Abstract
The present study aimed to investigate the association between the expression of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) long non-coding RNA (lncRNA) and the recurrence of non-small cell lung cancer (NSCLC) and to elucidate the potential mechanisms of MALAT1 in vitro. Between 1 June 1, 2010 and December 30, 2016, NSCLC tumor tissues and adjacent non-cancerous tissues were obtained from 120 patients with NSCLC, who had undergone surgical resection at Taizhou Hospital of Wenzhou Medical University (Linhai, China). The total RNA of tissues and cells were extracted and the expression of MALAT1 was determined using a wound healing assay and reverse transcription quantitative polymerase chain reaction. In addition, MALAT1 expression in A549 cells was silenced using small interfering RNA. The proliferation, migration and invasion of cells were then assessed using a CellTiter 96 kit and Transwell assays. MALAT1 expression was significantly increased in NSCLC samples compared with expression in adjacent non-cancerous tissues. Furthermore, the expression of MALAT1 in patients with NSCLC that exhibited recurrence was markedly higher than in those that did not. The results of the present study also demonstrated significant associations between high expression of MALAT1 and female sex, Tumor-Node-Metastasis advanced stage, vessel invasion, pathological differentiation and recurrence of patients with NSCLC. The proliferative, migratory and invasive abilities of MALAT1-silenced A549 cells were significantly decreased compared with those of control cells. MALAT1 expression was significantly increased in NSCLC tissues and was revealed to serve a role in the progression of NSCLC.
Keywords: non-small cell lung cancer, long non-coding RNA, metastasis-associated lung adenocarcinoma transcript 1, clinical characteristic, migration, invasion
Introduction
Lung cancer is the most common type of cancer worldwide (1), and 85% of lung cancer cases are diagnosed as non-small cell lung cancer (NSCLC) (2). In China alone, ~7,333,000 patients were diagnosed with NSCLC and, of these, ~6,102,000 patients succumbed to mortality in 2015 (3). With increased levels of environmental pollution, the incidence of NSCLC and the mortality rate of patients with this disease have increased significantly (1), resulting in increased health and economic burdens on patients and society. Although NSCLC treatments have improved with the development of medical technology, certain clinically applied drugs targeting late-stage NSCLC (2,4) are ineffective due to the presence of multiple gene mutations and comprehensive pathogenesis (5,6). Therefore, it is important to obtain a greater understanding of the underlying mechanism of NSCLC development in order to improve the efficacy of clinical treatment.
Long non-coding RNAs (lncRNAs), comprising >200 nucleotides, are a class of transcripts with no protein-coding capacity (7). Previous studies have demonstrated that lncRNAs perform diverse functions in cancer cells, including proliferation, migration, invasion and apoptosis (8,9). The roles of lncRNAs have also been assessed in patients with NSCLC. Shi et al (10) identified that growth arrest specific 5 lncRNA serves critical roles in the proliferation and apoptosis of NSCLC cells via p53-dependent and -independent pathways and may serve as a diagnostic marker for NSCLC. In addition, Yang et al (11) demonstrated that PVT1 oncogene lncRNA is significantly upregulated in patients with NSCLC and its expression level is associated with lymph node metastasis and a poorer prognosis. Kruer et al (12) also indicated that maternally expressed 3 lncRNA is involved in the regulation of lung cancer cell proliferation via the retinoblastoma pathway. In addition, a previous study indicated that urothelial cancer associated 1 lncRNA may serve as an oncogene in NSCLC by targeting microRNA (miR)-193a-3p (13), which has been demonstrated to suppress the metastasis of NSCLC by downregulating the Erb-B2 receptor tyrosine kinase 4/phosphoionositide-3-kinase regulatory subunit 3/mechanistic target of the rapamycin/ribosomal protein S6 kinase B2 signaling pathway (14). The results of these studies indicated that lncRNAs serve important roles in the regulation of NSCLC pathogenesis.
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT-1), also known as MALTA1 or nuclear-enriched abundant transcript 2, is the first identified lncRNA to be associated with lung cancer. Since its discovery over a decade ago, a large number of studies have demonstrated the associations between MALAT1 and cancer progression (15–17). Recently, a study reported that MALAT1 may promote bone metastasis in patients with NSCLC (18). However, associations between MALAT1 and NSCLC clinical outcome or occurrence have rarely been addressed (19). Therefore, the present study aimed to elucidate the associations between MALAT1 and the clinical characteristics of patients with NSCLC and to assess the potential effect of MALAT1 on the progression of NSCLC.
Materials and methods
Patients and samples
A total of 120 patients with NSCLC, who had undergone surgical resection at the Taizhou Hospital of Wenzhou Medical University (Linhai, China) and were diagnosed by biopsy, were enrolled in the present study between June 1, 2010 and December 30, 2016. Patients were excluded if they exhibited any of the following: Hepatitis infection, autoimmune disease, human immunodeficiency virus infection or psychosis. During enrollment, patient clinical data, including sex, age, vessel invasion, clinical stage, tumor type, tumor differentiation, tumor diameter and recurrence, were collected and are presented in Table I. Tumor-Node-Metastasis (TNM) stage evaluation was performed according to 2014 8th edition of the TNM classification (20).
Table I.
Associations between MALAT1 expression and the clinical characteristics of patients with non-small cell lung cancer.
| MALAT1 | ||||
|---|---|---|---|---|
| Term | Low-expression | High-expression | χ2 | P-value |
| Sex | ||||
| Male | 69 | 40 | 5.467 | 0.019 |
| Female | 3 | 8 | ||
| Age, year | ||||
| ≤50 | 25 | 24 | 0.025 | 0.838 |
| >50 | 37 | 34 | ||
| Maximum diameter, cm | ||||
| ≤5 | 38 | 33 | 0.629 | 0.504 |
| >5 | 24 | 25 | ||
| Tumor number | ||||
| Single | 21 | 19 | 0.012 | 0.905 |
| Multiple | 43 | 37 | ||
| TNM stage | ||||
| I–II | 32 | 20 | 5.172 | 0.016 |
| III–IV | 20 | 48 | ||
| Vessel invasion | ||||
| No | 35 | 45 | 6.483 | 0.032 |
| Yes | 8 | 32 | ||
| Pathological differentiation | ||||
| High-medium | 23 | 65 | 12.383 | 0.013 |
| Low | 0 | 32 | ||
| Recurrence | ||||
| No | 30 | 15 | 9.542 | 0.006 |
| Yes | 24 | 51 | ||
MALAT1, metastasis-associated lung adenocarcinoma transcript 1; TNM, tumor, node, metastasis. P<0.05 was considered to indicate a statistically significant difference.
During surgical resection, paired tumor tissues and adjacent non-cancerous tissues were collected from patients with NSCLC. Tissue sections were preserved at −80°C or in liquid nitrogen for subsequent analysis. The present study was approved by the Ethics Committee of Taizhou Hospital of Wenzhou Medical University (Linhai, China) and written informed consent was obtained from all patients prior to enrollment.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. RNA quality was assessed using the Nanodrop N-1000 (Agilent Technologies, Inc., Santa Clara, CA, USA) and 2 µg total RNA was reverse transcribed into cDNA using the Applied Biosystems® TaqMan® mRNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to manufacturer's protocol. qPCR was subsequently performed using SYBR Green (Applied Biosystems; Thermo Fisher Scientific, Inc.) on an ABI 7900 Real-Time PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the following thermocycling conditions: 95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C for 31 sec. GAPDH was used as the internal control. The gene primers utilized were as follows: MALAT1 forward, 5′-AGGCGTTGTGCGTAGAGGA-3′ and reverse, 5′-GGATTTTACCAACCACTCGC-3′; and GAPDH forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′. Each experiment was performed in triplicate and was quantified using the 2−ΔΔCq method (21).
Cell culture and transfection
The NSCLC A549 cell line was purchased from ATCC (Manassas, VA, USA) and cells cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere with 5% CO2.
Small interfering RNA (siRNA) targeting MALAT1 (10 nmol/l) and its corresponding control were transfected onto a 6-well plate with a cell confluence of 70% in each well using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The sequences for siRNA were designed as follows: si-MALAT1, forward, 5′-CACAGGGAAAGCGAGUGGUUGGUAATT-3′, and reverse, 5′-UUACCAACCACUCGCUUUCCCUGUGTT-3′; and si-control, forward, 5′-UUCUCCGAACGUGUCACGUTT-3′, and reverse 5′-ACGUGACACGUUCGGAGAATT-3′. The siRNA sequences were synthetized by Shanghai GenePharma Co., Ltd (Shanghai, China). After 48 h, stable cell strains were screened using puromycin (Thermo Fisher Scientific, Inc.) and the expression of MALAT1 was evaluated using RT-qPCR was performed as aforementioned using the human tissue.
Cell proliferation assay
Following the determination of the effect of silenced MALAT1, transfected A549 cells were seeded onto a 96-well plate at a density of 5×103 cells/well. Subsequently, CellTiter 96 (Promega Corporation, Madison, WI, USA) was added to each well, according to the manufacturer's protocol. Following culture at 37°C, the absorbance of cells and the index of cellular proliferation were measured at 490 nm using a Bio-Rad plate-reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) every 24 h. A total of 100 µl DMEM was utilized as a blank control. Each sample was conducted with five repeats and experiments were independently repeated twice.
Wound healing assay
For the wound healing assay, cells were seeded onto 6-cm plates and were cultured in DMEM supplemented with 10% FBS. Once the cells had reached 80% confluency, they were scraped with a 200 µl tip to wound the monolayer and the starting width of the wound (200 µl) was recorded. Cells were then cultured in DMEM medium (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h. Images of the migrated distance of cells were captured and measured using a light microscope at magnification, ×100, 0 and 48 h after scraping.
Invasion assay
For the invasion assay, 1×104 cells in 200 µl FBS-free DMEM were seeded into an upper Matrigel-coated chamber (BD Biosciences, Franklin Lakes, NJ, USA). The lower chamber was filled with 500 µl RPMI-1640 medium containing 10% FBS to induce cell invasion. Following a 24-h incubation, cells on the upper filter were removed using cotton swabs. Cells on the lower filter, namely the invading cells, were fixed with 95% ethanol at 4°C for 1 h, stained with 0.5% crystal violet at room temperature for 12 min, and monitored using an inverted microscope at a magnification of ×200. For each sample, cells in ≥5 random microscopic visual fields were counted and the average number was used as the final result.
Statistical analyses
In the present study, SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Continuous variables are presented as the mean ± standard deviation. Comparisons between two groups were analyzed using Student's t-test, and pair-wise comparisons among multiple groups were determined using a one-way analysis of variance followed by the Least Significant Difference test. Associations between MALAT1 and clinical characteristics were determined using the χ2 test. The receiver operative characteristics (ROC) curve analysis in SPSS was utilized to create a summary ROC curve in order to evaluate the predicative ability of MALAT1 in NSCLC recurrence. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of MALAT1 in clinical NSCLC tissues
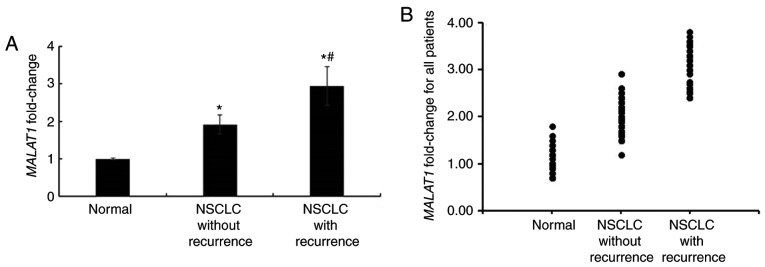
Following the collection of clinical samples, the expression levels of MALAT1 in tumor and adjacent non-cancerous tissues were determined using RT-qPCR. The results demonstrated that the expression of MALAT1 was significantly increased in NSCLC tumor tissues when compared with non-tumor adjacent tissues (P<0.05, data not shown). Additionally, the expression of MALAT1 in patients with recurrent NSCLC was markedly higher than that in NSCLC patients without recurrence (Fig. 1).
Figure 1.
Expression of MALAT1 in NSCLC tissue samples. (A) MALAT1 expression in tissue samples as determined using reverse transcription-quantitative polymerase chain reaction. (B) MALAT1 expression in tissue samples obtained from all patients. *P<0.05 vs. the normal group; #P<0.05 vs. the non-recurrence group. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; NSCLC, non-small cell lung cancer.
Association between MALAT1 and clinical characteristics
Following the assessment of MALAT1 expression in tissue samples, associations between MALAT1 and clinical characteristics were evaluated (Table I). The results demonstrated that a high expression of MALAT1 was significantly associated with female sex (P=0.019), TNM stage (P=0.016), vessel invasion (P=0.032), pathological differentiation (P=0.013) and recurrence (P=0.006) in patients with NSCLC. However, no significant associations were identified between the expression of MALAT1 and age, tumor maximum diameter or tumor number (P>0.05). In addition, the diagnostic value of MALAT1 in the recurrence of NSCLC was also estimated (Fig. 2). The area under curve value was 0.76 and the Q index was 0.71, indicating that MALAT1 has potential diagnostic value for the predication of NSCLC recurrence.
Figure 2.
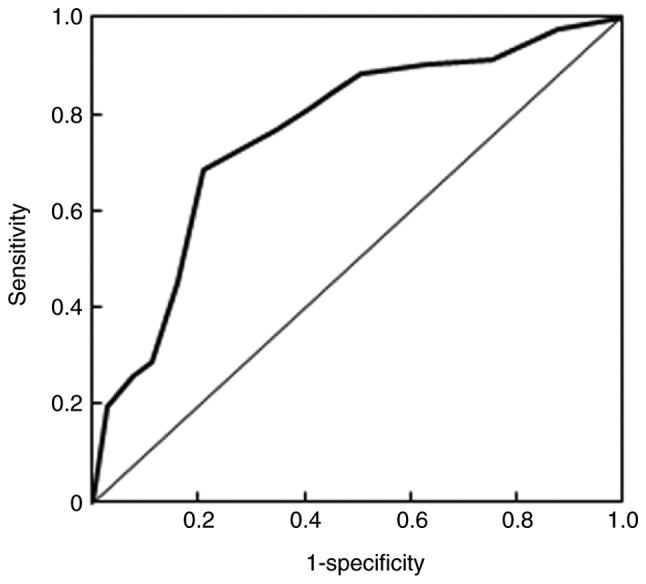
Receiver operative characteristic curve analysis for the ability of MALAT1 to predict non-small cell lung cancer recurrence. The area under curve value was 0.76 and the Q index was 0.71. MALAT1, metastasis-associated lung adenocarcinoma transcript 1.
Effects of silenced MALAT1 on cell proliferation
In order to further elucidate the underlying mechanism of MALAT1, its expression was silenced using siRNA in A549 cells and the efficacy was confirmed using RT-qPCR (Fig. 3). Once silencing had been successful, the influence of MALAT1 on cell proliferation was assessed. The results demonstrated that A549 cellular proliferation was significantly decreased in si-MALAT1 A549 cells compared with the controls at all time points (P<0.05; Fig. 4). These results indicated that MALAT1 promoted the proliferation of A549 cells.
Figure 3.
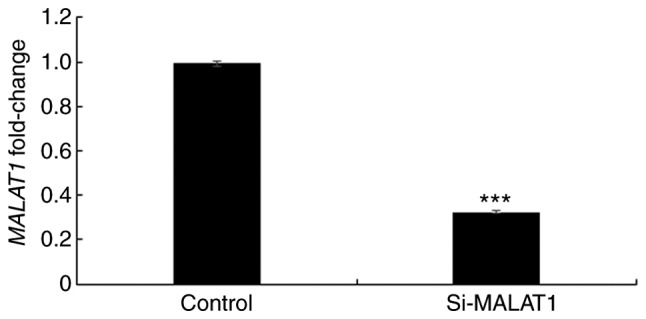
Verification of si-MALAT1 using reverse transcription-quantitative polymerase chain reaction in A549 cells. ***P<0.001 vs. the control group. si-MALAT1, silenced metastasis-associated lung adenocarcinoma transcript 1.
Figure 4.
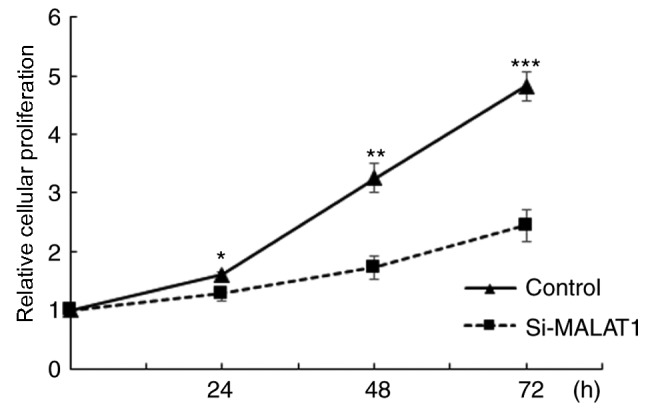
Comparison of relative cellular proliferation between control and si-MALAT1 treated A549 cells. *P<0.05, **P<0.01, and ***P<0.001 vs. the control group. si-MALAT1, silenced metastasis-associated lung adenocarcinoma transcript 1.
Effects of si-MALAT1 on cell migration and invasion
The influence of si-MALAT1 on A549 cell migration and invasion were assessed using wound healing and Matrigel assays, respectively. The results of the wound healing assay demonstrated that migratory abilities were markedly decreased in si-MALAT1 A549 cells compared with control cells (Fig. 5). In addition, Transwell migration demonstrated that the invasive ability of si-MALAT1 A549 cells was also markedly inhibited compared with control cells (Fig. 6). These results indicated that MALAT1 may improve the migration and invasion ability of A549 cells.
Figure 5.
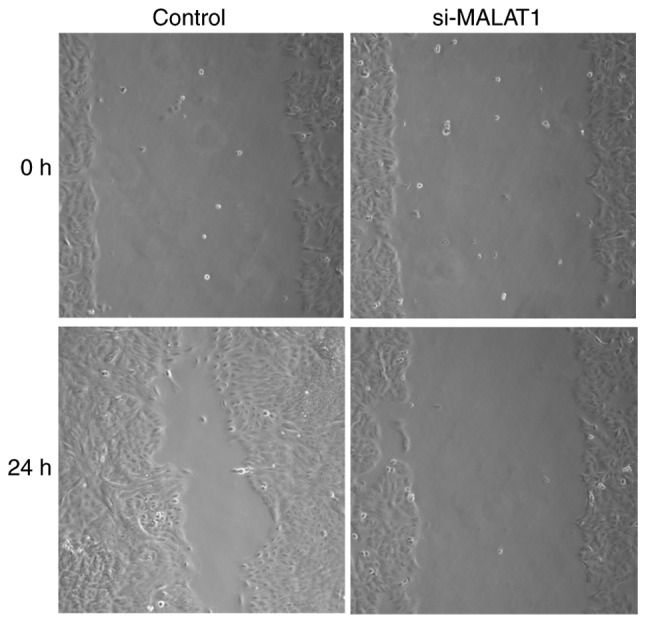
Wound healing assay demonstrates the migratory ability of control and si-MALAT1-treated A549 cells under a light microscope at magnification, ×100. si-MALAT1, silenced metastasis-associated lung adenocarcinoma transcript 1.
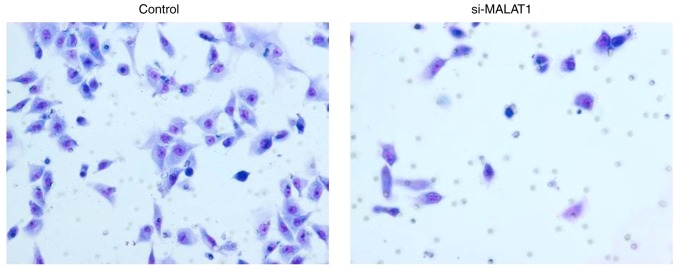
Figure 6.
Matrigel invasion assay verifies the invasiveness of control and si-MALAT1 A549 cells. si-MALAT1, silenced metastasis-associated lung adenocarcinoma transcript 1.
Discussion
The present study aimed to assess the associations between MALAT1 expression and the clinical characteristics of patients with NSCLC, and to reveal the potential underlying mechanism of MALAT1 in NSCLC. The results of the present study demonstrated that the expression of MALAT1 was significantly increased in patients with NSCLC, particularly in those with recurrent malignancy. Furthermore, the silencing of MALAT1 markedly decreased the proliferability, migration and invasiveness of A549 cells. Therefore, MALAT1 served a crucial role in the occurrence of NSCLC.
It has been demonstrated that lncRNA serves important roles in various processes involved in cancer pathogenesis, including proliferation, differentiation, migration, invasion and apoptosis (22). As an important lncRNA discovered a decade ago, the abnormal expression of MALAT1 has been exhibited in certain malignancies (23). Lai et al (24) demonstrated that MALAT1 expression was significantly increased in hepatocellular carcinoma clinical tissues and cell lines, serving as a novel biomarker for tumor recurrence following liver transplantation. Ying et al (25) also revealed that MALAT1 is abnormally upregulated in bladder cancer and that it promotes cellular migration by inducing epithelial-mesenchymal transition. Furthermore, Schmidt et al (26) observed that MALAT1 expression is increased in NSCLC and interacts with B cell lymphoma 2 to regulate lung cancer cell apoptosis. In addition, Liu et al (18) demonstrated that MALAT1 promotes bone metastasis in patients with NSCLC; however, the study only compared NSCLC tissues and NSCLC cell lines with and without bone metastasis. The results of the present study indicated that there was a significant increase in the expression of MALAT1 in NSCLC and that the expression of MALAT1 in patients with NSCLC that exhibited recurrence was markedly higher than that in those who did not. These results therefore indicate that MALAT1 serves a crucial role in the development and recurrence of NSCLC and that it may act as an onco-lncRNA to regulate the migration, differentiation and apoptosis of cancer cells as malignancies progress.
Considering the effect of MALAT1 in cancer, associations between MALAT1 and the clinical characteristics and survival rates of patients with NSCLC were assessed. Shen et al (27) demonstrated that MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. The results of the present study demonstrated that the invasive and migratory abilities of MALAT1-silenced A549 cells were significantly decreased. This may be the reason for the significant association between a high expression of MALAT1 and low pathological differentiation, as well as vessel invasion, as identified in the present study. Furthermore, a study undertaken by Tano et al (28) revealed that MALAT1 facilitates lung adenocarcinoma cell motility by regulating the expression of motility-related genes, including CTHRC1, CCT4, ROD1 and HMMR. Therefore, these results indicated that MALAT1 serves a crucial role in NSCLC metastasis.
However, an in vivo study further demonstrated that MALAT1-deficient cells exhibit an impaired migration and form fewer tumor nodules in a mouse xenograft (29). In the present study, it was demonstrated that si-MALAT1 also significantly suppresses A549 cell proliferation and migration. In addition, Schmidt et al (30) revealed that a high-expression of MALAT1 indicates a poor prognosis in patients with NSCLC. These observations may indicate that MALAT1 promotes the proliferation of NSCLC cells. MALAT1 has also been revealed to serve a role in the migration and invasion of breast cancer and osteosarcoma cells, and was demonstrated to be significantly inhibited by 17β-Estradiol (31,32). In line with this, significant associations were identified between a high expression of MALAT1 and TNM stage, as well as recurrence. The results of the present study also indicated that MALAT1 may be able to predict NSCLC recurrence. These results indicate that MALAT1 serves an important role in the progression of NSCLC and that it may serve as a potential biomarker for the development and recurrence of NSCLC.
The present study exhibited certain limitations. In order to confirm the association between MALAT1 expression and NSCLC recurrence, further studies are required. The present study also only utilized a histological method to confirm NSCLC diagnosis, and histological analysis of NSCLC tissue was not conducted to assess the effects of MALAT1. Further study would therefore be required in order to gain a greater understanding of this.
In conclusion, to the best of our knowledge, the present study was the first to demonstrate that MALAT1 was associated with NSCLC recurrence. MALAT1 was revealed to be an important onco-lncRNA involved in the pathogenesis of NSCLC. Inhibiting the expression of MALAT1 significantly decreased the proliferation, migration and invasion of tumor cells. According to these results, MALAT1 may serve as a potential treatment target and a possible diagnostic biomarker of NSCLC. However, the detailed underlying mechanism of MALAT1 in NSCLC remains to be elucidated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LL wrote the manuscript and conducted a majority of the experiments. HL, YZ and SH collected and analyzed the data. HG designed the study and approved the manuscript for submission.
Ethics approval and consent to participate
Written informed consent was obtained from all participants and the present study was approved by the Research Committee of Taizhou Hospital of Wenzhou Medical University (Linhai, China).
Consent for publication
Written informed consent was obtained from all patients.
Competing interests
The authors declare that there are no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Friese-Hamim M, Bladt F, Locatelli G, Stammberger U, Blaukat A. The selective c-Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c-Met activation in NSCLC models. Am J Cancer Res. 2017;7:962–972. [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev. 2013;27:2065–2071. doi: 10.1101/gad.228122.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner SA, Beli P, Serve H. Abstract 3888: Systematic characterization of aberrant signaling induced by oncogenic fusions in non-small cell lung cancer. Cancer Res. 2016;76(14 Suppl):S3888–S3888. doi: 10.1158/1538-7445.AM2016-3888. [DOI] [Google Scholar]
- 7.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Shi SJ, Wang LJ, Yu B, Li YH, Jin Y, Bai XZ. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6:11652–11663. doi: 10.18632/oncotarget.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 10.Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F, Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2015;54(Suppl 1):E1–E12. doi: 10.1002/mc.22120. [DOI] [PubMed] [Google Scholar]
- 11.Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:6929–6935. [PMC free article] [PubMed] [Google Scholar]
- 12.Kruer TL, Dougherty SM, Reynolds L, Long E, de Silva T, Lockwood WW, Clem BF. Expression of the lncRNA maternally expressed gene 3 (MEG3) contributes to the control of lung cancer cell proliferation by the Rb pathway. PloS One. 2016;11:e0166363. doi: 10.1371/journal.pone.0166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao X, Chen WS, Li B. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Yu T, Li J, Yan M, Liu L, Lin H, Zhao F, Sun L, Zhang Y, Cui Y, Zhang F, et al. MicroRNA-193a-3p and −5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34:413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Zhang X, Wang H, Wang L, Liu T, Du L, Yang Y, Wang C. MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol Cancer Ther. 2017;16:739–751. doi: 10.1158/1535-7163.MCT-16-0591. [DOI] [PubMed] [Google Scholar]
- 16.Zhang TH, Liang LZ, Liu XL, Wu JN, Su K, Chen JY, Zheng QY, Huang HZ, Liao GQ. Long non-coding RNA MALAT1 interacts with miR-124 and modulates tongue cancer growth by targeting JAG1. Oncol Rep. 2017;37:2087–2094. doi: 10.3892/or.2017.5445. [DOI] [PubMed] [Google Scholar]
- 17.Malakar P, Shilo A, Mogilevsky A, Stein I, Pikarsky E, Nevo Y, Benyamini H, Elgavish S, Zong X, Prasanth KV, Karni R. Long non-coding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77:1155–1167. doi: 10.1158/0008-5472.CAN-16-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Sun W, Liu Y, Dong X. The role of lncRNA MALAT1 in bone metastasis in patients with non-small cell lung cancer. Oncol Rep. 2016;36:1679–1685. doi: 10.3892/or.2016.4909. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Xia Y, Wang Z, Zheng J, Chen Y, Li X, Wang Y, Ming H. Serum long non-coding RNA MALAT-1 protected by exosomes is upregulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490:406–414. doi: 10.1016/j.bbrc.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 20.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-(Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Lu X, Yuan L. LncRNA: A link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Xue Y, Teng YQ, Zhou JD, Rui YJ. Prognostic value of long noncoding RNA MALAT1 in various carcinomas: Evidence from nine studies. Tumor Biol. 2016;37:1211–1215. doi: 10.1007/s13277-015-3915-z. [DOI] [PubMed] [Google Scholar]
- 24.Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY, Zhang F, Wu LM, Chen LM, Zheng SS. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29:1810–1816. doi: 10.1007/s12032-011-0004-z. [DOI] [PubMed] [Google Scholar]
- 25.Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8:2289–2294. doi: 10.1039/c2mb25070e. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt LH, Görlich D, Spieker T, Rohde C, Schuler M, Mohr M, Humberg J, Sauer T, Thoenissen NH, Huge A, et al. Prognostic impact of Bcl-2 depends on tumor histology and expression of MALAT-1 lncRNA in non-small-cell lung cancer. J Thorac Oncol. 2014;9:1294–1304. doi: 10.1097/JTO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015;121:101–108. doi: 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 28.Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, Ijiri K, Akimitsu N. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–4580. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt LH, Spieker T, Koschmieder S, Schäffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Chen C, Liu Y, Wu C. 17β-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level. Biochem Biophys Res Commun. 2014;445:388–393. doi: 10.1016/j.bbrc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Fang D, Yang H, Lin J, Teng Y, Jiang Y, Chen J, Li Y. 17β-estradiol regulates cell proliferation, colony formation, migration, invasion and promotes apoptosis by upregulating miR-9 and thus degrades MALAT-1 in osteosarcoma cell MG-63 in an estrogen receptor-independent manner. Biochem Biophys Res Commun. 2015;457:500–506. doi: 10.1016/j.bbrc.2014.12.114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.