Abstract
Exposure to mercury has detrimental effects on the cardiovascular system, particularly the vascular endothelium. The present study aimed to investigate the effects of ergothioneine (EGT) on endothelial dysfunction induced by low-dose mercury chloride (HgCl2). Agonist-induced contractions and relaxations were evaluated in isolated aortic rings from 3-month-old male Wistar rats treated by intra-muscular injection to caudal hind leg muscle with HgCl2 (first dose, 4.6 µg/kg; subsequent doses, 0.07 µg/kg/day for 15 days) and optionally with EGT (2 µg/kg for 30 days). Reactive oxygen species (ROS) in aortic rings were measured by means of lucigenin- and luminol-enhanced chemiluminescence. The protein level of endothelial nitric oxide synthase was evaluated by ELISA. Blood glutathione (GSH) and catalase levels, lipid peroxidation and total nitrite were measured spectrophotometrically. The results indicated that low-dose HgCl2 administration impaired acetylcholine (ACh)-induced relaxation and potentiated phenylephrine- and serotonin-induced contractions in rat aortas. In addition, HgCl2 significantly increased the levels of ROS in the aortic tissue. EGT prevented the loss of ACh-induced relaxations and the increase in contractile responses. These effects were accompanied by a significant decrease in ROS levels. EGT also improved the ratio of reduced GSH to oxidized GSH and catalase levels with a concomitant decrease in lipid peroxidation. In conclusion, to the best of our knowledge, the present study was the first to report that EGT prevents endothelial dysfunction induced by low-dose HgCl2 administration. EGT may serve as a therapeutic tool to reduce mercury-associated cardiovascular complications via improving the antioxidant status.
Keywords: mercury toxicity, ergothioneine, endothelial dysfunction
Introduction
Chronic exposure to heavy metals poses a serious health threat. Among these, mercury, considered by the World Health Organization as one of the top ten chemicals of major public health concern (1), has been used for numerous years for a variety of purposes (2). Exposure mostly occurs through consumption of fish and fishery products contaminated with organic mercury, inhalation of mercury vapour from dental amalgams and from vaccines containing thiomersal (3). Fish and sea mammals are increasingly becoming a source of mercury toxicity (4). For a long time, mercury was principally thought to affect central nervous system, thus leading to degenerative diseases (5). However, as extensively reviewed by Fernandes Azevedo et al (6), mercury also produces profound cardiovascular toxicity. Mercury has been demonstrated to induce endothelial dysfunction in experimental models using low doses of mercury (7–11), attaining the blood mercury concentration just above the safe level recommended by the Environmental Protection Agency (12). In these studies, reduction of nitric oxide (NO) bioavailability and increased oxidative stress consistent with high levels of reactive oxygen species (ROS) were noted as major causes of endothelial dysfunction observed in low-dose mercury toxicity. In the light of the above, antioxidants may have therapeutic potential in the prevention of mercury-induced endothelial dysfunction. This notion is further supported by a study by Rizetti et al (9), which demonstrated that apocynin improves endothelial dysfunction in aortas of rats exposed to nanomolar concentrations of mercury.
Ergothioneine (EGT) is an ubiquitous histidine derivative occurring in higher-order plants and animals (13). In humans, EGT accumulates in cells and tissues, which are frequently exposed to oxidative stress, including the liver, bone marrow, lens of the eye, seminal fluid and blood (14–16). Organic cation transporter, novel, type 1, encoded by the gene solute carrier family 22, member 4, mediates the cellular uptake of EGT (17). In contrast to the major tissue antioxidant glutathione (GSH), EGT is resistant to autoxidation and does not form disulphides under physiological conditions (18,19). Several lines of in vitro evidence suggest that EGT is a potent scavenger of ROS (20–26). Furthermore, a previous study by our group reported for the first time that EGT produces relaxation in isolated rat aortas by inactivating superoxide anions (27). This result and those of further studies, which indicate a potential role for EGT in the protection of endothelium (28–30), prompted us to examine its effects on mercury-induced endothelial dysfunction. The present study was performed to evaluate the effects of EGT on vascular reactivity in aortic rings from rats, which were treated with nanomolar concentrations of mercury chloride.
Materials and methods
Animals
Male Wistar rats (Lemali Ltd., Ankara, Turkey; weight, 150–175 g; age, 3 months; n=18) were used in the present study. The protocol for the animal experiment was approved by the Ethics Committee of Dokuz Eylül University (Izmir, Turkey; approval no. B.30.2/DEU/0.01/9402). The rats were provided pelleted food and water ad libitum and were maintained under constant temperature (22±2°C) and at a relative humidity level of 50% with a 12-h light/dark cycle. Animals were divided into three groups (n=6 in each) and treated for 30 days as follows: i) Control [0.9% NaCl, 0.5 ml administered by intramuscular (IM) injection]; ii) Mercury chloride (HgCl2) (first dose, 4.6 µg/kg; maintenance doses, 0.07 µg/kg/day, IM, to make up for daily loss) (10); and iii) HgCl2 + EGT (2 mg/kg, IM).
Reagents
EGT, potassium chloride (KCl), acetylcholine hydrochloride (ACh), phenylephrine hydrochloride (PE), serotonin hydrochloride (5-HT) and HgCl2 were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). All drugs were dissolved in saline (0.9% NaCl).
Preparation of samples
Blood samples were collected by cardiac puncture under anesthesia with ketamine (100 mg/kg)/xylazine (10 mg/kg) administered by intraperitoneal injection; the rats were then sacrificed by decapitation. The thoracic aorta was removed, cleaned of fat and loose connective tissue, and cut into 2 mm-thick transverse rings. Aortic rings were suspended between two stainless hooks in 10-ml organ baths filled with Krebs solution gassed with 95% O2-5% CO2 at 37°C. The composition of Krebs solution was (in mM): NaCl, 118; KCl, 4.7; CaCl2 × 2H2O, 2.5; KH2PO4, 1.20; MgSO4 × 7H2O, 1.17; glucose, 11.1; NaHCO3, 25. A resting tension of 2 g was applied to the aortic rings, which were then allowed to equilibrate for 45 min prior to further experimentation. In this period, tissues were washed out with Krebs solution every 15 min. Isometrical changes in tension were processed with MLT0201/RAD force transducers (AD Instruments, Inc., Colorado Springs, CO, USA) and recorded on LabChart Pro (version 7.1; AD Instruments, Inc.).
Experimental protocol for vascular reactivity studies
Concentration-response curves to ACh (10−9-10−4 M) were recorded in aortic rings previously contracted with PE (10−6 M). After a washout period of 45 min, concentration-response curves to PE (10−9-10−4 M) and to 5-HT (10−9-10−4.5 M) were recorded, respectively.
Detection of ROS
Levels of ROS were determined according to the method described by Wang et al (31), with slight modifications. After a 30-min stabilization period in Krebs solution maintained at 37°C and gassed with 95% O2 - 5% CO2, aortic rings were transferred to solid white 96-well plates containing 200 µl HEPES-buffered Krebs solution (pH 7.4). Following addition of lucigenin or luminol (final concentration of either, 5 µmol/l), ROS were quantified using a multi-plate reader (Victor III-1420; Perkin Elmer, Inc., Waltham, MA, USA). Counts were obtained at 10-second intervals and corrected for wet tissue weight. Results were expressed as the area under curve (AUC) for a counting period of 10 min [AUC of relative light units/mg wet tissue].
Tissue homogenization
Aortic tissue was homogenized on ice in PBS (pH 7.4) using a sonicator (Bandelin Sonopuls, UW 2070; Bandelin, Berlin, Germany). Homogenate was centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was aliquoted and stored at −80°C for further evaluation. The protein content was determined by the method of Lowry et al (32).
Determination of total nitrite
Nitrite levels were determined after conversion of nitrate to nitrite by nitrate dehydrogenase (33). Aortic supernatant was mixed with an equal volume of Griess reagent (sulfanilamide 1% w/v; naphtylethylenediamine dihydrochloride, 0.1% w/v; and orthophosphoric acid, 25% v/v). Following incubation at 37°C for 10 min, the absorbance was read at 540 nm. Total nitrite levels were determined from a standard curve with increasing concentrations of sodium nitrite and normalized to the protein content of the aortic sample.
Determination of endothelial NO synthase (eNOS)
Protein levels of eNOS were determined in aortic supernatants by using an ELISA kit (cat. no. SEA868Ra; Wuhan USCN Business Co., Ltd., Wuhan, China) according to the manufacturer's protocols.
Measurement of oxidative stress markers
The levels of GSH (reduced form) and the ratio of GSH to oxidized glutathione (GSSG) in blood samples were determined using the Bioxytech® GSH/GSSG-412 assay (cat. no. 21040; Oxis International, Inc.; GT Biopharma, Inc., Washington, DC, USA) according to the manufacturer's protocols. The formation of malondialdehyde (MDA) and catalase activity in blood samples were determined using commercially available assay kits [Bioxytech® MDA-586 (cat. no. 21044) and Catalase-520 assay (cat. no. 21042), respectively; Oxis International, Inc.; GT Biopharma, Inc.] according to the manufacturer's protocols.
Statistical analysis
All values are expressed as the mean ± standard error of the mean. Relaxation responses to ACh are expressed as percentage (%) relaxation of the PE-induced tone. Contractile responses to PE and 5-HT are expressed as percentage (%) of the KCl-induced tone. Analysis of variance followed by Tukey's multiple comparisons test was performed using GraphPad Prism (Version 5.0 for Mac OS X; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Isometric tension recordings
Relaxations
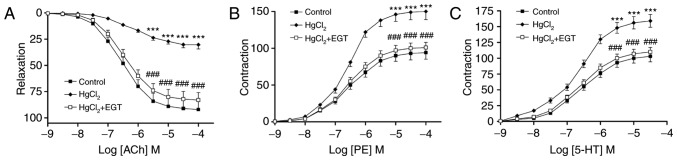
ACh induced concentration-dependent relaxations in PE-pre-contracted aortic rings from control and treated rats (Fig. 1A). Treatment with HgCl2 reduced ACh-induced relaxations by up to 67.1% compared with the control group (P<0.001) and shifted the concentration-response curve to the right (Fig. 1A). EGT inhibited the impairment of ACh-induced relaxation observed in aortic rings from HgCl2-treated rats (P<0.001; Fig. 1) and significantly increased the pD2 values (P<0.01; Table I) compared with HgCl2-treated rats without EGT treatment.
Figure 1.
Effects of EGT on vascular reactivity in aortas from HgCl2-treated rats. (A) Relaxation responses to ACh. (B) Contractile responses to PE. (C) Contractile responses to 5-HT. Relaxation responses to ACh are expressed as the percentage relaxation of PE-induced tone. Contractile responses to PE and 5-HT are expressed as the percentage of KCl-induced tone. Values are expressed as the mean ± standard error of mean (n=6). ***P<0.001 vs. Control. ###P<0.001 vs. HgCl2. EGT, ergothioneine; ACh, acetylcholine; PE, phenylephrine hydrochloride; 5-HT, serotonin hydrochloride.
Table I.
Effects of EGT on the sensitivity to ACh, PE and 5-HT in aortas from HgCl2-treated rats.
| Parameter | Control | HgCl2 | HgCl2+EGT |
|---|---|---|---|
| Ach | 6.55±0.06 | 6.16±0.07a | 6.46±0.05b |
| PE | 6.54±0.07 | 6.59±0.09 | 6.58±0.05 |
| 5-HT | 6.57±0.08 | 6.64±0.07 | 6.62±0.05 |
P<0.001 vs. Control.
P<0.001 vs. HgCl2. Values are expressed as the mean ± standard error of mean (n=6). EGT, ergothioneine; ACh, acetylcholine; PE, phenylephrine hydrochloride; 5-HT, serotonin hydrochloride.
Contractions
PE and 5-HT induced concentration-dependent contractions in aortic rings from control and treated rats (Fig. 1B and C). Treatment with HgCl2 significantly increased PE- and 5-HT-induced contractions compared with the control group (P<0.001; Fig. 1B and C) without affecting the sensitivity to either agent (Table I). Co-treatment with EGT prevented the increase in contractile response to PE and to 5-HT compared with HgCl2-treated rats without EGT treatment (P<0.001; Fig. 1B and C).
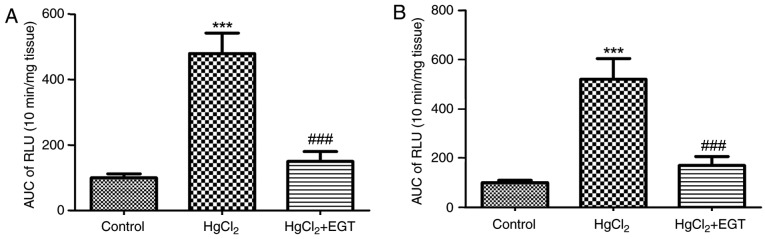
Levels of ROS
Low-dose HgCl2 significantly increased the levels of ROS in the rat thoracic aortas (Fig. 2A and B). Lucigenin- and luminol-enhanced chemiluminescence in aortas from HgCl2-treated rats were ~4.8 and ~5.2 times higher than in those of control tissues, respectively (for either, P<0.001). EGT significantly reduced the ROS levels increased by HgCl2 treatment in HgCl2+EGT-treated rats compared with HgCl2-treated rats without EGT treatment (P<0.001; Fig. 2A and B).
Figure 2.
Effects of EGT on the levels of reactive oxygen species in aortas from HgCl2-treated rats. (A) Lucigenin-enhanced chemiluminescence. (B) Luminol-enhanced chemiluminescence. Values are expressed as the mean ± standard error of mean (n=6). ***P<0.001 vs. Control. ###P<0.001 vs. HgCl2. AUC, area under curve; RLU, relative light units; EGT, ergothioneine.
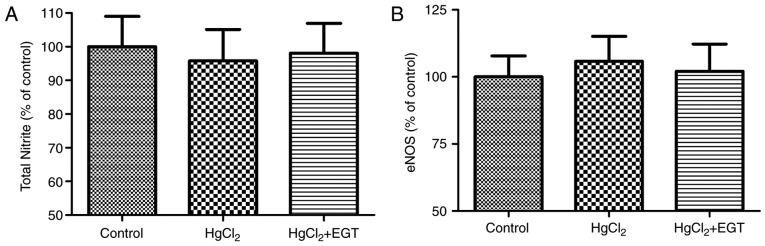
Total nitrite and eNOS levels
Levels of total nitrite and eNOS remained unchanged among the experimental groups (Fig. 3A and B).
Figure 3.
Effects of EGT on nitric oxide synthesis in aortas from HgCl2-treated rats. (A) Total nitrite levels. (B) eNOS levels. Values are expressed as the mean ± standard error of mean (n=6). EGT, ergothioneine; eNOS, endothelial nitric oxide synthase.
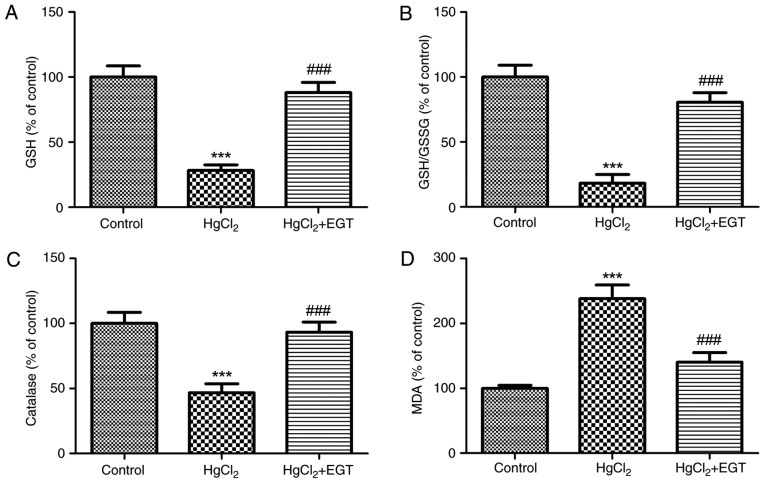
Antioxidant status
Fig. 4 summarizes the effects of EGT on the antioxidant status in the blood of rats. Low-dose HgCl2 caused a significant increase in oxidative stress and lipid peroxidation, and reduced catalase activity. When compared with the control group, GSH levels and the GSH/GSSG ratio were significantly lower in HgCl2-treated rats (P<0.001; Fig. 4A and B). Similarly, catalase activity decreased by 53.3% in HgCl2-treated rats compared with the control group (P<0.001; Fig. 4C). In addition, lipid peroxidation, as indicated by increased plasma MDA levels, increased by ~1.3-fold in HgCl2-treated rats compared with the control group (P<0.001; Fig. 4D). Co-treatment with EGT not only restored the antioxidant status, but also significantly reduced lipid peroxidation in HgCl2+EGT-treated rats compared with HgCl2-treated rats without EGT treatment (P<0.001; Fig. 4D).
Figure 4.
Effects of EGT on the blood antioxidant status from HgCl2-treated rats. (A) Levels of GSH, (B) GSH/GSSG ratio, (C) catalase levels and (D) MDA levels. Values are expressed as the mean ± standard error of the mean (n=6). ***P<0.001 vs. Control. ###P<0.001 vs. HgCl2. GSH, reduced glutathione; GSSG, oxidized glutathione; EGT, ergothioneine; MDA, malondialdehyde.
Discussion
Overall health effects of chronic exposure to mercury are a matter of serious concern and cardiovascular consequences of mercury toxicity remain an important area of research. As comprehensively reviewed by Houston (4), exposure to mercury is an underestimated risk factor for hypertension, coronary heart disease, myocardial infarction, reduction in heart rate variability, increase in carotid intima-media thickness and carotid obstruction, generalized atherosclerosis, renal dysfunction and proteinuria, and an overall increase in total and cardiovascular mortality. Garcia Gomez et al (34) reported that the occurrence of hypertension, stroke and total cardiovascular mortality in mercury mine workers is increased by 2.78-, 1.17- and 1.51-fold, respectively. It may simply be assumed that these consequences are most probably due to high levels of occupational or environmental exposure to mercury. However, evidence from animal models producing blood mercury levels similar to those of average human exposure suggest that low-dose mercury promotes endothelial dysfunction (8–11), a systemic pathological state of the endothelium, which is widely accepted as an early crucial event in cardiovascular diseases (35,36). The present study provides preliminary evidence that EGT, a ubiquitous, water soluble, sulphur-containing derivative of the amino acid histidine, prevents low-dose HgCl2-induced endothelial dysfunction.
The present results may be summarized as follows: i) Low-dose HgCl2 decreases the maximum value and sensitivity of the relaxation response to ACh and increases the maximum value of contractile responses to 5-HT and PE, ii) HgCl2 increases the levels of ROS in the thoracic aorta, iii) HgCl2 causes significant reductions in GSH and catalase levels and decreases the GSH/GSSG ratio, while markedly increasing MDA formation compared with that in the control group, and iv) EGT reverses the abovementioned HgCl2-induced alterations in antioxidant status and vascular reactivity.
In the present study, chronic low-dose HgCl2 administration to rats caused a marked decline in the relaxation response to ACh in isolated thoracic aortas by up to 67.1% and significantly decreased the sensitivity, which is consistent with the results of Wiggers et al (10). The present results indicate that the HgCl2-associated reduction in relaxant responses and sensitivity to ACh were almost completely reversed by EGT treatment. In addition, EGT suppressed the significant increases in contractile responses to 5-HT and PE in HgCl2-treated rats. A previous study by our group demonstrated that pre-treatment with EGT did not affect the ACh-induced relaxation responses in endothelium-intact rat aortic rings (27). However, in parallel experiments employing a model of oxidative stress, which is based on inhibition of endogenous Cu/Zn superoxide dismutase leading to the accumulation of superoxide anions, EGT recovered the impaired ACh relaxation (27). In addition, EGT elicited a concentration-dependent relaxation effect in aortic rings, which was blunted by endothelial denudation or by inhibition of NOS (27). Taking the above results into consideration, the present study first investigated the possibility that EGT interferes with NO synthesis to increase Ach-induced relaxation. According to the present results, EGT does not appear to affect NO synthesis as reflected by similar total nitrite and eNOS levels among groups. This result gives rise to the question whether alterations in ROS levels and/or antioxidant status underlie the ameliorative effects of EGT on NO-dependent relaxations. Decreased bioavailability of NO due to increased superoxide anion production by NADPH oxidase is regarded as a crucial aspect of endothelial dysfunction observed in chronic exposure to low concentrations of mercury (6,7,37). Superoxide anions not only participate in endothelial dysfunction, mainly owing to their rapid interaction with NO, but also produce direct biological effects and serve as a progenitor for numerous other ROS (38). Several studies revealed that EGT is a powerful scavenger of ROS and protects cells against a wide range of stressors (20,26,27). Indeed, in the present study, high levels of ROS, including superoxide anions, observed in aortas from HgCl2-treated rats were significantly diminished by EGT treatment. As is known, NO rapidly reacts with superoxide anions to form peroxynitrite, leading to decreased NO bioavailability (39). Furthermore, peroxynitrite, the end product of this reaction, may also lead to eNOS uncoupling and cause vasoconstriction (39). Thus, it is concluded that the superoxide scavenging effects of EGT may lead to increased NO bioavailability and/or reduced eNOS uncoupling to restore impaired ACh-induced relaxation without changing eNOS levels and/or NO production. In addition, EGT improved the antioxidant status by increasing GSH levels, the GSH/GSSG ratio and catalase activity, and by reducing lipid peroxidation. Apart from these results, the protective effects of EGT on vascular reactivity were also evident in contractile responses. EGT significantly reduced the increment in PE- and 5-HT-induced contractions. ROS, particularly superoxide anions, are regarded as endothelium-derived contracting factors, which have major roles in the regulation of the arterial tone (40). Furthermore, superoxide anions and hydrogen peroxide may be transformed into hydroxyl radicals, which was demonstrated to increase the synthesis of vasoconstrictor prostanoids (41). In this context, radical scavenging by and/or the antioxidant activity of EGT may account for the observed attenuation in contractile responses to PE and 5-HT.
In conclusion, the present study was the first to report that endothelial dysfunction induced by low doses of HgCl2 is prevented by EGT. It should be noted that EGT is acquired by humans through dietary means and accumulates in cells and tissues that are frequently exposed to oxidative stress (27). Taking into consideration that mercury-induced toxicity is often associated with poor prognosis due to limited treatment options, further studies evaluating the effects of EGT on the complications of mercury exposure may provide new insight for therapeutic intervention.
Acknowledgements
Not applicable.
Funding
This study was supported by the Scientific Research Fund of Ege University, Izmir, Turkey (grant no. 09-ECZ-024).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
GG conceived and designed the experiments. GG and MZA performed the experiments. GG, MZA and EE analyzed the results and wrote the paper. All authors have read and approved the final manuscript.
Ethical approval and consent to participate
The protocol for the animal experiment was approved by the Ethics Committee of Dokuz Eylül University (Izmir, Turkey; approval no. B.30.2/DEU/0.01/9402).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.World Health Organization, corp-author. Mercury and health. http://www.who.int/mediacentre/factsheets/fs361/en/ Fact sheet. 2016 Updated March 2017. [Google Scholar]
- 2.Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.CIR.91.3.645. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury-current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 4.Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich) 2011;13:621–627. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutter J, Naumann J, Sadaghiani C, Schneider R, Walach H. Alzheimer disease: Mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuro Endocrinol Lett. 2004;25:331–339. [PubMed] [Google Scholar]
- 6.Fernandes Azevedo B, Barros Furieri L, Peçanha FM, Wiggers GA, Frizera Vassallo P, Ronacher Simões M, Fiorim J, de Batista Rossi P, Fioresi M, Rossoni L, et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol. 2012;2012:949048. doi: 10.1155/2012/949048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furieri LB, Galán M, Avendaño MS, García-Redondo AB, Aguado A, Martínez S, Cachofeiro V, Bartolomé MV, Alonso MJ, Vassallo DV, Salaices M. Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. Br J Pharmacol. 2011;162:1819–1831. doi: 10.1111/j.1476-5381.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pecanha FM, Wiggers GA, Briones AM, Perez-Giron JV, Miguel M, Garcia-Redondo AB, Vassallo DV, Alonso MJ, Salaices M. The role of cyclooxygenase (COX)-2 derived prostanoids on vasoconstrictor responses to phenylephrine is increased by exposure to low mercury concentration. J Physiol Pharmacol. 2010;61:29–36. [PubMed] [Google Scholar]
- 9.Rizzetti DA, Torres JG, Escobar AG, Peçanha FM, Santos FW, Puntel RL, Alonso MJ, Briones AM, Salaices M, Vassallo DV, Wiggers GA. Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury. PLoS One. 2013;8:e55806. doi: 10.1371/journal.pone.0055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiggers GA, Peçanha FM, Briones AM, Pérez-Girón JV, Miguel M, Vassallo DV, Cachofeiro V, Alonso MJ, Salaices M. Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. Am J Physiol Heart Circ Physiol. 2008;295:H1033–H1043. doi: 10.1152/ajpheart.00430.2008. [DOI] [PubMed] [Google Scholar]
- 11.Wiggers GA, Stefanon I, Padilha AS, Pecanha FM, Vassallo DV, Oliveira EM. Low nanomolar concentration of mercury chloride increases vascular reactivity to phenylephrine and local angiotensin production in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:252–260. doi: 10.1016/j.cbpc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Rice DC. The US EPA reference dose for methylmercury: Sources of uncertainty. Environ Res. 2004;95:406–413. doi: 10.1016/j.envres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Cheah IK, Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta. 2012;1822:784–793. doi: 10.1016/j.bbadis.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Melville DB, Horner WH, Lubschez R. Tissue ergothioneine. J Biol Chem. 1954;206:221–228. [PubMed] [Google Scholar]
- 15.Shires TK, Brummel MC, Pulido JS, Stegink LD. Ergothioneine distribution in bovine and porcine ocular tissues. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;117:117–120. doi: 10.1016/S0742-8413(96)00223-X. [DOI] [PubMed] [Google Scholar]
- 16.Shukla Y, Kulshrestha OP, Khuteta KP. Ergothioneine content in normal and senile human cataractous lenses. Indian J Med Res. 1981;73:472–473. [PubMed] [Google Scholar]
- 17.Gründemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schömig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melville DB. Ergothioneine. Vitamin Horm. 1959;17:155–204. doi: 10.1016/S0083-6729(08)60271-X. [DOI] [Google Scholar]
- 19.Paul BD, Snyder SH. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010;17:1134–1140. doi: 10.1038/cdd.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motohashi N, Mori I. The role of ergothioneine in the oxidation of reduced nicotinamide adenine dinucleotide by metmyoglobin or methemoglobin. Chem Pharm Bull (Tokyo) 1983;31:1702–1707. doi: 10.1248/cpb.31.1702. [DOI] [PubMed] [Google Scholar]
- 21.Reglinski J, Smith WE, Sturrock RD. Spin-echo 1H NMR detected response of ergothioneine to oxidative stress in the intact human erythrocyte. Magn Reson Med. 1988;6:217–223. doi: 10.1002/mrm.1910060210. [DOI] [PubMed] [Google Scholar]
- 22.Hartman PE. Ergothioneine as antioxidant. Methods Enzymol. 1990;186:310–318. doi: 10.1016/0076-6879(90)86124-E. [DOI] [PubMed] [Google Scholar]
- 23.Akanmu D, Cecchini R, Aruoma OI, Halliwell B. The antioxidant action of ergothioneine. Arch Biochem Biophys. 1991;288:10–16. doi: 10.1016/0003-9861(91)90158-F. [DOI] [PubMed] [Google Scholar]
- 24.Aruoma OI, Whiteman M, England TG, Halliwell B. Antioxidant action of ergothioneine: Assessment of its ability to scavenge peroxynitrite. Biochem Biophys Res Commun. 1997;231:389–391. doi: 10.1006/bbrc.1997.6109. [DOI] [PubMed] [Google Scholar]
- 25.Mitsuyama H, May JM. Uptake and antioxidant effects of ergothioneine in human erythrocytes. Clin Sci (Lond) 1999;97:407–411. doi: 10.1042/cs0970407. [DOI] [PubMed] [Google Scholar]
- 26.Franzoni F, Colognato R, Galetta F, Laurenza I, Barsotti M, Di Stefano R, Bocchetti R, Regoli F, Carpi A, Balbarini A, et al. An in vitro study on the free radical scavenging capacity of ergothioneine: comparison with reduced glutathione, uric acid and trolox. Biomed Pharmacother. 2006;60:453–457. doi: 10.1016/j.biopha.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Gokce G, Arun MZ. Ergothioneine produces relaxation in isolated rat aorta by inactivating superoxide anion. Eur Rev Med Pharmacol Sci. 2014;18:3339–3345. [PubMed] [Google Scholar]
- 28.Sit ASM, Ho EYW, Li RWS, et al. Ergothioneine shows protective effect on endothelial cells in oxidative stress. Faseb J. 2011;25:630–633. [Google Scholar]
- 29.Martin KR. The bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J Med Food. 2010;13:1340–1346. doi: 10.1089/jmf.2009.0194. [DOI] [PubMed] [Google Scholar]
- 30.Li RW, Yang C, Sit AS, Kwan YW, Lee SM, Hoi MP, Chan SW, Hausman M, Vanhoutte PM, Leung GP. Uptake and protective effects of ergothioneine in human endothelial cells. J Pharmacol Exp Ther. 2014;350:691–700. doi: 10.1124/jpet.114.214049. [DOI] [PubMed] [Google Scholar]
- 31.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr Al, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Majithiya JB, Paramar AN, Balaraman R. Pioglitazone, a PPARgamma agonist, restores endothelial function in aorta of streptozotocin-induced diabetic rats. Cardiovasc Res. 2005;66:150–161. doi: 10.1016/j.cardiores.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Gomez Garcia M, Boffetta P, Klink Caballero JD, Espanol S, Quintana Gomez J. Cardiovascular mortality in mercury miners. Med Clin (Barc) 2007;128:766–771. doi: 10.1157/13106327. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 35.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/S0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 36.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 37.Massaroni L, Rossoni LV, Amaral SM, Stefanon I, Oliveira EM, Vassallo DV. Haemodynamic and electrophysiological acute toxic effects of mercury in anaesthetized rats and in langendorff perfused rat hearts. Pharmacol Res. 1995;32:27–36. doi: 10.1016/S1043-6618(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 38.Guzik TJ, Channon KM. Measurement of vascular reactive oxygen species production by chemiluminescence. Methods Mol Med. 2005;108:73–89. doi: 10.1385/1-59259-850-1:073. [DOI] [PubMed] [Google Scholar]
- 39.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- 41.Vanhoutte PM. Endothelium-derived free radicals: For worse and for better. J Clin Invest. 2001;107:23–25. doi: 10.1172/JCI11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.