Abstract
The aim of the present study was to was investigate the treatment efficacy of hyperbaric oxygen (HBO) combined with nimodipine on diffuse brain injury. AA total of 80 patients with diffuse brain injury were randomly divided into four groups: Group A, conventional treatment; Group B, conventional treatment + nimodipine; Group C, conventional treatment + HBO therapy and Group D, conventional treatment + nimodipine + HBO therapy. The Glasgow Coma Scale (GCS) score and serum tumor necrosis factor (TNF)-α and interleukin (IL)-1β levels were assessed before treatment and at 8, 24, 48 and 72 h after treatment. The bilateral middle cerebral arterial blood flow velocity (VmMCA) was measured by transcranial Doppler ultrasound. The results indicated that serum TNF-α and IL-1β were significantly decreased in all groups at 24, 48 and 72 h after treatment, compared with 8 h after treatment (P<0.05), with Group D exhibiting the largest decrease. The serum TNF-α, IL-1β and VmMCA peaked at 8 h and gradually decreased over the treatment period. VmMCA was decreased in Group B and D compared with Group A and C, and the decrease rate was higher in Group D compared with Group B (P<0.05). GCS scores were significantly increased in all groups at 24, 48 and 72 h after treatment compared with 8 h after treatment (P<0.05), with Group D exhibiting the largest increase. Serum TNF-α and IL-1β levels were positively correlated with VmMCA (P<0.05) and negatively correlated with GCS (P<0.05). Punctate hemorrhage was observed in all groups on CT before treatment, with a value of 66±3 HU. Punctate hemorrhage was observed to decrease over time in CT images, and CT values were significantly decreased in all groups at 8, 24, 48 and 72 h compared with before treatment (P<0.05). CT values were significantly lower in group D compared with groups A, B and C (P<0.05) at all time points. Serum TNF-α and IL-1β levels were positively correlated with CT value (P<0.05). In conclusion, HBO combined with nimodipine exhibited increased efficacy in the treatment of brain injury compared with either treatment alone.
Keywords: tumor necrosis factor-α, interleukin-1β, hyperbaric oxygen, nimodipine, diffuse brain injury
Introduction
Traumatic brain injury has one of the highest incidences among all types of trauma, with mortality rate as high as 30–50% (1). Brain injury can be categorized into four types: Diffuse axonal injury, diffuse brain swelling, diffuse microvascular injury and hypoxic brain damage (2). At the early stage of brain injury, local nerve cells and tissues are damaged, leading to blood-brain barrier damage and multiple cytokine releases (3). Cytokines serve key roles in the pathogenesis and outcome of brain injury (4).
Insufficient supply of oxygen (5) and inflammation (4) are vital in the development of brain injury. An insufficient supply of oxygen is hard to correct at normal temperature and pressure (6). However, in hyperbaric oxygen (HBO) therapy, the diffused oxygen content can increase up to 20 times, with increased oxygen difference between alveoli and pulmonary vein (7,8). Therefore, brain tissue oxygen partial pressure and oxygen storage capacity are significantly improved, thereby reducing cerebral hypoxia, preventing cerebral hypoxic-ischemic brain damage and improving brain circulation (9). It has previously been demonstrated that oxygen supply is closely associated with prognosis after brain injury (10).
Brain injury produces multiple cytokines from the site of injury or the whole brain, including interleukins (ILs) (11), tumor necrosis factor-α (TNF-α) (12), interferons (13) and basic fibroblast growth factor (14). These cytokines serve critical roles at different stages of brain injury. For example, TNF-α can directly activate and induce leukocyte endothelial cell adhesion at the site of injury (15). Then, the leukocytes release a series of chemical factors, causing tissue damage (16).
In the present study, the levels of serum TNF-α and IL-1β were detected before and after HBO combined with nimodipine treatment in patients with diffuse brain injury. The efficacy of HBOHBO combined with nimodipine as a target intervention in the early stages of trauma was evaluated.
Materials and methods
Subjects
A total of 80 patients with brain injury were recruited from October 2013 to October 2015 within 24 h after injury. Patients included 62 males and 18 females, with ages ranging from 18 to 45 years. The characteristics of included subjects are shown in Table I. Patients were included if they: i) were diagnosed by CT or MRI; ii) had no other injuries; iii) had a Glasgow Coma Scale (GCS) score of 5–8 (17); iv) had no history of major underlying diseases at admission; and v) gave consent for treatment. Patients were excluded if they: i) were <18 years or >45 years old; ii) had any underlying brain diseases, such as brain tumors; iii) had a history of major chronic diseases, including hypertension, coronary heart disease, diabetes or liver and kidney dysfunction; iv) were pregnant or breastfeeding; v) had a history of epilepsy; vi) had multiple drug allergies; and vii) had other factors that could influence GCS score, including mental retardation. Prior written and informed consent was obtained from every patient. The study was approved by the ethics review board of the Second Hospital of Hebei Medical University (Shijiazhuang, China).
Table I.
General characteristics of subjects.
| Characteristic | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| n | 20 | 20 | 20 | 20 |
| Sex (male/female) | 14/6 | 17/3 | 15/5 | 16/4 |
| Age (years) | 39.15±3.65 | 40.25±3.14 | 38.26±2.78 | 40.19±2.25 |
| Time from injury to admission (h) | 5.15±2.04 | 4.84±2.16 | 4.32±2.28 | 4.60±2.14 |
| GCS at admission | 6.03±1.34 | 6.42±1.56 | 6.27±1.48 | 6.24±1.85 |
| Cause of injury (n) | ||||
| Car accident | 13 | 12 | 11 | 15 |
| Fall | 2 | 2 | 2 | 1 |
| Crashing | 3 | 4 | 5 | 3 |
| Crash | 1 | 1 | 2 | 1 |
| Other | 1 | 1 | 0 | 0 |
| Type of brain injury (n) | ||||
| Brain contusion and laceration | 7 | 8 | 6 | 6 |
| Intracranial hematoma | 5 | 6 | 6 | 5 |
| Subarachnoid hemorrhage | 5 | 4 | 4 | 4 |
| Primary brain stem injury | 1 | 0 | 1 | 1 |
| Diffuse axonal injury | 2 | 2 | 3 | 4 |
GCS, Glasgow Coma Scale.
Grouping and treatment
All patients were randomly divided into four groups (n=20 in each group): Group A, conventional treatment; Group B, conventional treatment + nimodipine; Group C, conventional treatment + HBO therapy; and Group D, conventional treatment + nimodipine + HBO therapy.
Patients in Group A received conventional treatment, which included dehydration agents, corticoids, vitamins, hemostatic agents and neurotrophic drugs, including 250 ml mannitol (20%), i.v., for consecutive 5 days (18), and aescine sodium (10–20 mg in 0.9% NaCl solution, in a total volume of 100 ml) via i.v., once or twice each day (19). Hematoma or decompression craniectomy procedures were performed if necessary.
In addition to conventional therapy, patients in Group B received infusion of nimodipine (8 mg in 500 ml of 5% glucose) after exclusion of further bleeding and hematoma formation by computed tomography (CT) scan. The nimodipine was administered at 1 mg/h within the first 2 h, and this was increased to 2 mg/h if vital signs remained stable for a full course of 14 days. If the vital signs were unstable, the observation was suspended.
In addition to conventional therapy, patients in Group C received HBO therapy. Patients inhaled pure oxygen for 30 min through an oxygen mask at a pressure of 0.2 MPa in an HBO chamber. After a break of 10 min, patients re-inhaled pure oxygen for 20 min. Then, the pressure was reduced to 0.15 MPa and patients inhaled pure oxygen for 10 min. The pressure then continued decreasing to 0.1 MPa over 15 min. The HBO therapy was administered once a day for 20 days.
In addition to conventional therapy, patients in Group D received the conventional therapy combined with HBO therapy and infusion of nimodipine (8 mg in 500 ml of 5% glucose) after exclusion of further bleeding and hematoma formation by CT scan. Detailed procedures were the same as those for Group B.
Sampling and detection of serum TNF-α and IL-1β
Blood samples were drawn before treatment and at 8, 24, 48 and 72 h after injury. The blood sample was placed in 45°C water bath for 10 min, followed by centrifugation at 955.78 × g for 5 min. Detection of TNF-α and IL-1β levels was performed using Human TNF-α and IL-1β ELISA kits (ml028611 mibio and ml001543 mibio, Hengyuan Biotech, Shanghai, China). All samples were detected immediately. Briefly, all samples were reacted at 37°C for 30 min; enzyme-labeled reagents were added after washing 5 times and reacted at 37°C for 30 min. Subsequently, chromogenic reagents were added after washing 5 times and reacted at 37°C for 10 min. The OD value at 450 nm was recorded 15 min after the buffer was added.
CT values
Head CT was performed using a Brilliance iCT machine (Philips Medical Systems B.V., Eindhoven, The Netherlands). The CT values were recorded.
Detection of cerebral artery blood flow velocity (VmMCA) and GCS score
Transcranial Doppler ultrasound was used to measure bilateral middle VmMCA and GCS scores were calculated for all patients at 8, 24, 48, and 72 h after treatment. GCS scores were based on evaluation of eye movement, language ability and body movements, and were categorized into 3 types: Light coma (13–14 points), moderate coma (9–12 points) and deep coma (3–8 points) (5).
Statistical analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Quantitative data are expressed as the mean ± standard deviation and were analyzed using independent-sample student's t-tests. Group data were compared using one-way analysis of variance, and pairwise comparisons were performed using the Student-Newman-Keuls test. Qualitative data were analyzed using the χ2 test. Correlations were analyzed using Pearson's correlation coefficient. P<0.05 was considered to indicate a statistically significant difference.
Results
Serum TNF-α content
To determine the dynamic changes in TNF-α level, serum TNF-α level was detected before treatment and at 8, 24, 48 and 72 h after treatment. As shown in Table II, at all time points, the serum TNF-α level was the lowest in Group D. Compared with Group A, the level of TNF-α was significantly lower in Groups B, C and D at 8, 24, 48 and 72 h after treatment (P<0.05). In all groups, the serum TNF-α level was low before treatment, peaked at 8 h after treatment and then gradually decreased at 24, 48 and 72 h after treatment. Compared with at 8 h, TNF-α levels were increased in the early stage of trauma and decreased after HBO therapy combined with nimodipine treatment. These results indicated that the TNF-α levels decreased after HBO therapy combined with nimodipine treatment, which suggested that the TNF-α may be involved in the pathgensesis of brain injury.
Table II.
Serum tumor necrosis factor-α content (µg/l).
| Group | Before treatment | 8 h after treatment | 24 h after treatment | 48 h after treatment | 72 h after treatment |
|---|---|---|---|---|---|
| Group A | 120.2±3.5a | 135.4±2.6 | 125.8±2.5a | 110.4±3.5a | 102.4±2.7a |
| Group B | 115.3±2.4a,b | 129.1±2.3b | 120.3±2.5a,b | 102.3±2.5a,b | 95.6±2.2a,b |
| Group C | 113.7±2.3a,b | 125.3±2.1b | 110.3±2.2a,b | 97.5±1.8a,b | 88.7±2.4a,b |
| Group D | 110.5±2.3a,b | 119.8±3.2b | 98.2±2.2a,b | 89.5±2.1a,b | 75.5±2.2a,b |
P<0.05 vs. 8 h
P<0.05 vs. group A.
Serum IL-1β content
To determine the dynamic changes in IL-1βs, serum IL-1β level was detectedbefore before treatment and at 8, 24, 48 and 72 h after treatment. As shown in Table III, the serum IL-1β level was significantly decreased in Groups B, C and D at alltime pointsall scompared with Group A (P<0.05). In all groups, the serum IL-1β level peaked at 8 h after treatment and gradually decreased at 24, 48 and 72 h after treatment. Compared with at 8 h, IL-1β level was significantly lower before treatment and at 24, 48 and 72 h after treatment (P<0.05). These results indicated that IL-1β increases in the early stage of trauma and may be involved in the pathogenesis of brain injury.
Table III.
Serum interleukin-1β content (µg/l).
| Group | Before treatment | 8 h after treatment | 24 h after treatment | 48 h after treatment | 72 h after treatment |
|---|---|---|---|---|---|
| Group A | 125.2±2.5a | 140.2±2.6 | 132.8±2.1a | 119.4±2.5a | 110.4±2.3a |
| Group B | 120.3±2.4a,b | 135.1±2.5b | 128.3±2.4a,b | 110.3±2.3a,b | 105.6±2.5a,b |
| Group C | 115.7±2.1a,b | 129.3±2.4b | 120.3±2.5a,b | 104.5±2.8a,b | 100.7±2.2a,b |
| Group D | 110.5±2.2a,b | 120.8±2.2b | 115.2±2.3a,b | 98.5±2.3a,b | 89.5±2.1a,b |
P<0.05 vs. 8 h
P<0.05 vs. group A.
VmMCA comparisons
To determine the vascular contraction after acute brain injury, VmMCA was measured before treatment and at 8, 24, 48 and 72 h after treatment. In each group, the VmMCA was highest at 24 h (Table IV). At all time points, the VmMCA was significantly lower in Groups B, C and D compared with Group A (P<0.05). In addition, VmMCA was significantly lower before treatment and at 48 and 72 h after treatment compared with 8 h after treatment (P<0.05). VmMCA was significantly higher at 24 h after treatment compared with 8 h after treatment (P<0.05). These findings suggest that nimodipine could dilate cerebral blood vessels, relieve spasms and slow the blood flow in the bilateral middle cerebral arteries.
Table IV.
Comparisons of cerebral arterial blood flow velocity (cm/s).
| Group | Before treatment | 8 h after treatment | 24 h after treatment | 48 h after treatment | 72 h after treatment |
|---|---|---|---|---|---|
| Group A | 78.2±1.5a | 125.12±2.1 | 168.8±1.1a | 108.24±2.2a | 86.4±2.1a |
| Group B | 77.1±2.1a–c | 103.1±2.1b | 141.3±1.4a–c | 85.3±1.3a–c | 77.6±1.5a–c |
| Group C | 82.5±1.1a,b | 114.3±1.4b | 158.3±1.5a,b | 96.5±1.8a,b | 82.7±1.2a,b |
| Group D | 75.5±1.2a–c | 91.8±1.2b | 130.2±1.3a–c | 76.5±1.3a,b,c | 74.5±2.1a–c |
P<0.05 vs. 8 h
P<0.05 vs. group A
P<0.05 vs. Group C.
GCS score
To determine the severity of brain damage, GCS score was evaluated before treatment and at 8, 24, 48 and 72 h after treatment. At all time points, the GCS score in Groups B, C and D was significantly increased compared with Group A (P<0.05; Table V). The largest increases were in Group D. In all groups, the GCS score gradually increased over time, with the exception of Groups B, C and D at 72 h, which exhibited slight decreases compared with 48 h. In all groups, the GCS score at 24 h, 48 h and 72 h after treatment was significantly higher compared with 8 h after treatment (P<0.05). These results indicated that patient brain damage gradually recovered after brain injury. Furthermore, when compared with the conventional therapy, the bilateral carotid blood flow velocity is faster in the craniocerebral injury when treated with nimodipine combined with HBO therapy.
Table V.
Glasgow Coma Scale scores.
| Group | Before treatment | 8 h after treatment | 24 h after treatment | 48 h after treatment | 72 h after treatment |
|---|---|---|---|---|---|
| Group A | 7.03±1.2a | 7.41±2.1 | 8.80±1.1a | 9.52±2.2a | 10.05±1.1a |
| Group B | 6.24±1.3a,b | 7.65±1.7b | 9.92±1.4a,b | 11.15±1.3a,b | 10.96±1.3a,b |
| Group C | 6.53±1.3a,b | 7.92±1.4b | 10.19±1.5a,b | 11.36±1.3a,b | 11.20±1.2a,b |
| Group D | 6.58±1.2a,b | 8.26±1.2b | 12.21±1.4a,b | 13.44±1.3a,b | 12.65±2.1a,b |
P<0.05 vs. 8 h
P<0.05 vs. group A.
Correlation between serum TNF-α/IL-1β and VmMCA or GCS
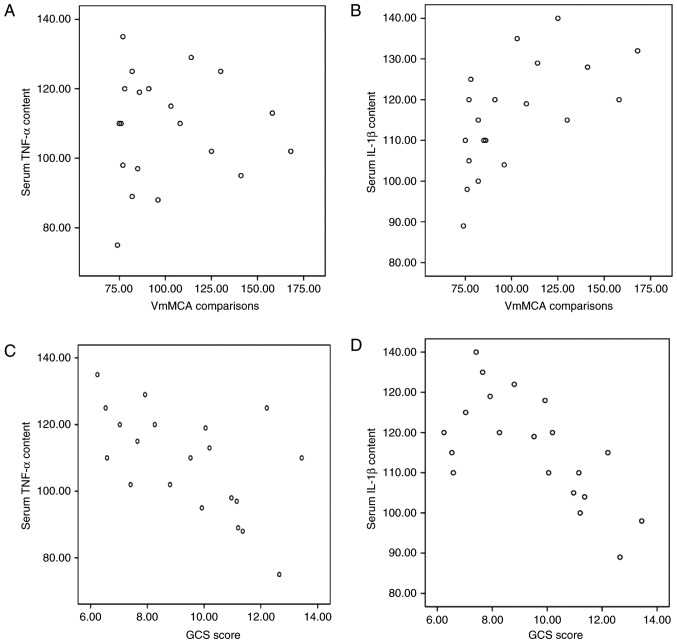
Correlation analyses were performed to evaluate the association between serum TNF-α/IL-1β and VmMCA or GCS. Serum TNF-α and IL-1β levels were positively correlated with VmMCA (r=0.38 and r=0.73, respectively; P<0.05; Fig. 1A and B). Serum TNF-α and IL-1β levels were negatively correlated with GCS (r=−0.89 and r=−and 0.78, respectively; P<0.05; Fig. 1C and D). These results indicate that serum TNF-α and IL-1β levels are positively correlated with VmMCA and negatively correlated with GCS.
Figure 1.
Correlation analysis of serum TNF-α and IL-1β levels, VmMCA and GCS score. (A) Correlation analysis of TNF-α and VmMCA (r=0.38; P<0.05). (B) Correlation analysis of IL-1β and VmMCA (r=0.73; P<0.05). (C) Correlation analysis of TNF-α and GCS score (r=−0.89; P<0.05). (D) Correlation analysis of IL-1β and GCS score (r=−0.78; P<0.05). TNF, tumor necrosis factor; IL, interleukin; VmMCA, cerebral arterial blood flow velocity; GCS, Glasgow Coma Scale.
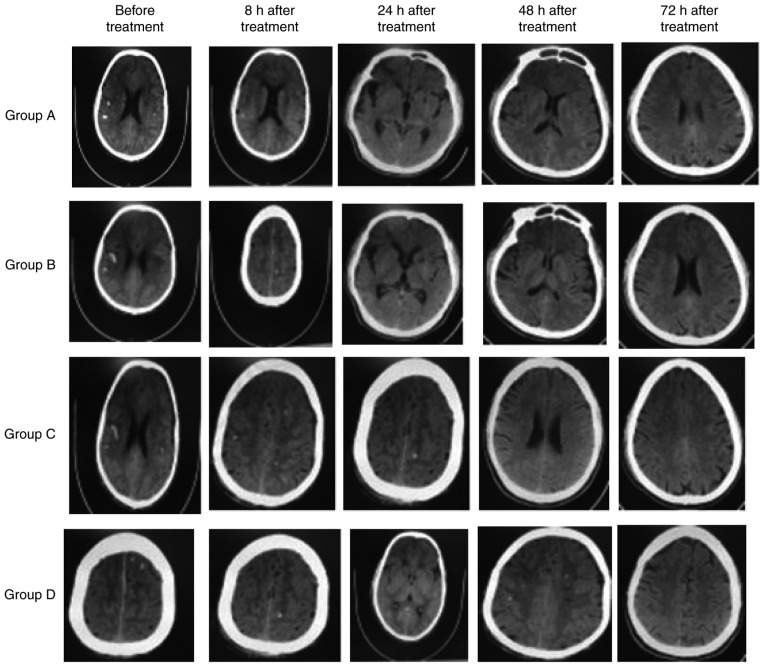
Head CT
To determine brain structural change, head CT was performed before treatment and at 8, 24, 48 and 72 h after treatment. Punctate hemorrhage was observed in all groups on CT with a value of 68±2.37 HU before treatment (Table VI). Over time, punctate hemorrhage was reduced in all groups (Fig. 2). CT values also decreased over time in all groups. Compared with before treatment, the CT values at 8, 24, 48 and 72 h after treatment were significantly decreased (P<0.05; Table VI). Furthermore, Groups A, B and C exhibited significantly higher CT values at all time points compared with group D (P<0.05). These results indicated that patients treated with nimodipine and HBO therapy exhibit fewer brain structural changes compared with either treatment alone.
Table VI.
Computed tomography values (HU).
| Group | Before treatment | 8 h after treatment | 24 h after treatment | 48 h after treatment | 72 h after treatment |
|---|---|---|---|---|---|
| Group A | 68.2±2.3a | 61.2±2.3a,b | 55.6±2.1a,b | 52.4±2.5a,b | 45.4±2.1a,b |
| Group B | 67.3±2.1a | 58.1±2.1a,b | 52.3±2.2a,b | 48.2±2.2a,b | 42.6±2.7a,b |
| Group C | 66.7±2.5a | 55.3±2.2a,b | 50.3±2.1a,b | 45.5±2.4a,b | 40.7±2.2a,b |
| Group D | 67.5±2.2 | 52.4±2.4b | 48.2±2.5b | 42.3±2.3b | 37.5±2.3b |
P<0.05 vs. Group D
P<0.05 vs. before treatment.
Figure 2.
Head computed tomography images. Punctate hemorrhage was indicated in all groups before treatment, with a value of 68±2.37 HU. The extent of punctate hemorrhage decreased over time. Group D exhibited the largest decrease.
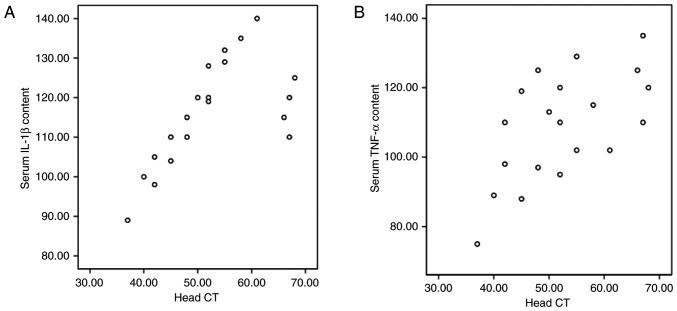
Correlation between serum TNF-α/IL-1β and CT values
Correlation analyses were performed to evaluate the association between serum TNF-α/IL-1β and CT values. Serum TNF-α and IL-1β were positively correlated with CT value (r=0.73 and r=0.89, respectively; P<0.05), as shown in Fig. 3. These results indicated that serum TNF-α and IL-1β levels are positively correlated with brain structural damage. Furthermore, the findings suggest that HBO therapy could alleviate the hypoxic condition in the craniocerebral injury, and nimodipine could improve the circulation and reduce the production of TNF-α.
Figure 3.
Correlation analysis of TNF-α and IL-1β levels and CT values. (A) Correlation analysis of IL-1β and CT values (r=0.89; P<0.05). (B) Correlation analysis of TNF-α and CT values (r=0.73; P<0.05). TNF, tumor necrosis factor; IL, interleukin; CT, computed tomography.
Discussion
Cerebral ischemia, hypoxia and inflammation are key factors in brain damage after brain injury (20). Overexpression of inflammatory factors after injury leads to environmental changes for neurons, resulting in neuronal degeneration (21). TNF-α elevates early after injury and is involved in the inflammatory response (22). TNF-α is produced by activated monocytes or macrophages in nerve tissues such as astrocytes, microglia and neurons (22). The elevated TNF-α level in cerebrospinal fluid and plasma after brain injury is closely associated with disease severity (13). Leukocyte accumulation and inflammation infiltration significantly impact the scope and extent of brain damage (23). TNF-α can regulate the activity of vascular endothelial cells and vascular inflammation (12) and promote the expression of adhesion molecules in endothelial cells. This leads to leukocyte adhesion, aggregation and migration from capillaries to the brain, thereby activating microglia and subsequently causing damage (24).
HBO therapy is the inhalation of pure oxygen at greater than normal atmospheric pressure (9). HBO treatment increases the oxygen content and storage in brain tissue and cerebrospinal fluid, reduces cerebral edema and intracranial pressure, promotes functional recovery of neurons, particularly dilate vertebral arteries to increase cerebral blood flow, restores and maintains cell pump function and improves microcirculation (25–29). In the present study, punctate hemorrhage was detected in all groups before treatment. Over the treatment period, punctate hemorrhage and CT values gradually decreased, with Group D exhibiting the largest decrease. The serum levels of TNF-α and IL-1β were significantly lower in Groups B, C and D compared with Group A. Higher CT values were identified to be correlated with higher TNF-α and IL-1β levels. These results suggest that HBO treatment can enhance hematoma absorption to repair brain damage.
Calcium antagonists can disrupt calcium flow from the outside to the inside of vascular smooth muscle cells, reduce the cerebral vasospasm to regulate vascular muscle tension, increase blood flow for brain tissue perfusion, prevent secondary damage, and improve microcirculation to protect brain tissue (30). Nimodipine is a type of fat-soluble dihydropyridine calcium channel blocker that can permeate the blood-brain barrier, block calcium channels to prevent calcium influx, and promote Ca2+ release, thereby reducing the intracellular calcium concentration (31). In the present study, the VmMCA was lower in Group B and D compared with Group A and C, and VmMCA was positively correlated with serum TNF-α and IL-1β decrease. These results are indicative of the selective duration of dilation and protection effects of nimodipine on cerebral blood vessels (32). GCS scores increased in all groups over the treatment period, with Group D exhibiting the largest increase. GCS scores were positively correlated with serum TNF-α and IL-1β levels. These results indicate that that HBO with nimodipine may influence the serum content of TNF-α and IL-1β. Thus, early cytokine intervention may be beneficial in improving prognosis after diffuse brain injury.
Physiological changes in patients with diffuse brain injuries include secondary cerebral ischemia and hypoxia, brain tissue edema, release of inflammatory cell media and microcirculation disorder. The pathological changes mainly include cerebral contusion, axonal injury and increased intracranial pressure. Notably, oxygen supply and circulation improvement are important factors involved in the recovery of damaged brain tissue. In the present study, the results suggested that nimodipine combined with HBO therapy could effectively alleviate cerebral ischemia and hypoxia, improve the cerebral circulation, and greatly elevate the salvage rate. Because nimodipine could relieve the cerebral vasospasm and increase the cerebral blood flow, its treatment inevitably leads to increased cerebral blood volume and cerebral edema. However, HBO therapy does have limitations due to its association with barotrauma, oxygen poisoning and compressed-airsickness in the treatment of coma patients (33), particularly those with mechanical ventilation. Therefore, further in-depth studies are still required to optimize the treatment options for the diffuse brain injury, particularly for coma patients.
Acknowledgements
The authors would like to thank Professor Xiaolu Wang at The Second Hospital of Hebei Medical University (Shijiazhuang, China) for his technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FW and JW made substantial contributions to conception and design. JS performed the trial and was a major contributor in writing the manuscript. JZ obtained the patient data regarding the diffuse brain injury. GZ made substantial contributions to analysis and interpretation of data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Prior written and informed consent was obtained from every patient. The study was approved by the ethics review board of the Second Hospital of Hebei Medical University (Shijiazhuang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li Yu, Du Yanli. High field magnetic resonance diffusion tensor imaging in the evaluation of severe traumatic brain injury. Zhonghua Lin Chuang Yi Shi Za Zhi (Dian zi ban) 2013;9:4103–4104. (In Chinese) [Google Scholar]
- 2.Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–651. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- 3.Vink R, Nimmo AJ, Cemak I. An overview of new and novel pharmaco theraies for use in traumatic brain injury. Clin Exp Pharmacol Physiol. 2001;28:919–921. doi: 10.1046/j.1440-1681.2001.03548.x. [DOI] [PubMed] [Google Scholar]
- 4.He XS. Recognizing the study on inflammatory responses post traumatic brain injury. J Fourth Military Med University. 2004;25:769. [Google Scholar]
- 5.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical seale. Lancet. 1974;2:81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 6.Hardy P, Johnston KM, De Beaumont L, Montgomery DL, Lecomte JM, Soucy JP, Bourbonnais D, Lassonde M. Pilot case study of the therapeutic potential of hyperbaricoxygen therapy on chronic brain injury. J Neurol Sci. 2007;253:94–105. doi: 10.1016/j.jns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Yang HW, Liao SC. The clinical analysis of earlyhyperbaric oxygen treatment for postoperative acute brain injury. J Yanan University (Medical Sciences) 2011;9:25–28. (In Chinese) [Google Scholar]
- 8.Yu SN, He GS, Cai SZ. The clinical analysis of earlyhyperbaric oxygen treatment for postoperative severe brain injury. Chin J Clin Neurosurgery. 2013;18:306–308. (In Chinese) [Google Scholar]
- 9.Sukoff MH. Effect of hyperbaric oxygenation. J Neurosurg. 2001;95:544–546. doi: 10.3171/jns.2001.95.3.0544. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Peterson PL, Lee CP. Alterations in cerebral energy metabolism induced by traumatic brain injury. Neurol Res. 2001;23:129–138. doi: 10.1179/016164101101198460. [DOI] [PubMed] [Google Scholar]
- 11.Suehiro E, Fujisawa H, Akimura T, Ishihara H, Kajiwara K, Kato S, Fujii M, Yamashita S, Maekawa T, Suzuki M. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: Influence of hypothermic therapy. J Neumtrauma. 2004;21:1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- 12.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesion: Helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 13.Feng B, Tan ZM, Wang HS. The clinical importance of tumor necrosis factor in acute brain injury. Chin J Critical Care Med. 1997;17:14–16. (In Chinese) [Google Scholar]
- 14.Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammotory cytokines in serum of patients with acute cerebral ischemia: Kinetics of secretion and relation to extent of brain damage and outcome of disease. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510X(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhu T, Yao Z, Yuan HN. The changes of TNF-α, IL-1β and IL-6 levels in postoperative rats. Chin J Trauma. 1998;14:367–369. (In Chinese) [Google Scholar]
- 16.Si QJ. The function of Tumor necrosis factor in myocardial ischemia/reperfusion injury. J Chin PLA Postgraduate Med School. 1994;15:220–222. (In Chinese) [Google Scholar]
- 17.Li H, Yang X, Shi W, Ma Z, Feng G, Wang Q, Shen L, Xie C. Protective effects of nimodipine on cerebrovascular function in chronic alcoholic encephalopathy. Int J Mol Med. 2014;33:201–208. doi: 10.3892/ijmm.2013.1540. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Zhang Y, Wang Z, Wang S, Gao M, Xu R, Liang C, Zhang H. Attenuation of acute phase injury in rat intraxranial hemorrhage by cerebrolysin that inhibits brain edema and inflammatory response. Neurochem Res. 2016;41:748–757. doi: 10.1007/s11064-015-1745-4. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Edwards NJ, Choi HA, Chang TR, Jo KW, Lee K. Treatment strategies to attenuate perihematomal edema in patients with intracerebral hemeorrhage. World Neurosurg. 2016;94:32–41. doi: 10.1016/j.wneu.2016.06.093. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell N. Interleukin-1 and neuronal injury: Mechanisms, modification, and therapeutic potential. Brain Behav Immun. 2003;17:152–157. doi: 10.1016/S0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- 21.Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann NY Acad Sci. 2003;992:39–47. doi: 10.1111/j.1749-6632.2003.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 22.Schoami E, Gallily R, Mechonlam R, Bass R, Ben-Hur T. Cytokine production the brain following closed head injury: Dexanabinol (HU-211)isnovel TNF-alpha inhibitor and effective neuroprotectant. J Neuroimmuno1. 1997;72:169–177. doi: 10.1016/S0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 23.Fassbender K, RossoI S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: Kinetics of secretion and relation to extent of brain damage and outcome of disease. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510X(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy P, Johnston KM, De Beaumont L, Montgomery DL, Lecomte JM, Soucy JP, Bourbonnais D, Lassonde M. Pilot case study of the therapeutic potential of hyperbaric oxygen therapy on chronic brain injury. J Neurol Sci. 2007;253:94–105. doi: 10.1016/j.jns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Philippon B, Munsch RC. Variations of the cerebral blood flow after hyperbaric oxygenation in traumatic coma. Neurochirurgie. 1975;21:483–492. (In French) [PubMed] [Google Scholar]
- 27.Isakov IuV, Anan'ev GV, Aide KhB, Korol'kov IuI. Use of hyperbaric oxygenation in various complications of craniocerebral injury in the acute period. Zh Vopr Neirokhir Im N N Burdenko. 1981;4:15–18. (In Russian) [PubMed] [Google Scholar]
- 28.Ren H, Wang W, Ge Z. Glasgow coma scale, brain electric activity mapping and Glasgow outcome seale after hyperbaric oxygen treatment of severe brain injury. Chin J Traumatol. 2001;4:239–241. [PubMed] [Google Scholar]
- 29.Fan JZ, Xue L. Hyperbaric oxygen therapy for pediatric brain injury. Chin J Rehabilitation. 2001;16:74–75. (In Chinese) [Google Scholar]
- 30.Wang GT. Efficacy analysis of Nimodiping combined with compound Danshen injection for treatment of 35 cases with severe brain injury and encephaledema. Chin Mod Med. 2009;16:80–84. (In Chinese) [Google Scholar]
- 31.Chen ZB, Zhang MG, Lin QX. Effective observation of nimodiping combined with hyperbaric oxygen in severe injury of brain. Hainan Medical J. 2007;18:2–3. (In Chinese) [Google Scholar]
- 32.Cai ZC, Li M, Li JZ. Protective effect of Nimodipine on porcine basilar artery oxidative stress injury induced by hydrogen peroxide. Chin J Clin Pharmacol Ther. 2007;12:906–910. (In Chinese) [Google Scholar]
- 33.Xu L, Li B, Yang C, Li C, Peng Y. Clinical research on postoperative efficacy and related factors of early simulation hyperbaric oxygen therapy for severe craniocerebral injury. Pak J Pharm Sci. 2016;29(1 Suppl):S273–S280. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.