Abstract
Nucleobindin 2 (NUCB2) is mainly expressed in the hypothalamic nuclei and has a proven role in energy homeostasis. It has also been recently reported to have a key role in tumor progression. However, the clinical significance of NUCB2 in colorectal cancer (CRC) remains unknown. In the present study, the level of NUCB2 mRNA was quantified by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in 34 paired fresh tissues from patients with CRC. RT-qPCR was followed by immunohistochemical (IHC) staining of NUCB2 protein in tissue microarrays of 251 samples to evaluate the clinical significance of NUCB2 in CRC. The RT-qPCR indicated an upregulation of NUCB2 mRNA in CRC tissues compared with normal tissues (P=0.027). IHC staining indicated a positive association between elevated NUCB2 expression and lymph node metastasis or tumor-node-metastasis (TNM) stage. Patients with CRC and lymph node metastasis demonstrated a higher expression of NUCB2 (49.5%, 50/101) compared with those without lymph node metastasis (36.7%, 55/150; P=0.043). Furthermore, NUCB2 expression was also higher in patients with CRC and TNM stage III–IV compared with those with TNM stage I–II (50.9% vs. 35.0%; P=0.011). However, Kaplan-Meier analysis indicated no significant association between NUCB2 expression and disease-free survival of patients. Additionally, multivariate analysis did not identify the upregulation of NUCB2 as an independent prognostic predictor in patients with CRC (P=0.755). In conclusion, the present study demonstrated that upregulation of NUCB2 is significantly associated with CRC metastasis, indicating that NUCB2 may be a cancer-associated oncogene associated with the aggressive progression of CRC.
Keywords: nucleobindin 2, metastasis, cancer progression, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer type, and it is also the third highest cause of cancer-associated mortalities globally (1). Of all cases of CRC, ~20–25% are metastatic and 50–60% of the remainder eventually develop metastases as per statistical estimation (2,3). Therefore, it is important to identify novel CRC biomarkers to enhance the efficiency of early diagnosis and improve therapeutic strategies.
Nucleobindin 2 (NUCB2), a precursor of the hypothalamic neuropeptide nesfatin-1, is mainly expressed in the hypothalamic nuclei, and it has specific roles in energy homeostasis (4). It is reportedly distributed in the central nervous system, gastrointestinal system, reproductive organs and adipose tissue (5). Additionally, NUCB2 has been indicated to serve an important role in cancer progression and metastasis (6–10). High levels of NUCB2 mRNA and protein are associated with shorter recurrence-free survival time in prostate cancer (6,7) and increased migration of prostate cancer cells compared with low expression of NUCB2 (8). High expression of NUCB2 is also associated with metastasis and reduced overall survival in clear cell renal cell carcinoma (9). In addition, NUCB2 has been reported to be a potent prognostic factor for primary breast carcinoma as it is associated with its metastasis (10).
These findings strongly indicate that NUCB2 could serve a vital role in inducing metastasis in various types of cancer. However, a number of studies have also demonstrated that NUCB2 inhibited the proliferation of human adrenocortical carcinoma and ovarian epithelial carcinoma cells (11,12). These conflicting results point towards a possible tissue-specific regulatory function of NUCB2. Kan et al (8) indicated that NUCB2 promoted the migration, invasion and epithelial-mesenchymal transition (EMT) CRC cells in vitro and in vivo. Nevertheless, the exact underlying mechanism of NUCB2's action in CRC remains unclear. In the present study, the expression levels of NUCB2 mRNA and protein were analyzed in CRC tissues and adjacent non-cancerous tissues via reverse transcription-quantitation polymerase chain reaction (RT-qPCR) and immunohistochemistry (IHC), respectively. The association between NUCB2 expression and the clinicopathological parameters of CRC was also evaluated to determine its clinical significance.
It was predicted that utilizing a larger sample size would generate more consistent data that would help improve the understanding of the role of NUCB2 in the pathological progression of CRC.
Materials and methods
Patients and tissue samples
The project was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China), and each patient was required to provide written informed consent. Samples of cancerous and adjacent non-cancerous colorectal tissue were collected between January 2010 and December 2010 from 34 patients (age range 45–68 years, mean age 55.7 years), comprising of 19 males and 15 females) with CRC admitted to the First Affiliated Hospital, Zhejiang University School of Medicine.
Tissue microarrays (TMA) with 251 CRC paraffin-embedded specimens were purchased from Shanghai Biochip Co. Ltd. (Shanghai, China), and were used for detection of NUCB2 expression by IHC staining. The TMAs included samples from 138 males and 112 females, and 1 of unknown sex, with a median age of 66 years (range, 27–91) at the time of operation. Tumor node metastasis (TNM) stage was classified using AJCC cancer staging manual (13). All patients were followed-up for >5 years following surgery, and the survival time was calculated from the date of surgery until the deadline for follow-up, or until the date of mortality. An additional 30 samples of normal colorectal tissues were also collected from patients with CRC between January 2014 and December 2014 (16 males and 14 females, age range 38–70 years, mean age 58.6 years) at the First Affiliated Hospital, Zhejiang University School of Medicine (Zhejiang, China).
RT-qPCR
Total RNA was extracted from fresh tissues using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the concentration was determined using Nanodrop™ 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). The mRNA samples were stored at −80°C. RT-qPCR was performed with iTaq™ Universal One-Step RT-qPCR kit (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer's protocol. The NUCB2 primers used were: Forward, 5′-TCTTGGAGCCAGATAGCTGG-3′ and reverse, 5′-AGCTTCTGAGCCTCCAGTTG-3′. GAPDH was used as an internal control with the following primers: Forward, 5′-TGAAGGTCGGAGTCAACGG-3′ and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′. All primers were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The following cycling conditions were used: 10 min at 50°C for cDNA synthesis plus 1 min at 95°C for reverse transcription inactivation and Taq polymerase activation, followed by 35 cycles of 10 sec at 95°C denaturation and 30 sec at 58°C for extension and data collection. The NUCB2 mRNA level was calculated relative to the internal GAPDH control with the ΔΔCq method (14). For each sample, the PCR was run in duplicates every time and was repeated for a total of three times.
Haematoxylin and eosin staining
Sections were stained with haematoxylin and eosin (H&E) prior to IHC staining. First, sections were de-paraffinized with xylene, rehydrated in a graded alcohol series (100, 95 and 75%), and then washed briefly in distilled water and stained with hematoxylin for 8 min at room temperature. Following this, the sections were washed in running tap water for 5 min and differentiated in 1% acid alcohol for 30 sec at room temperature. The sections were washed in running tap water for 5 min and counterstained in eosin-phloxine solution for 30 sec to 1 min at room temperature. Then the sections were dehydrated and mounted.
IHC staining
Tissue were fixed by 10% formaldehyde for 24 h at room temperature, and TMA sections was cut to 3–4 µm thicknesses and then used for IHC. Briefly, TMA sections were first de-paraffinized with xylene, rehydrated in graded alcohol (100, 95, 85 and 75% at room temperature) and autoclaved for 3 min in 0.01 M citrate buffer (pH 6.0) for antigen retrieval. The sections were then incubated with 3% (v/v) H2O2 for 10 min at room temperature in order to block endogenous peroxidase. This was followed by incubation with 10% (v/v) normal goat serum (Thermo Fisher Scientific, Inc.) for 15 min at room temperature to reduce nonspecific binding. Subsequently, the slides were incubated overnight with rabbit polyclonal antibody against human NUCB2 (dilution, 1:1,000; catalog no. HPA008395; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) at 4°C. Following rinsing with phosphate-buffered saline (PBS) three times, the sections were incubated with biotin-labeled secondary antibody contained within the Histostain-Plus kit (HRP, Broad Spectrum) for IHC staining (ready-to-use, 859043; Thermo Fisher Scientific, Inc.) at room temperature for 20 min, followed by horseradish peroxidase-conjugated goat anti-rat antibody (ready-to-use, 859043; Thermo Fisher Scientific, Inc.) for an additional 20 min at room temperature. Finally, the sections were stained with 3,3-diaminobenzidine for 3 min at room temperature, counterstained with hematoxylin, dehydrated with graded alcohol (75, 85, 95 and 100%) and then mounted. For the negative control, PBS was used instead of the primary antibody.
Evaluation of IHC staining
The degree of immunostaining was semi-quantitatively evaluated by two independent expert pathologists who were blinded to the clinical data. The pathologists scored number of positively stained cells per field under a light microscope and viewed five fields under ×200 magnification. The level of NUCB2 expression was calculated on the basis of the intensity of staining and the percentage of positively stained cells. The staining intensity was graded according to the following criteria: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. To determine the percentage of stained cells, the number of stained and unstained cells was counted per field under ×200 magnification, and the average of five fields was calculated. Based on the percentage of positive cells, the tumor score was graded as follows: 0, ≤5% positively stained tumor cells; 1, 6–25% positive tumor cells; 2, 26–50% positive tumor cells; and 3, ≥51% positive tumor cells. The staining index was calculated by multiplying the staining intensity score with the percentage of positive cells. For the final evaluation, a staining index score of ≤3 was defined as low NUCB2 expression, and a staining index score of ≥4 was defined as high NUCB2 expression.
NUCB2 staining in the tumor cells was also quantified as integrated optical density (IOD) by estimating the area of the objects and medium pixel intensity per object using the Image-Pro Plus software version 6 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA) was used to perform all statistical analyses. For quantitative values with normal distribution, the paired-sample Student's t-test was used for comparison, and if the values were not distributed normally, the Wilcoxon Sign Rank test was used to compare two groups of paired values. χ2 or Fisher's exact test was used to analyze categorical data and evaluate the associations between the expression of NUCB2 and the clinicopathological parameters of CRC. The Kaplan-Meier method was used to perform univariate survival analysis, and the log-rank test was used to calculate differences between the survival curves. Multivariate survival analysis and Cox proportional hazards regression model were used to assess predictors associated with prognosis. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of NUCB2 mRNA expression level
To detect NUCB2 mRNA expression level, a total of 34 paired fresh CRC specimens and their surrounding normal mucosal tissues were analyzed using RT-qPCR. NUCB2 upregulation was defined when the NUCB2 mRNA level was higher in the cancer tissue compared with the non-cancerous tissue from the same patient. Conversely, downregulation of NUCB2 was defined when a lower NUCB2 expression was detected in the cancer tissue compared with the non-cancerous tissue.
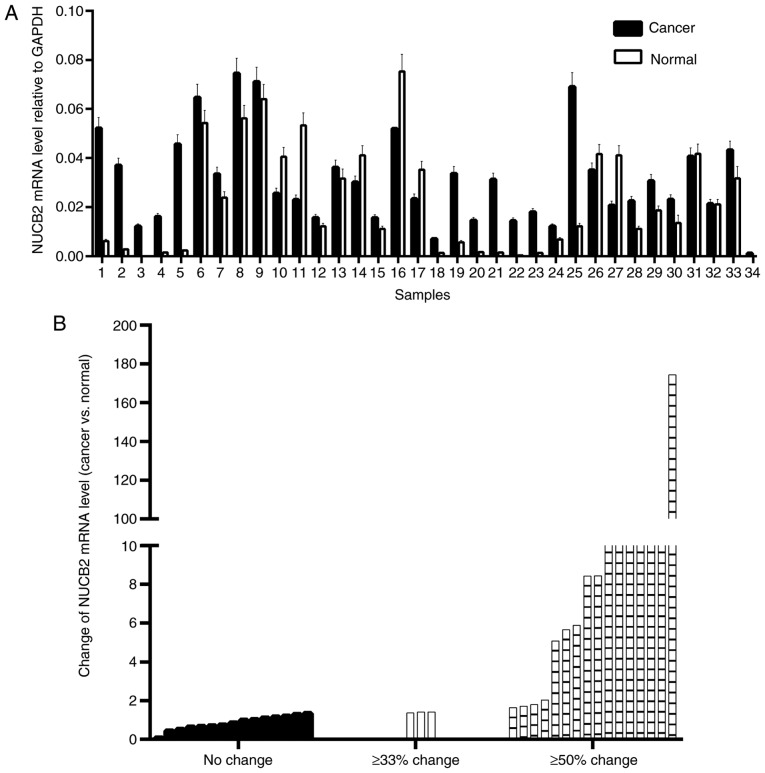
NUCB2 mRNA levels were upregulated in 73.5% of CRC (25/34) samples and downregulated in the remaining 26.5% of samples (9/34) (Fig. 1A). Paired Student's t-test demonstrated that the mean level of NUCB2 mRNA was upregulated in CRC tissues compared with the normal tissues (P=0.018). Depending on the extent of the relative change in NUCB2 mRNA level in the cancer tissues, the samples were divided into three groups (no change, ≥33% change and ≥50% change) and nearly half of the samples indicated a ≥50% change (Fig. 1B).
Figure 1.
Gene expression of NUCB2 in CRC and adjacent normal tissues. (A) The relative mRNA levels of NUCB2 normalized to GAPDH in 34 paired specimens. (B) NUCB2 mRNA level normalized to GAPDH in CRC compared with normal tissues. CRC, colorectal cancer; NUCB2, nucleobindin 2.
Association of NUCB2 expression with clinicopathological features of CRC
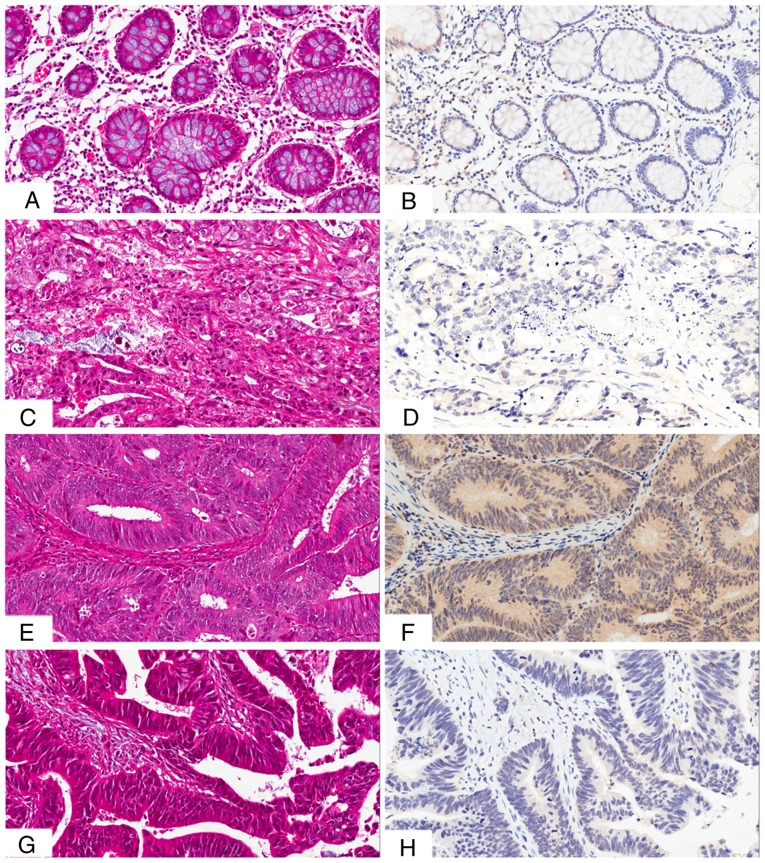
The presence and distribution of NUCB2 in the tissues was assessed via IHC staining. The NUCB2 protein was primarily localized in the cytoplasm and, to a lesser extent, in the membrane of the cancer cells (Fig. 2). High expression of NUCB2 was detected in 5/30 (16.7%) normal tissue samples, and in 105/251 (41.8%) CRC tissue samples, indicating a significant difference (χ2=7.124, P=0.008).
Figure 2.
IHC staining of NUCB2 in CRC tissues. (A) H&E staining of low expression of NUCB2 in normal tissues. (B) Low expression of NUCB2 in normal tissues. (C) H&E staining of low NUCB2 expression in poorly differentiated CRC tissues. (D) Low NUCB2 expression in poorly differentiated CRC tissues. (E) H&E staining of high expression of NUCB2 in moderately differentiated CRC. (F) High expression of NUCB2 in moderately differentiated CRC. (G) H&E staining of the negative control of where the primary antibody was replaced by phosphate-buffered saline (H) Negative control of IHC where primary antibody was replaced by phosphate-buffered saline. CRC, colorectal cancer; NUCB2, nucleobindin 2; IHC, immunohistochemistry. All images are recorded at ×200 magnification.
In order to assess the association between NUCB2 expression and colon cancer progression, the clinicopathological parameters of colon cancer was analyzed in samples that exhibited high levels of NUCB2. The results indicated a positive association between NUCB2 expression and lymph node metastasis and TNM stage (13) (Table I). Patients with lymph node metastasis had a higher expression of NUCB2 (49.5%, 50/101) compared with those without lymph node metastasis (36.7%, 55/150; P=0.043). In addition, patients with TNM stage III–IV exhibited significantly higher NUCB2 expression compared with those with TNM stage I–II (50.9% vs. 35.0%; P=0.011). The quantification of NUCB2 staining also indicated that the IOD values in tumors with lymph node metastasis and TNM stage III–IV were higher compared with those in tumors without lymph node metastasis and TNM stage I–II (Fig. 3; P<0.05).
Table I.
Association between NUCB2 expression and clinicopathological features of colorectal cancer.
| NUCB2 expression | ||||
|---|---|---|---|---|
| Clinical parameters | Negative (%) | Positive (%) | χ2 | P-value |
| Sex | 0.011 | 0.917 | ||
| Male | 81 (58.3) | 58 (41.7) | ||
| Female | 66 (58.9) | 46 (41.1) | ||
| Age, years | 0.077 | 0.781 | ||
| <60 | 37 (59.7) | 25 (40.3) | ||
| ≥60 | 109 (57.7) | 80 (42.3) | ||
| Tumor diameter, cm | 2.522 | 0.112 | ||
| <20 | 76 (63.3) | 44 (36.7) | ||
| ≥20 | 70 (53.4) | 61 (46.6) | ||
| Differentiation | 0.918 | 0.632 | ||
| High | 32 (64.0) | 18 (36.0) | ||
| Moderate | 85 (56.3) | 66 (43.7) | ||
| Poor | 29 (58.0) | 21 (42.0) | ||
| TNM stage | 6.442 | 0.011 | ||
| I + II | 93 (65.0) | 50 (35.0) | ||
| III + IV | 53 (49.1) | 55 (50.9) | ||
| Lymph node metastasis | 4.088 | 0.043 | ||
| No | 95 (63.3) | 55 (36.7) | ||
| Yes | 51 (50.5) | 50 (49.5) | ||
| Distant metastasis | 2.366 | 0.171 | ||
| No | 143 (59.1) | 99 (40.9) | ||
| Yes | 3 (33.3) | 6 (66.7) | ||
| Liver metastasis | 1.558 | 0.212 | ||
| Negative | 144 (58.8) | 101 (41.2) | ||
| Positive | 2 (33.3) | 4 (66.7) | ||
TNM, tumor-node-metastasis; NUCB2, nucleobindin 2.
Figure 3.
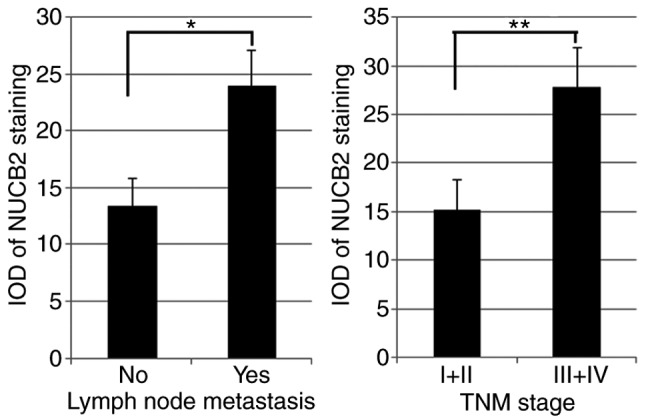
Quantification of NUCB2 staining in colorectal cancer tissues as detected with IOD. *P<0.05, **P<0.01. NUCB2, nucleobindin 2; IOD, integrated optical density; TNM, tumor-node-metastasis.
Clinical significance of NUCB2 expression in prognosis of CRC
Univariate survival analysis demonstrated that the 3- and 5-year cumulative survival rates of patients with high expression of NUCB2 were 64.3 and 52.0%, and the 3- and 5-year cumulative survival rates were 71.9 and 61.6% in those with no NUCB2 expression, respectively. The mean survival duration of patients with CRC with a high NUCB2 expression was 56.27±3.42 months, and the mean survival duration of patients with low NUCB2 expression was 62.59±2.79 months (Fig. 4). In addition, the analysis indicated no significant association of NUCB2 expression with overall survival (Fig. 4; χ2=2.044, P=0.153).
Figure 4.
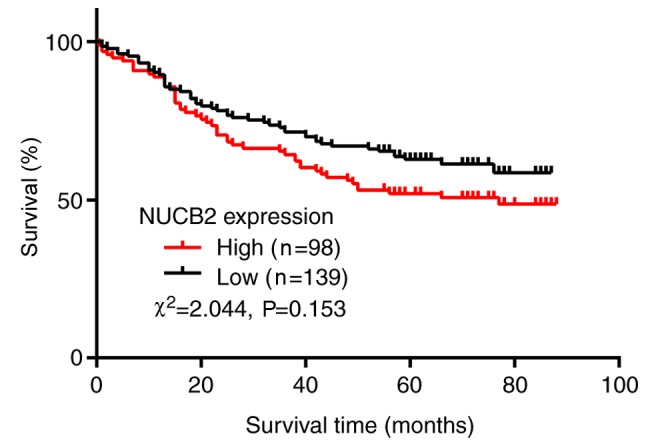
Kaplan-Meier survival curve analysis in patients with NUCB2 expression. There was no significant association between NUCB2 expression and overall survival (P=0.153). NUCB2, nucleobindin 2.
Discussion
CRC is one of the most frequently occurring cancer types globally (15). Metastasis in patients with colon cancer is associated with poor prognosis at early stages of the disease and subsequent mortality (16). Therefore, it is vital to investigate the underlying molecular mechanisms of metastasis and develop therapeutic strategies specifically targeting this process (17). NUCB2 has a widespread expression pattern in the body and mainly participates in physiological processes, including nocturnal feeding and regulation of body weight (18). Recent reports have demonstrated the diverse functions of NUCB2 in various tissues and in different cancer types (11–13,19). Takagi et al (19) reported that NUCB2 expression was positively correlated with Ki67 expression, and the knockdown of NUCB2 significantly inhibited the proliferation and migration of tumor cells in endometrial carcinoma. By contrast, NUCB2 also inhibited cell proliferation in adrenocortical and ovarian epithelial carcinoma (11,12). Notably, Kan et al (8) determined that NUCB2 enhanced migration, invasion and EMT of cancer cells in colon cancer. Nevertheless, the clinical significance of NUCB2 in colon cancer remains unclear.
In the present study, RT-qPCR indicated an upregulation of NUCB2 mRNA levels in CRC tissues compared with the adjacent non-cancerous tissues, which was consistent with previous reports on prostate cancer (6,20). The association of NUCB2 protein expression with CRC progression was further investigated on the basis of a large clinical sample cohort. The results demonstrated an upregulation of NUCB2 in a large proportion of patients with CRC, and elevated NUCB2 protein level was associated with the TNM stage and lymph node metastasis. These results support the hypothesis that NUCB2 may act as an oncogene in CRC with a key role in metastasis and progression.
A number of studies have also indicated an interaction between NUCB2 and the mechanistic target of rapamycin (mTOR) or AMP-activated protein kinase (AMPK) pathways (8,21). For example, treatment with Nestafin-1/NUCB2 enhanced the phosphorylation of AMPK and TORC2 in the rat brain (21). Kan et al (8) also indicated that NUCB2 enhanced cell migration and invasion via the liver kinase B1/AMPK/transducer of CREB protein 1/ZEB1 pathways in colon cancer. Therefore, the AMPK and mTOR pathways may serve an important role in metastasis.
A previous study by Zhang et al (7), which focused on the role of NUCB2 in cancer prognosis, demonstrated that high levels of the NUCB2 protein in prostate cancer was significantly associated with the biochemical recurrence-free survival rate, and multivariate analysis also indicated that high NUCB2 levels could be an independent prognostic factor in patients with prostate cancer. NUCB2 expression level was also reported as an independent prognostic predictor in patients with renal cell carcinoma (9,22). However, in the present study, Kaplan-Meier analysis indicated no significant association between disease-free survival of patients and NUCB2 expression. Several studies have demonstrated poorer prognosis in younger patients with CRC compared with older patients (23,24), whilst other studies have indicated the opposite (25,26). This may be due to certain characteristics of the younger patients, which vary across different regions (27) and ethnicities (28). For instance, a higher frequency of colorectal neoplasia was observed among 40–49 year-old African Americans when compared with Hispanic Americans suggesting an increased susceptibility to CRC risk in this population (28). In the present study, it was determined that there was no significant association between NUCB2 expression and the age of patients with CRC as well as clinical prognosis. It is possible that the clinicopathological features of the tissue samples are responsible for this finding. Further studies are required to continuously collect more clinical data, and larger samples are also needed in order to yield more accurate and consistent results in the future.
Furthermore, in the present study, multivariate analysis demonstrated that the upregulation of NUCB2 was also not an independent prognostic predictor in patients with CRC. In view of the limitations of the present study, caused by the sample size and collection, more clinical studies are being considered for future studies, which would include a larger cohort to investigate the role of NUCB2 in CRC prognosis.
In conclusion, a significant association was detected between high NUCB2 expression level and metastasis or poor clinical outcome of CRC. This strongly indicated that NUCB2 is a cancer-associated oncogene, which is associated with aggressive progression in CRC. Additionally, NUCB2 may be useful a novel biomarker for the diagnosis, prognosis and as a potential therapeutic target for CRC. However, these results are based on a single Chinese cohort, and therefore further studies are required to validate the results.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
WC was responsible for study conception and design and revised the manuscript, JX performed experiments and drafted the manuscript, LC analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Approval for the present study was obtained by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China).
Consent for publication
All patients admitted to the study provided informed consent for their participation of the present study and the publication of this data.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Oliveira J. ESMO Guidelines Working Group: Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:61–63. doi: 10.1093/annonc/mdp130. [DOI] [PubMed] [Google Scholar]
- 3.Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202–207. doi: 10.3816/CCC.2006.n.036. [DOI] [PubMed] [Google Scholar]
- 4.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Galiano D, Navarro VM, Gaytan F, Tena-Sempere M. Expanding roles of NUCB2/nesfatin-1 in neuroendocrine regulation. J Mol Endocrinol. 2010;45:281–290. doi: 10.1677/JME-10-0059. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Qi C, Li L, Luo F, Xu Y. Clinical significance of NUCB2 mRNA expression in prostate cancer. J Exp Clin Cancer Res. 2013;32:56. doi: 10.1186/1756-9966-32-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Qi C, Wang A, Yao B, Li L, Wang Y, Xu Y. Prognostication of prostate cancer based on NUCB2 protein assessment: NUCB2 in prostate cancer. J Exp Clin Cancer Res. 2013;32:77. doi: 10.1186/1756-9966-32-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ, Ho YW, Kuo PL. Nesfatin-1/Nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget. 2016;7:31336–31349. doi: 10.18632/oncotarget.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi C, Ma H, Zhang HT, Gao JD, Xu Y. Nucleobindin 2 expression is an independent prognostic factor for clear cell renal cell carcinoma. Histopathology. 2015;66:650–657. doi: 10.1111/his.12587. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Takagi K, Miki Y, Onodera Y, Akahira J, Ebata A, Ishida T, Watanabe M, Sasano H, Suzuki T. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2012;103:136–143. doi: 10.1111/j.1349-7006.2011.02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanjaneya M, Tan BK, Rucinski M, Kawan M, Hu J, Kaur J, Patel VH, Malendowicz LK, Komarowska H, Lehnert H, et al. Nesfatin-1 inhibits proliferation and enhances apoptosis of human adrenocortical H295R cells. J Endocrinol. 2015;226:1–11. doi: 10.1530/JOE-14-0496. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Pang X, Dong M, Wen F, Zhang Y. Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation in vitro. Biochem Biophys Res Commun. 2013;440:467–472. doi: 10.1016/j.bbrc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committie on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field K, Lipton L. Metastatic colorectal cancer-past, progress and future. World J Gastroenterol. 2007;13:3806–3815. doi: 10.3748/wjg.v13.i28.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. Metastasis: New perspectives on an old problem. Mol Cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X, Liu XM, Zhou LH. Recent progress in research on the distribution and function of NUCB2/nesfatin-1 in peripheral tissues. Endocr J. 2013;60:1021–1027. doi: 10.1507/endocrj.EJ13-0236. [DOI] [PubMed] [Google Scholar]
- 19.Takagi K, Miki Y, Tanaka S, Hashimoto C, Watanabe M, Sasano H, Ito K, Suzuki T. Nucleobindin 2 (NUCB2) in human endometrial carcinoma: A potent prognostic factor associated with cell proliferation and migration. Endocr J. 2016;63:287–299. doi: 10.1507/endocrj.EJ15-0490. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Qi C, Wang A, Li L, Xu Y. High expression of nucleobindin 2 mRNA: An independent prognostic factor for overall survival of patients with prostate cancer. Tumour Biol. 2014;35:2025–2028. doi: 10.1007/s13277-013-1268-z. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Zhang Z, Wang C, Li K, Li S, Boden G, Li L, Yang G. Nesfatin-1 action in the brain increases insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced insulin resistance. Diabetes. 2012;61:1959–1968. doi: 10.2337/db11-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Wang Z, Xie H, Dai B, Xu J, Ye D. High NUCB2 expression level represents an independent negative prognostic factor in Chinese cohorts of non-metastatic clear cell renal cell carcinoma patients. Oncotarget. 2017;8:35244–35254. doi: 10.18632/oncotarget.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan MA, Isikdogan A, Gumus M, Arslan UY, Geredeli C, Ozdemir N, Koca D, Dane F, Suner A, Elkiran ET, et al. Childhood, adolescents, and young adults (≤25 y) colorectal cancer: Study of Anatolian Society of Medical Oncology. J Pediatr Hematol Oncol. 2013;35:83–89. doi: 10.1097/MPH.0b013e31827e7f20. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Bao F, Yan J, Liu H, Li T, Chen H, Li G. Poor prognosis of young patients with colorectal cancer: A retrospective study. Int J Colorectal Dis. 2017;32:1147–1156. doi: 10.1007/s00384-017-2809-5. [DOI] [PubMed] [Google Scholar]
- 25.Taggarshe D, Rehil N, Sharma S, Flynn JC, Damadi A. Colorectal cancer: Are the ‘young’ being overlooked? Am J Surg. 2013;205:312–316. doi: 10.1016/j.amjsurg.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Yeo SA, Chew MH, Koh PK, Tang CL. Young colorectal carcinoma patients do not have a poorer prognosis: A comparative review of 2,426 cases. Tech Coloproctol. 2013;17:653–661. doi: 10.1007/s10151-013-0977-z. [DOI] [PubMed] [Google Scholar]
- 27.You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: Is it time to pay attention? Arch Intern Med. 2012;172:287–289. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 28.Ashktorab H, Paydar M, Namin HH, Sanderson A, Begum R, Brim H, Panchal H, Lee E, Kibreab A, Nouraie M, Laiyemo AO. Prevalence of colorectal neoplasia among young African Americans and hispanic Americans. Dig Dis Sci. 2014;59:446–450. doi: 10.1007/s10620-013-2898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.