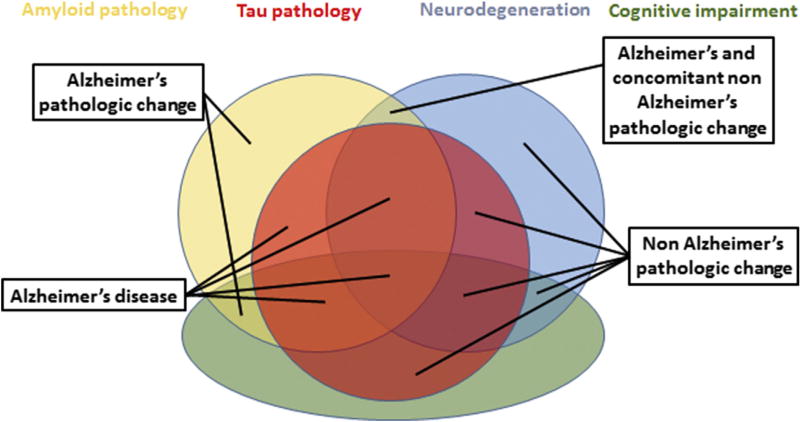
Every research participant has both a biomarker profile and a cognitive stage. Many researchers prefer to retain traditional descriptive terms from 2011 that combined these two sources of information. In Table 4, we illustrate descriptive terminology combining a biomarker profile and a cognitive stage, which retains nomenclature from 2011 but does depart from 2011 naming in some ways (Text Box 4). For example, in the research framework, the label “Alzheimer’s disease with MCI” is used rather than “MCI due to Alzheimer’s disease (2011)”. By this, we indicate that although the person has an AD biomarker profile, we cannot know if the cognitive deficit is attributable to AD alone or to other potential comorbidities in addition. In Table 4, we further recognize contributions of comorbidities for individuals with an A+T−N+ biomarker profile with the descriptive phrase “Alzheimer’s and concomitant suspected non-Alzheimer’s pathologic change”. By this, we imply that in an A+T−(N)+ MCI individual, both Alzheimer’s and non-Alzheimer’s pathologic change may be contributing to the individual’s impairment (Fig. 3). In addition to carrying forward the NIA-AA 2011 terminology, we also incorporate the term “prodromal AD” from the IWG, which many investigators find useful (Table 4). Fig. 4 is a Venn diagram illustrating a simplified schema of Table 4.
Table 4.
Descriptive nomenclature: Syndromal cognitive staging combined with biomarkers
| Cognitive stage | ||||
|---|---|---|---|---|
| Cognitively Unimpaired | Mild Cognitive Impairment | Dementia | ||
| Biomarker Profile | A− T− (N)− | normal AD biomarkers. cognitively unimpaired | normal AD biomarkers with MCI | normal AD biomarkers with dementia |
| A+ T (N) | Preclinical Alzheimer’s pathologic change | Alzheimer’s pathologic change with MCI | Alzheimer’s pathologic change with dementia | |
| A+ T+ (N)− | Preclinical Alzheimer’s disease | Alzheimer’s disease with MCI(Prodromal AD) | Alzheimer’s disease with dementia | |
| A+ T+(N)+ | ||||
| A+ T (N)+ | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change, cognitively unimpaired | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change with MCI | Alzheimer’s and concomitant suspected non Alzheimer’s pathologic change with dementia | |
| A− T+(N)− | non-Alzheimer’s pathologic change, cognitively unimpaired | non-Alzheimer’s pathologic change with MCI | non-Alzheimer’s pathologic change with dementia | |
| A− T− (N)+ | ||||
| A−T+(W+ | ||||
Abbreviations: AD, Alzheimer disease; MCI, mild cognitive impairment.
NOTE. Formating denotes three general biomarker “categories” based on biomarker profiles: those with normal AD biomarkers (no color), those with non-AD pathologic change (dark grey), and those who are in the Alzheimer’s continuum (light grey).
Fig. 4.
Descriptive nomenclature Venn diagram. As an adjunct to Table 4, we illustrate how AT(N) biomarker grouping and cognitive status interact for classification of research participants in this Venn diagram. For simplicity, MCI and dementia are combined into a single (cognitively impaired) category and the A−T−(N)− groups are not shown. Also “Alzheimer’s and concomitant non-Alzheimer’s pathologic change” [A+T−(N)+] in cognitively impaired is not shown in this figure. Abbreviation: MCI, mild cognitive impairment.
Table 4 illustrates the principle that biomarker profile and cognitive staging represent independent sources of information. For a given cognitive stage (i.e., a given column in Table 4), different biomarker profiles will be present in the population. Likewise, different cognitive stages may be present in the population among people with the same biomarker profile (i.e., along a given row in Table 4). Many effects can blur the relationship between neuropathologic severity and cognitive symptoms at the individual level. These include protective factors, such as cognitive reserve [181–183], and risk factors, such as comorbid pathologic processes [184–186].
Table 5 illustrates the principle that a greater number of abnormal AT(N) groups (i.e., more severe pathologic stage) indicate a greater risk of short-term cognitive decline, and the cognitive stage provides additional independent information about the risk of future cognitive decline.
Table 5.
Risk of short-term cognitive decline based on the biomarker profile and cognitive stage
Non-Alzheimer’s continuum profiles are not included in table because the risk associated with different combinations of T+(N)-, T+(N)+, T-(N)+ among A- individuals has not been established
 rate of short term clinical progression expected to be low
rate of short term clinical progression expected to be low
 rate of short term clinical progression expected to be high
rate of short term clinical progression expected to be high
10.3. Alternative naming, avoiding the term “Alzheimer’s disease ”
While many investigators prefer the descriptive terms in the cells of Table 4, others indicated a preference to avoid terms that have any reference to AD because of historic controversies associated with these terms. The NIA-AA research framework provides an alternative to descriptive names in the cells of Table 4, which is to simply combine AT(N) biomarker profile and cognitive stage without using descriptive phrases (Text Box 5). That is, combine the row and column names from Table 4 without the descriptive phrases in the cells of the table; for example, “A+T+(N) + dementia” instead of “Alzheimer’s disease with dementia”. Some groups may prefer this “row and column” naming approach. Similarly, some investigators may prefer to not use the biomarker category terminology in Table 2 but instead simply report biomarker profile; for example, A+T+(N)+ instead of AD.
Text Box 5 Alternative naming, avoiding the term Alzheimer’s disease.
Some investigators may prefer to not use the biomarker category terminology in Table 2 but instead simply report biomarker profile (i.e., A+T+(N)+ instead of Alzheimer’s disease). Similarly, some investigators may prefer to avoid using descriptive names in the cells of Table 4, including the term Alzheimer’s disease. An alternative is to combine the row and column names from Table 4 without the descriptive phrases in the cells of the table; for example, “A+T+(N)+ with dementia” instead of “Alzheimer’s disease with dementia”.
10.4. Numeric clinical staging
The committee also created a “numeric clinical staging scheme” (Table 6) that avoids traditional syndromal labels and is applicable for only those in the Alzheimer’s continuum. This staging scheme reflects the sequential evolution of AD from an initial stage characterized by the appearance of abnormal AD biomarkers in asymptomatic individuals. As biomarker abnormalities progress, the earliest subtle symptoms become detectable. Further progression of biomarker abnormalities is accompanied by progressive worsening of cognitive symptoms, culminating in dementia. A useful application envisioned for this numeric cognitive staging scheme is interventional trials. Indeed, the NIA-AA numeric staging scheme is intentionally very similar to the categorical system for staging AD outlined in recent FDA guidance for industry pertaining to developing drugs for treatment of early AD [187]. As the FDA guidance notes, the categorical staging definitions are intimately related to appropriate outcome measure selection in interventional trials, and it was our belief that harmonizing this aspect of the framework with FDA guidance would enhance cross fertilization between observational and interventional studies, which in turn would facilitate conduct of interventional clinical trials early in the disease process.
Table 6.
Numeric clinical staging—Applicable only to individuals in the Alzheimer’s continuum
| Stage 1 |
| Performance within expected range on objective cognitive tests. Cognitive test performance may be compared to normative data of the investigators choice, with or without adjustment (the choice of the investigators) for age, sex, education, etc.* |
| Does not report recent decline in cognition or new onset of neurobehavioral symptoms of concern. |
| No evidence of recent cognitive decline or new neurobehavioral symptoms by report of an observer (e.g., study partner) or by longitudinal cognitive testing if available. |
| Stage 2 |
| Normal performance within expected range on objective cognitive tests. |
| Transitional cognitive decline: Decline in previous level of cognitive function, which may involve any cognitive domain(s) (i.e., not exclusively memory). |
| May be documented through subjective report of cognitive decline that is of concern to the participant. |
| Represents a change from individual baseline within past 1–3 years, and persistent for at least 6 months. |
| May be corroborated by informant but not required. |
| Or may be documented by evidence of subtle decline on longitudinal cognitive testing but not required. |
| Or may be documented by both subjective report of decline and objective evidence on longitudinal testing. |
| Although cognition is the core feature, mild neurobehavioral changes—for example, changes in mood, anxiety, or motivation—may coexist. In some individuals, the primary compliant may be neurobehavioral rather than cognitive. Neurobehavioral symptoms should have a clearly defined recent onset, which persists and cannot be explained by life events† |
| No functional impact on daily life activities |
| Stage 3 |
| Performance in the impaired/abnormal range on objective cognitive tests. |
| Evidence of decline from baseline, documented by the individual’s report or by observer (e.g., study partner) report or by change on longitudinal cognitive testing or neurobehavioral behavioral assessments. |
| May be characterized by cognitive presentations that are not primarily amnestic‡ |
| Performs daily life activities independently, but cognitive difficulty may result in detectable but mild functional impact on the more complex activities of daily life, that is, may take more time or be less efficient but still can complete, either self-reported or corroborated by a study partner. |
| Stage 4 |
| Mild dementia |
| Substantial progressive cognitive impairment affecting several domains, and/or neurobehavioral disturbance. Documented by the individual’s report or by observer (e.g., study partner) report or by change on longitudinal cognitive testing. |
| Clearly evident functional impact on daily life, affecting mainly instrumental activities. No longer fully independent/requires occasional assistance with daily life activities. |
| Stage 5 |
| Moderate dementia |
| Progressive cognitive impairment or neurobehavioral changes. Extensive functional impact on daily life with impairment in basic activities. No longer independent and requires frequent assistance with daily life activities. |
| Stage 6 |
| Severe dementia |
| Progressive cognitive impairment or neurobehavioral changes. Clinical interview may not be possible. |
| Complete dependency due to severe functional impact on daily life with impairment in basic activities, including basic self-care. |
For stages 1–6: Cognitive test performance may be compared to normative data of the investigators choice, with or without adjustment (choice of the investigators) for age, sex, education, etc.
For stages 2–6: Although cognition is the core feature, neurobehavioral changes—for example, changes in mood, anxiety, or motivation—may coexist.
For stages 3–6: Cognitive impairment may be characterized by presentations that are not primarily amnestic.
It is apparent that numeric stages 1–6 (Table 6) bear a close resemblance to the Global Deterioration Scale [188], with the important distinction that the Global Deterioration Scale was created before the development of disease-specific AD biomarkers. Stage 1 (Table 6) is defined by biomarker evidence of the Alzheimer’s continuum in asymptomatic individuals. Stage 2 describes the earliest detectable clinical consequence of the Alzheimer’s continuum and is similar to “stage 3 preclinical AD” in the 2011 NIA-AA guidelines [4]. Stage 3 describes cognitive impairment that is not severe enough to result in significant functional loss. Stages 4–6 describe progressively worse functional loss. The nature of decline or impairment in stages 2–6 may involve any cognitive domain(s)—not only memory. We suspect that finding individuals in stages 3–6 with (N) – profiles will be uncommon, as clinical symptoms are typically associated with evidence of neurodegeneration. However, these biomarker profiles are included in all 6 numeric stages for sake of completeness.
The syndromal categories in Table 3 and numeric stages in Table 6 obviously point to similar constructs. A CU individual who also has no subjective or objective evidence of subtle decline (Table 3) and stage 1 (Table 6) both describe an asymptomatic state. A CU individual who has subjective or objective evidence of subtle decline (Table 3) is similar to stage 2 (Table 6). MCI (Table 3) and stage 3 (Table 6) both describe cognitive impairment short of dementia. Mild, moderate, and severe dementia (Table 3) is identical to stages 4–6 (Table 6).
However, because the two staging systems address different needs, there are important differences between them. First, numeric staging is only applicable to those in the Alzheimer’s continuum, whereas syndromal categorical staging includes all biomarker profiles. Second, stage 2 is called out as a distinct transitional stage between asymptomatic (stage 1) and mildly impaired (stage 3) in the numeric scheme (Table 6), but there is no separate category between clinically unimpaired and MCI in the syndromal categorical scheme. Our reasoning was that if an individual is in the Alzheimer’s continuum, then it is reasonable to label subjective complaints or evidence of subtle cognitive decline as a transitional stage attributable to the pathologic process. However, in the syndromal categorical scheme (Table 3) where abnormal biomarkers are not required, it is not reasonable to assume that subjective complaints (which are very common in aging) represent a symptom of any specific disease(s). Third, neurobehavioral symptoms are treated differently between the two staging systems. While cognitive symptoms represent the core clinical feature of AD, in some individuals, the initial presentation may be neurobehavioral (e.g., depression, anxiety, and apathy) rather than cognitive [189]. Therefore, in the numeric scheme, an individual may be placed into stage 2 on the basis of neurobehavioral symptoms alone, that is, without evident cognitive decline. To reflect this, we use the term “clinical staging” rather than cognitive staging to recognize that early clinical manifestations of AD may be either cognitive or neurobehavioral. Individuals must have cognitive impairment to be placed into numeric stages 3–6 [190]. Our position is that without biomarker abnormalities indicating the presence of a neurodegenerative disease, it is not reasonable to classify patients with isolated neurobehavioral symptoms as having MCI or dementia. Consequently, cognitive symptoms are required for inclusion in these categories in the syndromal staging scheme, which is not limited to individuals in the Alzheimer’s continuum.
Because only four biomarker profiles are included in numeric staging, the committee saw an opportunity to streamline nomenclature. In this shorthand naming scheme, the four Alzheimer’s continuum biomarker profiles are labeled a-d: (a) A+T−(N)−; (b) A+T+(N)−; (c) A+T+(N)+; and (d) A+T−(N)+. Thus, individuals can be fully described by a single number/letter combination denoting numeric clinical stage and biomarker profile—stage 1 a, stage 2c, etc. Some investigators may wish to treat participants with an A+T−(N)+ profile (i.e., d above) differently from the other three Alzheimer’s continuum profiles because A+T−(N)+ indicates Alzheimer’s and concomitant suspected non-Alzheimer’s pathologic change (Table 4, Fig. 3).
11. Implementation
The committee avoided making specific recommendations for many implementation details. Our objective was to outline a general research framework that could be adapted by individual research groups to their own research goals and environment. For example, different research groups will use cognitive testing batteries and cut points that best fit their own research samples.
PET or MRI images may be evaluated by visual interpretation or by quantitative methods. Methods of image quantification vary among research groups and are constantly being refined [191,192]. Cut points must be determined, and age norming biomarker cut points is controversial. Arguments have been made that neurodegenerative biomarkers should be age normed because loss of neuropil is closely tied with aging. By contrast, a strong argument can be made that any amyloid or pathologic tau detected by a biomarker is abnormal regardless of age, and thus age-norming biomarker cut points is inappropriate. The distinction between normal aging and age-related disease has been debated for decades [193–195], and we do not presume to settle this here. Cut points should be selected to fit the specific question(s) of interest. It is quite conceivable that the field will ultimately settle on the concept of multiple cut points. For example, lenient cut points would be useful if the research question centered on the earliest evidence of Alzheimer’s pathologic change. In contrast, more conservative cut points might be appropriate if the research questions required high diagnostic certainty.
For amyloid imaging, where over a decade of data are available, different ligands, methods of image acquisition, and image processing can result in different thresholds when compared to neuropathologic standards [21,22,196]. These issues are currently less understood for pathologic tau imaging, but the questions are equally tractable. The committee avoided taking a proscriptive approach to these methodologic issues under the assumption that this was best left to expert work groups and individual research centers.
Initiatives to standardize imaging and CSF biomarker measures exist, for example, the Centiloid Project [197], EADC-ADNI Harmonized Protocol for hippocampal segmentation [198], Alzheimer’s Association Global Biomarkers Standardization Consortium [199], and International Federation of Clinical Chemistry Working Group for CSF proteins [200]. These efforts are the subject of ongoing research, but universal standards have not yet been established [201].
12. Genetics
Genetics is not formally included in the research framework because our concept of disease rests on neuropathologic change (that can be detected by biomarkers). In contrast, gene variants do not measure pathologic change but rather indicate an individual’s risk for developing pathologic change. For example, inheritance of an APOE ε4 allele neither defines the presence of Alzheimer’s pathologic change or AD nor indicates any particular stage of the disease.
The penetrance of the classic autosomal dominant mutations in APP, PSEN1, or PSEN2 is essentially 100%, and for this reason, it could be argued that these mutations confer a pathologic state that exists from conception. Moreover, one can be almost certain that a symptomatic autosomal dominant mutation carrier has AD neuropathologic change without the use of biomarkers. However, also in this specific instance, our definitions of AD pathologic change and AD are based on biomarker evidence of disease.
13. Comparison to IWG
In addition to the NIA AA, the other group that has established diagnostic guidelines for AD that incorporate biomarkers is the IWG [64,74,75]. In the most recent formal IWG document, published in 2014 [75], the diagnosis of AD required the presence of cognitive symptoms plus an AD biomarker signature. This could be either an abnormal amyloid PET study or both abnormal CSF Aβ and tau. The NIA-AA research framework aligns with these criteria in recognizing that neither hypometabolism nor atrophy are specific for AD and thus cannot be used to support a diagnosis of AD. One difference though is that we regard CSF T-tau as a nonspecific marker of neuronal injury, while the IWG 2014 treats the combination of elevated T-tau and low Aβ42 as a biomarker signature that is specific for AD. In addition, tau PET was not available in 2014 and thus was not included in the 2014 IWG criteria. In addition to an AD biomarker signature, cognitive symptoms (specifically either a typical or a known atypical AD phenotype) were also required to diagnose AD in IWG 2014. Individuals with symptoms that fell short of dementia were labeled prodromal AD. CU individuals with an abnormal amyloid PET study or a CSF study demonstrating both abnormal Aβ and tau were labeled “asymptomatic at risk for AD”. The most significant difference between the 2014 IWG criteria and the NIA-AA research framework is that, with the exception of genetically determined AD, the 2014 IWG diagnosis of AD in living persons required both biomarker and clinical findings and therefore was not purely a biological construct.
In an article on preclinical AD (published in 2016 [15] that may be considered part of the IWG series), the diagnosis of AD was extended to include asymptomatic individuals with biomarker evidence of both Aβ and tau. In contrast to IWG 2014, symptoms were no longer required to reach a diagnosis of AD. Some differences with the NIA-AA research framework remain, however. Preclinical AD in IWG 2016 [15] defines a CU individual with an abnormal Aβ biomarker and normal tau (A+T−) as “at risk for AD, asymptomatic A+” and one with A−T+ as “at risk for AD, asymptomatic T+”. We label the former Alzheimer’s pathologic change and the latter suspected non-Alzheimer’s pathologic change (in keeping with the NIA-AA pathologic definition of primary age-related tauopathy as not AD [105,106]). Importantly, the NIA-AA research framework uses “at risk” in a much different connotation, referring to asymptomatic individuals with biomarker evidence of AD as having AD but being “at risk” of subsequent cognitive decline (as opposed to “at risk” for AD). While differences remain, IWG 2016 and the NIA research framework are aligned on the key issue that the combination of an abnormal Aβ and tau biomarker constitutes AD regardless of cognitive symptoms, and thus AD is a biologically defined entity throughout its continuum. This is an important step toward harmonization.
14. Clinical research without biomarkers or with incomplete biomarker information
While the main thesis of this research framework focuses on a biological definition of AD, we stress that for some types of studies, incorporation of biomarkers is not necessary. PET and CSF biomarkers can be difficult to acquire in some types of studies and in some geographic locations PET may not be possible. This is particularly true for large population- and community-based cohort studies. Such studies typically seek to identify risk factors for cognitive or other clinically determined outcomes. For these studies, high participant engagement is essential for internal validity and many rely on home visits to achieve both high participation and high follow-up rates. Such studies would have more limited participation and greater expense if PET or CSF biomarkers were required. Thus, research without biomarkers remains a significant enterprise and will continue to find risk factors for clinically defined syndromes or for resilience indices. The extent to which these risk factors are associated with AD will require complementary studies with imaging or biofluid biomarkers, or brain autopsy. While imaging/CSF biomarker data on subsets of individuals from well-designed community-based cohorts would provide additional research value, incorporating biomarkers on a large scale in many settings will require low-cost and minimally invasive biomarkers (e.g., blood or saliva) that are now emerging [202–205].
Another issue is that the vast majority of data from PET and CSF are from selected participants recruited through tertiary care dementia centers [176]. It is widely recognized that clinic-based participants differ from community-based studies, for example, the amount, type, and distribution of neuropathologic changes differ by the source of a participant [206,207]. There are limited data on CSF and PET AD biomarkers from population-based studies. Therefore, incorporating biomarkers into these studies is highly warranted to increase our understanding of the biology of AD, but only when such inclusion does not compromise the overarching scientific goals of the parent project. Importantly, there are less data from diverse populations. As with population-based studies, we encourage the inclusion of AD biomarkers in studies of diverse populations that use this research framework [208], but it is clearly premature to recommend that all such studies incorporate biomarkers.
The issues of clinical research without biomarkers and defining AD as amnestic dementia are often conflated, but it is important to recognize the distinction. Clinical research without biomarkers provides valuable information about the societal burden of cognitive disability and risk factors for cognitive impairment. However, amnestic multidomain dementia and other classic syndromal variants are not synonymous with the presence of Aβ deposition and neurofibrillary degeneration. In addition, the absence of amnestic dementia is clearly not synonymous with the absence of these hallmark lesions of AD. AD neuropathologic change is documented in approximately 80% of cases with a traditional clinical diagnosis of “AD dementia” (see Fig. 5 for an example of clinical misdiagnosis of “AD dementia”) [50–52,162,185,209–211]. However, preclinical AD cannot be ascertained without biomarkers. Up to 60% of CU individuals over age 80 years have AD neuropathologic changes at autopsy or by biomarkers [60,152,212–214]. Thus, using a clinical diagnosis of “AD” to ascertain absence of disease is associated with an error rate exceeding 50% in the elderly.
Fig. 5.
Non-Alzheimer’s pathologic change with dementia. An 86-year-old female with progressive amnestic dementia. The patient had been diagnosed clinically (i.e., without biomarkers) as “Alzheimer’s disease dementia” by several physicians before enrolling in the Mayo Alzheimer’s Disease Research Center. Imaging performed for research purposes revealed a normal amyloid PET (Pittsburgh compound B, left), normal tau PET with flortaucipir (middle), and severe medial temporal atrophy on MRI (right). The biomarker profile [A−T−(N)+] suggests the patient has non-Alzheimer’s pathologic change. Based on her biomarker profile, hippocampal sclerosis was suspected antemortem, and hippocampal sclerosis with TDP43 (and without Alzheimer’s disease) was later confirmed at autopsy.
Valuable clinical research will continue in contexts that do not use biomarkers where the outcome that is ascertained is a multi-(or single-) domain amnestic syndrome or a classic syndromal variant. Historically though such individuals have been labeled “probable or possible AD” [1,2] and in practice this is more often than not shortened to simply “AD” and this is problematic. Labeling individuals “AD” who do not have biomarker evidence of AD undermines the major theme of this framework. But, we also recognize the deeply engrained historic use of the term “Alzheimer” to denote particular syndromes. Thus, we strongly recommend that a clinically ascertained syndrome consistent with what has historically been labeled “probable or possible AD” be referred to as Alzheimer’s clinical syndrome, but not as AD or some modified form of AD (e.g., “possible or probable AD”). This terminology applies to both mildly impaired and demented individuals and is consistent with our position that a syndrome is not a disease, while at the same time recognizing the deeply engrained use of the term Alzheimer. This terminology is also consistent with the frontotemporal lobar degeneration field where corticobasal syndrome refers to the syndrome and corticobasal degeneration refers to a specific disease.
Studies without biomarkers that infer biological associations with “AD” can cause confusion. For example, in studies without biomarkers, diabetes has been claimed to be a risk factor for probable AD, when AD was defined as an amnestic dementia [215]. In contrast, in clinical-autopsy studies, diabetes was associated with cognitive impairment (and the clinical diagnosis of probable AD); however, the pathologic basis for this association was vascular brain injury and not Aβ plaques and neurofibrillary degeneration [50,216]. Population-based studies using biomarkers also show that mid-life risk factors (obesity, smoking, diabetes, hypertension, and cardiac disease) commonly found to be associated with cognitive decline and clinically ascertained impairment that is labeled “AD” dementia are associated with neurodegeneration but not amyloid pathology [183]. Certain genome-wide association studies provide another example of this phenomenon. When loci that were associated with clinically defined AD are examined against autopsy-defined AD, many of these loci have no association with neuritic plaques and neurofibrillary degeneration but rather with other pathologic findings such as cerebrovascular disease [217]. Apparently, conflicting conclusions like these in the literature create confusion in the medical field and in the general public, which highlights the need to investigate the relationship of risk factors to AD biomarkers or neuropathologic change to understand the biologic basis of such associations. Thus, non-biomarker studies can establish robust and valid associations between risk factors and Alzheimer’s clinical syndrome, but the biologically based studies are needed to determine if these associations are with AD.
An issue related to research without biomarkers is that many studies will ascertain some but not all biomarker groups in study participants. Because tau PET is relatively new, incomplete biomarker information will occur in studies that use imaging for amyloid and neurodegenerative biomarker characterization but lack tau PET. A missing biomarker group is denoted *; missing T would therefore be T* (Table 2). Participants in these studies may be categorized on the basis of information that is available, that is, A+T* places the participant in the “Alzheimer’s continuum,” and A−T*(N)+ is suspected non-AD pathologic change (Table 2). Another common situation will be studies with MRI but without either PET or CSF molecular biomarkers for amyloid and tau. In this situation, while MRI cannot be used as a biomarker of the Alzheimer’s continuum, it is useful as a measure of cerebrovascular disease and of nonspecific neurodegeneration, which in turn is a predictor of future clinical decline.
15. Hypothesis testing using the research framework
This framework is a flexible platform to generate and test hypotheses concerning the interactions among different pathologic processes (denoted by biomarkers) and cognitive symptoms. Abundant human and animal data implicate A and T in the primary pathogenesis of AD [9,100], including the observation that the age-related exponential increase in prevalence of A (by biomarkers and neuropathology) anticipates the age-related exponential increase in prevalence of clinically defined possible/probable “AD” by around 15 years [53,212]. However, we point out the potential distinction between possible cause(s) of AD and a biologically based definition of AD. This framework does not depend on A and T being causal in AD pathogenesis. The AT(N) biomarker system is an unbiased system for grouping biomarkers and classifying research participants on the basis of biomarker profiles. Thus, this framework can serve as a hypothesis testing platform for disease models where A and T are present as epiphenomena and models where they are causal. We emphasize though that A and T proteinopathies define AD as a unique disease among the many that can lead to dementia. As a consequence, disease models where A and T are not in the primary causal pathway must provide a mechanistic explanation for the development of both of these diagnostic proteinopathies, as well as neurodegeneration and clinical symptoms.
Many in the field are convinced that amyloidosis induces or facilitates the spread of pathologic tau (perhaps by promoting pathologic tau strains [218,219]), pathologic tau is immediately proximate to neurodegeneration, and neurodegeneration is the proximate cause of cognitive decline (C). If this “modified amyloid cascade hypothesis” were correct, then the logical biomarker sequence of AD pathogenesis would be that denoted in Fig. 6A [153,220,221]. Indeed, Fig. 6A maps onto the definitions outlined in Table 2. However, other biomarker sequences are possible and can be investigated through this framework. T could induce A (Fig. 6B), although if this were true, individuals with primary tauopathies (particularly MAPT mutations that produce 3R/4R fibrillar pathological tau that is morphologically identical to tau deposits in AD) would be expected to develop Aβ plaques, which is not the case. Both A and T could arise spontaneously and independently with the combination of both required to induce (N) (Fig. 6C). A and T could arise simultaneously due to a common upstream pathologic process (W) (Fig. 6D). For example, it is possible that cell senescence [222] or age-related breakdown of systems involved in immune surveillance or clearance of proteinaceous debris could be the upstream etiology for both A and T accumulation. A and T could be promoted by different and independent upstream pathologic processes (X and Y) [223] (Fig. 6E). For example, an age-related decrease in the rate of Aβ turnover could represent mechanism X in Fig. 6E [224]. “X” could also be a complement component receptor-1 variant that may influence Aβ clearance [164]. A yet unknown or unproven upstream pathologic process (Z) could induce A, T, and (N), with A and T being epiphenomena that are not in the causal pathway of (N) and (C). “Z” could represent many different possible mechanisms, for example, immune function, over or under activation of inflammatory pathways [165], and network failure [225,226] (Fig. 6F). It is also possible that mechanisms exist that lead to A and T but never lead to (N) and (C). Ultimately, proof of causality requires that mechanistically targeted interventions alter the natural history of the disease. If interventions that prevent A and T do not prevent (N) and (C), then this would be evidence that neither A nor T is central to the pathogenesis of AD. The research framework provides a platform to test these hypotheses.
Fig. 6.
Hypothesis testing using the research framework. In this figure, we outline various possible mechanistic pathways that involve A, T, (N), and (C). We believe current evidence most strongly supports the “modified amyloid cascade hypothesis” pathway denoted in (A), and this is reflected in the terminology in Table 2. However, we illustrate several alternatives that could be tested using the research framework. These are discussed in the text. This is not intended to represent an exhaustive list of all possible pathways but rather an illustration of some possible mechanistic pathways where A and Tare and are not causal in AD pathogenesis. In each of these models, the final common pathway is (N) → (C), which is based on the assumption that in neurodegenerative diseases, neuronal/ synaptic damage is the histopathologic feature that is most proximate to cognitive impairment. Abbreviation: AD, Alzheimer disease.
Another possible scenario is that the same pathologic process has different effects in different people. It might be that the pathway outlined in Fig. 6A is operative in some individuals, but other individuals have a factor Q (which could be genetic or environmental) that blocks the effect of A on T. In these individuals, A could accumulate harmlessly without leading to downstream events. If Q were discovered, then its effect on T, (N), and (C) given A could be tested empirically with the framework. However, this issue needs to be approached thoughtfully. The fact that individuals die with A without developing T, (N), or (C) in their lifetimes does not prove the existence of factor Q. Because of increasing death rates with age and the long preclinical period of AD [227], it cannot be known if that person would have developed T, (N), or (C) had they lived longer.
For conceptual completeness, we have outlined what undoubtedly seems like a complex system, but it is important to note that the design of this framework poses many questions that are readily testable using subsets of the population. Many research questions may use only a few of the cells in Table 4, and thus large research cohorts are not necessary to evaluate many aspects of this framework. For example, are rates of cognitive decline different for different manifestations of transitional cognitive decline (subjective report, subtle decline on testing, or neurobehavioral symptoms)? How do cognitive outcomes differ among various biomarker profiles? What is the influence of age on these relationships? Is the prevalence of cerebrovascular disease different among the three suspected non-AD pathologic change biomarker profiles [A−T+(N)−, A−T−(N) + , and A−T+(N)+]?
16. Future directions
The NIA-AA research framework defines AD biologically, by neuropathologic change or biomarkers, and treats cognitive impairment as a symptom/sign of the disease rather than the definition of the disease. This approach should enhance efforts to understand both the biology of AD and the multifactorial etiology of dementia, which has been obscured to some extent in the past by equating amnestic multidomain dementia with the presence of AD neuropathologic changes, and by equating the absence of the prototypical dementia syndrome with the absence of AD neuropathologic changes. The notion of providing a common language with which researchers can communicate is important. If one research group defines AD as Aβ plaques and pathologic tau (either by biomarkers or neuropathology) and a different group defines AD as the presence of amnestic dementia (see Fig. 5), then the findings from the two groups point to different entities, and the conclusions are not directly comparable.
We recognize that current biomarkers used in AD research are either expensive or invasive. The current generation of biomarkers is invaluable for research; however, widespread, use will be facilitated by the development of less-expensive and less-invasive biomarkers. For example, new ultrasensitive immunoassay techniques may enable measurement of minute amounts of brain-specific proteins in blood samples [228]. Candidate blood biomarkers such as neurofilament light protein [204] and plasma tau [205] show promise as non-disease-specific tools to identify neurodegeneration. Plasma Aβ measures now show promise [202,203]. In the future, less-invasive/less-expensive blood-based biomarker tests along with genetics, clinical, and demographic information will likely play an important screening role in selecting individuals for more-expensive/ more-invasive biomarker testing. This has been the history in other biologically defined diseases such as cardiovascular disease (see, e.g., the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults) [229].
This unifying research framework is a natural descendant of the 2011 NIA-AA preclinical AD recommendations that were based on the concept that AD, identified by biomarkers, can exist in the absence of symptoms [4]. The present research framework extends this concept throughout the entire Alzheimer’s continuum (Text box 4); however, it will also need to be updated at some point in the future when a modified or different conceptual approach to AD is needed to accommodate scientific advances.
Supplementary Material
Footnotes
The authors’ conflict of interest statements can be viewed online at https://doi.org/10.1016/j.jalz.2018.02.018.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2018.02.018.
References
- 1.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Assocation Workgroup. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association Workgroup. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Assocation workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carillo M, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–15. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monsell SE, Mock C, Hassenstab J, Roe CM, Cairns NJ, Morris JC, et al. Neuropsychological changes in asymptomatic persons with Alzheimer disease neuropathology. Neurology. 2014;83:434–40. doi: 10.1212/WNL.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzinger TL, Blazey T, Jack CR, Jr, Koeppe RA, Su Y, Xiong C, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci USA. 2013;110:E4502–9. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gutierrez Gomez M, Langois CM, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol. 2015;72:316–24. doi: 10.1001/jamaneurol.2014.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 13.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–92. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3007901. 226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–45. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–11. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 19.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 20.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of Florbetapir-PET for Imaging B-Amyloid Pathology. JAMA. 2011;305:275–83. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain. 2015;138:1370–81. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thal DR, Beach TG, Zanette M, Heurling K, Chakrabarty A, Ismail A, et al. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: Specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015;11:975–85. doi: 10.1016/j.jalz.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomovic M, Abrahamson E, Kofler J, Paljug W, Debnath ML, Price J, et al. Neuropathology and biochemical correlations of [F-18]AV-1451 and [C-11]PiB PET imaging in a subject with Alzheimer’s disease. In: Johnson KA, Jagust WE, Klunk WE, Mathis CA, editors. 11th Human Amyloid Imaging. Miami, Florida: 2017. p. 157. Available at: www.worldeventsforum.com/hai. [Google Scholar]
- 24.Seo SW, Ayakta N, Grinberg LT, Villeneuve S, Lehmann M, Reed B, et al. Regional correlations between [11C]PIB PET and post-mortem burden of amyloid-beta pathology in a diverse neuropathological cohort. Neuroimage Clin. 2017;13:130–7. doi: 10.1016/j.nicl.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blennow K, Mattsson N, Scholl M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36:297–309. doi: 10.1016/j.tips.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC. Tau imaging: early progress and future directions. Lancet Neurol. 2015;14:114–24. doi: 10.1016/S1474-4422(14)70252-2. [DOI] [PubMed] [Google Scholar]
- 27.Villemagne VL, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Hodges J, Harada R, et al. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2014;41:816–26. doi: 10.1007/s00259-013-2681-7. [DOI] [PubMed] [Google Scholar]
- 28.Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-Tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–68. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 29.Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci. 2013;33:5553–63. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri R, Lowe VJ, et al. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol. 2013;73:472–80. doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–85. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–65. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Harten AC, Smits LL, Teunissen CE, Visser PJ, Koene T, Blankenstein MA, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81:1409–16. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 34.Caroli A, Prestia A, Galluzzi S, Ferrari C, van der Flier WM, Ossenkoppele R, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology. 2015;84:508–15. doi: 10.1212/WNL.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15:1044–53. doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- 36.Vos SJ, Gordon BA, Su Y, Visser PJ, Holtzman DM, Morris JC, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. doi: 10.1016/j.neurobiolaging.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mormino EC, Papp KV, Rentz DM, Schultz AP, LaPoint M, Amariglio R, et al. Heterogeneity in suspected non-Alzheimer disease pathophysiology among clinically normal older individuals. JAMA Neurol. 2016;73:1185–91. doi: 10.1001/jamaneurol.2016.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik PJ, Knopman DS, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74:199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wisse LE, Butala N, Das SR, Davatzikos C, Dickerson BC, Vaishnavi SN, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36:3152–62. doi: 10.1016/j.neurobiolaging.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, et al. Longitudinal beta-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non-alzheimer disease pathophysiology. JAMA Neurol. 2016;73:1192–200. doi: 10.1001/jamaneurol.2016.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, Landau SM, et al. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70:1512–9. doi: 10.1001/jamaneurol.2013.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo JB, Weiner MW, Wolk DA, Da X, Chen K, Arnold SE, et al. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun. 2014;2:26. doi: 10.1186/2051-5960-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prestia A, Caroli A, van der Flier WM, Ossenkoppele R, Van Berckel B, Barkhof F, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80:1048–56. doi: 10.1212/WNL.0b013e3182872830. [DOI] [PubMed] [Google Scholar]
- 45.Vos SJ, Verhey F, Frolich L, Kornhuber J, Wiltfang J, Maier W, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138:1327–38. doi: 10.1093/brain/awv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene JA. The abnormal and the pathological: Cholesterol, statins, and the threshold of disease. In: Tone A, Siegel Watkins E, editors. Medicating Modern America: Prescription Drugs in History. New York: University Press; 2007. pp. 193–228. [Google Scholar]
- 47.Karlawish J. Desktop medicine. JAMA. 2010;304:2061–2. doi: 10.1001/jama.2010.1624. [DOI] [PubMed] [Google Scholar]
- 48.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002609. 111 cm 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol. 2013;9:54–8. doi: 10.1038/nrneurol.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121:571–87. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serrano-Pozo A, Qian J, Monsell SE, Blacker D, Gomez-Isla T, Betensky RA, et al. Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol. 2014;75:597–601. doi: 10.1002/ana.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85:528–34. doi: 10.1212/WNL.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 55.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–33. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 58.Zwan MD, Bouwman FH, Konijnenberg E, van der Flier WM, Lammertsma AA, Verhey FR, et al. Diagnostic impact of [18F]flutemetamol PET in early-onset dementia. Alzheimers Res Ther. 2017;9:2. doi: 10.1186/s13195-016-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ossenkoppele R, Prins ND, Pijnenburg YA, Lemstra AW, van der Flier WM, Adriaanse SF, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9:414–21. doi: 10.1016/j.jalz.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, et al. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–95. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 65.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid pet positivity in dementia syndromes: A meta-analysis. JAMA. 2015;313:1939–49. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 68.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 69.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BE, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–95. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 70.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 71.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donohue MC, Jacqmin-Gadda H, Le Goff M, Thomas RG, Raman R, Gamst AC, et al. Estimating long-term multivariate progression from short-term data. Alzheimers Dement. 2014;10:S400–10. doi: 10.1016/j.jalz.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid Abeta42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–7. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 75.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 76.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 77.Villain N, Chetelat G, Grassiot B, Bourgeat P, Jones G, Ellis KA, et al. Regional dynamics of amyloid-beta deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: a voxelwise PiB-PET longitudinal study. Brain. 2012;135:2126–39. doi: 10.1093/brain/aws125. [DOI] [PubMed] [Google Scholar]
- 78.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 79.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 80.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–27. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 81.Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–41. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 82.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf2362. 338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chhatwal JP, Schultz AP, Marshall GA, Boot B, Gomez-Isla T, Dumurgier J, et al. Temporal T807 binding correlates with CSF tau and phospho-tau in normal elderly. Neurology. 2016;87:920–6. doi: 10.1212/WNL.0000000000003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 85.Seab JP, Jagust WJ, Wong ST, Roos MS, Reed BR, Budinger TF. Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magn Reson Med. 1988;8:200–8. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- 86.Fox NC, Cram WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 87.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 88.Besson FL, La Joie R, Doeuvre L, Gaubert M, Mezenge F, Egret S, et al. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J Neurosci. 2015;35:10402–11. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri R, Lowe VJ, et al. Selective worsening of brain injury biomarker abnormalities in cognitively normal elderly persons with beta-amyloidosis. JAMA Neurol. 2013;70:1030–8. doi: 10.1001/jamaneurol.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuro-pathol. 2013;126:365–84. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 93.Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R, et al. Predicting Alzheimer disease with beta-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74:905–13. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 94.Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104–14. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–76. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- 96.Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–4. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA. 2017;317:2305–16. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73:85–92. doi: 10.1001/jamaneurol.2015.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Papp KV, Rentz DM, Mormino EC, Schultz AP, Amariglio RE, Quiroz Y, et al. Cued memory decline in biomarker-defined preclinical Alzheimer disease. Neurology. 2017;88:1431–8. doi: 10.1212/WNL.0000000000003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chabrier MA, Blurton-Jones M, Agazaryan AA, Nerhus JL, Martinez-Coria H, Laferla FM. Soluble abeta promotes wild-type tau pathology in vivo. J Neurosci. 2012;32:17345–50. doi: 10.1523/JNEUROSCI.0172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 102.Mortimer JA, Snowdon DA, Markesbery WR. The effect of APOE-epsilon4 on dementia is mediated by Alzheimer neuropathology. Alzheimer Dis Assoc Disord. 2009;23:152–7. doi: 10.1097/wad.0b013e318190a855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young AL, Oxtoby NP, Daga P, Cash DM, Fox NC, Ourselin S, et al. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain. 2014;137:2564–77. doi: 10.1093/brain/awu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiong C, Jasielec MS, Weng H, Fagan AM, Benzinger TL, Head D, et al. Longitudinal relationships among biomarkers for Alzheimer disease in the Adult Children Study. Neurology. 2016;86:1499–506. doi: 10.1212/WNL.0000000000002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Landau SM, Horng A, Fero A, Jagust WJ. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86:1377–85. doi: 10.1212/WNL.0000000000002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 109.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. Brain beta-amyloid measure and magnetic resonance imaging atophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13:205–16. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klunk WE, Cohen A, Bi W, Weissfeld L, Aizenstein H, McDade E, et al. Alzheimer’s Association International Conference 2012. Vancouver, British Columbia, Canada: Alzheimer’s Associaton; 2012. Why we need two cutoffs for amyloid-imaging: Early versus Alzheimer’s-like amyloid-positivity; pp. P453–4. [Google Scholar]
- 112.Denoix P. Enquete permanente dans les centres anticancereux. Bull Inst Natl Hyg. 1946;1:12–7. [PubMed] [Google Scholar]
- 113.Brierly JD, Gospodarowicz MK, Wittekind C. 2017 Chichester. 8. West Sussex, UK: Wiley-Blackwell; 2017. TNM Classification of Malignant Tumours, 8th Edition. [Google Scholar]
- 114.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 115.Gordon BA, Friedrichsen K, Brier M, Blazey T, Su Y, Christensen J, et al. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139:2249–60. doi: 10.1093/brain/aww139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol. 2013;74:826–36. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Minthon L, Blennow K, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–9. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–9. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alexopoulos P, Kriett L, Haller B, Klupp E, Gray K, Grimmer T, et al. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimers Dement. 2014;10:684–9. doi: 10.1016/j.jalz.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 120.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 121.Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease. Brain. 2015;138:772–83. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palmqvist S, Mattsson N, Hansson O. Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain. 2016;139:1226–36. doi: 10.1093/brain/aww015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vlassenko AG, McCue L, Jasielec MS, Su Y, Gordon BA, Xiong C, et al. Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann Neurol. 2016;80:379–87. doi: 10.1002/ana.24719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattsson N, Scholl M, Strandberg O, Smith R, Palmqvist S, Insel PS, et al. 18F–AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer’s disease. EMBO Mol Med. 2017;9:1212–23. doi: 10.15252/emmm.201707809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Makaretz SJ, Quimby M, Collins J, Makris N, McGinnis S, Schultz A, et al. Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2017 doi: 10.1136/jnnp-2017-316409. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marquie M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol. 2017;81:117–28. doi: 10.1002/ana.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marquie M, Siao Tick Chong M, Anton-Fernandez A, Verwer EE, Saez-Calveras N, Meltzer AC, et al. [F-18]-AV-1451 binding correlates with postmortem neurofibrillary tangle Braak staging. Acta Neuropathol. 2017;134:619–28. doi: 10.1007/s00401-017-1740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–58. doi: 10.1002/ana.24711. [DOI] [PubMed] [Google Scholar]
- 131.Johnson KA, Shultz A, Betensky RA, Becker JA, Sepulcre J, Rentz DM, et al. Tau positron emission tomographic imaging in aging and early Alzheimer’s disease. Ann Neurol. 2016;79:110–9. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET Imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–82. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lowe V, Wiste HJ, Pandey M, Senjem M, Boeve B, Josephs KA, et al. Tau-PET imaging with AV-1451 in Alzheimer’s disease. In: Johnson KA, Jagust W, Klunk W, Mathis C, editors. Human Amyloid Imaging. Miami Beach, FL: World Events Forum, Inc; 2016. p. 114. [Google Scholar]
- 134.Schwarz AJ, Yu P, Miller BB, Shcherbinin S, Dickson J, Navitsky M, et al. Regional profiles of the candidate tau PET ligand 18F–AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016;139:1539–50. doi: 10.1093/brain/aww023. [DOI] [PubMed] [Google Scholar]
- 135.Cho H, Choi JY, Lee SH, Lee JH, Choi Y-C, Ryu YH, et al. Excessive tau accumulation in the parieto-occipital cortex characterizes early-onset Alzheimer’s disease. Neurobiol Aging. 2017;53:103–11. doi: 10.1016/j.neurobiolaging.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 136.Phillips JS, Das SR, McMillan CT, Irwin DJ, Roll EE, Da Re F, et al. Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease. Human Brain Mapping. 2018;39:691–708. doi: 10.1002/hbm.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S, Jogi J, Ohlsson T, et al. Distinct 18F–AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain. 2017;140:2286–94. doi: 10.1093/brain/awx171. [DOI] [PubMed] [Google Scholar]
- 138.Nasrallah IM, Chen YJ, Hsieh MK, Philips JS, Ternes K, Stockbower G, et al. 18F–Flortaucipir PET-MRI correlations in non-amnestic and amnestic variants of Alzheimer Disease. J Nucl Med. 2018;59:299–306. doi: 10.2967/jnumed.117.194282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xia C, Makaretz SJ, Caso C, McGinnis S, Gomperts SN, Sepulcre J, et al. Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 2017;74:427–36. doi: 10.1001/jamaneurol.2016.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marks SM, Lockhart SN, Baker SL, Jagust WJ. Tau and beta-amyloid are associated with medial temporal lobe structure, function, and memory encoding in normal aging. J Neurosci. 2017;37:3192–201. doi: 10.1523/JNEUROSCI.3769-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pontecorvo MJ, Devous MD, Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–63. doi: 10.1093/brain/aww334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hostetler ED, Walji AM, Zeng Z, Miller P, Bennacef I, Salinas C, et al. Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J Nucl Med. 2016;57:1599–606. doi: 10.2967/jnumed.115.171678. [DOI] [PubMed] [Google Scholar]
- 143.Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 144.Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 145.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 146.Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 147.Ost M, Nylen K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelso C, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–4. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 148.Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 2014;71:476–83. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- 149.Buerger K, Otto M, Teipel SJ, Zinkowski R, Blennow K, DeBernardis J, et al. Dissociation between CSF total tau and tau protein phosphorylated at threonine 231 in Creutzfeldt-Jakob disease. Neurobiol Aging. 2006;27:10–5. doi: 10.1016/j.neurobiolaging.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 150.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 151.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 152.Jack CR, Jr, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, et al. Age-specific and sex-specific prevalence of cerebral beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017 doi: 10.1016/S1474-4422(17)30077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95:721–5. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 155.Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barkhof F, Polvikoski TM, van Straaten EC, Kalaria RN, Sulkava R, Aronen HJ, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology. 2007;69:1521–7. doi: 10.1212/01.wnl.0000277459.83543.99. [DOI] [PubMed] [Google Scholar]
- 157.van Rossum IA, Vos SJ, Burns L, Knol DL, Scheltens P, Soininen H, et al. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology. 2012;79:1809–16. doi: 10.1212/WNL.0b013e3182704056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chetelat G, Ossenkoppele R, Villemagne VL, Perrotin A, Landeau B, Mezenge F, et al. Atrophy, hypometabolism and clinical trajectories in patients with amyloid-negative Alzheimer’s disease. Brain. 2016;139:2528–39. doi: 10.1093/brain/aww159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roberts BR, Lind M, Wagen A, Rembach A, Frugier TJ, Li OX, et al. Biochemically-defined pools of Aβ-amyloid in Alzheimer’s disease: correlation with Aβ-PET imaging and a first approximation of accumulation rates of Aβ. Brain. 2017;140:1486–98. doi: 10.1093/brain/awx057. [DOI] [PubMed] [Google Scholar]
- 161.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012;72:599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 163.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016 doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thambisetty M, An Y, Nails M, Sojkova J, Swaminathan S, Zhou Y, et al. Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol Psychiatry. 2013;73:422–8. doi: 10.1016/j.biopsych.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry. 2010;68:903–12. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Piccio L, Deming Y, Del-Aguila JL, Ghezzi L, Holtzman DM, Fagan AM, et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131:925–33. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Parbo P, Ismail R, Hansen KV, Amidi A, Marup FH, Gottrup H, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain. 2017;140:2002–11. doi: 10.1093/brain/awx120. [DOI] [PubMed] [Google Scholar]
- 168.Rizzo G, Veronese M, Tonietto M, Bodini B, Stankoff B, Wimberley C, et al. Generalization of endothelial modelling of TSPO PET imaging: considerations on tracer affinities. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17742004. 271678X17742004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bevan-Jones WR, Surendranathan A, Passamonti L, Vazquez Rodriguez P, Arnold R, Mak E, et al. Neuroimaging of Inflammation in Memory and Related Other Disorders (NIMROD) study protocol: a deep phenotyping cohort study of the role of brain inflammation in dementia, depression and other neurological illnesses. BMJ Open. 2017;7:e013187. doi: 10.1136/bmjopen-2016-013187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010;1362:13–22. doi: 10.1016/j.brainres.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 171.Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 2015:1–7. doi: 10.1001/jamaneurol.2015.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73:60–7. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–13. doi: 10.1016/S1474-4422(07)70057-1. [DOI] [PubMed] [Google Scholar]
- 174.Lim SM, Katsins A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F-FDG PET cingulate island sign and comparison to 1231-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45. doi: 10.2967/jnumed.109.065870. [DOI] [PubMed] [Google Scholar]
- 175.Mormino EC, Papp KV, Rentz DM, Donohue MC, Amariglio R, Quiroz YT, et al. Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated amyloid-beta. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–85. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lopez OL, Klunk WE, Mathis C, Coleman RL, Price J, Becker JT, et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83:1804–11. doi: 10.1212/WNL.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 180.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Washington, D.C.: 2013. [Google Scholar]
- 181.Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133:2196–209. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–64. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72:730–8. doi: 10.1002/ana.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 185.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, Montine KS, et al. Ecology of the aging human brain. Arch Neurol. 2011;68:1049–56. doi: 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dunn B. Services USDoHaH, Administration FaD, (CDER) CfDEaR, (CBER) CfBEaR. Silver Spring, MD: Office of the Federal Register, National Archives and Records Administration; 2018. Early Alzheimer’s Disease: Developing Drugs for Treatment; Draft Guidance for Industry; pp. 7060–1. [Google Scholar]
- 188.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 189.Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fischer CE, Qian W, Schweizer TA, Ismail Z, Smith EE, Millikin CR, et al. Determining the impact of psychosis on rates of false-positive and false-negative diagnosis in Alzheimer’s disease. Alzheimers Dement. 2017;3:385–92. doi: 10.1016/j.trci.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448–63. doi: 10.1016/j.neuroimage.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Southekal S, Devous MD, Sr, Kennedy I, Navitsky M, Lu M, Joshi AD, et al. Flortaucipir F 18 Quantitation using a Parametric Estimate of Reference Signal Intensity (PERSI) J Nucl Med. 2017 doi: 10.2967/jnumed.117.200006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 193.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77:219–34. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Walhovd KB, Krogsrud SK, Amlien IK, Bartsch H, Bjornerud A, Due-Tonnessen P, et al. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc Natl Acad Sci USA. 2016;113:9357–62. doi: 10.1073/pnas.1524259113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–33. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klunk WE, Koeppe RA, Price JC, Benzinger T, Devous M, Jagust W, et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dementia. 2015;11:1–15. doi: 10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Frisoni GB, Jack CR, Jr, Bocchetta M, Bauer C, Frederiksen KS, Liu Y, et al. The EADC-ADNI Harmonized Protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimers Dement. 2015;11:111–25. doi: 10.1016/j.jalz.2014.05.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carrillo MC, Blennow K, Soares H, Lewczuk P, Mattsson N, Oberoi P, et al. Global standardization measurement of cerebral spinal fluid for Alzheimer’s disease: an update from the Alzheimer’s Association Global Biomarkers Consortium. Alzheimers Dement. 2013;9:137–40. doi: 10.1016/j.jalz.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 200.Kuhlmann J, Andreasson U, Pannee J, Bjerke M, Portelius E, Leinenbach A, et al. CSF Abeta1-42-an excellent but complicated Alzheimer’s biomarker - a route to standardisation. Clin Chim Acta. 2017;467:27–33. doi: 10.1016/j.cca.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 201.Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59:1152–60. doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–9. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018 doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 204.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–66. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dage JL, Wennberg AM, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12:1226–34. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brenowitz WD, Keene CD, Hawes SE, Hubbard RA, Longstreth WT, Jr, Woltjer RL, et al. Alzheimer’s disease neuropathologic change, Lewy body disease, and vascular brain injury in clinic- and community-based samples. Neurobiol Aging. 2017;53:83–92. doi: 10.1016/j.neurobiolaging.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ighodaro ET, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, et al. Challenges and Considerations Related to Studying Dementia in Blacks/African Americans. J Alzheimers Dis. 2017;60:1–10. doi: 10.3233/JAD-170242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Au R, Seshadri S, Knox K, Beiser A, Himali JJ, Cabral HJ, et al. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9:673–86. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 213.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 214.Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12:882–9. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Beecham GW, Hamilton K, Naj AC, Martin ER, Huentelman M, Myers AJ, et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014;10:el004606. doi: 10.1371/journal.pgen.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM, et al. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability. In Vivo. Neuron. 2016;92:796–812. doi: 10.1016/j.neuron.2016.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li AM, et al. Distinct Tau Prion Strains Propagate in Cells and Mice and Define Different Tauopathies. Neuron. 2014;82:1271–88. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s Disease. Neuron. 2013;80:1347–58. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Latorre E, Birar VC, Sheerin AN, Jeynes JCC, Hooper A, Dawe HR, et al. Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. BMC Cell Biol. 2017;18:31. doi: 10.1186/s12860-017-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vemuri P, Knopman DS, Lesnick TG, Przybelski SA, Mielke MM, Graff-Radford J, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neural. 2017;74:718–26. doi: 10.1001/jamaneurol.2017.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neural. 2015;78:439–53. doi: 10.1002/ana.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, et al. Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2016;139:547–62. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14:121–9. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Andreasson U, Blennow K, Zetterberg H. Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimers Dement. 2016;3:98–102. doi: 10.1016/j.dadm.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.