Abstract
Objectives
Iodine deficiency during pregnancy is associated with obstetric and neonatal adverse outcomes. Serum thyroglobulin (sTg) and thyroid volume (TV) are optional tools to urinary iodine concentration (UIC) for defining iodine status. This cross-sectional study aims to evaluate the iodine status of pregnant women living in iodine-adequate area by spot UIC and correlation with sTg, TV and thyroid function.
Methods
Two hundred and seventy-three pregnant women were evaluated at three trimesters. All had no previous thyroid disease, no iodine supplementation and negative thyroperoxidase and thyroglobulin antibodies. Thyroid function and sTg were measured using electrochemiluminescence immunoassays. TV was determined by ultrasonography; UIC was determined using a modified Sandell–Kolthoff method.
Results
Median UIC was 146 µg/L, being 52% iodine deficient and only 4% excessive. TSH values were 1.50 ± 0.92, 1.50 ± 0.92 and 1.91 ± 0.96 mIU/L, respectively, in each trimester (P = 0.001). sTg did not change significantly during trimesters with median 11.2 ng/mL and only 3.3% had above 40 ng/mL. Mean TV was 9.3 ± 3.4 mL, which positively correlated with body mass index, but not with sTg. Only 4.5% presented with goitre.
When pregnant women were categorized as iodine deficient (UIC < 150 µg/L), adequate (≥150 and <250 µg/L) and excessive (≥250 µg/L), sTg, thyroid hormones and TV at each trimester showed no statistical differences.
Conclusions
Iodine deficiency was detected frequently in pregnant women living in iodine-adequate area. sTg concentration and TV did not correlate to UIC. Our observation also demonstrated that the Brazilian salt-iodization programme prevents deficiency, but does not maintain iodine status within adequate and recommended ranges for pregnant women.
Keywords: iodine, pregnancy, thyroglobulin, thyroid function tests
Introduction
Iodine status is a global health concern, particularly in developing countries, and emphasis should be placed on diagnosis and correction at the community level rather than the individual due to the high impact in child neurological development and pregnancy outcomes (1, 2).
During pregnancy, daily iodine requirement increases by nearly 50% because of an increase in renal iodine excretion, increased thyroid hormone production and foetal iodine requirements (3). In iodine-sufficient areas, pregnant women maintain stable total body iodine levels throughout pregnancy. However, in mild-to-moderate iodine-deficient areas, total body iodine stores decline gradually from the first to the third trimester of pregnancy, and the potential changes in the mother’s thyroid function during pregnancy are uncertain (4).
The recommended method to evaluate iodine nutrition status in a community of pregnant women is by measuring the urinary iodine concentration (UIC), thyroid size and serum thyroglobulin (sTg) (5). The UIC is most often used, but it indicates current iodine nutrition while thyroid size and sTg concentrations reflect iodine nutrition over a period of months or years. Measurements of UIC in randomly collected urine samples have proven to be useful, and consequently, iodine nutrition is defined as mildly-to-moderately iodine-deficient groups of pregnant women whose median UICs are between 50 and 150 µg/L (5). The median UIC values for United States pregnant women was 129 µg/L, which is consistent with a mild iodine deficiency (6). The American Thyroid Association (ATA) recommends that women who are planning to become pregnant or who are pregnant should supplement their diets with 150 µg/day of iodine (3).
Thyroid size is a sensitive marker for severe iodine deficiency because a goitre can be easily assessed by a physical examination and by a thyroid ultrasound. During pregnancy, the thyroids of mothers in iodine-deficient (moderate to low) areas will increase in size due to autoregulatory mechanisms of iodine on thyroid growth (7). There are limited data from pregnant women living in iodine-sufficient areas.
sTg also is a sensitive marker of iodine status as it reflects thyroid activity and hyperplasia (8). In iodine-deficient infants and children, the sTg concentrations are high, more often than are the serum TSH concentrations (9). Therefore, the sTg levels should be a good indicator of iodine nutrition in the general population and also in pregnant women (10).
Since 1953, salt iodization has been mandatory in Brazil, which is now reported to be an iodine-intake-adequate area with median UIC 276 µg/L, which was previously categorized as ‘more than adequate’ category (http://www.ign.org). However, iodine deficiency is still observed in a significant proportion of schoolchildren, especially from poor and uneducated families and rural regions (11). Previous small studies carried out in Brazil demonstrated an insufficient iodine intake in pregnant women, alerting about the adequacy of the iodine supply in Brazil, especially regarding the new determinations of the Brazilian government that recently reduced the concentrations of iodine in table salt to 15–45 mg/kg of salt (12).
We conducted this study to evaluate iodine status according to the median UIC, sTg and thyroid volume (TV) by ultrasound and thyroid function in pregnant women living in iodine-adequate areas during the three trimesters of pregnancy.
Materials and methods
This cross-sectional study included 312 pregnant women who were consecutively recruited from the University Hospital Obstetric Outpatient Clinic and from the Einstein programme at Paraisopolis Community, both located in Sao Paulo (Sao Paulo State, Brazil) between April 2012 and June 2016. The exclusion criteria were ages less than 18 years, twin pregnancies, high-risk pregnancies (kidney disorders, hypertension, diabetes and HIV-positive), preeclampsia and known thyroid disease. Women taking iodine-containing supplements and thyroxine or drugs that interfere with thyroid function (amiodarone, glucocorticoid, lithium, biotin, metformin) also were excluded from the study.
The first analysis excluded pregnant women with thyroperoxidase antibody-positive and/or thyroglobulin antibody (TgAb)-positive measured by electrochemiluminescence immunoassays (ECLIA) methods (Roche Diagnostics, Mannheim, Germany and Beckman Coulter, Fullerton, USA). Pregnant women with TSH > 10 mIU/L were also excluded. The remaining 273 pregnant women were analyzed according to thyroid function, sTg, ultrasound examination of thyroid gland and UIC at each trimester.
The study was approved by the Institutional Ethical Committee of our Institution (Hospital das Clinicas HCFMUSP, CAPPesq nº 01503912.8.0000.0068). Written consent was obtained from all participants.
Biochemical and hormonal measurements
TSH, total and free T3 (TT3 and fT3), total and free T4 (TT4 and fT4), sTg and UIC were measured in all pregnant women. Blood and urine samples were collected between 08:00 and 10:00 h and were maintained at −20°C until measurement. The ECLIA were used to measure serum thyroid hormones (Roche Diagnostics, Mannheim, Germany and Beckman Coulter, Fullerton, USA), and all samples were measured in duplicate.
According to the manufacturer, the reference values for the non-pregnant population were 0.27–4.20 mIU/L for TSH; 1.3–3.1 nmol/L for TT3; 3.1–6.8 pmol/L for fT3; 66–181 nmol/L for TT4 and 12.0–22.0 pmol/L for fT4.
Spot morning urine samples (10 mL) were collected after overnight fasting from all participants in universal containers and transferred to Monovette tubes for transport and storage at −20°C until analysis. The adapted Sandell–Kolthoff reaction was used to determine the UIC, and all samples were measured in duplicate (13).
Thyroid ultrasonography
A thyroid ultrasound was performed on each pregnant woman by the same physician (Ana Carolina de Castro Nassif Gomes Monteiro) using a 7.5–12 MHz linear probe with a colour Doppler ultrasound programme (Philips IU-22, USA). Volumes of each thyroid lobe were calculated according to the rotation ellipsoid model: total TV (mL) = right lobe volume + left lobe volume + isthmus volume, considering depth (cm) × width (cm) × length (cm) × π/6 for each lobe. The thyroid echogenicity was characterized as normal or hypoechoic in comparison to cervical muscle echogenicity.
Statistical analysis
Data were analysed using IBM SPSS statistics for windows (Version 22.0.) and Minitab 17 statistical software (Version 2010; State College, PA, USA). Results were expressed as mean ± s.d. The normality tests used were Kolmogorov–Smirnov or Shapiro–Wilk. Comparisons between the numerical variables were performed by Student’s t-test and one-way ANOVA while non-normal distribution was performed using Mann–Whitney and Kruskal–Wallis tests. The Bonferroni post hoc-test was applied to adjust for multiple comparisons. A P value of less than 0.05 was considered statistically significant.
Results
A total of 273 pregnant women were evaluated, with mean ages of 28.1 ± 6.5 years, mean BMI of 26.2 ± 4.9 kg/m2, and 40% were primigravida. Clinical characteristics of the study population according to each trimester of pregnancy are shown in Table 1.
Table 1.
Demographic characteristics of the pregnant women in each trimester.
| Parameter | Trimester | |||
|---|---|---|---|---|
| First (n = 79) (<13th week) | Second (n = 108) (13–27th week) | Third (n = 86) (≥27th week) | ||
| Age (years) | 27.7 ± 6.5 | 28.5 ± 6.6 | 28.0 ± 6.5 | P = 0.738 |
| BMI (kg/m2) | 25.2 ± 4.8 | 25.8 ± 4.9 | 27.8 ± 4.7 | P < 0.001 |
| Median UIC (μg/L) | 135 | 155 | 140 | P = 0.132 |
| Min–Max | 69–285 | 27–403 | 41–316 | |
| 25th–75th | 107–180 | 117–204 | 104–184 | |
| % < 150 μg/L | 53% | 47% | 58% | |
| TSH (mIU/L) | 1.50 ± 0.92 | 2.03 ± 1.03 | 1.91 ± 0.96 | *P = 0.001 |
| TT3 (nmol/L) | 2.55 ± 0.43 | 2.94 ± 0.49 | 2.96 ± 0.52 | *P < 0.001 |
| TT4 (nmol/L) | 144 ± 25.3 | 152 ± 34.0 | 146 ± 29.3 | P = 0.350 |
| fT3 (pmol/L) | 4.59 ± 0.64 | 4.37 ± 0.52 | 4.14 ± 0.56 | *P < 0.001 |
| fT4 (pmol/L) | 15.6 ± 1.8 | 13.3 ± 2.1 | 12.5 ± 1.6 | *P < 0.001 |
| sTg (μg/L) | 13.9 ± 14.9 | 14.5 ± 14.0 | 12.8 ± 8.7 | P = 0.701 |
| TV (mL) | 9.1 ± 4.2 | 9.2 ± 3.2 | 9.5 ± 2.9 | P = 0.213 |
P value <0.05 was considered statistically significant.
*P values for the comparison between first and second trimester and first and third trimester.
sTg, serum thyroglobulin; TV, thyroid volume; UIC, urinary iodine concentration.
The median UIC of all pregnant women was 144 µg/L (range 27.3–403), which is indicative of iodine deficiency according to the World Health Organization (WHO) criteria for pregnant women (5). A UIC of <150 µg/L was found in 52% of the women, and a UIC of <50 µg/L was found in only 2%. Adequate iodine status (150–249 µg/L) was observed in 44% of the women, and only 4% had a UIC of ≥250 µg/L. The UIC showed no statistic differences among the trimesters of pregnancy, also considering percentiles 25th and 75th. Iodine deficiency status was more frequent in the third trimester, but the lowest median UIC was found in the first trimester (135 µg/L).
The mean TSH values were 1.50 ± 0.92, 2.03 ± 1.03 and 1.91 ± 0.96 mIU/L, in the first, second and third trimesters, respectively (P = 0.001 for all trimesters). The fT4 and fT3 values decreased significantly during pregnancy (P < 0.001 for all trimesters) (Table 1). The TT4 and TT3 concentrations were higher during pregnancy. The TT4 concentrations remained stable throughout pregnancy, but the TT3 concentrations increased during consecutive trimesters, which was 29% higher than the reference values for the non-pregnant population.
The sTg levels did not change significantly during the trimesters (13.9 ± 14.9, 14.5 ± 14.0 and 12.8 ± 8.7 ng/mL, respectively). The median sTg was 11.2 ng/mL, and sTg above 40 ng/mL was found in only 3.3% of studied patients.
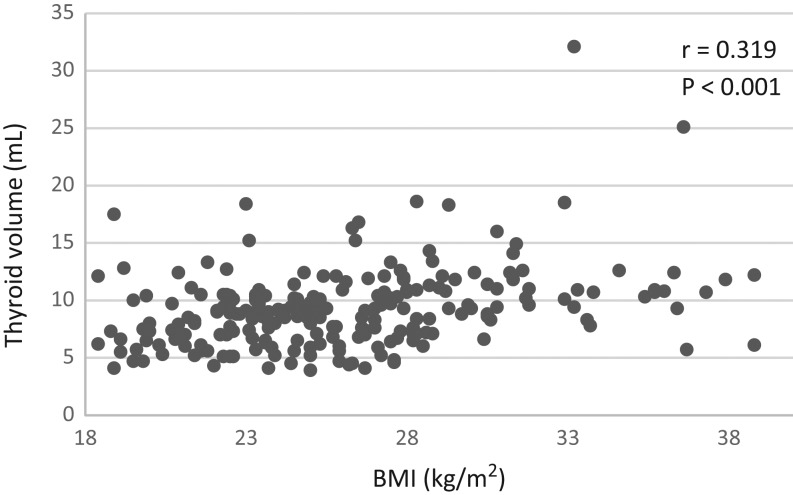
The mean TV was 9.3 ± 3.4 mL, and only 4.5% had TV exceeding 15 mL. The TV did not differ during pregnancy and positively correlated with the BMI (r = 0.319, P < 0.001) (Fig. 1), but not with the sTg and TSH. We found no correlation between TV and UIC. A thyroid nodule was detected in 19% of all pregnant patients.
Figure 1.
Relationship between body BMI and TV in pregnant women from an adequate iodine area. TV, thyroid volume.
When pregnant women were categorized as iodine deficient (UIC < 150 µg/L), iodine adequate (UIC ≥ 150 and <250 µg/L) and iodine excessive (UIC ≥ 250 µg/L), concentrations of sTg, thyroid hormones and TV at each trimester had no statistical difference according to the iodine status groups (Table 2).
Table 2.
Thyroid parameters according to iodine status in the pregnant women.
| Iodine status | First | P | Second | P | Third | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± s.d. | N | Mean ± s.d. | N | Mean ± s.d. | |||||
| TT3 (nmol/L) | Deficiency | 42 | 2.60 ± 0.42 | 0.360 | 51 | 3.04 ± 0.48 | 0.077 | 50 | 2.89 ± 0.54 | 0.408 |
| Adequate | 36 | 2.51 ± 0.44 | 49 | 2.83 ± 0.46 | 32 | 2.99 ± 0.50 | ||||
| TT4 (nmol/L) | Deficiency | 42 | 148 ± 26.8 | 0.402 | 51 | 156 ± 33.4 | 0.432 | 49 | 140 ± 20.3 | 0.075 |
| Adequate | 36 | 141 ± 23.4 | 49 | 149 ± 35.7 | 32 | 151 ± 34.6 | ||||
| fT3 (pmol/L) | Deficiency | 42 | 4.62 ± 0.59 | 0.760 | 51 | 4.31 ± 0.55 | 0.696 | 50 | 4.15 ± 0.57 | 0.898 |
| Adequate | 36 | 4.49 ± 0.68 | 49 | 4.23 ± 0.49 | 32 | 4.15 ± 0.58 | ||||
| fT4 (pmol/L) | Deficiency | 42 | 15.6 ± 1.7 | 0.999 | 51 | 13.4 ± 2.2 | 0.586 | 49 | 12.3 ± 1.5 | 0.552 |
| Adequate | 36 | 15.6 ± 1.8 | 49 | 13.2 ± 2.1 | 32 | 12.7 ± 1.7 | ||||
| TSH (mIU/L) | Deficiency | 42 | 1.63 ± 0.95 | 0.376 | 51 | 2.27 ± 1.05 | 0.062 | 50 | 1.85 ± 0.91 | 0.154 |
| Adequate | 36 | 1.34 ± 0.88 | 49 | 1.79 ± 1.01 | 32 | 1.95 ± 1.06 | ||||
| sTg (µg/L) | Deficiency | 42 | 14.7 ± 17.9 | 0.727 | 51 | 15.8 ± 17.0 | 0.597 | 50 | 12.5 ± 8.7 | 0.617 |
| Adequate | 36 | 13.2 ± 10.8 | 48 | 13.4 ± 11.1 | 32 | 12.9 ± 8.6 | ||||
| TV (mL) | Deficiency | 38 | 8.5 ± 2.9 | 0.447 | 48 | 9.2 ± 2.7 | 0.946 | 43 | 9.4 ± 2.9 | 0.996 |
| Adequate | 29 | 9.8 ± 5.4 | 45 | 9.2 ± 3.8 | 30 | 9.5 ± 2.8 | ||||
Values are expressed as mean ± s.d. N = number of pregnant women evaluated. Epidemiological criteria for assessing iodine nutrition in pregnant women according to World Health Organization (WHO): iodine deficiency UIC < 150 μg/L and iodine adequate UIC > 150 and <249 μg/L. P value for the comparison between iodine deficiency and adequate status in the trimesters. sTg, serum thyroglobulin; TV, thyroid volume; UIC, urinary iodine concentration.
Discussion
In the present study, we support that iodine deficiency is frequent in pregnant women living in an area of adequate iodine consumption. According to WHO recommendation, in our cohort, iodine deficiency is considered mild, with a median of 144 µg/L. Several reports described iodine-deficient pregnant women even in iodine-sufficient areas, despite the existence of an iodization programme, and pregnant women are considered a risk group (3, 12). In Brazil, the salt iodization programme is successful at combating iodine deficiency, but ongoing monitoring is mandatory as Brazil is a continental country with vulnerable regions, such as rural areas and lower economic conditions (11). Recently, the Brazilian government’s salt iodization programme reduced the iodine concentration in salt from 40–60 mg/kg to 15–45 mg/kg based on previous reports pointing to excessive iodine intake (14). Our data were collected before the lower iodine concentration in salt was enacted, reinforcing the high risk of iodine deficiency in pregnant women in the next few years. Brazil also has no iodine supplementation programme for pregnant and lactating women as suggested in recent ATA guidelines, which recommend iodine-containing supplements (3, 12, 15).
Few reports analysed the UIC, thyroid function and TV with sTg in each trimesters of pregnancy. Thyroid hormonal findings here reflect the well-known physiological change: TSH increments, high levels of TT3 and TT4 and trend to lower fT4 and fT3 in the second and third trimesters of pregnancy. No differences in thyroid function were found according to iodine intake as previously described (10, 16).
The sTg is speculated to be a sensitive indicator of both low and excessive iodine intake (17). Zimmermann et al. suggested a median sTg of <13 µg/L and/or <3% of sTg values >40 µg/L indicates an iodine sufficiency in the population (18). Recently, Bath et al. performed sTg in pregnant women and found a negative association between iodine status (measured by the iodine-to-creatinine ratio) and sTg concentrations (10). The sTg was higher in the iodine-deficient group compared to the iodine-sufficient group, especially in later pregnancy. It is noteworthy that 15.7, 8.7 and 18.4% of women had a sTg > 40 µg/L in each trimester, independent of iodine status, and the median sTg was 21, 19 and 23 µg/L, respectively, in each trimester. In our study, the median sTg was 10.8, 11.3 and 11.2 µg/L, respectively, in each trimester, and only 3.3% of pregnant women had a sTg > 40 µg/L (3 patients in the first trimester, 5 in the second and 1 in the third). Therefore, sTg concentrations were not useful to determine iodine status in our cohort.
Thyroid enlargement also is an indicator of insufficient iodine intake, and 10–15% of pregnant women in areas with low iodine intake develop a goitre, besides the physiologic increment due to normal pregnancy (19). In the present study, we did not observe increments in TV during pregnancy or a higher frequency of goitres with no increment of sTg, probably because most women were mild iodine deficient so no differences in TSH concentrations were found. Although we found no difference in TV during pregnancy, TV was positively related with BMI, independent of the trimester. The effect of TSH on thyroid hyperplasia must be discreet since TSH levels remained in the normal range, and BMI was not correlated with iodine status and TSH. Our data indicate that a higher BMI increases TV mostly due to extracellular fluid expansion and blood volume increments that occur during normal pregnancy (20).
In the present paper, a 97% TSH concentration was 4.21 mIU/L, similar to previously described iodine-sufficient pregnant populations, and our frequency of subclinical hypothyroidism was 3.6% when considering a TSH of <4 mIU/L (3).
Our study had several limitations, as a dietary intake of iodine survey was not performed as well as the limited number of pregnant women evaluated in each trimester. Although the median UIC is a well-known indicator of iodine intake for the population, individual intakes are highly variable, and a low UIC on any given day might be found despite adequate daily intakes to maintain thyroidal iodine stores (21). Our study did not evaluate the socioeconomic and educational statuses that are associated with iodine deficiency (11).
Caution in accepting the necessity of iodine supplementation has been expressed, especially in areas where iodized salt is already in use (22). However, our findings reinforce a worldwide concern about the adequacy of the iodine intake of pregnant women. The iodine status evaluation in pregnant women must be reassessed with UIC, as it is the most appropriate method, in regular intervals to assess adequate intake and recommend supplementation if necessary. The Brazilian salt iodization programme prevents iodine deficiency but does not maintain iodine status within an adequate and recommended range for pregnant women, and ongoing vigilance is mandatory after the recent reduction of iodine in table salt (12).
In conclusion, iodine deficiency was found in 52% of pregnant women living in iodine-adequate areas. The sTg concentration and TV were not feasible to use as iodine status markers.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was supported by the Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP 2012/03732-0) and Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES).
Acknowledgements
We would like to thank the staff of Clinic of the Paraisopolis Community (Hospital Albert Einstein Project) for their assistance and Dr Raymundo Soares de Azevedo Neto for the statistical analysis.
References
- 1.Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutrition 2007. 10 1542–1546. ( 10.1017/S1368980007360886) [DOI] [PubMed] [Google Scholar]
- 2.de Escobar GM, Obregón MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutrition 2007. 10 1554–1570. ( 10.1017/S1368980007360928) [DOI] [PubMed] [Google Scholar]
- 3.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman W, Laurberg P, Lazarus JH, Mandel SJ, et al 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017. 27 315–389. ( 10.1089/thy.2016.0457) [DOI] [PubMed] [Google Scholar]
- 4.Brander L, Als C, Buess H, Haldimann F, Harder M, Hänggi W, Herrmann U, Lauber K, Niederer U, Zürcher T, et al Urinary iodine concentration during pregnancy in an area of unstable dietary iodine intake in Switzerland. Journal of Endocrinological Investigation 2003. 26 389–396. ( 10.1007/BF03345192) [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, United Nations Children’s Fund & Disorders ICfCoID. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination: A Guide for Programme Managers. Genova, Switzerland: WHO Press, 2007. [Google Scholar]
- 6.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 2011. 21 419–427. ( 10.1089/thy.2010.0077) [DOI] [PubMed] [Google Scholar]
- 7.Berghout A, Wiersinga W. Thyroid size and thyroid function during pregnancy: an analysis. European Journal of Endocrinology 1998. 138 536–542. ( 10.1530/eje.0.1380536) [DOI] [PubMed] [Google Scholar]
- 8.Ma ZF, Skeaff SA. Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid 2014. 24 1195–1209. ( 10.1089/thy.2014.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Carlé A, Pedersen IB, Rasmussen LB, Ovesen L, Jørgensen T. Thyroglobulin as a marker of iodine nutrition status in the general population. European Journal of Endocrinology 2009. 161 475–481. ( 10.1530/EJE-09-0262) [DOI] [PubMed] [Google Scholar]
- 10.Bath SC, Pop VJ, Furmidge-Owen VL, Broeren MA, Rayman MP. Thyroglobulin as a functional biomarker of iodine status in a cohort study of pregnant women in the United Kingdom. Thyroid 2017. 27 426–433. ( 10.1089/thy.2016.0322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ReO Campos, Reboucas SC, Beck R, de Jesus LR, Ramos YR, IoS Barreto, Marques TX, Cerqueira TL, Santos WA, Oliveira CA, et al Iodine nutritional status in schoolchildren from public schools in Brazil: a cross-sectional study exposes association with socioeconomic factors and food insecurity. Thyroid 2016. 26 972–979. ( 10.1089/thy.2015.0448) [DOI] [PubMed] [Google Scholar]
- 12.Ferreira SM, Navarro AM, Magalhães PK, Maciel LM. Iodine insufficiency in pregnant women from the State of São Paulo. Arquivos Brasileiros de Endocrinologia e Metabologia 2014. 58 282–287. ( 10.1590/0004-2730000002979) [DOI] [PubMed] [Google Scholar]
- 13.Carvalho AL, Meirelles CJ, Oliveira LA, Costa TM, Navarro AM. Excessive iodine intake in schoolchildren. European Journal of Nutrition 2012. 51 557–562. ( 10.1007/s00394-011-0239-7) [DOI] [PubMed] [Google Scholar]
- 14.Medeiros-Neto G. Iodine nutrition in Brazil: where do we stand? Arquivos Brasileiros de Endocrinologia e Metabologia 2009. 53 470–474. ( 10.1590/S0004-27302009000400014) [DOI] [PubMed] [Google Scholar]
- 15.ReO Campos, IoS Barreto, Maia LR, Rebouças SC, Cerqueira TL, Oliveira CA, Santos CA, Mendes CM, Teixeira LS, Ramos HE. Iodine nutritional status in Brazil: a meta-analysis of all studies performed in the country pinpoints to an insufficient evaluation and heterogeneity. Archives of Endocrinology and Metabolism 2015. 59 13–22. ( 10.1590/2359-3997000000004) [DOI] [PubMed] [Google Scholar]
- 16.Anaforoğlu İ, Algün E, İnceçayır Ö, Topbaş M, Erdoğan MF. Iodine status among pregnant women after mandatory salt iodisation. British Journal of Nutrition 2016. 115 405–410. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann MB, Aeberli I, Andersson M, Assey V, Yorg JA, Jooste P, Jukić T, Kartono D, Kusić Z, Pretell E, et al Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 μg/L: a UNICEF/ICCIDD study group report. Journal of Clinical Endocrinology and Metabolism 2013. 98 1271–1280. ( 10.1210/jc.2012-3952) [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann MB. The importance of adequate iodine during pregnancy and infancy. World Review of Nutrition and Dietetics 2016. 115 118–124. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann MB. Iodine deficiency. Endocrine Reviews 2009. 30 376–408. ( 10.1210/er.2009-0011) [DOI] [PubMed] [Google Scholar]
- 20.Vannucchi G, Covelli D, Vigo B, Perrino M, Mondina L, Fugazzola L. Thyroid volume and serum calcitonin changes during pregnancy. Journal of Endocrinological Investigation 2017. 40 727–732. ( 10.1007/s40618-017-0622-1) [DOI] [PubMed] [Google Scholar]
- 21.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. Journal of Nutrition 2011. 141 2049–2054. [DOI] [PubMed] [Google Scholar]
- 22.Menéndez Torre E, Delgado Alvarez E, Rabal Artal A, Suárez Gutiérrez L, Rodríguez Caballero MG, Ares Blanco J, Díaz Naya L, Fernández Fernández JC. Iodine nutrition in pregnant women from Oviedo area. Is iodine supplementation necessary? Endocrinología y Nutrición 2014. 61 404–409. [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a