Abstract
The aim of the present study was to evaluate the clinical utility of plasma chromogranin A (CgA) in patients diagnosed with early-stage pancreatic neuroendocrine tumors (PNETs) in terms of diagnostic value and treatment response. A total of 35 patients with PNETs were prospectively enrolled from August 2010 to April 2014. Demographic and clinicopathological data were collected, and serial plasma CgA levels were measured. Tumor responses were defined by the Response Evaluation Criteria In Solid Tumors criteria. Pearson's χ2 test was used for the analysis of the association between the plasma CgA level and various factors. Plasma CgA level was significantly associated with the size (P=0.03), metastasis (P=0.02) and tumor stage (P=0.03) of the PNETs. Using 126 U/l as the optimal cutoff value, the sensitivity and specificity were 87.5 and 81.5%, respectively. For localized tumors, the sensitivity of CgA for diagnosing PNETs was relatively low, even following a lowering of the cutoff values (29.6–51.9%). Plasma CgA level was correlated with therapeutic response in those patients with high baseline CgA levels (P=0.025), but not in the patients with low baseline CgA levels (P=0.587). In conclusion, plasma CgA level was associated with tumor size, metastasis and tumor stage in patients with PNET. For early-stage PNETs, CgA exhibited a limited role in diagnosis and treatment response evaluation in the population of the present study.
Keywords: chromogranin A, pancreatic neuroendocrine tumor, tumor marker
Introduction
Pancreatic neuroendocrine tumors (PNETs) are rare neoplasms, with an incidence of 0.48 cases/100,000 individuals each year between 2000 and 2012 in the United States of America (1–2). A trend towards increasing incidence and prevalence rates has been documented by previous studies (3,4). Although functional PNETs may be present in a variety of hormone syndromes and detected at an earlier stage, numerous non-functional PNETs are diagnosed late in the disease course, with symptoms associated with local mass effects or metastatic disease (5). A biomarker with a high sensitivity and specificity is mandatory for the early and accurate diagnosis of PNETs, particularly for those with vague symptoms.
Chromogranin A (CgA) is an acidic glycoprotein stored in the dense granules of the NETs and co-released with peptide hormones (6). CgA has been suggested to be a reliable biomarker for NETs in terms of diagnostic value, prognosis prediction and treatment response evaluation (7,8). In western countries, a low diagnostic accuracy but good prognostic value of CgA in patients with resectable non-functional PNETs has been demonstrated (9,10). Concerning differences in biomarker performance across racial groups, there are a small number of studies examining the diagnostic value of CgA in Asian populations. Plasma CgA has been suggested to be a useful biomarker for PNETs in Asian populations (11–14), but few studies have evaluated the diagnostic role of plasma CgA in early-stage PNETs. The present study aimed to evaluate the clinical utility of plasma CgA in the diagnostic confirmation and evaluation of treatment response in Asian patients with PNETs, particularly those with early-stage tumors.
Materials and methods
Study design and population
The study protocol was approved by the Institutional Review Board of National Taiwan University Hospital (Taipei, Taiwan). Written informed consent for participation in the study was obtained from all participants. From August 2010 to April 2014, 35 patients with PNETs according to tissue-based diagnosis were prospectively enrolled consecutively. The age, gender, clinical presentation and plasma CgA level were recorded prior to tissue-based diagnosis Patients presenting with symptoms suggestive of excessive hormone production were considered to have functional PNETs, and the other patients were considered to have non-functional tumors. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) cell blocks or surgical specimens were used for a definite diagnosis. The tissue coagulum clot method was adapted for cell block preparation (15). The steps included fixation by transferring tissue coagulum clot strips into the 10% formalin solution at room temperature for between 10 and 24 h, subsequent centrifugation, and final transfer of the pellet for paraffin embedding as a cell block. Each cell clock was examined via hematoxylin and eosin staining and immunohistochemical staining with antibodies against CgA and synaptophysin, as previously described (16). Histopathological characteristics, including tumor location, size, grade, Tumor-Node-Metastasis (TNM) status and tissue CgA immunoreactivity were also recorded. Tumor grade was classified according to the recommendations of the World Health Organization (WHO) (17). TNM status was based on the 7th edition of the American Joint Commission on Cancer Staging System (18). Inclusion criteria included patients aged ≥20 years old with tissue-based diagnosis of PNETs consecutively within the study period. Participants were recruited on a rolling basis as and when they were identified using tissue-based diagnosis. Exclusion criteria included end-stage renal disease, liver failure and the presence of any other malignancies. Serial CgA measurement and imaging studies were used to evaluate treatment response. Response Evaluation Criteria In Solid Tumors (RECIST) (19) was used to evaluate the treatment response of the image studies.
Measurement of plasma CgA
Blood samples were obtained following overnight fasting and collected prior to definite tissue-based diagnosis either by EUS-FNA or by surgical resection. The plasma CgA level was measured with a commercial kit (Chromoa R assay; CIS Bio International S.A., Saclay, France; cat. no. CGA-ELISA), according to the manufacturer's protocol. The recommended cutoff value was set at 94 U/l, according to a previous study (9).
Statistical analysis
Comparison of values from independent groups was performed using a Mann-Whitney test. Pearson's χ2 test was used to measure the strength of the association between pairs of variables without specifying dependencies. To determine whether CgA level was an independent predictor of metastasis, hazard ratios were calculated using the Cox proportional hazards model. The sensitivity and specificity of CgA level to discriminate metastatic from localized PNETs were analyzed using the receiver operating characteristic (ROC) curve, and the optimal cutoff value was determined. Kendall's τ correlation test was applied to estimate the correlation between change of CgA level and treatment response by RECIST. Data were analyzed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of enrolled patients
Among the 35 patients with PNET, there were 18 males and 17 females. The mean age of these patients was 53.0 (range, 31–80) years. A total of 29 patients received surgical resection only, and 1 patient received surgical resection and additional targeted therapy. A total of 4 patients received systemic antitumor therapies, including target therapy and cytotoxic chemotherapy, and 1 patient received best supportive care. The CgA levels among patients with PNET with different clinicopathological characteristics were compared (Table I). There was no association between sex and CgA value. The patients usually presented asymptomatically, and those PNETs were identified incidentally by health examination (21/35, 60%). The majority of these tumors were well differentiated (G1; 22/35, 63%) and localized tumors (27/35, 77%). Among patients with the localized disease, the majority of the tumors were >2 cm in diameter (16/27, 59%). The majority of the tumors were located in single areas of the pancreatic body or tail where they were more easily detected by routine abdominal sonography, with the exception of 3 cases of multiple endocrine neoplasia type 1 (MEN-1), which presented with multiple tumors from the pancreatic head to tail. The majority of the patients with PNETs were immunoreactive to tissue CgA.
Table I.
Plasma CgA levels in patients with PNETs.
| Variables | n | Median CgA level (range), U/l | P-value | |
|---|---|---|---|---|
| Sex | 0.22 | |||
| Female | 17 | 84.4 (12.1–16465.7) | ||
| Male | 18 | 66.5 (33.6–3117.5) | ||
| Age, years | 0.26 | |||
| <50 | 17 | 67.9 (12.1–16465.7) | ||
| ≥50 | 18 | 107.5 (33.6–3117.5) | ||
| Clinical symptoms | 0.71 | |||
| Absent | 21 | 53.5 (12.1–3117.5) | ||
| Present | 14 | 70.5 (99.5–16465.7) | ||
| Proton pump inhibitor | 0.39 | |||
| Absent | 33 | 84.4 (12.1–3117.5) | ||
| Present | 2 | 8264.4 (63.0–16465.7) | ||
| MEN-1 | 0.68 | |||
| Absent | 32 | 85.0 (12.1–3117.5) | ||
| Present | 3 | 67.9 (59.9–16465.7) | ||
| Pancreatic locationa | 0.62 | |||
| Body + tail | 21 | 72.7 (33.6–3117.5) | ||
| Head + neck | 11 | 86.5 (12.1–2637.5) | ||
| Size, cm | 0.03 | |||
| ≤2 | 16 | 64.0 (12.1–1506.5) | ||
| >2 | 19 | 115.4 (33.6–16465.7) | ||
| Metastasis | 0.02 | |||
| Absent | 27 | 67.9 (12.1–16465.7) | ||
| Present | 8 | 308.7 (35.9–2637.5) | ||
| Pathological stage | 0.03 | |||
| I | 24 | 66.5 (12.1–16465.7) | ||
| II+III+IV | 11 | 221.0 (35.9–2637.5) | ||
| WHO grade | 0.59 | |||
| G1 | 22 | 72.7 (42.5–16465.7) | ||
| G2+G3 | 13 | 132.0 (12.1–3117.5) | ||
| Tissue CgA | 0.20 | |||
| Negative | 2 | 51.4 (35.9–67.9) | ||
| Positive | 33 | 85.5 (12.1–16465.7) |
A total of 3 MEN-1 cases were excluded due to multiple locations. PNET, pancreatic neuroendocrine tumor; CgA, chromogranin A; MEN-1, multiple endocrine neoplasia type 1; WHO, World Health Organization.
Univariate analysis of CgA levels based on the clinicopathological variables demonstrated that plasma CgA levels were significantly higher in patients with larger tumor size (size >2 cm vs. size ≤2 cm: 115.4 vs. 64.0 U/l, P=0.03; Fig. 1A), distant metastasis (metastasis vs. non-metastasis: 308.7 vs. 67.9 U/l, P=0.02; Fig. 1B) and advanced pathological stage (stage II/III/IV vs. stage I: 221.0 vs. 66.5 U/l, P=0.03; Fig. 1C). CgA level did not differ in terms of sex, age, clinical symptoms, proton pump inhibitor use, MEN-1 presence, tumor location, tumor grade or tissue CgA immunoreactivity.
Figure 1.
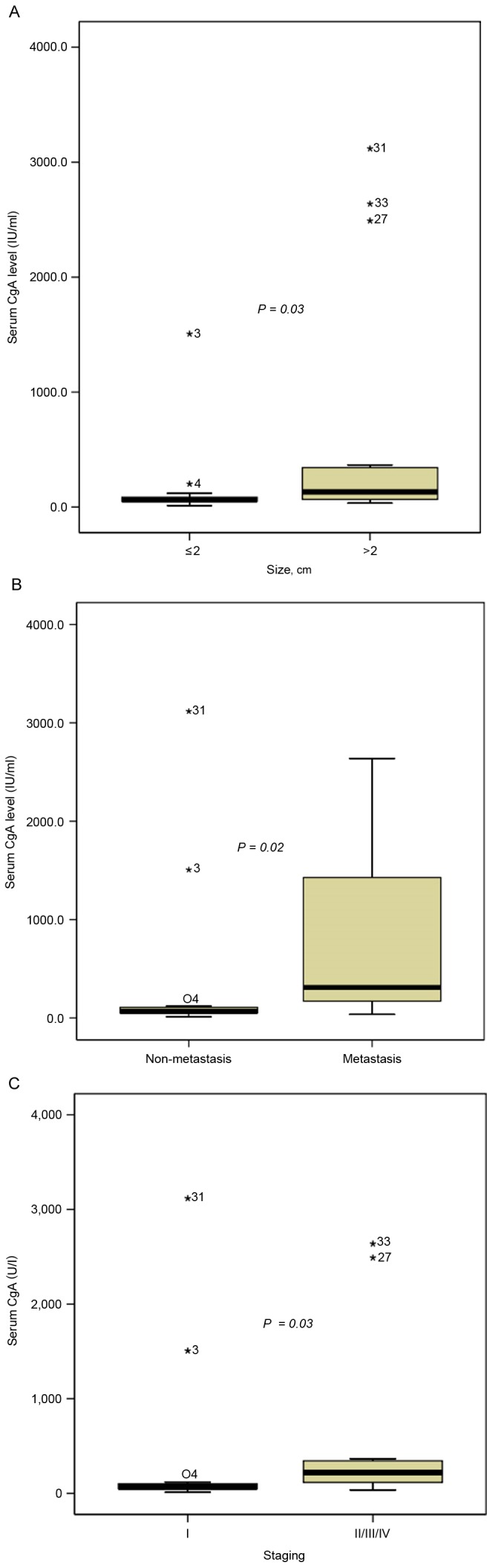
Plasma CgA levels according to tumor characteristics in pancreatic neuroendocrine tumors. Plasma CgA is significantly associated with (A) size, (B) metastasis and (C) staging. Asterisks denote outliers, with case numbers in brackets. CgA, chromogranin A.
Diagnostic value of CgA
Table II summarizes the sensitivities of plasma CgA levels of PNETs under the different cutoff values suggested by previous studies (8,12,16). When the cutoff value was 94 U/l, the overall sensitivity of plasma CgA was only 42.9%. Subgroup analysis indicated that the sensitivity of plasma CgA for patients with metastatic tumors was 87.5%, while the sensitivity for patients with localized tumors was 29.6%. On evaluation under different cutoff values, all the sensitivities for the patient with localized PNETs were poor (74 U/l, 40.7%; 65.7 U/l, 51.9%).
Table II.
Sensitivity of CgA under different cutoff values.
| Tumor location | |||
|---|---|---|---|
| CgA cutoff level, U/l | Localizeda | Metastasisa | Overall sensitivitya |
| 94 | 29.6 (8/27) | 87.5 (7/8) | 42.9 (15/35) |
| 74 | 40.7 (11/27) | 87.5 (7/8) | 51.4 (18/35) |
| 64.3 | 51.9 (14/27) | 87.5 (7/8) | 60.0 (21/35) |
Sensitivity data presented as % (n/total n). CgA, chromogranin A.
CgA as a predictor of metastasis
Table III summarizes the univariate and multivariate analyses of predictors of metastasis. Larger tumor size (P=0.019), WHO grade 2 or 3 (P=0.006) and higher plasma CgA level (≥94 U/l; P=0.014) were associated with a higher risk of metastasis according to the univariate analysis. Multivariate analysis of significant factors indicated that only higher plasma CgA level was significantly associated with a higher risk of metastasis (P=0.045). To distinguish between patients with localized disease or metastasis, a ROC curve analysis was applied (Fig. 2). When the cutoff value was 94 U/l, the sensitivity and specificity were 87.5 and 70.4%, respectively. Using 126 U/l as the optimal cutoff value, the sensitivity and specificity were 87.5 and 81.5%, respectively.
Table III.
Univariate and multivariate analyses of predictors of metastasis.
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male vs. female | 0.93 (0.19–4.50) | 0.927 | ||
| Age, years | ||||
| ≥50 vs. <50 | 0.93 (0.19–4.50) | 0.927 | ||
| Clinical symptoms | ||||
| Present vs. absent | 3.33 (0.65–17.18) | 0.150 | ||
| Location | ||||
| Head/neck vs. body/tail | 0.53 (0.09–3.18) | 0.490 | ||
| Size, cm | ||||
| >2 vs. ≤2 | 2.05 (1.13–3.74) | 0.019 | 1.72 (0.66–4.50) | 0.266 |
| WHO grade | ||||
| G1 vs. G2/3 | 0.04 (0.004–0.40) | 0.006 | 0.09 (0.57–202.8) | 0.112 |
| Plasma CgA, U/l | ||||
| ≥94 vs. <94 | 16.63 (1.75–158.09) | 0.014 | 18.31 (1.073–312.56) | 0.045 |
HR, hazard ratio; CI, confidence interval; CgA, chromogranin A; WHO, World Health Organization.
Figure 2.
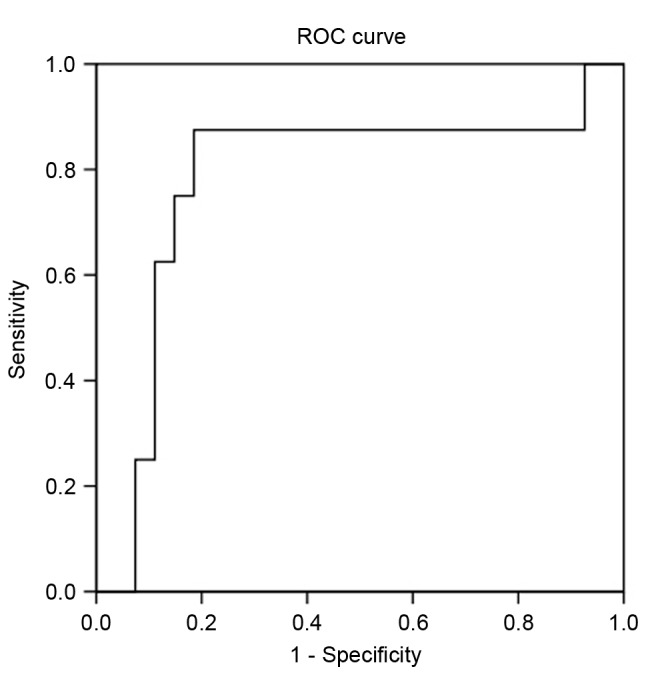
ROC curve of CgA obtained from 8 patients with metastasis and 27 patients without metastases. ROC, receiver operating characteristic.
Correlation between plasma CgA change and treatment response
A total of 29 out of 35 patients underwent serial CgA measurement and imaging studies prior and subsequent to treatment (6 patients did not receive serial CgA measurement as they were lost to follow-up). A total of 13 patients exhibited high baseline plasma CgA levels (≥94 U/l) and 16 patients exhibited low baseline levels (<94 U/l). Among the patients with high baseline CgA levels, 7 patients achieved a complete response and 3 patients demonstrated a partial response. A total of 3 patients exhibited progressive disease. Serial plasma CgA change was correlated with treatment response. A decrease of ≥30% in CgA levels was observed in patients with complete and partial responses (P=0.025; Fig. 3A). Among the patients with low baseline CgA levels, 15 patients achieved complete response and 1 patient exhibited progressive disease. However, serial CgA level was not correlated with treatment response in this patient group (P=0.587; Fig. 3B).
Figure 3.
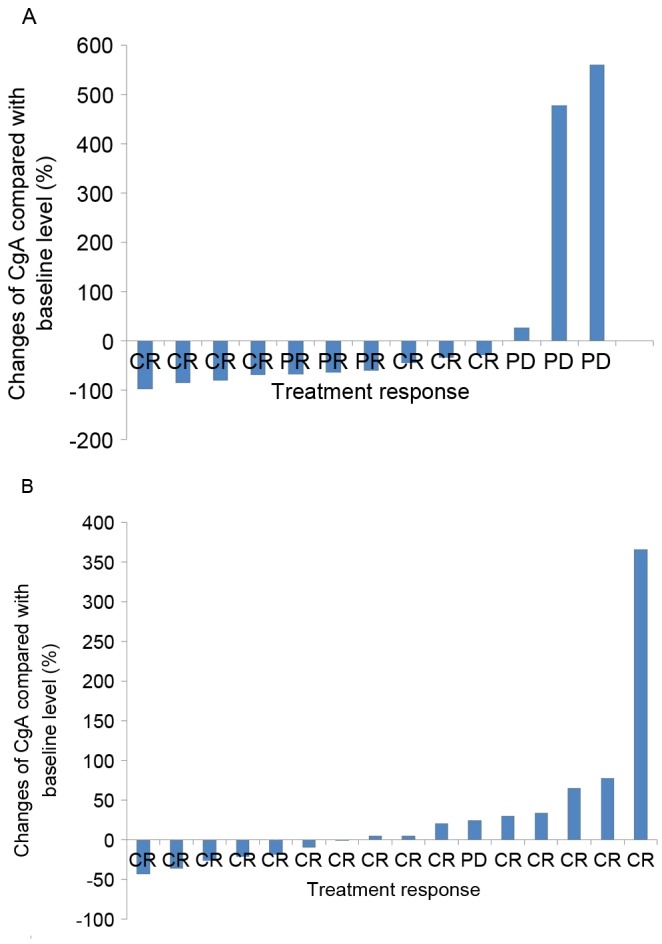
Correlation between plasma CgA change and treatment response among (A) high baseline group (r=0.523, P=0.025) and (B) low baseline group (r=0.118, P=0.587). CR, complete response; PD, progressive disease; CgA, chromogranin A.
Discussion
The present study demonstrated that plasma CgA exhibited limited diagnostic value in early PNETs. Smaller and localized PNETs tended to exhibit lower plasma CgA levels. Although plasma CgA change was correlated with treatment response, this result was limited to the patients with high baseline CgA levels (≥94 U/l).
CgA is an acidic glycoprotein stored in the dense granules of NETs and co-released with peptide hormones (6). CgA is considered the most accurate tumor marker in the diagnosis of gastro-entero-pancreatic NETs (GEP-NETs), in comparison with other tumor markers, including urinary 5-hydroxyindoleacetic acid, neuron-specific enolase and carcinoembryonic antigen (7). The sensitivity and specificity of CgA differs and depends on numerous factors, such as the type of assay used, the cutoff value, tumor burden and organs involved (20). A small number of studies concerning the diagnostic value of plasma CgA in Asian populations are summarized in Table IV (11–14). The sensitivities and specificities of plasma CgA were 53.6–86.0 and 78.6–91.9% respectively. However, these studies only enrolled a small number of early-stage PNETs. In the present study, the overall sensitivity was only 42.9%, and the sensitivity of localized PNETs was even lower (29.6%). The low sensitivity of plasma CgA identified in the patients was probably due to the small size of the tumors and the early stage. Among the localized tumors, the majority were >2 cm (59%). A previous study indicated that small tumors may be associated with normal CgA levels (8). In concordance with previous studies, the present study demonstrated that plasma CgA levels were significantly associated with tumor size. Smaller tumors tended to exhibit lower plasma CgA levels. Conversely, the sensitivity of plasma CgA for patients with metastatic cancer was 87.5%, which was comparable with a previous study conducted in Asia (11). CgA levels and their associated sensitivities depended on metastasis (21). Previous studies suggested that plasma CgA level was correlated with tumor mass and disease extent. CgA levels were higher in patients with extensive liver metastases compared with localized disease (22,23). The present study also indicated that those patients with metastatic tumors tended to exhibit higher plasma CgA levels. The sensitivity of plasma CgA for patients with metastatic PNETs was relatively good in comparison to patients with localized PNETs.
Table IV.
Summary of previous studies on the diagnostic value of CgA in Asian populations.
| First author | Study design | Assay type | Cutoff value, U/l | Sensitivity, % | Specificity, % | (Ref.) |
|---|---|---|---|---|---|---|
| Han et al | Non-functional PNET (n=51) | Chromoa assay, ELISA | 64.3 | 63.2 | 91.2 | (12) |
| Qiao et al | Non-insulinoma PNET (n=32) | Chromoa assay, ELISA | 74.0 | 65.6 | 91.9 | (14) |
| Chou et al | GEP-NET (n=44) | Chromoa assay, ELISA | 94.0 | 86.0 | 88.0 | (11) |
| Hijioka et al | PNET (n=69) | Chromoa assay, ELISA | 78.7 | 53.6 | 78.6 | (13) |
PNET, pancreatic neuroendocrine tumor; GEP, gastro-entero-pancreatic; CgA, chromogranin A.
Distant metastasis predicts a higher risk of mortality in PNETs (24,25). Identification of reliable predictors of metastasis has been the aim of numerous studies. Tumor size was the most commonly documented clinical predictor of metastasis (26). The risk of metastasis increased significantly if the tumor size exceeded 15 mm. A small number of studies evaluated the association between the plasma CgA level and the risk of metastasis. Paik et al demonstrated that a high level of CgA (>156.5 U/l) predicted distant metastasis in those patients with PNETs (27). In concordance with this study, the present study also demonstrated that plasma CgA was a reliable indicator of metastasis. When the cutoff value was 94 U/l, the sensitivity and specificity were 87.5 and 70.4%, respectively. Using 126 U/l as the best cutoff value, the sensitivity and specificity were 87.5 and 81.5%, respectively. Identification of any metastatic tumors should thus be performed carefully once the PNET patient has demonstrated high CgA levels.
Korse et al (28), demonstrated that a higher sensitivity of plasma CgA was noted in patients with well-differentiated tumors. The sensitivity was ~68% for G1 NETs and 74% for G2 NETs. A much lower sensitivity (37%) was noted in the patients with poorly differentiated NETs, which was probably due to the relative lack of large dense-core granules. The present study did not indicate a significant association between grade and plasma CgA level. This may be as only 2 cases of G3 NETs were enrolled in the series. The single patient with metastatic PNET and lower plasma CgA level exhibited grade G3 NET. Although plasma CgA was revealed to be useful in the detection of metastatic NETs (11), it may not diagnose those patients with poorly differentiated NETs.
Plasma CgA has been shown to be valuable in evaluating the treatment response of different therapies (2,29). For patients with hepatic metastases from functional carcinoid tumors, Jensen et al (29), demonstrated that a reduction in CgA of ≥80% following cytoreductive surgery was predictive of the stabilization of disease. For patients with advanced PNETs, Yao et al (2) revealed that those patients with an early CgA response following treatment of everolimus experienced longer progression-free survival. Similar to previous studies, the present study also indicated that changes in plasma CgA were correlated with treatment response in those patients with high baseline CgA levels. However, this was not true for those patients with low baseline CgA levels, which implied that the role of CgA in evaluating treatment efficacy may be limited to patients with high baseline CgA levels.
Functional PNETs may present with various hormone syndromes, so they are usually detected at an earlier stage. By contrast, non-functional PNETs usually present with symptoms following local mass effects or metastatic disease at more advanced stages (5). In the present study, the majority of PNETs were non-functional and detected incidentally (60%), with a localized (77%) status. This is likely due to the widespread use of abdominal ultrasound in Taiwan. Routine ultrasound screening for patients with chronic hepatitis or abnormal liver function tests is extremely popular. PNETs localized in the body or tail may be easily detected by screening ultrasounds at an earlier stage.
A previous study indicated that high CgA levels (150-fold higher compared with the normal upper limit) in GEP-NETs were associated with MEN-1 (6). However, low diagnostic accuracy of plasma CgA in the detection of PNETs in patients with MEN-1 has been demonstrated by two other studies (30,31). The plasma CgA test cannot replace the other established diagnostic tools in screening for early PNETs among patients with MEN-1. The results of the present study also indicated a limited use of plasma CgA in patients with MEN-1, although only 3 patients with MEN-1 were included and only 1 of these exhibited a higher CgA level.
There are a number of non-neoplastic causes of CgA elevation, including renal insufficiency, chronic hepatitis and drug use. Certain adenocarcinomas may also account for CgA elevation (6,7). In the present study, the patients with liver failure, renal failure and other types of cancer were excluded. Although proton pump inhibitor use has been suggested to be a common cause for CgA elevation (32), only 2 cases in the present study were administered proton pump inhibitors and they exhibited different CgA responses (63.0 and 16,465.7 U/l). Additional interpretation with subgroup analysis was not feasible due to a relatively small number of patients with PNET in the present study. As PNET is an uncommon disease, future studies with multi-center cooperation may provide a more comprehensive view.
To conclude, the present study demonstrated that plasma CgA levels were associated with tumor size, metastasis status and tumor stage in diagnosing patients with PNET. Changes in plasma CgA levels were correlated with treatment response only in those patients with high baseline CgA levels. For early-stage PNETs, CgA exhibited a limited role in the diagnosis and evaluation of treatment response.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CgA
chromogranin A
- EUS-FNA
endoscopic ultrasound-guided fine-needle aspiration
- NET
neuroendocrine tumor
- GEP-NET
gastro-entero-pancreatic NET
- MEN-1
multiple endocrine neoplasia type 1
- PNET
pancreatic NET
- RECIST
Response Evaluation Criteria In Solid Tumors
- ROC
receiver operating characteristic
- WHO
World Health Organization
Funding
Pinancial support was provided by Novartis (Taiwan) Co., Ltd., (Taipei, Taiwan) for the CgA test.
Availability of data and materials
All the datasets generated and analyzed in the present study are included in this published article.
Authors' contributions
CMT designed the study, reviewed the article (Tables. 2 and 4) and drafted the manuscript. TYC drafted and revised the manuscript, collected data (FNA and tissue staining) and performed statistic analysis (Tables. 1 and 3). T-BC performed statistical analysis and revised the figures and tables (Tables. 1 and 3). YWT contributed to data collection (surgical tissue) and provided technical support. C-CC contributed to data collection (plasma chromogranin A) and was involved in the interpretation of data. JTL revised the study protocol and revised the manuscript. H-PW designed the study, contributed to data collection (patient's background) and performed critical revisions of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of National Taiwan University Hospital (Taipei, Taiwan). Written informed consent for participation in the study was obtained from all participants.
Consent for publication
All study participants provided consent for the data to be published.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Pavel M, Phan AT, Kulke MH, Hoosen S, St Peter J, Cherfi A, Öberg KE. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–3749. doi: 10.1210/jc.2011-0666. [DOI] [PubMed] [Google Scholar]
- 3.Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012;24:46–55. doi: 10.1097/CCO.0b013e32834c554d. [DOI] [PubMed] [Google Scholar]
- 4.Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in taiwan: A nation-wide cancer registry-based study. PLoS One. 2013;8:e62487. doi: 10.1371/journal.pone.0062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns WR, Edil BH. Neuroendocrine pancreatic tumors: Guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(111–134):viii. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Vinik AI, Woltering EA, Warner RR, Caplin M, O'Dorisio TM, Wiseman GA, Coppola D, Go VL. North American Neuroendocrine Tumor Society (NANETS): NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 9.Jilesen AP, Busch OR, van Gulik TM, Gouma DJ, van Dijkum Nieveen EJ. Standard pre- and postoperative determination of chromogranin a in resectable non-functioning pancreatic neuroendocrine tumors-diagnostic accuracy: NF-pNET and low tumor burden. Dig Surg. 2014;31:407–414. doi: 10.1159/000370007. [DOI] [PubMed] [Google Scholar]
- 10.Shanahan MA, Salem A, Fisher A, Cho CS, Leverson G, Winslow ER, Weber SM. Chromogranin A predicts survival for resected pancreatic neuroendocrine tumors. J Surg Res. 2016;201:38–43. doi: 10.1016/j.jss.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Chou WC, Hung YS, Hsu JT, Chen JS, Lu CH, Hwang TL, Rau KM, Yeh KY, Chen TC, Sun CF. Chromogranin A is a reliable biomarker for gastroenteropancreatic neuroendocrine tumors in an Asian population of patients. Neuroendocrinology. 2012;95:344–350. doi: 10.1159/000333853. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Zhang C, Tang M, Xu X, Liu L, Ji Y, Pan B, Lou W. The value of serum chromogranin A as a predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. Eur J Gastroenterol Hepatol. 2015;27:527–535. doi: 10.1097/MEG.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 13.Hijioka M, Ito T, Igarashi H, Fujimori N, Lee L, Nakamura T, Jensen RT, Takayanagi R. Serum chromogranin A is a useful marker for Japanese patients with pancreatic neuroendocrine tumors. Cancer Sci. 2014;105:1464–1471. doi: 10.1111/cas.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao XW, Qiu L, Chen YJ, Meng CT, Sun Z, Bai CM, Zhao DC, Zhang TP, Zhao YP, Song YL, et al. Chromogranin A is a reliable serum diagnostic biomarker for pancreatic neuroendocrine tumors but not for insulinomas. BMC Endocr Disord. 2014;14:64. doi: 10.1186/1472-6823-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yung RC, Otell S, Illei P, Clark DP, Feller-Kopman D, Yarmus L, Askin F, Gabrielson E, Li QK. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120:185–195. doi: 10.1002/cncy.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatzipantelis P, Salla C, Konstantinou P, Karoumpalis I, Sakellariou S, Doumani I. Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: A study of 48 cases. Cancer. 2008;114:255–262. doi: 10.1002/cncr.23637. [DOI] [PubMed] [Google Scholar]
- 17.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system: World Health Organization. 2010 [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC Cancer Staging Manual. New York, NY: Springer; 2010. [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Vinik AI, Silva MP, Woltering EA, Go VL, Warner R, Caplin M. Biochemical testing for neuroendocrine tumors. Pancreas. 2009;38:876–889. doi: 10.1097/MPA.0b013e3181bc0e77. [DOI] [PubMed] [Google Scholar]
- 21.Nölting S, Kuttner A, Lauseker M, Vogeser M, Haug A, Herrmann KA, Hoffmann JN, Spitzweg C, Göke B, Auernhammer CJ. Chromogranin a as serum marker for gastroenteropancreatic neuroendocrine tumors: A single center experience and literature review. Cancers (Basel) 2012;4:141–155. doi: 10.3390/cancers4010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, Tomassetti P. Chromogranin A: Is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967–1973. doi: 10.1200/JCO.2006.10.1535. [DOI] [PubMed] [Google Scholar]
- 23.Walter T, Chardon L, Chopin-laly X, Raverot V, Caffin AG, Chayvialle JA, Scoazec JY, Lombard-Bohas C. Is the combination of chromogranin A and pancreatic polypeptide serum determinations of interest in the diagnosis and follow-up of gastro-entero-pancreatic neuroendocrine tumours? Eur J Cancer. 2012;48:1766–1773. doi: 10.1016/j.ejca.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Cherenfant J, Stocker SJ, Gage MK, Du H, Thurow TA, Odeleye M, Schimpke SW, Kaul KL, Hall CR, Lamzabi I, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2013;154:785–793. doi: 10.1016/j.surg.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishi Y, Shimada K, Nara S, Esaki M, Hiraoka N, Kosuge T. Basing treatment strategy for non-functional pancreatic neuroendocrine tumors on tumor size. Ann Surg Oncol. 2014;21:2882–2888. doi: 10.1245/s10434-014-3701-y. [DOI] [PubMed] [Google Scholar]
- 27.Paik WH, Ryu JK, Song BJ, Kim J, Park JK, Kim YT, Yoon YB. Clinical usefulness of plasma chromogranin a in pancreatic neuroendocrine neoplasm. J Korean Med Sci. 2013;28:750–754. doi: 10.3346/jkms.2013.28.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korse CM, Taal BG, Vincent A, van Velthuysen ML, Baas P, Buning-Kager JC, Linders TC, Bonfrer JM. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of Chromogranin A, Neuron specific enolase, Progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48:662–671. doi: 10.1016/j.ejca.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Jensen EH, Kvols L, McLoughlin JM, Lewis JM, Alvarado MD, Yeatman T, Malafa M, Shibata D. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780–785. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]
- 30.de Laat JM, Pieterman CR, Weijmans M, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes B, et al. Low accuracy of tumor markers for diagnosing pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1 patients. J Clin Endocrinol Metab. 2013;98:4143–4151. doi: 10.1210/jc.2013-1800. [DOI] [PubMed] [Google Scholar]
- 31.Granberg D, Stridsberg M, Seensalu R, Eriksson B, Lundqvist G, Oberg K, Skogseid B. Plasma chromogranin A in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1999;84:2712–2717. doi: 10.1210/jcem.84.8.5938. [DOI] [PubMed] [Google Scholar]
- 32.Giusti M, Sidoti M, Augeri C, Rabitti C, Minuto F. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol. 2004;150:299–303. doi: 10.1530/eje.0.1500299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the datasets generated and analyzed in the present study are included in this published article.


