Abstract
The peritoneal metastasis-associated phosphatase of regenerating liver-3 (PRL-3) is upregulated in gastric cancer. The phosphatidylinositol 3-kinase (PI3K)/RAC serine/threonine-protein kinase (AKT) signaling pathway acts downstream of PRL-3 in gastric cancer. However, the exact PRL-3 signaling mechanisms are poorly understood. The present study investigated whether PRL-3 facilitates the peritoneal metastasis of gastric cancer via the PI3K/AKT pathway in vivo and in vitro. Nude mouse models of peritoneal metastasis were established using SGC7901/PRL-3 cell lines. The results confirmed that the invasion and migration abilities of SGC7901/PRL-3 cells were significantly increased in these models. Furthermore, western blotting demonstrated that the expression of p-AKT, matrix metallopeptidase-2 (MMP-2) and −9 proteins increased in SGC7901/PRL-3 cells. These effects were suppressed in SGC7901 cell lines when PI3K was inhibited by LY294002. Furthermore, tumors derived from the peritoneal injection of SGC7901/PRL-3 cells were significantly smaller when the cells were grown in the presence of LY249002, compared with cells grown in its absence. These results indicated that targeted inhibition of the PI3K/AKT signaling pathway decreased the effects of PRL-3 on metastasis in vivo. Collectively, the results of the present study indicated that PRL-3 acts via the PI3K/AKT pathway to promote peritoneal metastasis and invasion of gastric cancer cells in vitro and in vivo.
Keywords: gastric cancer, peritoneal metastasis, phosphatidylinositol 3-kinase/RAC serine/threonine-protein kinase, phosphatase of regenerating liver-3, nude mice models of peritoneal metastasis
Introduction
Gastric cancer is the fourth most common cause of cancer-associated mortality worldwide and accounts for ~10% of the annual incidences of cancer-associated mortality (1,2). Almost two-thirds gastric cancer cases occur in less developed regions (3). Research has been performed to improve the clinical outcomes of gastric cancer; this has included improvement in large-scale nationwide screening processes, radical surgery, chemotherapy and radiation therapy (4–6). Despite medical progress, Eastern Asia has the highest gastric cancer mortality rate worldwide (7). The primary cause of mortality due to gastric cancer is metastasis, which is often resistant to conventional therapy (8). Peritoneal metastasis is one of the main causes of gastric cancer progression and accounts for the majority of gastric cancer-induced mortalities (9). Therefore, the development of novel strategies to prevent the peritoneal metastasis of gastric cancer is required.
Members of the phosphatase of regenerating liver (PRL) protein family have been associated with numerous cancer types (10,11), and PRL-3 has been identified as a potentially critical biomarker of gastric cancer (12). A previous study demonstrated that PRL-3 expression was significantly increased in primary gastric cancer with peritoneal metastasis compared with the corresponding primary gastric cancer without metastasis (13). Additionally, PRL-3 has been demonstrated to induce the proliferation, invasion, migration, tumorigenesis and metastasis of malignant neoplasms (14,15), including gastric cancer (16).
The mechanisms by which PRL-3 promotes the motility, metastasis and invasion of gastric cancer cells are not fully understood. Dysregulated phosphatidylinositol 3-kinase (PI3K)/RAC serine/threonine-protein kinase (AKT) signaling has been demonstrated to cause abnormal cell growth and cellular transformation in malignant neoplasms, including gastric cancer (17–19). PRL-3 regulates PTEN expression and signals through PI3K/AKT to promote the epithelial-mesenchymal transition (20). Furthermore, PRL-3 has been demonstrated to promote the peritoneal metastasis of gastric cancer through PI3K/AKT signaling in vitro (21). However, these results have not been confirmed at a whole-animal level. To analyze the in vivo roles of PRL-3 and PI3K/AKT signaling, a nude mouse model of peritoneal metastasis was established and treated with LY294002 (LY), a potent and specific PI3K inhibitor (22), to test whether inhibition of the PI3K/AKT signaling pathway prevents PRL-3-mediated progression of tumorigenesis in vivo.
Materials and methods
Cell lines and animals
The human gastric adenocarcinoma cancer SGC7901 cell line was purchased from the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM (Biological Industries, Kibbutz Beit-Haemek, Israel) supplemented with 10% fetal bovine serum (FBS; Shanghai Li Rui Biological Technology Co., Ltd., Shanghai, China) and penicillin/streptomycin, and maintained in a humidified atmosphere with 5% CO2 at 37°C. Specific pathogen-free male BALB/c-nu mice (n=24, 5 weeks old, weighing 16–17 g) were purchased from the Hunan Slack King of Laboratory Animal Co., Ltd. (Changsha, China) [Production permit no. SCXK (XIANG) 2013-0004]. All mice were housed in the same environment (room temperature was maintained at 26±2°C, relative humidity was kept between 40 and 60%, and the light regimen was a 12/12-h dark: light cycle) and allowed free access to water and food. Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the present study was approved by the Ethics Committee of Nanchang University (Nanchang, China).
Cell proliferation analysis
To analyze SGC7901 cell proliferation, a Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer's instructions. SGC7901 cells were plated into 96-well plates and incubated for 24 h with different concentrations of LY294002 (0, 5, 10, 20 or 40 µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following a 24-h incubation at 37°C in 5% CO2, 10 µl CCK-8 was added to each well, and incubated for 4 h at 37°C. The absorbance values were detected at 450 nm using an enzyme-labeled instrument (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Generating a stable EGFP-PRL-3/SGC7901 cell line
EGFP-PRL-3 plasmid construction was performed as previously described (21). The EGFP-PRL-3 vector was transfected into SGC7901 cells using the Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Western blot analysis
Total protein extraction and bicinchoninic acid protein assay kits were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Polyvinylidene difluoride membranes were purchased from Merck KGaA. Antibodies against PRL-3 (ab50276; 1:400), AKT (ab8805; 1:500), p-AKT S473 (ab81283; 1:1,000), MMP-2 (ab97779; 1:1,000) and MMP-9 (ab137867; 1:1,000) were purchased from Abcam (Cambridge, UK). The antibody for β-actin (60008-1-Ig; 1:5,000) was purchased from Wuhan Sanying Biotechnology (Wuhan, China). These antibodies were used in the present study was described previously (23–26). Membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (ab6721; 1:3,000) or goat anti-mouse secondary antibody (Wuhan Sanying Biotechnology; SA00012-6; 1:2,000) and the western blotting protocol were performed as previously described (21). Reactive protein was detected by an enhanced chemiluminescence kit (EMD Millipore, Billerica, MA, USA). Band intensity was quantified by using the Quantity One image analysis software version 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell migration and invasion assays
The migratory and invasive abilities of SGC7901 cells transfected with PRL-3/LY (LY294002) or empty control vector/LY were determined using a Transwell assay. A total of 1.0×105 cells in serum-free DMEM medium were seeded in the upper chamber of an 8-µm pore, 6.5-mm polycarbonate filter (Corning Incorporated, Corning, NY, USA) for cell migration. For cell invasion, the upper chamber was coated with 20 µg Matrigel and the following protocols were the same as those for cell migration. DMEM containing 10% FBS was added to the lower chamber. The following steps were performed as previously described (21). All assays were independently repeated ≥3 times. Cells in five microscopic fields (magnification, ×200) were counted and photographed. Each assay was done at least in triplicate. Images were captured using a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Nude mouse peritoneal metastasis model
Specific pathogen-free grade male BALB/c nude mice (n=24, 5 weeks old, weighing 16–17 g) were housed in the Laboratory Animal Center of the First Affiliated Hospital of Nanchang University (Nanchang, Jiangxi, China). The 5-week old nude mice were randomly separated into 4 groups of 6. SGC7901/PRL-3/dimethyl sulfoxide (DMSO, SGC7901/PRL-3/LY294002 and SGC7901/vector/DMSO cells were injected into the abdominal cavity at 2.0×107 cells/mouse. The control group contained nude mice injected with an equivalent volume of phosphate buffered saline. After 1 week, twice-weekly intraperitoneal injections of 50 mg/kg LY294002 were performed, as described previously (27), for 4 weeks. The mice were then sacrificed by cervical dislocation and the peritoneal cavity was opened for visual inspection and photography.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as the mean ± standard deviation. The χ2 test was used to analyze the relationship between the expression levels of PRL-3. Differences between control and experiment groups were analyzed using a one-way analysis of variance test followed by least significance difference post-hoc test. Each experiment was repeated ≥3 times. P<0.05 was considered to indicate a statistically significant difference.
Results
LY294002-mediated PI3K/AKT inhibition decreases SGC7901 cell proliferation
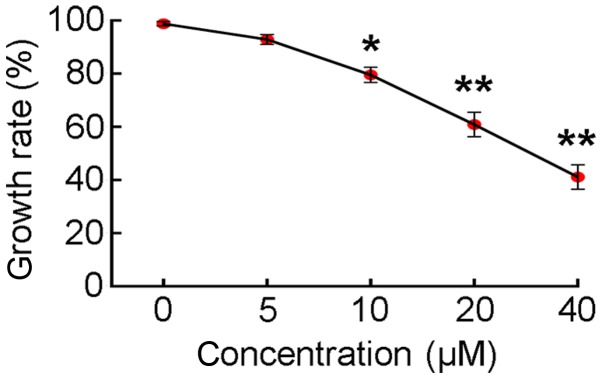
A CCK-8 assay was used to assess effect of LY294002-mediated inhibition of PI3K/AKT activity on SGC7901 cell proliferation. When SGC7901 cells were cultured in medium containing 0, 5, 10, 20 or 40 µM LY294002 for 24 h, the rate of cell proliferation decreased in a dose-dependent manner (Fig. 1). Treatment with ≥10 µM LY294002 reduced the rate of cell proliferation to ≤80%. Therefore, a dosage of 10 µM LY294002 was selected for use in subsequent experiments.
Figure 1.
SGC7901 cell proliferation with and without LY294002 treatment. Each plot represents the mean ± standard deviation of 3 independent experiments. *P<0.05, **P<0.001 vs. the control group.
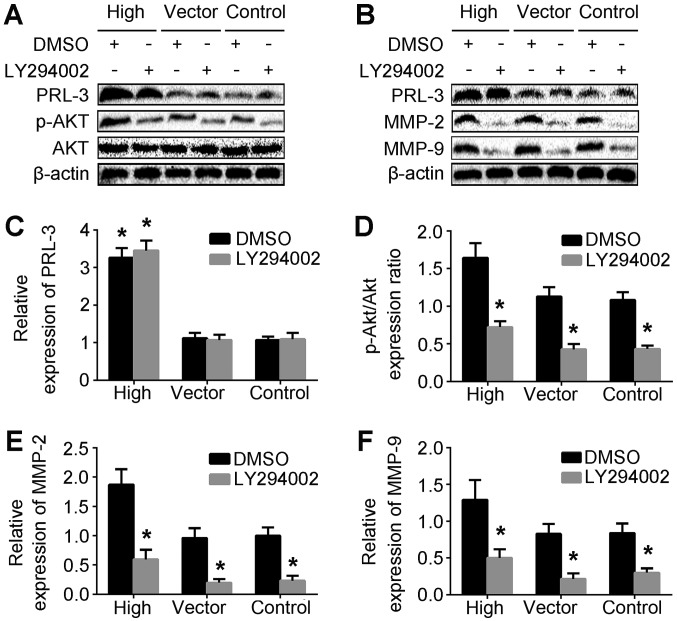
LY294002 inhibits the expression of p-AKT (Ser473), MMP-2 and MMP-9 proteins in pEGFP-PRL-3-SGC7901 cells
To investigate the association between PRL-3 and p-AKT levels in the SGC7901/PRL-3 and LY/SGC7901/PRL-3 treatment groups, western blotting was performed to determine the protein levels of PRL-3, AKT and p-AKT (Fig. 2A). MMPs, including MMP-2 and MMP-9, can promote cells to migrate through the basement membrane (28). To dissect the function and gelatinolytic activities of MMP-2 and MMP-9 in PRL-3-promoted cell invasion, the protein expression of MMP-2 and MMP-9 in the LY/SGC7901/PRL-3 and DMSO/SGC7901/PRL-3 treatment groups was analyzed by western blotting (Fig. 2B). The levels of p-AKT, MMP-2 and MMP-9 protein in the LY/SGC7901/PRL-3 treatment group were significantly lower than those in control groups (Fig. 2D-F). Taken together, these results indicated that PRL-3 may upregulate MMP-2 and MMP-9 expression via the PI3K/AKT pathway.
Figure 2.
p-AKT, MMP-2 and MMP-9 are involved in PRL-3-mediated invasion and migration of gastric cancer cells. (A) Levels of PRL-3, AKT and p-AKT were analyzed in SGC7901 cells (control), SGC7901 cells transfected with empty vector, and plasmid SGC7901/EGFP-PRL-3 cells (high) by western blotting. (B) The expression of MMP-2 and MMP-9 were also analyzed by western blotting. (C) The relative expression of PRL-3 was quantified relative to β-actin. (D) The ratio of p-AKT/AKT was quantified relative to the control group. (E) The expression of MMP-2 was quantified relative to β-actin. (F) The expression of MMP-9 was quantified relative to β-actin. Each bar represents the mean ± standard deviation of 3 independent experiments. *P<0.05 vs. the control group. p-AKT, phosphorylated-RAC serine/threonine-protein kinase; MMP-2, matrix metallopeptidase-2; MMP-9, matrix metallopeptidase-9; PRL-3, phosphatase of regenerating liver-3; DMSO, dimethyl sulfoxide.
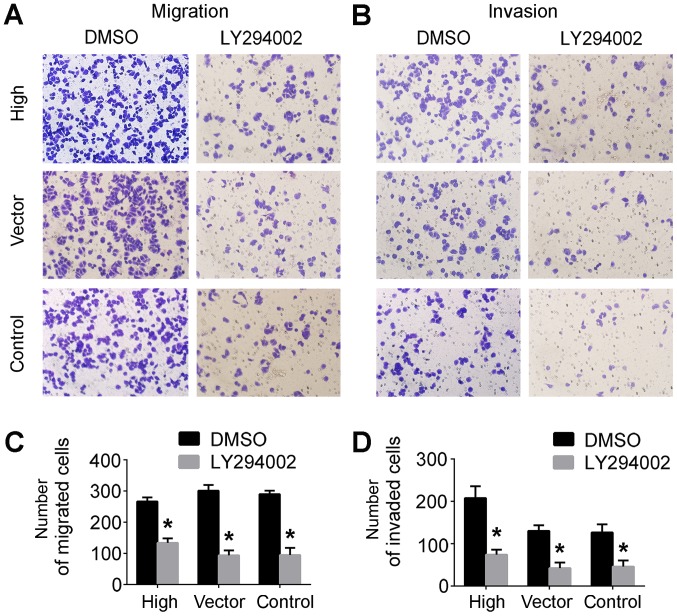
Migration and invasion abilities conferred by PRL-3 are reduced by LY249002
Transwell assays were performed to assess cell migratory and invasive capabilities. Compared with the control groups, the cells in the lower Transwell chamber were significantly decreased in the SGC7901/LY249002 group compared with controls (Fig. 3A and B). Taken together, these results demonstrated that LY294002 inhibited PRL-3-conferred invasive and migratory abilities of SGC7901 cells (Fig. 3C and D), indicating that PRL-3 is involved in cell migration and invasion via the PI3K/AKT signaling pathway in vitro.
Figure 3.
The PI3K/AKT pathway is involved in the PRL-3-mediated invasion and migration of gastric cancer. (A) Migration and (B) invasion were analyzed by Transwell assays. Quantification of (C) migration and (D) invasion prior to and following the addition of LY294002. Each histogram bar indicates the number of stained cells. The experiments were independently repeated ≥3 times. Magnification, ×200. *P<0.05 vs. the control group. Control, SGC7901 cells; vector, SGC7901 cells transfected with control empty vector; high, plasmid SGC7901/EGFP-PRL-3 cells. PI3K, phosphatidylinositol 3-kinase; AKT, RAC serine/threonine-protein kinase; PRL-3, phosphatase of regenerating liver-3; DMSO, dimethyl sulfoxide.
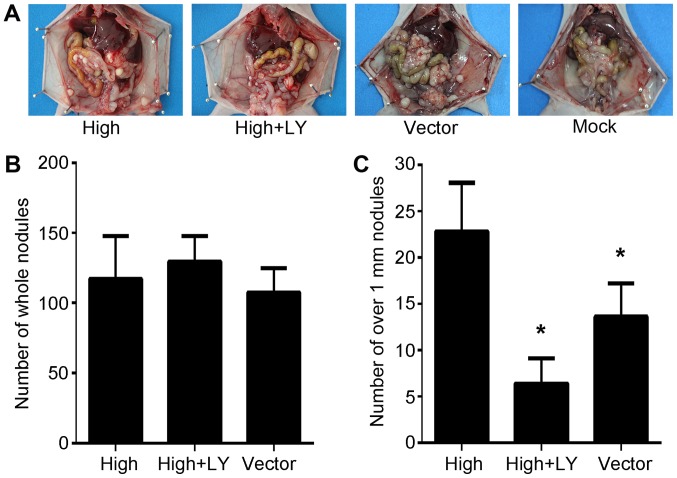
PRL-3 promotes peritoneal spreading via PI3K/AKT signaling in vivo
The in vivo effects of PRL-3 overexpression on peritoneal spreading and metastasis via the PI3K/AKT pathway were investigated in the present study. SGC7901/PRL-3, SGC7901/PRL-3/LY294002 and SGC7901/Vector cells were injected into the abdomens of nude mice. Extensive peritoneal spread was observed in all three groups (Fig. 4A). Differences in the total number of metastatic nodules on the peritoneal surface were not statistically significant (Fig. 4B). However, the number of metastatic nodules exceeding 1.0 mm3 in size significantly differed between treatment groups (P<0.05; Fig. 4C). These results are consistent with those previously reported (27,29), and indicate that the PI3K inhibitor effectively suppresses PRL-3-mediated invasion in this peritoneal metastasis model.
Figure 4.
PRL-3 promotes peritoneal spreading via the PI3K/AKT signaling pathway in vivo. (A) Representative peritoneal dissemination of SGC7901 cells in LY294002-treated mice. Mice were sacrificed 4 weeks post-injection and the peritoneum was observed. (B) The total number of metastatic nodules on the peritoneal surface in each treatment group. (C) The number of metastatic nodules >1 mm3 in each treatment group. The number of metastatic nodules was significantly lower in the high+LY group. The bars represent the mean ± standard deviation (n=6/group). *P<0.05 vs. the control group. Mock, normal nude mice; vector, SGC7901 transfected with control empty vector; high, SGC7901/EGFP-PRL-3 cells; high+LY, plasmid SGC7901/EGFP-PRL-3/LY294002 cells. PRL-3, phosphatase of regenerating liver-3; AKT, RAC serine/threonine-protein kinase; PI3K, phosphatidylinositol 3-kinase.
Discussion
The current gastric cancer drugs are designed to inhibit or alter PI3K/AKT signaling to prevent metastasis (30–32). PRL-3 is a regulator of the PI3K/AKT signaling pathway (20). To a certain extent, blocking the PI3K/AKT pathway influences the tumorigenic effects of PRL-3 (21). The present study aimed to confirm this at a whole-animal level. LY294002 effectively inhibits the growth of a variety of tumor cells in vitro and in vivo through the inhibition of the PI3K/AKT pathway (33–35). Therefore, in the case of peritoneal metastasis, it is possible that LY294002 treatment had a negative effect on PRL-3-mediated tumorigenesis.
Peritoneal metastasis accounts for the majority of incidences of gastric cancer-associated mortality (9). Previous in vitro results have demonstrated that PRL-3 is involved in peritoneal metastasis via the PI3K/AKT signaling pathway (21). The growth of malignant tumors is the result of interactions between tumor cells and the local microenvironment, which is affected by a variety of cytokines and other complex factors that influence in vivo growth (36). Therefore, in vitro experiments do not reliably reflect the in vivo environmental context. To determine whether PRL-3 can influence tumor growth via the PI3K/AKT pathway in vivo, a mouse model of gastric cancer peritoneal metastasis was established. Significant differences between the number of metastatic nodules on the peritoneal surface >1 mm3 were observed between the PRL-3/DMSO and PRL-3/LY groups. This is likely to be due to the fact that the growth of solid tumors >1 mm3 is dependent on specific oncogenes (37). Furthermore, it was indicated that PI3K inhibition might prevent PRL-3-mediated peritoneal metastasis. However, treatment with LY294002 did not completely inhibit tumor growth. Therefore, it is possible that the PI3K/AKT signaling pathway was only partially inhibited by LY294002 treatment, which represent a limitation of the present study. Other pathways may also be involved in this process, which may function similarly to the PI3K/AKT pathway. This speculation requires further investigation.
Invasion and metastasis are key steps in tumor development. MMPs serve notable roles in tumor cell migration, metastasis and invasion (38,39). Additionally, the expression of MMPs increase when the PI3K/AKT pathway is activated (40–42). MMP-2 and MMP-9 are closely associated with the lymphatic metastasis of gastric cancer (43). In the present study, a significant difference in the level of MMP-2 and MMP-9 expression was identified between transfected gastric tumor cells treated with LY249002 and control (P<0.05). This result indicates that the LY249002-mediated inhibition of MMP-2, MMP-9 and p-AKT expression occurred via PRL-3. These results indicated that LY249002 has the potential to inhibit PRL-3-mediated invasion and metastasis in vitro and in vivo.
Targeted therapies provide the most efficacious and selective treatment to suppress tumor development (44). The present study demonstrated that PRL-3 promoted the peritoneal metastasis of gastric cancer via the PI3K/AKT pathway in vivo and in vitro. These results may provide a novel candidate for gene-targeted therapy, and are likely to translate into improved strategies for the treatment of gastric cancer.
Acknowledgements
The authors thank the Key Laboratory of Basic Pharmacology of Nanchang University and the Department of Histology and Embryology of Medical College of Nanchang University (Nanching, China) for technical assistance in the experiments.
Funding
The present study was supported by the National Science Foundation of China (grant no. 81360362/81160304), the Education Department of Jiangxi Province Science and Technology Research Projects (grant no. GJJ13126), the Training Program for Young Scientists of Jiangxi Province (grant no. 20133BCB23020) and the Graduate Student Innovation Special Fund Project of Nanchang University (grant no. cx2016383).
Availability of data and materials
The datasets used or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
HC, ZL, YZ, XF and ZJ conceived and designed the experiment. YZ and XF performed the experiments. HC, YZ and XF analyzed the data. JX, GZ, XL, KL, YC, ZH, FW, LX and GD contributed reagents, materials and analysis tools. HC, ZL, YZ, XF and ZJ contributed to the writing of the manuscript. The final version of the manuscript has been read and approved by all authors.
Ethics approval and consent to participate
Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the present study was approved by the Ethics Committee of Nanchang University (Nanchang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Best LM, Mughal M, Gurusamy KS. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst Rev. 2016;3:Cd011389. doi: 10.1002/14651858.CD011389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P. Gastric cancer: Overview. Gastroenterol Clin North Am. 2013;42:211–217. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KS, Oh DK, Han MA, Lee HY, Jun JK, Choi KS, Park EC. Gastric cancer screening in Korea: Report on the national cancer screening program in 2008. Cancer Res Treat. 2011;43:83–88. doi: 10.4143/crt.2011.43.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259–267. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 6.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, Lee JS. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa K, Iwase K, Aono T, Yoshida H, Nomura M, Tamagawa H, Matsuda C, Deguchi T, Kawada J, Higashi S, et al. Efficacy of capecitabine/cisplatin chemotherapy after failure of all conventional therapies in patients with advanced gastric cancer. Gan To Kagaku Ryoho. 2013;40:57–60. (In Japanese) [PubMed] [Google Scholar]
- 9.Turaga KK, Gamblin TC, Pappas S. Surgical treatment of peritoneal carcinomatosis from gastric cancer. Int J Surg Oncol. 2012;2012:405652. doi: 10.1155/2012/405652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessette DC, Qiu D, Pallen CJ. PRL PTPs: Mediators and markers of cancer progression. Cancer Metastasis Rev. 2008;27:231–252. doi: 10.1007/s10555-008-9121-3. [DOI] [PubMed] [Google Scholar]
- 11.Slordahl TS, Abdollahi P, Vandsemb EN, Rampa C, Misund K, Baranowska KA, Westhrin M, Waage A, Rø TB, Børset M. The phosphatase of regenerating liver-3 (PRL-3) is important for IL-6-mediated survival of myeloma cells. Oncotarget. 2016;7:27295–27306. doi: 10.18632/oncotarget.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miskad UA, Semba S, Kato H, Yokozaki H. Expression of PRL-3 phosphatase in human gastric carcinomas: Close correlation with invasion and metastasis. Pathobiology. 2004;71:176–184. doi: 10.1159/000078671. [DOI] [PubMed] [Google Scholar]
- 13.Li ZR, Wang Z, Zhu BH, He YL, Peng JS, Cai SR, Ma JP, Zhan WH. Association of tyrosine PRL-3 phosphatase protein expression with peritoneal metastasis of gastric carcinoma and prognosis. Surg Today. 2007;37:646–651. doi: 10.1007/s00595-006-3437-9. [DOI] [PubMed] [Google Scholar]
- 14.Dong Q, Ding X, Chang B, Wang H, Wang A. PRL-3 promotes migration and invasion and is associated with poor prognosis in salivary adenoid cystic carcinoma. J Oral Pathol Med. 2016;45:111–118. doi: 10.1111/jop.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato H, Semba S, Miskad UA, Seo Y, Kasuga M, Yokozaki H. High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: A predictive molecular marker of metachronous liver and lung metastases. Clin Cancer Res. 2004;10:7318–7328. doi: 10.1158/1078-0432.CCR-04-0485. [DOI] [PubMed] [Google Scholar]
- 16.Dai N, Lu AP, Shou CC, Li JY. Expression of phosphatase regenerating liver 3 is an independent prognostic indicator for gastric cancer. World J Gastroenterol. 2009;15:1499–1505. doi: 10.3748/wjg.15.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruman DA, Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vara Fresno JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P, Roa JC. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465:25–33. doi: 10.1007/s00428-014-1588-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:2922–2926. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 21.Xiong J, Li Z, Zhang Y, Li D, Zhang G, Luo X, Jie Z, Liu Y, Cao Y, Le Z, et al. PRL-3 promotes the peritoneal metastasis of gastric cancer through the PI3K/Akt signaling pathway by regulating PTEN. Oncol Rep. 2016;36:1819–1828. doi: 10.3892/or.2016.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 23.Liu Y, Zheng P, Liu Y, Ji T, Liu X, Yao S, Cheng X, Li Y, Chen L, Xiao Z, et al. An epigenetic role for PRL-3 as a regulator of H3K9 methylation in colorectal cancer. Gut. 2013;62:571–581. doi: 10.1136/gutjnl-2011-301059. [DOI] [PubMed] [Google Scholar]
- 24.Mollevi DG, Aytes A, Padulles L, Martínez-Iniesta M, Baixeras N, Salazar R, Ramos E, Figueras J, Capella G, Villanueva A, et al. PRL-3 is essentially overexpressed in primary colorectal tumours and associates with tumour aggressiveness. Br J Cancer. 2008;99:1718–1725. doi: 10.1038/sj.bjc.6604747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayinuer A, Yasen M, Mogushi K, Obulhasim G, Xieraili M, Aihara A, Tanaka S, Mizushima H, Tanaka H, Arii S. Upregulation of protein tyrosine phosphatase type IVA member 3 (PTP4A3/PRL-3) is associated with tumor differentiation and a poor prognosis in human hepatocellular carcinoma. Ann Surg Oncol. 2013;20:305–317. doi: 10.1245/s10434-012-2395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khapare N, Kundu ST, Sehgal L, Sawant M, Priya R, Gosavi P, Gupta N, Alam H, Karkhanis M, Naik N, et al. Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis. PLoS One. 2012;7:e38561. doi: 10.1371/journal.pone.0038561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Fan D, Zhou G, Li X, Deng H. Phosphatidylinositol 3-kinase inhibitor (LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:34. doi: 10.1186/1756-9966-29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y, Liang F, Zhang ZY. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry. 2009;48:1838–1846. doi: 10.1021/bi8020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuoka T, Yashiro M, Nishioka N, Hirakawa K, Olden K, Roberts JD. PI3K/Akt signalling is required for the attachment and spreading, and growth in vivo of metastatic scirrhous gastric carcinoma. Br J Cancer. 2012;106:1535–1542. doi: 10.1038/bjc.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Liu L, Liu X, Wang Y, Li M, Yao L, Yang J, Ji G, Guo C, Pan Y, et al. MGr1-Ag/37LRP induces cell adhesion-mediated drug resistance through FAK/PI3K and MAPK pathway in gastric cancer. Cancer Sci. 2014;105:651–659. doi: 10.1111/cas.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almhanna K, Strosberg J, Malafa M. Targeting AKT protein kinase in gastric cancer. Anticancer Res. 2011;31:4387–4392. [PubMed] [Google Scholar]
- 32.Jia Y, Sun H, Wu H, Zhang H, Zhang X, Xiao D, Ma X, Wang Y. Nicotine Inhibits Cisplatin-Induced Apoptosis via regulating α5-nAChR/AKT signaling in human gastric cancer cells. PLoS One. 2016;11:e0149120. doi: 10.1371/journal.pone.0149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957–1963. [PubMed] [Google Scholar]
- 34.Hu L, Zaloudek C, Mills GB, Gray J, Jaffe RB. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002) Clin Cancer Res. 2000;6:880–886. [PubMed] [Google Scholar]
- 35.Zhao K, Zhu BS, Gong W, Zhu ML, Gao ZT, Wu YY, Chen Q, Yang XD, Xing CG. SN50 enhances the effects of LY294002 on cell death induction in gastric cancer cell line SGC7901. Arch Med Sci. 2013;9:990–998. doi: 10.5114/aoms.2013.39790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci. 2013;70:1335–1356. doi: 10.1007/s00018-012-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 38.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 39.Foda HD, Zucker S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov Today. 2001;6:478–482. doi: 10.1016/S1359-6446(01)01752-4. [DOI] [PubMed] [Google Scholar]
- 40.Furuya F, Hanover JA, Cheng SY. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc Natl Acad Sci USA. 2006;103:1780–1785. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL, Kim JS, Yoo YA. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-κB pathway, and MMP-9 expression. Exp Cell Res. 2011;317:1746–1762. doi: 10.1016/j.yexcr.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 43.Xu JEC, Yao Y, Ren S, Wang G, Jin H. Matrix metalloproteinase expression and molecular interaction network analysis in gastric cancer. Oncol Lett. 2016;12:2403–2408. doi: 10.3892/ol.2016.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2012;64:206–212. doi: 10.1016/j.addr.2012.09.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the present study are available from the corresponding author on reasonable request.