Abstract
Papillary thyroid carcinoma (PTC) is the most frequently occurring subtype of thyroid cancer. A certain portion of PTCs can progress to recurrent metastatic cancer. Currently, there remains no effective molecular target therapy for PTCs. As a subunit of the chaperonin containing TCP1 (CCT) complex, CCT3 is involved in various biological processes. CCT3 has been reported to drive the proliferation of hepatocellular carcinoma cells. Nevertheless, it remains unknown whether CCT3 regulates the development of PTC. The present study examined CCT3 protein expression in 30 PTC samples from patients undergoing thyroidectomy. A significant increase was observed in CCT3 expression in the PTC samples compared with the matched adjacent normal thyroid tissues. Lentiviral-mediated small interfering RNAs were used to knock down CCT3 in K1 cells. It was observed that the expression of CCT3 was significantly suppressed in K1 cells infected with lentivirus containing a CCT3-targeting short hairpin RNA. Our results showed that CCT3 knockdown markedly decreased the proliferation and cell cycle progression of K1 cells. In addition, the knockdown of CCT3 induced apoptosis of K1 cell. Taken together, the findings of the present study indicated that CCT3 presents as a potential molecular marker of PTC and regulates the development of PTC in humans.
Keywords: papillary thyroid carcinoma, chaperonin containing TCP1 subunit 3, K1 cells, proliferation, apoptosis
Introduction
Thyroid cancer is the most frequently occurring endocrine malignancy, the incidence of which has increased worldwide in the past three decades (1). As the most common subtype of thyroid cancer, papillary thyroid carcinoma (PTC) accounts for 74–80% of thyroid cancer (2). Although the majority of cases of PTC are curable through thyroidectomy and conservative radioactive iodine therapy, a certain proportion of PTCs progresses to life-threatening recurrent metastatic cancer (3–5). Hence, a deeper understanding of the molecular markers of PTC is required to provide diagnostic and prognostic information on the tumors and to facilitate optimal decision-making regarding treatment (6). In recent years, several chromosomal rearrangements and BRAF mutations have been identified in PTCs (7–9). Nevertheless, the molecular mechanism of PTC pathogenesis remains incompletely understood and there is a lack of effective molecular targeted therapy for PTCs. Hence, the identification of reliable molecular markers of PTC is required.
Chaperonin containing TCP1 (CCT), also known as the TCP1 ring complex (TRiC), is a double-ring-shaped chaperonin complex composed of eight different subunits (CCT1-CCT8) (10). Approximately 10% of cytosolic peptides are folded with the assistance of CCT to properly form functional proteins (11). In addition to cytoskeletal proteins, including actin and tubulin, a number of cell cycle regulators, such as cadherin 1, cyclin E and cell decision cycle 20, and tumor suppressors, such as von Hippel-Lindau (VHL) factor, are also the substrates of CCT (12–15). Although the function of several CCT members, including CCT1, CCT2, CCT4 and CCT8, in cell proliferation, has been studied (16–18), few studies have assessed the effect of CCT3 on tumorigenesis. Recently, CCT3 was revealed to be highly expressed in hepatocellular carcinoma (HCC) (19). Overexpression of CCT3 is predictive of a poor prognosis in patients with HCC (20,21). Despite the emerging detrimental effect of CCT3 on HCC progression, it remains unknown whether CCT3 is also involved in the tumorigenesis of other types of cancer, including PTC.
The present study examined CCT3 expression in human PTC samples and investigated the functions of CCT3 in PTC cell proliferation, cell cycle progression and apoptosis. The results indicated that CCT3 expression was markedly increased in human PTC tissues compared with the matched normal adjacent tissues. The lentivirus-mediated knockdown of CCT3 reduced the mitotic progression and induced apoptosis in K1 cells. Taken together, the findings of the present study indicated that CCT3 increased PTC cell proliferation, implicating CCT3 as a promising molecular marker of PTC.
Materials and methods
Human samples
In total, 30 patients with papillary thyroid carcinoma were enrolled between June and December 2015 in the Inner Mongolia Autonomous Region People's Hospital (Hohhot, China). The median age of patients was 44 years (range, 26–65 years) at the time of surgery. Adjacent normal tissues were taken from the area >2 cm away from primary neoplasms. The study was approved by the Ethics Committee of Inner Mongolia Autonomous Region People's Hospital. Written informed consent was obtained from all patients.
Immunohistochemistry (IHC)
A total of 30 paired formalin-fixed, paraffin-embedded samples were subjected to immunohistochemistry staining of CCT3. Briefly, 4 µm sections of tissues were deparaffinized in xylene and rehydrated in descending alcohol series. Antigen-retrieval was performed by incubating 0.01 M boiled citrate buffer in a microwave for 20 min. After cooling to room temperature, slides were rehydrated in double distilled H2O for 10 min. The slides were then blocked with 10% goat serum (cat. no. C0265; Beyotime Institute of Biotechnology, Haimen, China) at room temperature for 30 min, followed by incubating with primary anti-CCT3 antibody (1:50; cat. no. ab174255; rabbit polyclonal; Abcam, Cambridge, UK) at 4°C overnight. Then, the slides were washed with TBS and incubating with the secondary antibodies (1:200; cat. no. sc-2004; goat anti-rabbit IgG-horse radish peroxidase (HRP); or 1:200; cat. no. sc-2005; goat anti-mouse IgG-HRP Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 60 min. After washing with TBS, the sections were stained by Vulcan Fast Red Chromogen kit 2 for 15 min at room temperature (Biocare, Shanghai, China). Inverted microscope (SDX-100; Caikon, Shanghai, China) was used for photographing with two magnifications (×200 and ×400). The extent and intensity of CCT3 immunostaining were taken into consideration. The intensity of extent of CCT3 expression was graded as follows: 0, negative; 1, weak; 2, moderate; and 4, strong. The extent of staining was grouped according to the percentage of high-staining cells in the cancer nest: 0, negative; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The final quantitation of each staining was obtained by multiplying the two scores. Immunoreactivity was assessed independently by two expert pathologists blinded to all clinical data.
Cell lines and cell culture
K1, a mixed cell line of thyroid gland papillary carcinoma cells (22) were obtained from the European Collection of Authenticated Cell Cultures (Public Health England, Porton Down, UK). B-CPAP cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). K1 cells were cultured in Dulbecco's modified Eagle's medium, nutrient mixture F-12 (1:1), supplemented with 10% fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Life Technologies; Thermo Fisher Scientific, Inc.). B-CPAP cells were cultured in RPMI 1640 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), completed by 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Life Technologies; Thermo Fisher Scientific, Inc.). All the cell cultures were maintained at 37°C in a humid atmosphere containing 5% CO2.
Packaging of lentivirus
The lentivirus vector system is composed of the vectors pGCSIL-GFP which stably expressed short hairpin RNA (shRNA) and green fluorescent protein (GFP), pHelper1.0 (gag/pol element) and pHelper2.0 (VSVG element). The vectors were purchased from Shanghai GeneChem Co., Ltd. (Shanghai, China). Target sequence of CCT3 shRNA, 5′-CAAGTCCATGATCGAAATT-3′; target sequence of the non-silencing control, 5′-GCGTCCTCATACCAGGATAAA-3′. The vectors were mixed and transfected into 293T cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After transfection for 48 h, pGCSIL-GFP supernatants were collected and centrifuged at 4°C at 75,000 × g for 2 h.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was obtained and purified using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Reverse transcription was performed using M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA). CCT3 expression was measured by qPCR using SYBR Advantage qPCR Premix (Takara Bio, Inc., Otsu, Japan), normalized to GAPDH and data analysis was conducted using the 2−ΔΔCq method (23). The qPCR primers used were as follows: CCT3 forward, 5′-TCAGTCGGTGGTCATCTTTGG-3′ and reverse, 5′-CCTCCAGGTATCTTTTCCACTCT-3′; and GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3. The thermocycling process of qPCR was as follows: 95°C for 30 sec, followed by 45 cycles of 95°C for 5 sec and 60°C for 30 sec.
Western blot analysis
CCT3 knockdown or control K1 cells were collected and lysed with Radioimmunoprecipitation Assay buffer (Beyotime Institute of Biotechnology) containing proteinase inhibitor cocktail (P8340; Sigma-Aldrich; Merck KGaA). Protein concentration was measured using a Pierce™ bicinchininc acid protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc., Waltham, MA, USA). A total of 60 µg of protein were subjected to 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Next the membranes were blocked in 5% fat-free milk for 2 h and incubated with anti-CCT3 (1:500; cat. no. ab174255; rabbit polyclonal, Abcam, Cambridge, UK) and anti-GAPDH (1:2,000; cat. no. sc-32233; mouse monoclonal; Santa Cruz Biotechnology, Inc.) primary antibodies overnight at 4°C. Following incubation with horseradish peroxidase conjugated goat anti-rabbit (1:2,000; cat. no. sc-2004) or goat anti-mouse IgG-HRP (1:2,000; sc-2005) secondary antibodies (all Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. The membranes were visualized using the ECL-Plus kit (GE Healthcare Life Sciences, Little Chalfont, UK).
High-content screening (HCS) for cell proliferation
Cell growth was measured using HCS. K1 cells were infected with negative control (NC) lentivirus or CCT3-shRNA lentivirus and cultured in 5% CO2 incubator at 37°C. Next, the cells were collected in the logarithmic growth phase and seeded in 96-well plates at a density of 4,000 per well. Cell images were captured and quantified everyday for 5 days using ArrayScan™ HCS 2.0 software (Cellomics; Thermo Fisher Scientific, Inc.).
MTT assay
MTT solution was used to measure cell growth with a microplate reader. Briefly, cells infected with NC lentivirus or CCT3-shRNA lentivirus were seeded in 96-well plates at a concentration of 2,000 cells per well and incubated in 5% CO2 incubator at 37°C for 1, 2, 3, 4 or 5 days. Next the cell supernatant was discarded and the cells were washed twice using ice-cold PBS and 5 mg/ml MTT solution was added to each well. After 4 h of incubation, remove the supernatants in each well and 100 µl dimethyl sulfoxide was added to solubilize the formazan salt. After 10 min, the optical density was measured at 490 nm by using a microplate reader.
Apoptosis assay
The Annexin V-Allophycocyanin (APC) apoptosis detection kit (eBioscience; Thermo Fisher Scientific, Inc.) was used to perform cell apoptosis analysis according to the manufacturer's instructions. Briefly, K1 cells were cultured in 6-well plates and infected with NC lentivirus or CCT3-shRNA lentivirus. Following incubation for 2 days, cells were collected and washed twice with ice-cold PBS. Cell densities were adjusted to 1×106-107/ml. Next 5 µl of Annexin V-APC was added into cell suspensions and cells were incubated at room temperature for 15 min. Cell apoptosis was measured using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and a BD FACStation™ 6.1 Software (BD Biosciences).
Cell cycle assay
Flow cytometry was used to determine the cell cycle distribution. Briefly, K1 cells were cultured in 6-well plates and were infected with NC lentivirus or CCT3-shRNA lentivirus in the logarithmic phase, as aforementioned. After incubation for 96 h, cells were seeded in 6-well culture plates and cultured to 80% confluence. Cell supernatants were removed and cells were fixed in 70% cold ethanol overnight at 4°C. Next, the cells were washed twice with ice-cold PBS and stained with propidium iodide (PI) buffer containing 10 mg/ml RNase at 37°C. Cell cycle distribution was then analyzed using a FACSCalibur flow cytometer (BD Biosciences) and a BD FACStation™ 6.1 Software (BD Biosciences).
Statistical analysis
The values are presented as the mean ± standard error of the mean of at least three independent experiments. Student's t-test was applied to analyze the difference between two groups and one-way analysis of variance to analyze the difference among three or more groups. All statistical analyses were performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicated a statistically significant difference.
Results
CCT3 is overexpressed in human papillary thyroid carcinoma
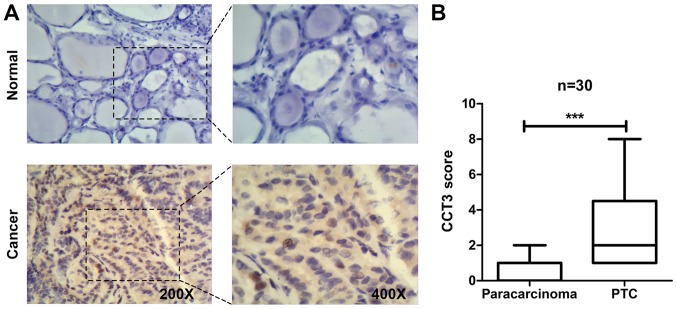
To investigate the involvement of CCT3 in PTC, CCT3 expression was examined in human PTC samples. A total of 30 samples were collected from PTC patients undergoing thyroidectomy. The expression of CCT3 was evaluated by IHC staining of CCT3 in these samples. The results revealed that there was little CCT3 staining in the normal paracarcinoma regions >2 cm away from the primary neoplasms. By contrast, in the PTC regions, CCT3 staining was high in the cytoplasm and in certain nuclei (Fig. 1A). Consistently, the quantitative scoring of CCT3 staining intensity revealed that CCT3 staining in PTCs was significantly higher than that in the matched adjacent normal tissues (Fig. 1B). These results indicated that CCT3 was overexpressed in the human PTC tissues.
Figure 1.
CCT3 is overexpressed in human papillary thyroid carcinoma. (A) CCT3 expression was examined by IHC staining of CCT3. Representative images of adjacent paracarcinoma tissues (upper) and PTC (lower) are shown. Original magnification, ×200; insets, ×400. (B) Quantification of CCT3 expression scoring based on the IHC images. n=30; ***P<0.001, paracarcinoma vs. PTC. CCT3, chaperonin containing TCP1 subunit 3; IHC, immunohistochemical; PTC, papillary thyroid carcinoma.
CCT3 is effectively knocked down in human papillary thyroid carcinoma cells
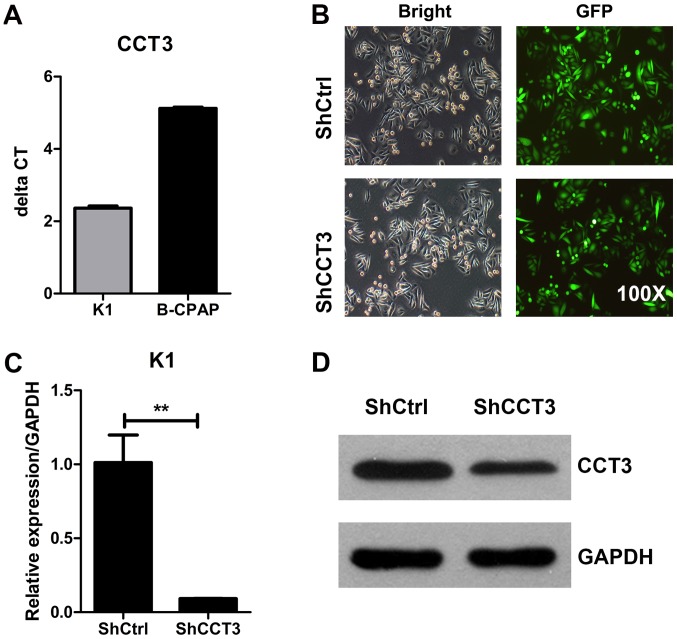
To investigate the role of CCT3 in the development of PTC, CCT3 was knocked down in human PTC cells and to investigate the cellular functions of CCT3. CCT3 mRNA expression was assessed in two human PTC cell lines, K1 (mixed thyroid gland papillary carcinoma cells) and B-CPAP cells. The results of this analysis revealed that the two cell lines expressed CCT3 mRNA, with K1 having a relatively higher level of CCT3 mRNA than B-CPAP cells (Fig. 2A). Hence, K1 cells were used to undergo knockdown of CCT3 expression.
Figure 2.
Knockdown of CCT3 in human papillary thyroid carcinoma cells. (A) The 2−ΔΔCq value indicating the relative expression of CCT3 in K1 and B-CPAP cells. (B) Infection efficiency as determined by light and fluorescence microscopy at 72 h Following lentiviral infection in K1 cells. Original magnification, ×200. Representative images of the cultures are shown. (C) At 5 days post-infection, CCT3 mRNA levels in K1 cells were measured by reverse transcription-quantitative polymerase chain reaction and normalized to GAPDH. n=3; data points are presented as the mean ± standard error of the mean. **P<0.01, shCtrl vs. shCCT3. (D) CCT3 protein expression was analyzed by western blot analysis in the shCtrl and shCCT3 K1 cells. GAPDH was used as an internal control. CCT3, chaperonin containing TCP1 subunit 3; shCtrl, control short hairpin RNA.
K1 cells were infected with lentivirus expressing shRNA targeting CCT3 (shCCT3) or a scramble control shRNA (shCtrl). Through the examination of the lentivirus-mediated GFP expression through fluorescence microscopy at 72 h after infection, >80% K1 cells in the shCCT3 and shCtrl groups were successfully infected (Fig. 2B). The results of RT-qPCR demonstrated that the expression of CCT3 mRNA in shCCT3 lentivirus-infected K1 cells was ~10% of that in the shCtrl lentivirus-infected K1 cells (Fig. 2C). Consistently, the results of western blot analysis revealed that CCT3 protein expression in K1 cells was markedly lower upon shCCT3 lentivirus infection than upon shCtrl virus infection (Fig. 2D). These results indicate the success of CCT3 knockdown using lentivirus in K1 cells.
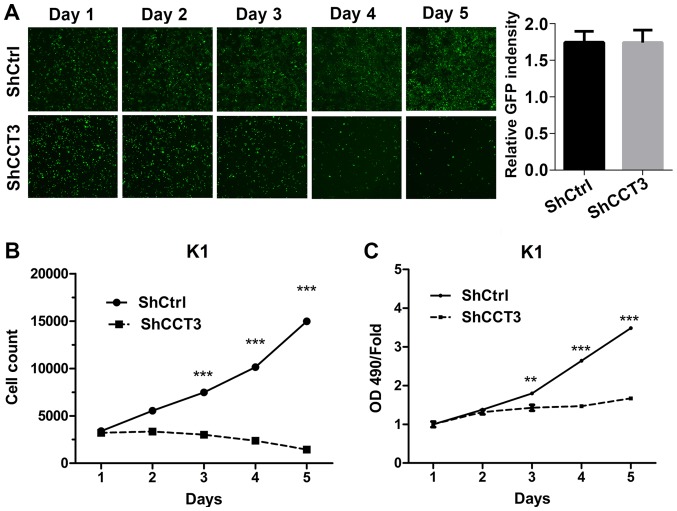
CCT3 knockdown suppresses the proliferation of papillary thyroid carcinoma cells
To investigate the effect of CCT3 knockdown on cell growth, shCtrl and shCCT3 lentivirus-infected K1 cells were seeded in 96-well plates at an equal concentration. The cell numbers were measured at days 1, 2, 3, 4 and 5. Using fluorescent microscopy, it was observed that the GFP-expressing cells were comparable in shCtrl and shCCT3 K1 cells in the first 2 days. However, the densities of GFP observed in shCCT3 K1 cells were lower than those in the shCtrl K1 cells from day 3 onwards (Fig. 3A). Cell counting results confirmed that the number of shCtrl K1 cells gradually increased, whereas the number of shCCT3 K1 cells slightly decreased, during the 5 days of culture (Fig. 3B). To confirm the suppressive effect of CCT3 knockdown on K1 cell proliferation, an MTT assay was performed. The results revealed that shCtrl K1 cells had steady growth throughout the 5 days (Fig. 3C). Although the survival number of shCCT3 K1 cells was similar to that of shCtrl K1 cells on the first 2 days, the growth of shCCT3 K1 cells was significantly reduced following this (Fig. 3C). Altogether, the cell counting and MTT assay results indicated that CCT3 knockdown suppressed the proliferation of K1 cells.
Figure 3.
CCT3 knockdown suppresses the proliferation of papillary thyroid carcinoma cells. (A) Representative fluorescence microscopy images of cell growth captured daily following lentivirus infection. (B) Post-infection daily cell counts were measured using an automated reader (n=3). (C) Post-infection cell proliferation as measured by MTT assays (n=3). Data points are presented as the mean ± standard error of the mean of absorbance values (OD 490 nm). **P<0.01, ***P<0.001, shCtrl vs. shCCT3. CCT3, chaperonin containing TCP1 subunit 3; shCtrl, control short hairpin RNA; OD, optical density; GFP, green fluorescent protein.
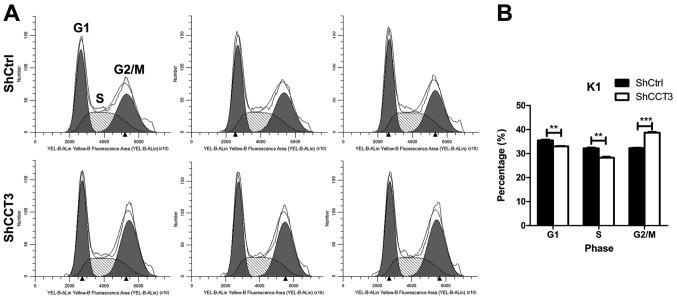
CCT3 knockdown affects cell cycle progression
To investigate the mechanism by which CCT3 knockdown suppresses the proliferation of K1 cells, the cell cycle distribution of K1 upon CCT3 knockdown was examined. After 96 h of shCCT3 or shCtrl lentivirus infection, K1 cells were stained with PI and analyzed by flow cytometry. The results revealed that the percentages of cells in G1 and S phase were lower in the shCCT3 group than in the shCtrl K1 group, whereas the percentage of G2/M phase in shCCT3 K1 cells was higher than that of shCtrl K1 cells (Fig. 4A and B). These results indicated that CCT3 knockdown affects the cell cycle progression of K1 cells, which may contribute to the suppression of K1 cell proliferation upon CCT3 knockdown.
Figure 4.
CCT3 knockdown affects cell cycle progression. (A) Cell cycle distribution of the K1 cells was analyzed by flow cytometry. Representative images for each group are shown. (B) Quantification of results shown in (A) (n=3). Data points are presented as the mean ± standard error of the mean. **P<0.01, ***P<0.001, shCtrl vs. shCCT3. CCT3, chaperonin containing TCP1 subunit 3; shCtrl, control short hairpin RNA.
CCT3 knockdown induces apoptosis
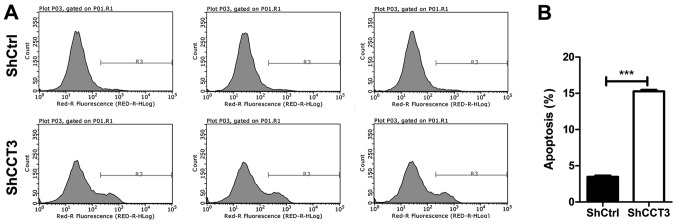
To determine whether CCT3 knockdown affected apoptosis, flow cytometry following Annexin V staining was performed in shCCT3 and shCtrl K1 cells. The results revealed that the percentage of apoptotic cells increased nearly four-fold in shCCT3 K1 cells in comparison with shCtrl K1 cells (Fig. 5A and B). These results indicated that CCT3 knockdown induced apoptosis in K1 cells.
Figure 5.
CCT3 knockdown induces apoptosis. (A) Apoptosis was determined by Annexin V staining followed by flow cytometry. Region R3 spans the fluorescence density of apoptotic cells. Representative images for each group are shown. (B) Quantification of results shown in (A) (n=3). ***P<0.001, shCtrl vs. shCCT3. CCT3, chaperonin containing TCP1 subunit 3; shCtrl, control short hairpin RNA.
Discussion
PTC is the most frequently occurring subtype of thyroid cancer. A certain portion of PTCs progresses to currently incurable recurrent metastatic cancer (1,24). However, there is currently no effective molecular targeted therapy to treat PTCs. The present study revealed that there was a significant increase in the expression of CCT3 in PTCs compared with adjacent tissue. Furthermore, knockdown of CCT3 decreased cellular proliferation and cell cycle progression and induced apoptosis in K1 cells. To the best of our knowledge, it is the first time to reveal the involvement of a CCT protein in the pathogenesis of PTC.
Recently, CCT3 was reported to be highly expressed in HCC, and the expression level of CCT3 was positively associated with the malignancy of the tumor (20). Notably, although CCT complex was deemed to function in the cytoplasm, CCT3 was observed in the nuclei of poorly differentiated HCC (20). Likewise, in the present study, it was observed that CCT3 expression was significantly increased in PTCs compared with the matched adjacent normal tissues. CCT3 was located at the cytoplasm and in certain nuclei of PTC cells. A prior study revealed that CCT3 has promise as a novel biomarker for HCC screening and diagnosis (21). Additionally, CCT3 was reported to support the proliferation of HCC cells (19). Overexpression of CCT3 was reported to be associated with the poor prognosis of patients with hepatocellular carcinoma (20). In the present study, it was found that an increase in CCT3 expression was associated with the tumor area of PTC. Knockdown of CCT3 reduced the proliferation of PTC cells. Hence, CCT3 might be a valuable PTC biomarker for diagnosis. Future study should verify whether CCT3 overexpression is also predictive of the prognosis of patients with PTC.
As a chaperonin complex, CCT was estimated to be responsible for the folding of ~10% of the proteome within the cell (25). Among the myriad substrates of CCT complex, there are a number of proteins associated with tumorigenesis, including cyclin E (13), the VHL tumor suppressor protein (26), cyclin B and p21 (27). Therefore, the CCT complex was shown to affect cancer cell proliferation (17). Specifically, as a subunit of CCT complex, CCT3 was reported to be required for the mitotic progression and proliferation of HCC cells (19). In the present study, CCT3 was successfully knocked down in K1 cells and found that CCT3 knockdown significantly reduced the proliferation and cell cycle progression of K1 cells. Cell cycle arrest at the G2/M was observed in CCT3-silenced K1 cells, which was accompanied with decreased G1 and S phase distribution. It is known that DNA damage checkpoint is a notable factor for G2/M arrest. This indicated that CCT3 silencing could lead to dysregulated DNA function and thereby the prevention of mitosis. In a previous report, CCT3 depletion was shown to enhance the sensitivity of HCC cells to vincristine treatment (19). In the present study, it was observed that CCT3 knockdown induced apoptosis in K1 cells. Furthermore, the enhanced G2/M cycle arrest could also result in increased apoptosis following CCT3 knockdown. Hence, CCT3 might be crucial for the survival and growth of PTC cells, and CCT3 may represent utilized as a molecular target for PTC treatment.
The present study revealed that CCT3 was involved in the pathogenesis of PTC. A marked increase in CCT3 expression in PTC tissues compared with the adjacent normal tissues. In addition, it was demonstrated that CCT3 knockdown compromised the proliferation and cell cycle progression of PTC cells. The present study also demonstrated that knockdown of CCT3 induced PTC cell apoptosis. Therefore, the findings of the current study implicate CCT3 as a notable oncogene of PTC and that CCT3 may be a potential candidate for the molecular diagnosis and treatment of PTC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- PTC
papillary thyroid carcinoma
- CCT
chaperonin containing TCP1
- TRiC
TCP1 ring complex
- VHL
von Hippel-Lindau
- IHC
immunohistochemistry
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- PI
propidium iodide
Funding
No funding received.
Availability of data and materials
The datasets generated/analyzed in the present study are available on reasonable request from the corresponding author.
Authors' contributions
WW conceived the study and carried out the experimental design, data interpretation, prepared and revised the manuscript. XS performed the majority of the experiments and SC performed the high-content screening assay.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Inner Mongolia Autonomous Region People's Hospital. Written informed consent was obtained from all patients.
Consent for publication
All study participants provided written informed consent for the publication of the present study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barollo S, Pezzani R, Cristiani A, Redaelli M, Zambonin L, Rubin B, Bertazza L, Zane M, Mucignat-Caretta C, Bulfone A, et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid. 2014;24:809–819. doi: 10.1089/thy.2013.0403. [DOI] [PubMed] [Google Scholar]
- 3.Pelizzo MR, Boschin Merante I, Toniato A, Pagetta C, Casal Ide E, Mian C, Rubello D. Diagnosis, treatment, prognostic factors and long-term outcome in papillary thyroid carcinoma. Minerva Endocrinol. 2008;33:359–379. [PubMed] [Google Scholar]
- 4.Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. 2008;113:48–56. doi: 10.1002/cncr.23515. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum MA, McHenry CR. Contemporary management of papillary carcinoma of the thyroid gland. Expert Rev Anticancer Ther. 2009;9:317–329. doi: 10.1586/14737140.9.3.317. [DOI] [PubMed] [Google Scholar]
- 6.Learoyd DL, Messina M, Zedenius J, Robinson BG. Molecular genetics of thyroid tumors and surgical decision-making. World J Surg. 2000;24:923–933. doi: 10.1007/s002680010164. [DOI] [PubMed] [Google Scholar]
- 7.Legakis I, Syrigos K. Recent advances in molecular diagnosis of thyroid cancer. J Thyroid Res. 2011;2011:384213. doi: 10.4061/2011/384213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 10.Liou AK, Willison KR. Elucidation of the subunit orientation in CCT (chaperonin containing TCP1) from the subunit composition of CCT micro-complexes. EMBO J. 1997;16:4311–4316. doi: 10.1093/emboj/16.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yam AY, Xia Y, Lin HT, Burlingame A, Gerstein M, Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol. 2008;15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brackley KI, Grantham J. Activities of the chaperonin containing TCP-1 (CCT): Implications for cell cycle progression and cytoskeletal organisation. Cell Stress Chaperones. 2009;14:23–31. doi: 10.1007/s12192-008-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won KA, Schumacher RJ, Farr GW, Horwich AL, Reed SI. Maturation of human cyclin E requires the function of eukaryotic chaperonin CCT. Mol Cell Biol. 1998;18:7584–7589. doi: 10.1128/MCB.18.12.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camasses A, Bogdanova A, Shevchenko A, Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol Cell. 2003;12:87–100. doi: 10.1016/S1097-2765(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 15.Melville MW, McClellan AJ, Meyer AS, Darveau A, Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol Cell Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Lin CY, Lei M, Yan S, Zhou T, Erikson RL. CCT chaperonin complex is required for the biogenesis of functional Plk1. Mol Cell Biol. 2005;25:4993–5010. doi: 10.1128/MCB.25.12.4993-5010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudiaf-Benmammar C, Cresteil T, Melki R. The cytosolic chaperonin CCT/TRiC and cancer cell proliferation. PLoS One. 2013;8:e60895. doi: 10.1371/journal.pone.0060895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Wang X, Cheng C, Cai J, He S, Wang H, Liu F, Zhu C, Ding Z, Huang X, et al. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. Apmis. 2014;122:1070–1079. doi: 10.1111/apm.12258. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang Y, Wei Y, Wu J, Zhang P, Shen S, Saiyin H, Wumaier R, Yang X, Wang C, Yu L. Molecular chaperone CCT3 supports proper mitotic progression and cell proliferation in hepatocellular carcinoma cells. Cancer Lett. 2016;372:101–109. doi: 10.1016/j.canlet.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Cui X, Hu ZP, Li Z, Gao PJ, Zhu JY. Overexpression of chaperonin containing TCP1, subunit 3 predicts poor prognosis in hepatocellular carcinoma. World J Gastroenterol. 2015;21:8588–8604. doi: 10.3748/wjg.v21.i28.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian EN, Han SY, Ding SZ, Lv X. Expression and diagnostic value of CCT3 and IQGAP3 in hepatocellular carcinoma. Cancer Cell Int. 2016;16:55. doi: 10.1186/s12935-016-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro FR, Meireles AM, Rocha AS, Teixeira MR. Conventional and molecular cytogenetics of human non-medullary thyroid carcinoma: Characterization of eight cell line models and review of the literature on clinical samples. BMC Cancer. 2008;8:371. doi: 10.1186/1471-2407-8-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Sabra MM, Dominguez JM, Grewal RK, Larson SM, Ghossein RA, Tuttle RM, Fagin JA. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab. 2013;98:E829–E836. doi: 10.1210/jc.2012-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitner A, Joachimiak LA, Bracher A, Mönkemeyer L, Walzthoeni T, Chen B, Pechmann S, Holmes S, Cong Y, Ma B, et al. The molecular architecture of the eukaryotic chaperonin TRiC/CCT. Structure. 2012;20:814–825. doi: 10.1016/j.str.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen WJ, Ohh M, Moslehi J, Kondo K, Kaelin WG, Welch WJ. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol. 2002;22:1947–1960. doi: 10.1128/MCB.22.6.1947-1960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melki R, Batelier G, Soulié S, Williams RC., Jr Cytoplasmic chaperonin containing TCP-1: Structural and functional characterization. Biochemistry. 1997;36:5817–5826. doi: 10.1021/bi962830o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated/analyzed in the present study are available on reasonable request from the corresponding author.