Abstract
The action mechanism of long non-coding ribonucleic acid-homeobox transcript antisense ribonucleic acid (lncRNA-HOTAIR) in the regulation of the Wnt signaling pathway on the drug resistance of non-small cell lung cancer was investigated. Forty eight patients with non-small cell lung cancer, who were treated with cisplatin (DDP) as neoadjuvant chemotherapy, were selected from the specimen bank of the Department of Pathology of Peking Union Medical College Hospital. Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the messenger RNA (mRNA) level of lncRNA-HOTAIR in cancer and cancer-adjacent tissues. The correlation curve of the expression of lncRNA-HOTAIR with the overall survival (OS) was plotted using the Kaplan-Meier method. NCI-H1299 DDP-resistant cell lines were constructed, and the half maximal inhibitory concentration (IC50) value was measured. The expression of lnc-HOTAIR in NCI-H1299/DDP cells was detected by the target interference of small interfering RNA (siRNA). The effect of si-HOTAIR on cell resistance was detected by Cell Counting Kit-8 (CCK-8). Western blot analysis was used to detect the effects of si-HOTAIR on multidrug resistance proteins, multidrug resistance-associated protein 1 (MRP1) and multidrug resistance 1 (MDR1), and Wnt signaling pathways, Wnt3a, adenomatous polyposis coli (APC) and β-catenin. The mRNA level of lncRNA-HOTAIR in cancer tissues was significantly higher than that in cancer-adjacent tissues (P<0.05), and the high expression of lncRNA-HOTAIR indicated that the OS of patients was shortened (P<0.05). The IC50 of NCI-H1299/DDP cells inhibiting DDP was 127.82 µM, which was significantly higher than that of parental NCI-H1299 cells (IC50=8.40 µM) (P<0.05). si-HOTAIR interference significantly decreased the sensitivity of cells to DDP, the IC50 of cells was decreased from 131.85 to 44.34 µM (P<0.05), the expression levels of MRP1 and MDR1 were significantly decreased, and the activation of Wnt signaling pathway was significantly inhibited (P<0.05). Thus, lncRNA-HOTAIR plays an important role in the occurrence and development of non-small cell lung cancer, and it may be an important factor in the clinical prognosis of patients with non-small cell lung cancer.
Keywords: lncRNA-HOTAIR, drug resistance, non-small cell lung cancer, Wnt signaling pathway
Introduction
Lung cancer is the most common cancer with the highest mortality rate worldwide, and it is estimated that there are 1.2 million new cases and 1.1 million deaths worldwide annually (1,2). Non-small cell lung cancer accounts for 80% of lung cancer cases (3).
For patients with advanced (Stage IIIB or IV) lung cancer who cannot undergo surgical resection, chemotherapy effectively prolongs their overall survival (OS) despite poor prognosis, in which the treatment with platinum-based compounds, cisplatin (DDP) or carboplatin, combined with gemcitabine, vinorelbine or taxane (paclitaxel or texotere) is the initial therapy of clinical non-small cell lung cancer. The International Adjuvant Lung Cancer Trial (IALT) showed that the 3-year survival rate of patients treated with DDP adjuvant chemotherapy was 41%, and the 5-year OS rate was 15% (4,5). However, the resistance of tumor cells to platinum drugs is the leading cause of clinical failure (6,7). Therefore, how to overcome drug resistance in order to improve the clinical efficacy of lung cancer patients is imperative.
Patients and methods
Collection of clinicopathological data
Fourty eight paired non-small cell lung cancer and cancer-adjacent tissues were selected from specimens receiving biopsy or surgical resection in the Department of Pathology of Peking Union Medical College Hospital (Beijing, China)from September, 2012 to May, 2014, and all specimens were diagnosed and confirmed by pathologists. The tested patients underwent cisplatin-based neoadjuvant chemotherapy at least 4 times prior to surgery as follows: PC (100 mg/m2 paclitaxel + 125 mg/m2 cisplatin; once every 3 weeks) or texotere + P (75 mg/m2 texotere + 125 mg/m2 cisplatin; once every 3 weeks). The mean age of the patients was 61.43±12.3 years, and all study subjects signed the informed consent. The study was approved by the Ethics Committee of Peking Union Medical College Hospital.
Reverse transcription-polymerase chain reaction (RT-PCR)
The total ribonucleic acid (RNA) was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA), and 1 µg RNA was reverse transcribed using the GenpCopoeia Reverse Transcription kit (GeneCopoeia, Inc., Guangzhou, China). RT-PCR was conducted at an annealing temperature of 55°C, and the amplification was repeated for 45 cycles using an SYBR® Premix Ex Taq™ II kit (Biomedical Technology Co., Ltd., Chendgu, China). The relative quantification of each gene was analyzed by 2−∆∆Cq with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal reference for correction. The relative expression level of messenger RNA (mRNA) of each index was calculated as: 2−∆∆Cq [∆∆Cq = Cq (target gene) - Cq (GAPDH)]. Primer sequences used were, long non-coding ribonucleic acid-homeobox transcript antisense ribonucleic acid (lncRNA-HOTAIR): forward, 5′-CGGAGTGAGTTTATCGCAG-3′, reverse, 5′-GGCGACCGGAGCTCATCTTACC-3′; GAPDH: forward 5′-ATTGATGGATGCTAFGAGTATT-3′, reverse, 5′-AGTCTTCTGGGTGGCAGTGAT-3′.
Cell culture
Human lung adenocarcinoma NCI-H1299 cell line was purchased from the Cell Bank, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. NCI-H1299/DPP was the DPP-resistant cell line constructed by our team (DDP with the final concentration of 100 µM maintained the drug resistance). The two cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% fetal bovine serum and 100 U/ml penicillin and streptomycin. The cell culture flask was incubated at 37°C with 5% CO2.
Detection of the sensitivity of cells to DDP by Cell Counting Kit-8 (CCK-8)
Cells were seeded in 96-well plates at the density of 1×104/ml, and the cells completely adhered to the wall 24 h later. Each well was added with 100 µl DDP at a final concentration of 0.1, 1, 10, 100 and 1,000 µM for incubation for 48 h, respectively. CCK-8 reagent (10 µl) (Biotool, Shanghai, China) was added to each well and incubated for 1 h in an incubator at 37°C. The optical density (OD) of each well at 450 nm was then measured using a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Target interference of small interfering RNA (siRNA) on lncRNA-HOTAIR
HOTAIR siRNA and negative control siRNA sequences were obtained from inernational high-scoring journals and were produced by Qiagen (Shanghai, China). Cells were transfected with Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols, and 48 h later, HOTAIR expression levels were significantly inhibited. The sequence of targeted interference with lncRNA-HOTAIR is as follows: 5′-UUUUCUACCAGGUCGGUAC-3′.
Western blot analysis
The cells in the drug treatment group and the control group were seeded in 6-well plates, and an appropriate amount of protease inhibitors and protein lysate were added. The cell lysate was aspirated and centrifuged at 12,000 × g at 4°C for 30 min. Total protein (40 µg) was electrophoresed in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) membrane and then transferred to a polyvinylidene difluoride (PVDF) membrane. The respective bands were cut according to the marker and incubated overnight with rabbit anti-human primary monoclonal antibodies including multidrug resistance associated protein (anti-MRP1), anti-Wnt3a, adenomatous polyposis coli (anti-APC), anti-β-catenin and anti-GAPDH (dilution,1:800; cat. nos. ab180960; ab172612; ab40778; ab32572 and ab181602, respectively; Abcam, Cambridge, UK) and rabbit anti-human multidrug resistance 1 (MDR1) polyclonal antibody (1:600; cat. no. 13978; Cell Signaling Technology, Danvers, MA, USA). Then it was incubated with the secondary antibody [goat anti-rabbit IgG (1:500; cat. no. ab6721, Abcam, Cambridge, UK)]. The membrane was visualized with an enhanced chemiluminescence (ECL) detection system (Bio-Rad lab, Hercules, CA, USA), and the gray scale analysis was performed using a gel analyzer. The relative content of the target protein was the ratio of the target protein to the gray value of corresponding internal reference bands.
Statistical analysis
The experimental results were analyzed using GraphPad Prism software (version 5.01; GraphPad Software, San Diego, CA, USA). The ANOVA test and Tukey post hoc test was used for intergroup comparisons. The Kaplan-Meier method was used to plot the patient's survival curve and examined using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of the expression level of lncRNA-HOTAIR in cancer and cancer-adjacent tissues
We extracted mRNAs from 48 pairs of lung cancer and cancer-adjacent tissues of patients and detected the expression level of lncRNA-HOTAIR. The results showed that the expression level of lncRNA-HOTAIR in cancer tissues was significantly higher than that in cancer-adjacent tissues (difference: 3.89-fold) (P<0.05) (Fig. 1).
Figure 1.
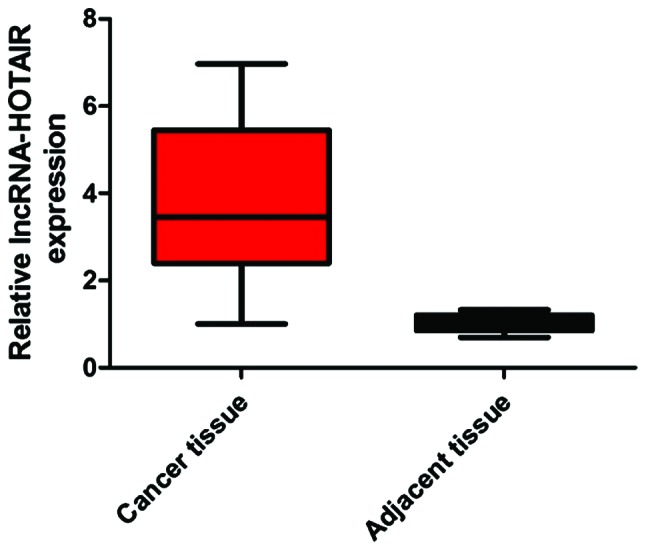
Detection of the expression level of lncRNA-HOTAIR mRNA in cancer and cancer-adjacent tissues of patients.
Expression level of lncRNA-HOTAIR and the OS of patients
The correlation curve of the expression of lncRNA-HOTAIR with the OS of patients was plotted using the Kaplan-Meier method. The results indicated that compared with the lncRNA-HOTAIR low expression group, the high expression represented shorter OS of patients (Fig. 2). Median survival time: lncRNA-HOTAIR low expression group vs. lncRNA-HOTAIR high expression group = 23 months vs. 17 months (log-rank test, P=0.0124).
Figure 2.
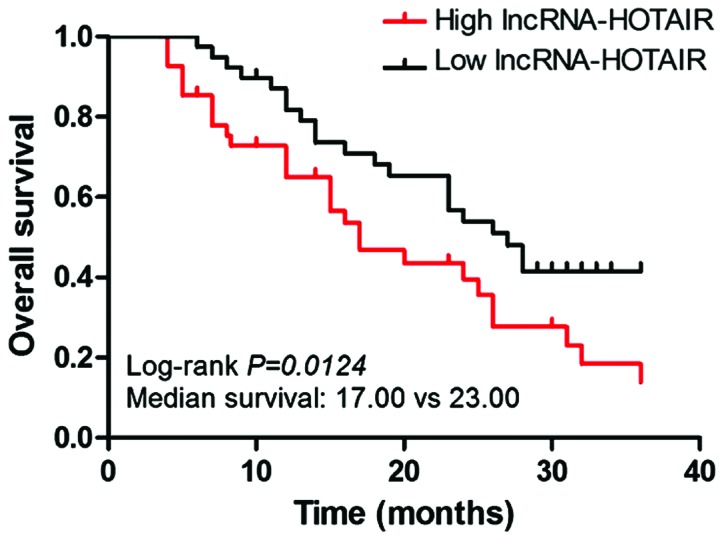
The relationship between the expression level of lncRNA-HOTAIR mRNA and the overall survival rate.
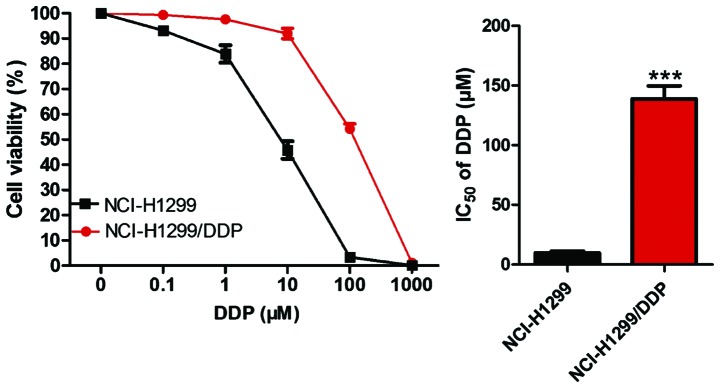
Construction and detection of drug-resistant cell lines
NCI-H1299 cells in the logarithmic growth phase were taken, and after the reaction with DDP for 48 h, the culture medium and dead cells were discarded. The cells were incubated in the normal cell culture medium until they grew to the logarithmic growth phase, and then the cells were incubated for 48 h after the drug concentration was elevated. The initial drug concentration was 0.1 µM, gradually increased to 100 µM with 10 µM each time, and then gradually increased to 1,000 µM with 100 µM each time. Through the progressive increase of the drug concentration and repeated intermittent induction, 4 months later, the human non-small cell lung cancer DDP-resistant cell line, named NCI-H1299/DDP, was obtained. The sensitivity of the NCI-H1299/DDP drug-resistant cell line and NCI-H1299 parental cells to cisplatin was detected by CCK-8 assay. As shown in Fig. 3, the half maximal inhibitory concentration (IC50) of NCI-H1299 parental cells was 8.40 µM, while that of NCI-H1299/DDP cells was 127.82 µM, which was significantly higher than that of parental cells (P<0.05).
Figure 3.
Detection of IC50 of parental cells and drug-resistant cells by CCK-8. ***P<0.001, compared with NC1-H1299.
Detection of the expression level of lncRNA-HOTAIR in cells by RT-PCR
We used RT-PCR to detect the mRNA levels of lncRNA-HOTAIR in normal lung fibroblast MRC-5, lung cancer parental NCl-H1299 and DDP-resistant NCl-H1299/DDP cells (Fig. 4).
Figure 4.

Detection of the expression level of lncRNA-HOTAIR in normal lung fibroblast, lung cancer parental and drug-resistant cells by RT-PCR. *P<0.05, ***P<0.001, compared with MRC-5.
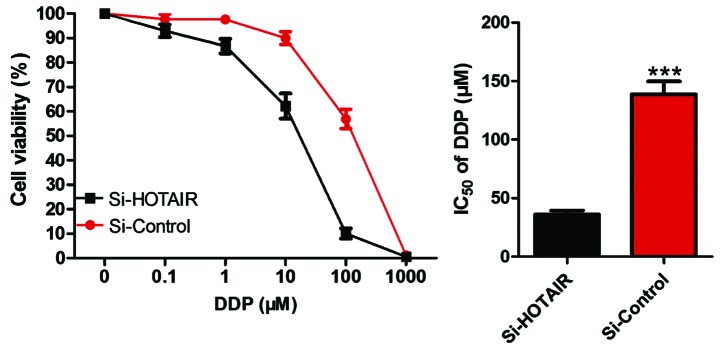
Detection of the effect of si-HOTAIR on IC50 of drug-resistant cell lines by CCK-8
After NCI-H1299/DDP cells were transfected by si-HOTAIR, CCK-8 was used to determine whether the sensitivity of cells to DDP was altered. The results (Fig. 5) revealed that compared with that in the control group, si-HOTAIR significantly decreased the IC50 value of NCI-H1299/DDP to DDP, and si-HOTAIR vs. si-control, i.e., 44.34 µM vs. 131.85 µM (P<0.05).
Figure 5.
Detection of the effect of si-HOTAIR on the IC50 of drug-resistant cell lines by CCK-8. ***P<0.001, compared with si-control.
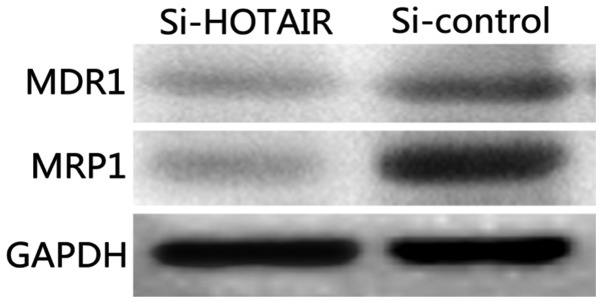
Detection of the effect of si-HOTAIR on drug-resistant proteins and proliferation indexes by western blot analysis
Classical multidrug-resistant proteins, MDR1 and MRP1, were selected as the detection indexes to analyze the effect of lncRNA-HOTAIR on the drug resistance of NCI-H1299/DDP cells. The results (Fig. 6) show that the protein levels of MDR1 and MRP1 in Si-HOTAIR cells were significantly decreased compared with those in the si-control group (P<0.05).
Figure 6.
Detection of the effect of si-HOTAIR on drug-resistant proteins by western blot analysis.
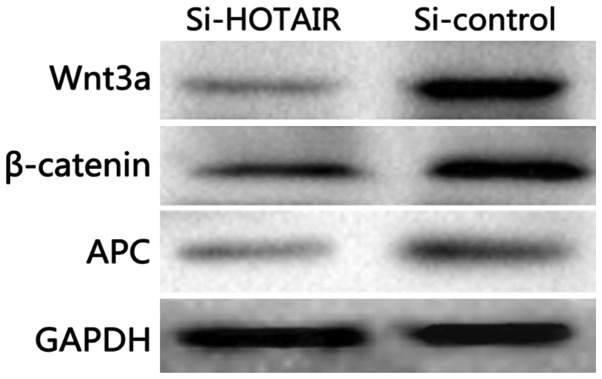
Detection of the effect of si-HOTAIR on the Wnt signaling pathway by western blot analysis
As shown in Fig. 7, compared with those in the si-control group, si-HOTAIR significantly decreased the protein expression levels of Wnt3a, β-actin and APC in NCI-H1299/DDP cells (P<0.05) and inhibited the activation of Wnt/β-catenin signaling pathway.
Figure 7.
Detection of the effect of si-HOTAIR on the Wnt signaling pathway by western blot analysis.
Discussion
Drug resistance is caused by genetic variation in patient's tumor cells (8). Previous reports have shown that the dormancy of cancer stem cells and epithelial-mesenchymal transition are associated with chemotherapy resistance (9); a common mechanism for obtaining resistance is the energy-dependent translocator expression, accelerated cell excretion of anticancer drugs, induction of drug detoxification and reduced susceptibility to apoptosis signals (10). Although the design of cancer chemotherapy becomes more and more complex, few drugs can avoid the emergence of drug resistance. Recent evidence suggests that lncRNA plays a key role in tumor resistance (11).
lncRNAs regulate gene expression and chromatin structure in the following ways: i) Bait effect: Altering its function with other RNAs and proteins (12); ii) Scaffold effect: Modifying chromatin so as to regulate protein and DNA junctions to form signals (13); and iii) post-transcriptional effect: Forming RNA dimers with mRNA sequences to block transcription-related sites, thus regulating the stability, cleavage and translation of protein-coding genes (14). lncRNA can silence or activate a gene or gene family by altering the chromatin structure. According to recent studies, lnc-HOTAIR, as a modular scaffold, assembles molecules and specific enzymes, which can regulate the combination of target genes. Further studies have shown that the chromatin of recombined HOTAIR enhances the invasion of pancreatic cancer cells, inhibits cell growth, regulates cell cycle progression and induces apoptosis in vitro. In addition, it enhances the transfer potential of liver, breast and gastric cancer cells (15,16).
The classical Wnt signaling pathway is involved in the development of the human body and various tumors (17). Among them, the release of a key protein of the Wnt signaling pathway, β-catenin is usually degraded by phosphorylation and targeted destruction of the Axin-APC-GSK3β-β-catenin complex. Subsequently, β-catenin migrates into the nucleus and binds to the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription site to stimulate the transcription of the Wnt target gene, thus affecting tumor cell proliferation, invasion and metastasis as well as drug sensitivity (18,19).
In this study, it was found that the expression of lnc-HOTAIR in lung cancer tissues of patients receiving neoadjuvant chemotherapy was significantly higher than that in cancer-adjacent tissues, and the high expression of lnc-HOTAIR was significantly correlated with poor prognosis. The result was consistent with the study of Bhan and Mandal, indicating that the, high expression of HOTAIR is closely related to breast cancer metastasis (20). Therefore, DDP-resistant cell lines were constructed, and the comparison between normal lung fibroblast cells and parental cells showed that the expression level of lnc-HOTAIR in drug-resistant cell lines was the highest. Then we used siRNA interference technology to silence lnc-HOTAIR expression, and interestingly NCI-H1299/DDP drug sensitivity was significantly improved, while the Wnt signaling pathway and resistance indexes were decreased significantly. In summary, we originally analyzed the expression of lnc-HOTAIR in cells of patients with lung cancer DDP resistance and found that the Wnt signaling pathway played a key role. Although the mechanism of lnc-HOTAIR specific control of chromosomes and targets has not yet been clarified, our experiments provide a potential treatment for lung cancer patients with a certain reference value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
FG wrote the manuscript and performed the cell culture. ZC performed and analyzed RT-PCR. HG analyzed and interpreted CCK-8 test. SL helped with western blot analysis and the conception of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (Beijing, China). All patients signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hill C. Cancer prevention and screening. Bull Cancer. 2013;100:547–554. doi: 10.1684/bdc.2013.1770. (In French) [DOI] [PubMed] [Google Scholar]
- 2.Dearing KR, Sangal A, Weiss GJ. Maintaining clarity: Review of maintenance therapy in non-small cell lung cancer. World J Clin Oncol. 2014;5:103–113. doi: 10.5306/wjco.v5.i2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, Govindan R, et al. NCCN (National Comprehensive Cancer Network): Non-small cell lung cancer. J Natl Compr Cancer Netw. 2012;10:1236–1271. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger DS, Akerley W, Borghaei H. Elemene increases autophagic apoptosis and drug sensitivity in human cisplatin (DDP)-resistant lung cancer cell line SPC-A-1/DDP by inducing Beclin-1 expression. Oncol Res Featuring Preclinical Clin Cancer Ther. 2017;4:502–510. doi: 10.3727/096504017X14954936991990. [DOI] [PubMed] [Google Scholar]
- 5.Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu Y, Feng J. Exosomes: Decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS One. 2014;9:e89534. doi: 10.1371/journal.pone.0089534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen W, Liang B, Yin J, Li X, Cheng J. Noscapine increases the sensitivity of drug-resistant ovarian cancer cell line SKOV3/DDP to cisplatin by regulating cell cycle and activating apoptotic pathways. Cell Biochem Biophys. 2015;72:203–213. doi: 10.1007/s12013-014-0438-y. [DOI] [PubMed] [Google Scholar]
- 7.Tang T, Song X, Liu YF, Wang WY. PEITC reverse multi-drug resistance of human gastric cancer SGC7901/DDP cell line. Cell Biol Int. 2014;38:502–510. doi: 10.1002/cbin.10169. [DOI] [PubMed] [Google Scholar]
- 8.Pastan I, Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med. 1987;316:1388–1393. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- 9.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 10.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Zhang J, Shi J, Guo Z, He C, Ding L, Tang JH, Hou Y. Role of long non-coding RNA in tumor drug resistance. Tumour Biol. 2016;37:11623–11631. doi: 10.1007/s13277-016-5125-8. [DOI] [PubMed] [Google Scholar]
- 12.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Yu B, Li J, Su L, Yan M, Zhu Z, Liu B. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Zhou X, Xu Y. Assessment of varied long noncoding RNA and messenger RNA expression levels in adolescent idiopathic scoliosis. Int J Clin Exp Med. 2016;5:8031–8038. [Google Scholar]
- 16.Furió-Tarí P, Tarazona S, Gabaldón T, Enright AJ, Conesa A. spongeScan: A web for detecting microRNA binding elements in lncRNA sequences. Nucleic Acids Res. 2016;44:W176–W180. doi: 10.1093/nar/gkw443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 19.Fifis T, Tran BM, Schwab RHM, Johanson TM, Warner N, Barker N, Vincan E. Wnt signaling regulation of tissue architecture (EMT and MET) and morphogenesis. In: Hoppler S, Moon RT, editors. Wnt Signaling in Development and Disease: Molecular Mechanisms and Biological Functions. John Wiley & Sons, Inc.; 2014. pp. 315–328. [DOI] [Google Scholar]
- 20.Bhan A, Mandal SS. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856:151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.