Abstract
Breast cancer stem cells (BCSCs) are a small subpopulation of breast cancer cells that have been proposed to be a primary cause of failure of therapies, including ionizing radiation (IR). Their embryonic stem-like signature is associated with poor clinical outcome. In the present study, the function of octamer-binding transcription factor 4 (Oct4), an embryonic stem cell factor, in the resistance of BCSCs to IR was investigated. Mammosphere cells exhibited increased expression of stemness-associated genes, including Oct4 and sex-determining region Y-box 2 (Sox2), and were more resistant to IR compared with serum-cultured monolayer cells. IR-resistant MCF7 cells also exhibited significantly increased expression of Oct4. To investigate the possible involvement of Oct4 in IR resistance of breast cancer cells, cells were transfected with Oct4. Ectopic expression of Oct4 increased the clonogenic survival of MCF7 cells following IR, which was reversed by treatment with small interfering RNA (siRNA) targeting Oct4. Oct4 expression decreased phosphorylated histone H2AX (γ-H2AX) focus formation and suppressed IR-induced premature senescence in these cells. Mammosphere, IR-resistant and Oct4-overexpressing MCF7 cells exhibited enhanced phosphorylation of signal transducer and activation of transcription 3 (STAT3) (Tyr705) and inhibitor of nuclear factor κB (NF-κB), and blockade of these pathways with siRNA against STAT3 and/or specific inhibitors of STAT3 and NF-κB significantly increased IR-induced senescence. Secretome analysis revealed that Oct4 upregulated interleukin 24 (IL-24) expression through STAT3 and NF-κB signaling, and siRNA against IL-24 increased IR-induced senescence, whereas recombinant human IL-24 suppressed it. The results of the present study indicated that Oct4 confers IR resistance on breast cancer cells by suppressing IR-induced premature senescence through STAT3- and NF-κB-mediated IL-24 production.
Keywords: radioresistance, breast cancer, octamer-binding transcription factor 4, senescence, signal transducer and activator of transcription 3, nuclear factor κB, interleukin 24
Introduction
Breast cancer is one of the most frequent malignancies in women, leading to >300,000 mortalities annually worldwide (1). Despite recent improvement in the mortality rate through drastic treatment strategies, including radiotherapy and chemotherapy, the patient survival rate has not improved owing to tumor relapse and metastasis (2). A growing body of evidence suggests that cancer recurrence and metastasis may be driven by a subpopulation of cells within the tumor termed cancer stem cells (CSCs) or cancer-initiating cells (3). As with normal stem cells, these cells have the ability to self-renew and differentiate into multiple lineages; therefore, they are considered to be responsible for tumorigenesis, therapeutic resistance, metastasis and recurrence (4). Stem-like breast cancer cells were first identified by Al-Hajj et al (5), who identified that a subpopulation of cells from human breast tumors and cluster of differentiation (CD)44+CD24−ESA+ pleural effusion cells of patients are responsible for breast cancer tumorigenicity. CD44+CD24− breast cancer stem cells (BCSCs) may be induced by promoting epithelial-mesenchymal transition via suppression of epithelial (E-)cadherin by short hairpin RNA, ectopic expression of an E-cadherin transcriptional suppressor such as Snail or Twist or treatment with transforming growth factor-β (6). BCSCs may be enriched by growth factor-enriched serum-free non-adherent sphere culture of primary cancer cells and established cell lines, including MCF7 breast cancer cells (7). Since CSCs are considered to be the primary reason for therapeutic failure, these cells are considered a candidate therapeutic target.
One of the hallmarks of CSCs is their resistance to therapy (3). Preclinical data indicate that BCSCs are more resistant to ionizing radiation (IR) compared with serum-cultured normal cancer cells. The radioresistance in BCSCs is based on their decreased production of reactive oxygen species in response to IR owing to enhanced expression of free radical-scavenging proteins (8,9). Additionally, upregulation of Notch ligand expression followed by activation of the Notch pathway by IR enriches BCSCs in MCF7 cells and maintains stemness in these cells (8). A previous study demonstrated that increased survival of MCF7 BCSCs in response to IR is mediated by downregulation of the senescence pathway, not apoptosis (10). Thus, targeting BCSCs may be a promising way to increase radiotherapeutic effectiveness in breast cancer.
The POU-domain transcription factor octamer-binding transcription factor 4 (Oct4) is one of the master regulators of maintenance of embryonic stem cells along with sex-determining region Y-box 2 (Sox2) and Nanog, and one of the key transcription regulators of stem cell pluripotency (11). Oct4 is expressed in various malignant tumor tissues and cell lines, including non-small cell lung cancer, liver cancer and glioma lines (12–14). Oct4 is also expressed in breast cancer tissues and BCSCs (15), and is associated with poorly differentiated high-grade estrogen receptor-negative tumors (16). Oct4 confers chemoresistance on liver cancer cells via protein kinase B (Akt)-mediated upregulation of ATP-binding cassette transporter G2 (ABCG2) (13). Additionally, Oct4 promotes colony formation of glioma cells (14), whereas Oct4 suppression leads to the induction of apoptosis in breast cancer cells (15). However, the function of Oct4 in the response of tumor cells to IR is poorly understood.
In the present study, the function of Oct4 was investigated in radioresistance of breast cancer cells. Using mammosphere and radioresistant cells derived from MCF7 cells, results indicated that radioresistance of breast cancer cells is associated with Oct4 expression. Mammosphere and radioresistant cells expressed an increased level of Oct4, and overexpression of Oct4 increased radioresistance of MCF7 cells. Importantly, Oct4 expression suppressed IR-induced premature senescence by enhancing IL-24 production through signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB (NF-κB) signaling pathways, which are associated with IR resistance of breast cancer cells.
Materials and methods
Reagents
Antibodies against Oct4 (cat. no. sc-5279), c-Myc (cat. no. sc-40), interleukin (IL)-24 (cat. no. sc-22769), Krüppel-like factor 4 (cat. no. sc-20691), p53 (cat. no. sc-126), phosphorylated (p)-p53 (Ser15) (cat. no. sc-101762), p21 (cat. no. sc-397), p27 (cat. no. sc-527), p16 (cat. no. sc-486), Sirt1 (cat. no. sc-15404) and β-actin (cat. no. sc-47778) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies against Bmi-1 (cat. no. 05-637), Nanog (cat. no. AB9220), phosphorylated histone H2AX (γ-H2AX; cat. no. 05-636) and Notch1 (cat. no. MAB5352) were from EMD Millipore (Billerica, MA, USA). Antibodies against p-extracellular-signal-regulated kinase 1/2 (cat. no. 4695), p-Akt (Ser473) (cat. no. 9271), STAT3 (cat. no. 9139), p-STAT3 (Ser727) (cat. no. 9134), p-STAT3 (Tyr705) (cat. no. 9145), p-c-Jun N-terminal kinase (cat. no. 2361), p-retinoblastoma protein (Ser807/811) (cat. no. 9308), non-p-β-catenin (cat. no. 8814), inhibitor of NF-κB (IκB) (cat. no. 4814) and p-IκB (cat. no. 2859) were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-Sox2 (cat. no. MAB2018) was from R&D Systems, Inc. (Minneapolis, MN, USA). Specific inhibitors of STAT3 (WP1066) and NF-κB (Bay 11-7082) were purchased from Calbiochem; Merck KGaA (Darmstadt, Germany).
Cell culture
Human breast cancer cells (MCF7 cells) (ATCC, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Welgen, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS; JR Scientific, Inc., Woodland, CA, USA) and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator. For collecting mammospheres, cells were trypsinized and cultured at 37°C in serum-free DMEM/Ham's F12 (Cellgro; Corning Incorporated, Corning, NY, USA) with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) (R&D Systems, Inc.) (10 ng/ml each). Mammospheres were collected following three passages of culture.
Sphere-forming and soft-agar clonogenic assays
For the sphere-forming assay, a single-cell suspension prepared by trypsinization was seeded in serum-free DMEM/Ham's F12 with EGF and bFGF (10 ng/ml each) at a density of 1×103 cells/ml. After 10 days of culture, spheres were attached to the plate by adding FBS (10%), stained using Diff-Quick solution (Sysmex Corporation, Kobe, Japan) and counted by eye. For the soft-agar clonogenic assay, cells were resuspended in the same volume of 0.7% agar with 10% FBS and plated onto the bottom agar layer (1:1 mixture of 1% agar and 2X DMEM/Ham's F12 with 10% FBS). After 14 days of incubation, colonies in five random fields/well were counted under a brightfield microscope (magnification, ×100).
Invasion and migration assays
Matrigel invasion assays were performed using Transwell chambers (Costar; Corning Incorporated) precoated with Matrigel (100 µg/cm2). Cells (105 cells in 100 µl serum-free DMEM) were seeded into the upper part of each chamber, whereas the lower compartments were filled with 600 µl DMEM with 1% FBS. Following incubation at 37°C for 18 h, non-invading cells on the upper surface of the filter were removed using a cotton swab, and the invading cells on the lower surface of the filter were fixed and stained using a Diff-Quick kit (Sysmex Corporation), according to the manufacturer's protocol. Invasiveness was determined by counting cells in five brightfield microscopic fields/well (magnification, ×100), and the extent of invasion was expressed as the mean number of cells/microscopic field. Transwell migration assays were performed using a similar procedure to that used for the invasion assay, except that the lower surfaces of filters were coated with gelatin (100 µg/cm2) and upper surfaces were not coated.
Western blot analysis
Cells (2×106) were lysed in lysis buffer. Following a brief sonication, the lysates were clarified by centrifugation at 12,000 g for 15 min at 4°C, and protein content was determined using the Bradford method. An aliquot (25 µg protein/lane) of total protein was separated by SDS-PAGE (10 or 12% gel) for 2 h at 80 V, blotted onto a nitrocellulose membrane (0.2 µm pore size; GE Healthcare, Chicago, IL, USA) for 1.5 h at 100 V. The membrane was blocked with 3% BSA in TBST [20 mmol/l Tris-HCl (pH 7.6), 137 mmol/l NaCl and 0.01% Tween-20] for 1 h at room temperature followed by incubation with primary antibody (1:1,000) for 2 h at room temperature. Following extensive washing with TBST, the membrane was probed with secondary antibody [rabbit immunoglobulin G (IgG) heavy and light chain antibody (cat. no. A120-101P) or mouse IgG heavy and light chain antibody (cat. no. A90-116P); 1:20,000; Bethyl Laboratories, Inc., Montgomery, TX, USA] conjugated with horseradish peroxidase for 1 h at room temperature. Following washing three times with TBST for 15 min, immunoblots were visualized using enhanced chemiluminescence (GE Healthcare), according to the manufacturer's protocol.
Reverse transcription-polymerase chain reaction (RT-PCR)
High-quality total RNA was isolated from cells (1×105) using TRIsure™ (Bioline; Meridian Bioscience Inc., London, UK), according to the manufacturer's protocol. cDNA was synthesized using a SentiFAST™ cDNA synthesis kit (cat. no. BIO-65054; Bioline; Meridian Bioscience Inc.). PCR for IL-6 (149-bp), IL-8 (194-bp), IL-24 (429-bp) and 18S (433-bp) was performed as described previously (22). Oligonucleotide primer sequences were as follows: IL-6, 5′-ACTCACCTCTTCAGAACGAATTG-3′ (forward) and 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ (reverse); IL-8, 5′-TTTTGCCAAGGAGTGCTAAAGA-3′ (forward) and 5′-AACCCTCTGCACCCAGTTTTC-3′ (reverse); IL-24, 5′-GACTTTAGCCAGCAGACCCTT-3′ (forward) and 5′-GGTTGCAGTTGTGACACGAT-3′ (reverse); 18S, 5′-AAACGGCTACCACATCCAAG-3′ (forward) and 5′-CGCTCCCAAGATCCAACTAC-3′ (reverse). The PCR products were analyzed on a 2% agarose gel, stained with ethidium bromide (Mbiotech, Inc., Hanam, Korea), and visualized under UV light using an image analyzer (ChemiDoc XRS; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunocytochemistry and γ-H2AX focus assay
Cells (1×105) were seeded in a chamber slide with DMEM supplemented with 10% FBS. The following day, the cells were fixed with 4% paraformaldehyde for 20 min and washed with PBS three times. Subsequently, the cells were incubated in blocking solution (5% BSA and 0.5% Triton X-100 in PBS) for 1 h at room temperature. The cells were stained with primary antibodies (against Oct4 and γ-H2AX) in blocking solution (1:100) for 2 h and washed with PBS three times. Subsequently, the cells were incubated with Alexa Fluor 488-labeled goat anti-rabbit and Alexa Fluor 594-labeled goat anti-mouse secondary antibodies (both 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h. Nuclei were stained using Vectashield mounting medium with DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA) for 5 min at room temperature and stained cells were viewed using a confocal laser-scanning microscope (magnification, ×100). γ-H2AX foci were determined in ≥50 cells.
Colony formation assay and selection of IR-resistant (IRR) cells
For determining IR resistance, 500 cells were seeded in 60-mm dishes and were exposed to γ-rays from a 137Cs γ-ray source (Atomic Energy of Canada, Korea Institute of Radiological and Medical Sciences, Seoul, Korea) at a dose rate of 3.81 Gy/min. After 7 days of incubation, the culture dishes were washed with PBS and the cells were stained with 0.05% crystal violet in 20% methanol. Following three washes with distilled water, colonies were counted by eye. For collection of IRR cells, cells (500 cells/60-mm dish) were exposed to IR at 5 or 7 Gy and cultured for 21 days. Emerging colonies were collected as IRR cells.
Transduction of adenoviruses and transfection of small interfering RNA (siRNA)
Adenoviral-Oct4 and control virus (adenoviral-luciferase) were a gift from Dr Hee-Choong Kwon (Korea Institute of Radiological and Medical Sciences). MCF7 cells (5×105) were infected with Adenoviruses (multiplicity of infection, 100) in the presence of 8 µl/ml polybrene. Cells (5×105) were transfected with a pool of siRNAs against Oct4 (5′-AUCACGUAAAGCUAGAAA-3′ and 5′-GGGAUCAUUUCUAGCUUU-3′; Integrated DNA Technologies, Coralville, IA, USA) and Stealth™ RNAi Negative Control Duplexes (Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 50 nM using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Cell-cycle and Annexin V-propidium iodide (PI) assays
For the Annexin V-PI assay, cells (5×105) were cultured in 60-mm dishes and exposed to IR (6 Gy). Cell death was quantified using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer's protocol. For the cell-cycle assay, cells (5×105) were suspended in 100% ice-cold ethanol at −20°C overnight. The cells were washed with PBS and stained with cell-cycle solution (50 µg/ml PI, 10 µg/ml ribonuclease A and 0.05% Triton X-100 in PBS) in the dark for 40 min. Following centrifugation at 200 × g for 5 min at 4°C, the cells were resuspended in PBS and analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Senescence-associated β-galactosidase (SA-β-gal) staining
Cells (1×104) were seeded in a 35-mm dish and were exposed to IR (4 or 6 Gy). After 4 days of incubation, the cells were washed with PBS and fixed with 2% formaldehyde (prepared in PBS) for between 4 and 5 min. The cells were washed with PBS twice, stained with SA-β-gal staining solution [NaCl, MgCl2 and X-gal in dimethylformamide, citric acid/sodium phosphate buffer (pH 6.0), potassium ferrocyanide and potassium ferricyanide] and incubated at 37°C for between 12 and 16 h. Following incubation, the cells were washed with PBS twice and blue colonies were counted under an inverted brightfield microscope (magnification, ×100).
Cytokine array-base analysis
Cytokine array-base analysis was performed using a Human Cytokine Array kit (cat. no. ARY022; R&D Systems, Inc.), according to the manufacturer's protocol.
Fluorescence-activated cell sorting (FACS)
Cells (2×105) were seeded in 60-mm dishes and were exposed to IR (4 or 6 Gy). After 4 days of incubation, the cells were washed with PBS and treated with bafilomycin A1 (100 nM) in fresh culture medium. After 1 h of incubation, 5-dodecanoylaminofluorescein di-D-galactopyranoside (C12FDG) solution (1 µl/ml) was added to the plate, and the cells were incubated further for between 2 and 3 h. The solution in the plate was removed and the cells were washed twice with PBS. The cells were harvested and analyzed by flow cytometry, according to a previously published experimental protocol (17).
Statistical analysis
The mean ± standard deviation of at least three independent experiments was calculated with reference to a control. Data analysis was performed using two-tailed Student's t-test to compare two experimental groups or an analysis of variance to compare ≤3 groups. Means were compared using an independent-samples t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Mammosphere and radioresistant cells exhibit increased Oct4 expression compared with parental breast cancer cells
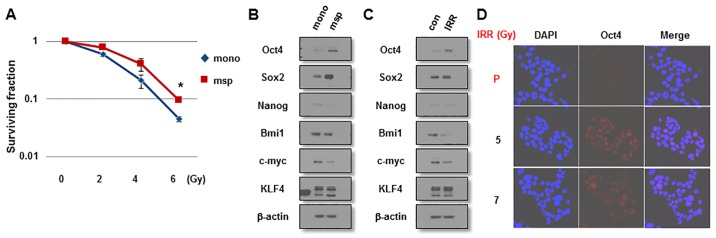
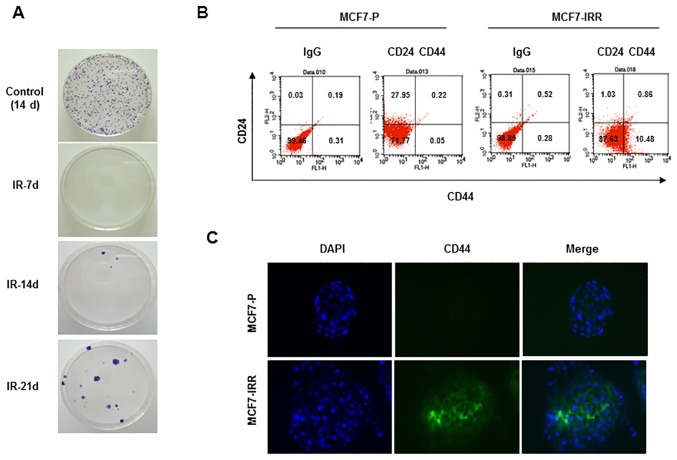
Since a number of studies have demonstrated that BCSCs are enriched in mammospheres and are the cause of IR resistance of MCF7 cells (16,18,19), it was first examined whether mammosphere-cultured breast cancer cells exhibit resistance to IR and a stem-like cell character. As presented in Fig. 1A, mammosphere-cultured MCF7 cells were more resistant to IR exposure compared with monolayer MCF7 cells cultured in serum-enriched medium as indicated by clonogenic survival assays. Mammosphere cells expressed increased levels of the stemness-associated factors, including Oct4 and Sox2, compared with monolayer cells (Fig. 1B). To investigate the involvement of these factors in the radioresistance of breast cancer cells, MCF7 cells were exposed to IR (6 Gy) and were allowed to regrow for 3 weeks. In line with these results, IRR cells expressed increased levels of Oct4, but not of Sox2 or other stemness-related factors, compared with parental cells (Fig 1C). The increased Oct4 expression was confirmed by immunocytochemical staining of IRR MCF7 cells (Fig. 1D). IRR MCF7 colonies began to emerge in the second week and were collected in the third week (Fig. 2A). Collected cells exhibited a larger CD44+CD24− cell fraction compared with parental cells, indicating that IRR cells exhibited BCSC traits (Fig. 2B). CD44+ IRR MCF7 cells were also confirmed using immunocytochemistry (Fig. 2C). On the basis of these results, it was decided to investigate the function of Oct4 in stemness and IR resistance of breast cancer cells.
Figure 1.
IR resistance of mammosphere cells and Oct4 expression in mammosphere and IRR breast cancer cells. (A) Clonogenic survival of irradiated serum-containing monolayer-cultured and serum-free mammosphere-cultured breast cancer cells. Western blot analysis of monolayer-cultured and mammosphere cells (B) and parental and IRR MCF7 cells (C) with the indicated antibodies. (D) Immunocytochemistry of Oct4 in parental and IRR MCF7 cells regrown following 5 and 7 Gy of IR exposure. Nuclei were stained with DAPI. *P<0.05. IR, ionizing radiation; Oct4, octamer-binding transcription factor 4; IRR, IR-resistant; msp, mammosphere; mono, monolayer-cultured; P, parental; Sox2, sex-determining region Y-box 2; KLF4, Krüppel-like factor 4; con, control.
Figure 2.
Enhancement of stem cell characteristics in IRR breast cancer cells. (A) Emergence of IR-resistant colonies at the indicated days following IR (6 Gy) in MCF7 cells. (B) Fluorescence-activated cell sorting analysis of parental and IRR MCF7 cells using the indicated antibodies. (C) Immunocytochemistry of CD44 in parental and IRR MCF7 cells. Nuclei were stained with DAPI. IRR, IR-resistant; IR, ionizing radiation; d, days; CD, cluster of differentiation; IgG, immunoglobulin G; P, parental.
Overexpression of Oct4 enhances stemness and IR resistance of breast cancer cells
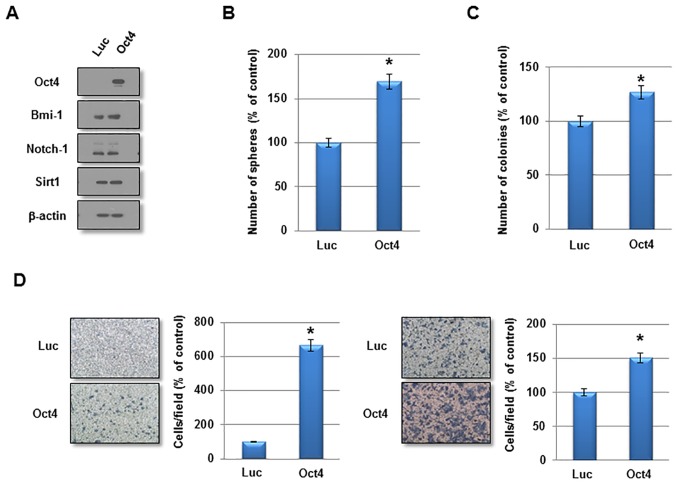
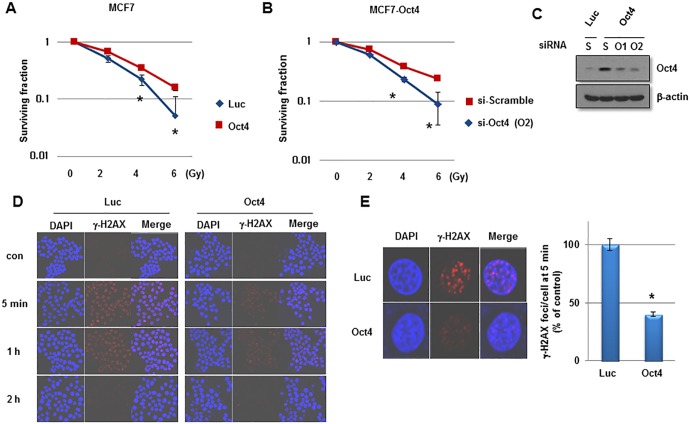
To investigate the function of Oct4 in the self-renewal activity and IR resistance of breast cancer cells, adenovirus harboring Oct4 was introduced into MCF7 cells. Overexpression of Oct4 was confirmed by western blotting (Fig. 3A). Other stem cell-associated factors were not altered significantly by Oct4 overexpression (Fig. 3A). In a sphere-forming assay, overexpression of Oct4 significantly enhanced mammosphere formation in MCF7 cells (Fig. 3B). Additionally, Oct4 promoted clonogenicity in soft agar and enhanced the invasive and migratory activities in MCF7 cells (Fig. 3C and D). Importantly, introduction of Oct4 significantly increased clonogenic survival upon IR exposure in MCF7 cells (Fig. 4A). To confirm the specific function of Oct4 in IR resistance of breast cancer cells, siRNA against Oct4 (siOct4) was introduced into Oct4-overexpressing MCF7 cells (Fig. 4B) and decreased clonogenic survival following IR observed in these cells (Fig. 4C). Next, it was determined whether Oct4 is involved in regulating the DNA damage response induced by IR in breast cancer cells. Immunocytochemical analysis revealed that focus formation of γ-H2AX following IR exposure was decreased in Oct4-overexpressing MCF7 cells compared with in vector control cells (Fig. 4D), which was identified to be a significant difference (Fig. 4E). Taken together, these results suggested that Oct4 serves a crucial function in self-renewal activity and IR resistance by decreasing DNA damage in breast cancer cells.
Figure 3.
Enhanced malignant characteristics of Oct4-overexpressing breast cancer cells. (A) Western blot analysis of MCF7 cells transduced with adenoviral-Luc and adenovira-Oct4 with the indicated antibodies. (B) Sphere-forming assay, (C) soft-agar clonogenic assay, and (D) migration and invasion assay of MCF7 cells transduced with adenoviral-Luc and adenoviral-Oct4. *P<0.05. Oct4, octamer-binding transcription factor 4; Luc, luciferase; Sirt1, sirtuin 1.
Figure 4.
Enhanced IR resistance by Oct4 overexpression in breast cancer cells. Clonogenic survival assays of MCF7 cells (A) infected with adenoviral-Luc and adenoviral-Oct4, and (B) transfected with scramble or Oct4-targeted siRNA. (C) Western blot analysis of Oct4 in MCF7 cells transfected with scramble or Oct4-targeted siRNA. (D) Immunocytochemical staining of γ-H2AX foci of MCF7 cells transduced with Luc and Oct4 following IR exposure (6 Gy) with anti-γ-H2AX at the indicated time-points. (E) Representative image of γ-H2AX foci (left) and quantification of foci/cell at 5 min after IR (right). *P<0.05. IR, ionizing radiation; Oct4, octamer-binding transcription factor 4; siRNA, small interfering RNA; γ-H2AX, phosphorylated histone H2AX; Luc, luciferase; con, control; si-Scramble, scramble siRNA; si-Oct4, Oct4-targeted siRNA; S, scramble; O1/2, Oct4-targeted siRNA 1/2.
Oct4 decreases IR-induced premature senescence in breast cancer cells
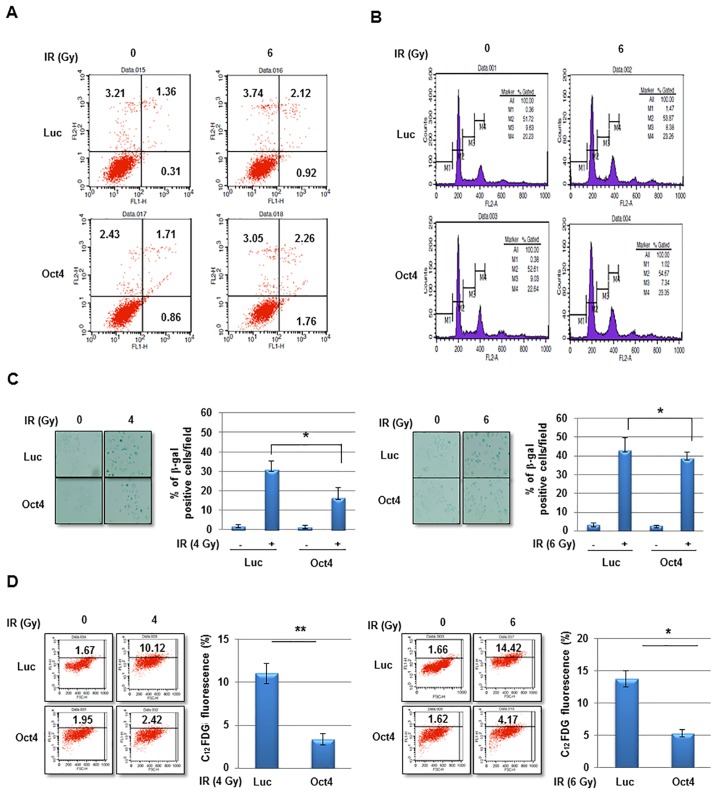
The potential mechanism of Oct4-mediated IR resistance of breast cancer cells was then investigated. IR exposure did not markedly induce cell death and did not affect cell-cycle distribution in MCF7 cells (Fig. 5A and B). However, exposure to IR at 4 and 6 Gy significantly increased senescence in MCF7 cells as revealed using the SA-β-gal assay and FACS (C12FDG fluorescence) analysis, which was significantly suppressed by overexpression of Oct4 (Fig. 5C and D). Whereas the Oct4-mediated suppression of IR-induced premature senescence was more evident at 4 Gy compared with at 6 Gy in the SA-β-gal assay (Fig. 5C), FACS analysis revealed similar suppressive effects under the two conditions (Fig. 5D). These results indicated that Oct4-mediated IR resistance is achieved by suppression of IR-induced premature senescence rather than by apoptosis or cell-cycle arrest.
Figure 5.
Suppression of IR-induced premature senescence in Oct4-overexpressing breast cancer cells. (A) Fluorescence-activated cell sorting and (B) cell-cycle analysis of MCF7 cells transduced with adenoviral-Luc and adenoviral-Oct4 following exposure to IR (6 Gy) for 48 h. (C) Representative image of SA-β-gal staining, and (D) fluorescence-based flow-cytometric assay results of IR-induced senescence in MCF7 cells transduced with adenoviral-Luc and adenoviral-Oct4 3 days after exposure to 4 (left panel) or 6 (right panel) Gy. *P<0.05, **P<0.01. IR, ionizing radiation; Oct4, octamer-binding transcription factor 4; SA-β-gal, senescence-associated β-galactosidase; Luc, luciferase; C12FDG, 5-dodecanoylaminofluorescein di-D-galactopyranoside.
STAT3 and NF-κB signaling is required for Oct4-mediated inhibition of IR-induced senescence in MCF7 cells
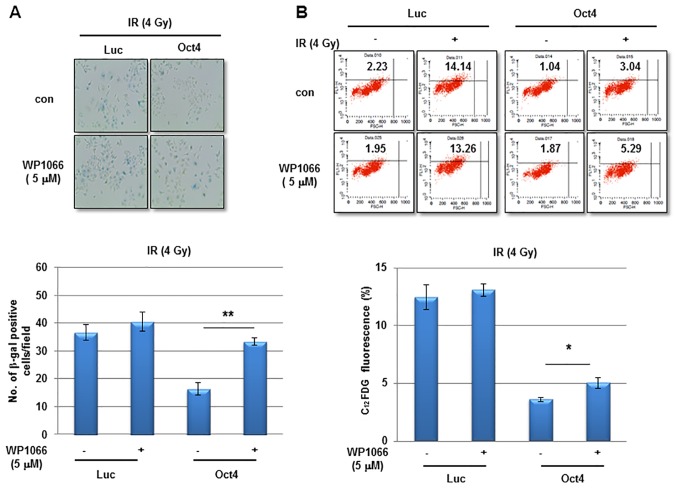
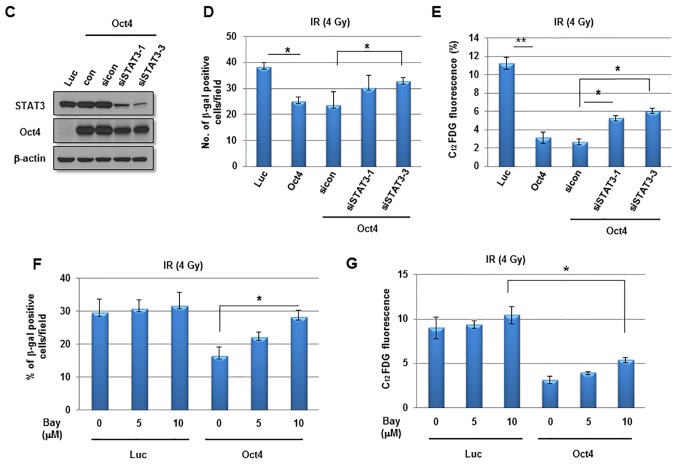
The next aim was to elucidate the signaling crucial for Oct4-mediated suppression of IR-induced premature senescence in breast cancer cells. Among the pivotal signaling molecules for cancer cell survival, STAT3 and NF-κB were commonly activated in mammosphere, IRR and Oct4-overexpressing MCF7 cells as was revealed by the enhanced phosphorylation of Tyr705 in STAT3 and of Ser32/36 in IκB (Fig. 6A). IR exposure of Oct4-overexpressing cells further increased Oct4 expression, and STAT3 (Tyr705) and IκB phosphorylation (Fig. 6B). To examine the involvement of STAT3 and NF-κB signaling in Oct4-mediated suppression of IR-induced premature senescence, the cells were pretreated with the STAT3-specific inhibitor WP1066 prior to IR. Pretreatment with WP1066 marginally affected IR-induced senescence in control cells; however, it significantly enhanced Oct4-mediated suppression of IR-induced senescence as determined using the SA-β-gal assay and FACS analysis (Fig. 7A and B). In addition, siRNA-mediated downregulation of STAT3 (Fig. 7C) inhibited the suppression of IR-induced senescence (Fig. 7D and E). Finally, pretreatment with the NF-κB-specific inhibitor Bay 11-7082 prevented Oct4-mediated suppression of IR-induced senescence (Fig. 7F and G). Taken together, these results indicated that Oct4-mediated inhibition of IR-induced premature senescence in MCF7 cells is achieved via activation of the STAT3 and NF-κB signaling pathways.
Figure 6.
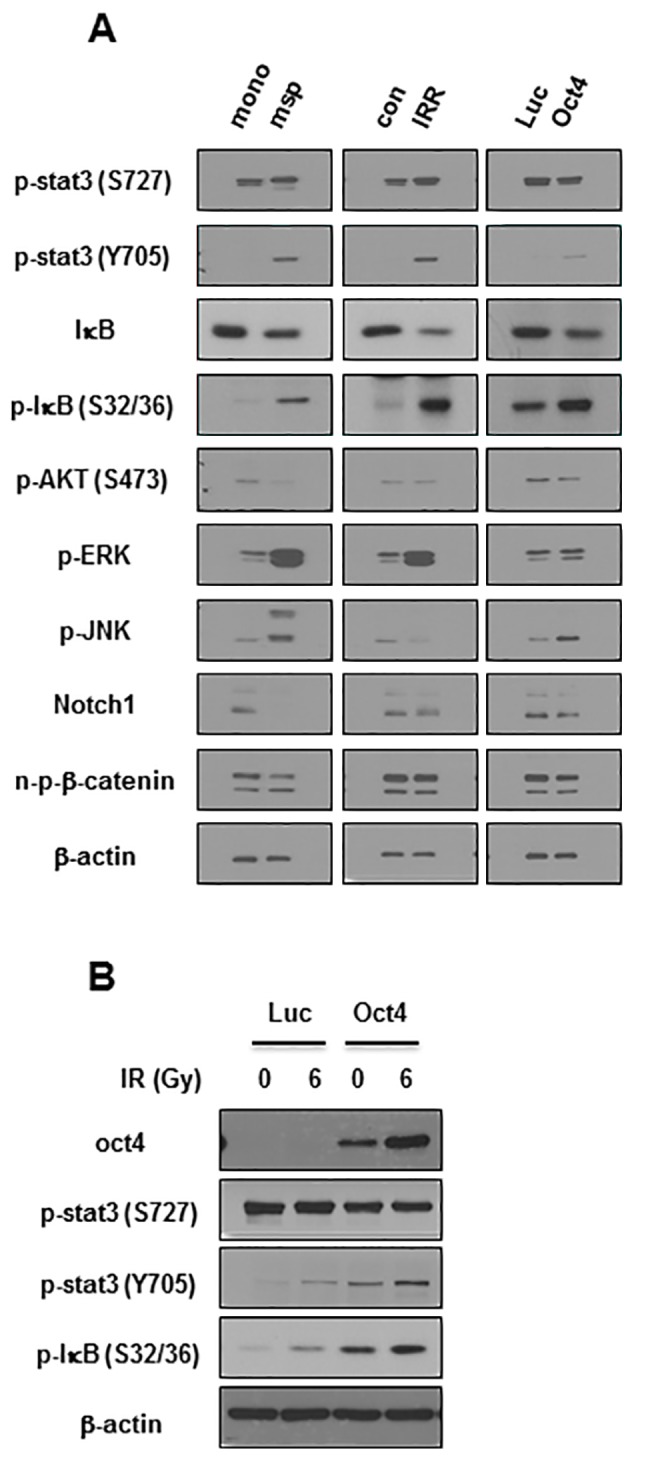
Increased activation of STAT3 and NF-κB in mammosphere, IRR, and Oct4-overexpressing breast cancer cells. (A) Western blot analysis of MCF7 cells with indicated antibodies. (B) Western blot analysis of MCF7 cells transduced with Luc and Oct4 following IR exposure (6 Gy). STAT3, signal transducer and activator of transcription 3; NF-κB, nuclear factor κB; Oct4, octamer-binding transcription factor 4; mono, serum-containing monolayer culture; msp, serum-free mammosphere culture; con, parental control cells; IRR, IR-resistant cells regrown following 6 Gy of IR exposure; Luc, luciferase; p-, phosphorylated; IκB, inhibitor of NF-κB; AKT, protein kinase B; ERK, extracellular-signal-regulated kinase; JNK, c-Jun N-terminal kinase; n-p-β-catenin, non-phosphorylated-β-catenin.
Figure 7.
Involvement of STAT3 and NF-κB signaling in Oct4-mediated suppression of IR-induced senescence. (A) Representative images of SA-β-gal assay (upper panel) and quantification (lower panel) and (B) flow cytometric assays (upper panel) and quantification (lower panel) of IR-induced senescence in MCF7 cells transduced with adenoviral-Luc and adenoviral-Oct4 3 days after exposure to 4 Gy of IR. Cells were pretreated with or without STAT3-specific inhibitor WP1066 (5 µM). (C) Western blot analysis of MCF7 cells transfected with sicon, siSTAT3-1 or -3 with the indicated antibodies. SA-β-gal assays (D) and flow cytometric assays (E) of MCF7 cells transfected with siSTAT3-1 and -3. SA-β-gal assays (F) and flow cytometric assays (G) of MCF7 cells treated with NF-κB-specific inhibitor Bay 11-7082 (5 and 10 µM) prior to IR exposure. *P<0.05, **P<0.01. STAT3, signal transducer and activator of transcription 3; NF-κB, nuclear factor κB; Oct4, octamer-binding transcription factor 4; SA-β-gal, senescence-associated β-galactosidase; IR, ionizing radiation; siSTAT3, small interfering RNA against STAT3; sicon, control small interfering RNA; Luc, luciferase; con, parental control; C12FDG, 5-dodecanoylaminofluorescein di-D-galactopyranoside.
Oct4 augments IL-24 expression through the STAT3 and NF-κB signaling pathways
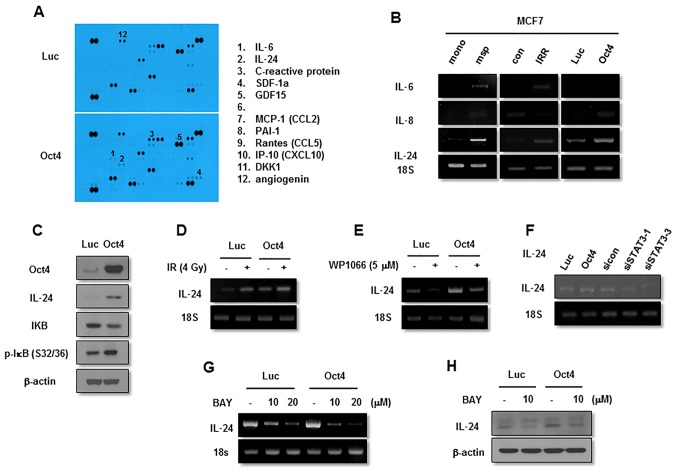
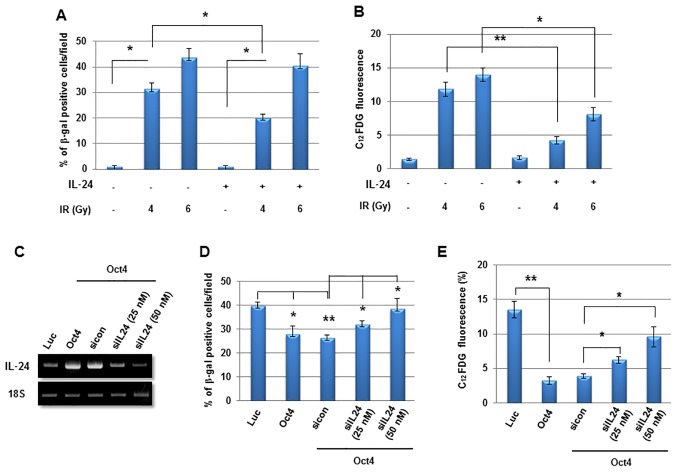
The next aim was to identify the effector molecule for Oct4-mediated inhibition of IR-induced senescence in MCF7 cells. Cytokine array-base analysis revealed that expression of IL-24, along with that of IL-6, C-reactive protein and SDF-1, was increased in Oct4-overexpressing cells (Fig. 8A). Among these proteins, IL-24 expression was commonly enhanced in mammosphere, IRR and Oct4-overexpressing cells compared with control cells as indicated using RT-PCR (Fig. 8B). The increase in IL-24 levels by Oct4 was confirmed by western blot analysis (Fig. 8C). IL-24 expression was increased further by IR in these cells (Fig. 8D). Treatment with STAT3 inhibitor and transfection of siRNA against STAT3 suppressed basal and Oct4-induced IL-24 expression (Fig. 8E and F). In addition, RT-PCR and western blot analysis demonstrated that NF-κB inhibition by Bay 11-7082 treatment also inhibited IL-24 expression (Fig. 8G and H). Taken together, these results suggested that IL-24 is an effector molecule of the Oct4-mediated STAT3 and NF-κB signaling axis.
Figure 8.
Function of IL-24 in Oct4-mediated suppression of IR-induced senescence. (A) Cytokine array of MCF7 cells. RT-PCR analysis of MCF7 cells (B) cultured as indicated with the indicated primers. (C) Western blot analysis of adenoviral-Luc- and adenoviral-Oct4-transduced MCF7 cells. RT-PCR analysis of MCF7 cells (D) exposed to 4 Gy of IR, (E) treated with STAT3 inhibitor WP1066 (5 µM), (F) transfected with siRNA-targeting STAT3 or (G) treated with NF-κB inhibitor Bay 11-7082 (10 and 20 µM) following transduction with adenoviral-Luc or adenoviral-Oct4. (H) Western blot analysis of adenoviral-Luc- and adenoviral-Oct4-transduced MCF7 cells pretreated with Bay 11-7082 (10 µM) with the indicated antibodies. IL, interleukin; Oct4, octamer-binding transcription factor 4; RT-PCR, reverse transcription-polymerase chain reaction; IR, ionizing radiation; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3; NF-κB, nuclear factor κB; IκB, inhibitor of NF-κB; SDF-1α, stromal cell-derived factor 1α; GDF15, growth differentiation factor 15; MCP-1, monocyte chemoattractant protein 1; CCL, CC chemokine ligand; PAI-1, plasminogen activator 1; Rantes, regulated on activation, normal T-cell expressed and secreted; IP-10, interferon γ-induced protein 10; CXCL10, CXC cytokine ligand 10; DKK1, dickkopf-related protein 10; msp, mammosphere; mono, monolayer-cultured; IRR, IR-resistant; Luc, luciferase; siSTAT3, small interfering RNA against STAT3; sicon, control small interfering RNA; p-, phosphorylated.
IL-24 serves a critical function in Oct4-mediated suppression of IR-induced senescence
Finally, to examine the function of IL-24 in Oct4-mediated suppression of IR-induced senescence further, MCF7 cells were treated with recombinant human IL-24 and it was identified to significantly suppress IR-induced senescence (Fig. 9A and B). In contrast, siRNA-mediated suppression of IL-24 expression (Fig. 9C) significantly restored IR-induced senescence in Oct4-overexpressing MCF7 cells (Fig. 9D and E). These results indicated that Oct4-mediated suppression of IR-induced premature senescence is achieved through IL-24 production via the STAT3 and NF-κB signaling pathways.
Figure 9.
Suppression of IR-induced senescence by recombinant human IL-24 treatment in breast cancer cells. (A) Senescence-associated β-gal and (B) flow cytometric assay of IR-induced senescence in MCF7 cells 3 days after exposure to 4 or 6 Gy of IR. *P<0.05, **P<0.01. (C) Reverse transcription-polymerase chain reaction analysis of adenoviral-Luc- and adenoviral-Oct4-transduced MCF7 cells probed with IL-24 primer following transfection with siIL-24. IR, ionizing radiation; IL-24, interleukin 24; β-gal, β-galactosidase; Oct4, octamer-binding transcription factor 4; C12FDG, 5-dodecanoylaminofluorescein di-D-galactopyranoside; Luc, luciferase; sicon, control small interfering RNA;siIL-24, small interfering RNA against IL-24.
Discussion
It has been identified previously that cancer cells are hierarchically organized in heterogeneous populations and contain minor subpopulations of stem-like cancer cells that are markedly capable of initiating tumor growth and resistance to conventional therapy, including IR (3). As accelerated relapse of cancer following sublethal therapy is thought to be caused by CSCs, eliminating CSCs may be a major step in improving the treatment of cancer. Consistent with this concept, the function of Oct4 was investigated in the maintenance of stemness and resistance to IR in breast cancer cells. It was identified that sphere-cultured cells were more resistant to IR exposure and exhibited increased Oct4 expression compared with monolayer-cultured breast cancer cells. In addition, IRR MCF7 cells exhibited more marked Oct4 expression compared with parental cells. Ectopic expression of Oct4 conferred IR resistance on breast cancer cells, whereas siOct4 reversed this effect. Oct4-mediated IR resistance was not due to the induction of apoptosis or cell-cycle arrest, but to IR-induced premature senescence. Oct4 expression enhanced the activation of STAT3 and NF-κB, and siRNA-mediated or pharmacological inhibition of these signaling molecules inhibited Oct4-mediated suppression of IR-induced senescence. Finally, the inhibition of IR-induced senescence by the activation of Oct4-mediated STAT3 and Oct4/NF-κB signaling was achieved through IL-24 production. These results suggested that the embryonic stem cell factor Oct4 is a stemness factor for BCSCs with IR-resistance capacity that acts through suppressing IR-induced premature senescence and it thus may be considered a potential target for eliminating CSCs in this devastating type of cancer.
Expression of Oct4 is thought to be a putative marker for CSCs in various types of cancer, including breast cancer (12–14). A number of studies have addressed the function of Oct4 in the maintenance of stemness and therapeutic resistance. In drug-resistant liver cancer cells, Oct4 mRNA expression is increased and overexpression of Oct4 results in chemoresistance of liver cancer cells via the upregulation of ABCG2 (14). Consistent with the results of the present study, CD133+ lung cancer cells maintain stemness and drug resistance by upregulating Oct4 expression (12). However, the function of Oct4 in IR resistance of CSCs is not well understood. The results of the present study indicated that ectopic expression of Oct4 enhanced IR resistance in breast cancer cells by suppressing IR-induced premature senescence. Thus, it is highly likely that the higher IR resistance of BCSCs enriched in mammospheres compared with that of differentiated monolayer-cultured cells is, at least in part, due to enhanced Oct4 expression.
In human epidermal growth factor receptor 2-positive breast cancer cells, activation of the STAT3 signaling pathway is crucial for radioresistance (18–20). Additionally, IR-induced NF-κB activation confers radioresistance on breast cancer cells (21–23). In the present study, STAT3 and NF-κB activation was common in mammosphere, radioresistant and Oct4-overexpressing MCF7 cells, and blockade of these molecules with specific siRNAs or through pharmacological inhibition sensitized these cells to IR-induced senescence. Therefore, the results of the present study suggest that Oct4-mediated activation of STAT3 and NF-κB signaling confers IR resistance on breast cancer cells.
IL-24, also known as melanoma differentiation-associated gene-7, is a secretory cytokine of the IL-10 family. Following identification in differentiated melanoma, it was demonstrated to serve a critical function in tumor inhibition (24–26). IL-24 induces cancer cell death by augmenting endoplasmic reticulum stress and mitochondrial dysfunction (27,28). It also stimulates autophagy in various cancer cells (29). In addition, IL-24 suppresses tumor angiogenesis directly by inhibiting tumor blood vessel formation and/or indirectly by suppressing the production of angiogenic growth factors, such as vascular endothelial growth factor, bFGF and IL-8 (30). Furthermore, IL-24 inhibits cancer cell invasion and metastasis by downregulating metastasis-associated genes (31). The present study identified another function of IL-24 in the suppression of IR-induced premature senescence. Ectopic expression of Oct4 increased IL-24 production in breast cancer cells, and IL-24 production was increased further by IR exposure in these cells. Although IL-24 reportedly enhances the IR effect in cancer cells (32,33), this appeared to be an effect of the adenoviral expression system. However, the results of the present study indicated that moderate expression of IL-24 was able to suppress IR-induced premature senescence in cancer cells.
In summary, the results of the present study provide the first evidence that Oct4-mediated STAT3 and NF-κB signaling serves an important function in resistance to IR by suppressing IR-induced premature senescence in breast cancer cells. Thus, controlling Oct4 is a promising approach for efficient suppression or elimination of BCSCs, and may be beneficial in radiotherapy for breast cancer.
Acknowledgments
Not applicable.
Abbreviations
- BCSC
breast cancer stem cell
- CSC
cancer stem cell
- IR
ionizing radiation
- IRR
IR-resistant
- Oct4
octamer-binding transcription factor 4
- Sox2
sex-determining region Y-box 2
- STAT3
signal transducer and activator of transcription 3
- IL
interleukin
- NF-κB
nuclear factor κB
- IκB
inhibitor of NF-κB
Funding
The present study was supported by the Korea Institute of Radiological and Medical Sciences, funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (grant nos. 1711045557, 1711045538 and 1711045554/50531-2017) and by the Basic Science Research Program Grant (grant no. NRF-2017R1A2B4003233) from the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, Republic of Korea.
Availability of data and materials
The analyzed datasets generated in the present study are available from the corresponding author on reasonable request.
Authors' contributions
JYK and JCK conducted experiments and data acquisition. JYL was involved in conceptualization interim discussions. MJP designed the present study and wrote the manuscript. All authors critically reviewed the manuscript content and agree with the submission of the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(SICI)1097-0215(19990315)80:6<827::AID-IJC6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 8.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 9.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AND, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karimi-Busheri F, Rasouli-Nia A, Mackey JR, Weinfeld M. Senescence evasion by MCF-7 human breast tumor-initiating cells. Breast Cancer Res. 2010;12:R31. doi: 10.1186/bcr2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, Lopez JP, Poon RTP, Fan ST. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology. 2010;52:528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- 14.Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, Cao X, Ling EA, Hao A. Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia. 2009;57:724–733. doi: 10.1002/glia.20800. [DOI] [PubMed] [Google Scholar]
- 15.Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. 2008;68:6533–6540. doi: 10.1158/0008-5472.CAN-07-6642. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4:1798–1806. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 18.Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18:6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS, Kim HA, Seong MK, Seol H, Oh JS, Kim EK, Chang JW, Hwang SG, Noh WC. STAT3-survivin signaling mediates a poor response to radiotherapy in HER2-positive breast cancers. Oncotarget. 2016;7:7055–7065. doi: 10.18632/oncotarget.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, III, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed KM, Zhang H, Park CC. NF-κB regulates radioresistance mediated by β1-integrin in three-dimensional culture of breast cancer cells. Cancer Res. 2013;73:3737–3748. doi: 10.1158/0008-5472.CAN-12-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veuger SJ, Hunter JE, Durkacz BW. Ionizing radiation-induced NF-kappaB activation requires PARP-1 function to confer radioresistance. Oncogene. 2009;28:832–842. doi: 10.1038/onc.2008.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunigal S, Lakka SS, Joseph P, Estes N, Rao JS. Matrix metalloproteinase-9 inhibition down-regulates radiation-induced nuclear factor-kappa B activity leading to apoptosis in breast tumors. Clin Cancer Res. 2008;14:3617–3626. doi: 10.1158/1078-0432.CCR-07-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeki T, Mhashilkar A, Swanson X, Zou-Yang XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21:4558–4566. doi: 10.1038/sj.onc.1205553. [DOI] [PubMed] [Google Scholar]
- 27.Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, Reed JC, Fisher PB. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138–8144. [PubMed] [Google Scholar]
- 28.Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC, et al. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, et al. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4:513–515. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- 31.Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510–518. doi: 10.1016/j.ymthe.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, Dent P, Reed JC, Fisher PB. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: Overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the anti-apoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- 33.Yacoub A, Mitchell C, Lebedeva IV, Sarkar D, Su ZZ, McKinstry R, Gopalkrishnan RV, Grant S, Fisher PB, Dent P. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol Ther. 2003;2:347–353. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated in the present study are available from the corresponding author on reasonable request.