Abstract
Sphingosine kinase 1 (SphK1) is a tumor-associated protein overexpressed in numerous types of cancer and is involved in the regulation of resistance to multiple chemotherapeutic agents. However, the role of SphK1 in the resistance of hepatocellular carcinoma (HCC) to oxaliplatin remains unclear. In the present study, the transcriptional levels of SphK1 were analyzed in 21 patients with HCC and the SphK1 expression levels were identified to be significantly upregulated in HCC tissue compared with that in adjacent normal tissue samples (P<0.001). High SphK1 expression correlated with shorter overall survival times in patients with HCC (P<0.05). Furthermore, SphK1 expression levels and activity were analyzed in a series of HCC cell lines and they were both demonstrated to be associated with resistance to oxaliplatin. Conversely, the knockdown of SphK1 protein expression resulted in decreased oxaliplatin resistance in SK-Hep1 and HCCLM3 cell lines. In addition, the results of the current study demonstrated that the downregulation of SphK1 decreased the levels of phosphorylated AKT serine/threonine kinase (Akt) and glycogen synthase kinase-3β (GSK3β), suggesting that SphK1 promotes oxaliplatin resistance of HCC cells via modulation of the Akt/GSK3β signaling pathway. To the best of our knowledge, the present study is the first to report that SphK1 is associated with poor prognosis and oxaliplatin resistance in HCC. Thus, the findings of the current study have provided a direction for the identification of novel therapeutic targets for the treatment of HCC.
Keywords: sphingosine kinase 1, hepatocellular carcinoma, oxaliplatin resistant, poor prognosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third most common cause of cancer-related deaths. Although the diagnosis and treatment of HCC have greatly improved, more than 1.23 million new cases are diagnosed each year and the 5-year survival rate is still <15% (1,2). Surgical resection is the important way to cure the HCC, unfortunately, most patients (approximately 80%) with HCC are unsuitable for surgery because of early invasion and extrahepatic metastases, underlying liver disease or comorbidities (3). Therefore, surgical resection combined with chemotherapy or chemotherapy alone was used as a hope to prolong the patient's life. However, abnormal metabolic environment of HCC cells will restrict the curative effect and survival, because of resistance to chemotherapy after long-term drugs used (4,5). For example, Etoposide, 5-Fluorouracil, Cisplatin, and their combinations have demonstrated multi-resistance for the treatment of HCC (6). Oxaliplatin, a third-generation platinum compound, has been widely used in the treatment of a variety of cancers and achieved excellent results in clinical applications (7). It is also considered to be a potential advantage drug of HCC because of the few cytotoxic in terms of nephrotoxicity and myelosuppression (8). Previous studies showed that oxaliplatin could increase the autophagy role of HCC cells, which eventually result in a tumor resistance response in HCC (8). However, the mechanism of oxaliplatin-resistance in HCC remains undetermined.
Sphingosine kinase is a conserved lipid kinase that catalyzes the phosphorylation of sphingosine to form sphingosine-1-phosphate (S1P), which potentially regulated the biological processes, including cell proliferation, motility, differentiation, apoptosis, and angiogenesis (9). Two isoforms of mammalian Sphingosine kinase 1 and 2 (SphK1 and SphK2) have been cloned and characterized (9). Recently, the role of SphKs in cancer (SphK1 in particular) has received considerable attention. Elevated expression of SphK1 has been observed in multiple types of cancer, and promoted cell survival and growth (10,11). Furthermore, high SphK1 expression has been associated with poor prognosis in patients with glioblastoma or breast cancer (12,13). Previous studies also found that blockade of the SphK1 signaling pathway may reprogram cellular responsiveness to tamoxifen and abrogate antiestrogen resistance in human breast cancer cells (14). SphK1 also involved the resistance of chemotherapy drugs in colorectal cancer and pancreatic cancer (15,16), but little is known about the SphK1 in HCC.
In the present study, we demonstrate that high SphK1 expression levels were correlated with shorter overall survival, and it is one of the important factors that facilitates resistance to the oxaliplatin in HCC. Furthermore, knockdown of SphK1 markedly suppressed the phosphorylations of AKT serine/threonine kinase and glycogen synthase kinase-3β (Akt and GSK3β) in SK-Hep1 cells, and suggested that SphK1 promote oxaliplatin resistance of HCC cell via modulating the Akt/GSK3β pathway. Thus, these findings suggest that the cellular response to oxaliplatin can be reprogrammed by manipulation of the SphK1 pathway, and may provide a new way to overcome oxaliplatin resistance in the treatment of HCC.
Materials and methods
Patients and tissue samples
A total of 21 paired human primary HCC tissues and adjacent non-tumors tissues (at least 3 cm from the edge of tumor) were obtained from patients who were recruited into a clinical trial at Xinchang People's Hospital between 2008 and 2010. The 21 HCC patients were classified into two groups according to the median level of SphK1 expression: The median level and above considered as high expression (n=11), and lower than the median level considered as low (n=10). All patients have no received irradiation or chemotherapy before surgery and the final diagnosis was based on the pathological diagnosis after surgery. The present study was approved by the ethics review board of Xinchang People's Hospital, and all patients signed informed consent forms.
Cell culture and transfection
Five HCC cell lines (Hep3B, HepG2, HCCLM3 and SK-Hep1) were obtained from the American Type Culture Collection (ATCC), and normal liver cell line (HL-7702) was kept at our laboratory. All cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen). For transfection, HCCLM3 and SK-Hep1 cells were seeded in 12-well plates (2×105 cells/well). After 18 h, the cells were transfected with 2 targeting SphK1 siRNA oligonucleotides or nonsilencing siRNA oligonucleotides (Qiagen AB, Sollentuna, Sweden) by using the HiPerFect Transfection Reagent (Qiagen) according to the manufacturer's protocol. After 72 h of incubation, cells were collected for western blotting and qRT-PCR analysis.
Measurement of mRNA expression level by quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues or cultured cells using TRIzol reagent (Invitrogen) as the manufacturer's protocol. For mRNA analyses, 1.0 µg of total RNA was used to synthesize cDNA using the RevertAid™ First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal amounts of cDNAs were taken for a real-time quantitative PCR (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using SYBR-Green as a dye (Roche, Mannheim, Germany). The relative quantity of SphK1 mRNA was normalized using the 18S rRNA as control. The following primers were used: SphK1 forward primer, 5′-CTGGCAGCTTCCTTGAACCAT-3′, and reverse primer, 5′-TGTGCAGAGACAGCAGGTTCA-3′; SphK2 forward primer, 5′-GCTCAACTGCTCACTGTTGC-3′, and reverse primer, 5′-GCAGGTCAGACACAGAACGA-3′; 18S rRNA forward primer, 5′-AAACGGCTACCACATCCAAG-3′, and reverse primer, 5′-CCTCCAATGGATCCTCGTTA-3′. All reactions were performed in triplicate.
Sphingosine kinase assay
SphK1 activity was measured by using Sphingosine Kinase 1 Assay kit (Novus Biologicals, Littleton, CO, USA) according to the manufacturer's instructions. Firstly, protein extracts (30 µg) were incubated in reaction buffer, 100 µM sphingosine and 10 µM ATP for 1 h at 37°C. Then, luminescence attached ATP detector was then added to stop the kinase reaction. Kinase activity was measured using BioTek Microplate Readers (BioTek, Winooski, VT, USA). The results were obtained from 3 independent experiments, each reactions were performed in triplicate.
MTT survival assay
Oxaliplatin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 100% dimethyl sulfoxide and diluted with DMEM to the desired concentration. The effects of oxaliplatin on HCC cells survival were determined by MTT (Sigma-Aldrich) assay. Cells were seeded into 96-well plates (5×103 cells/well) and cultured for 48 h with oxaliplatin concentrations between 0, 20, 40 and 60 µg/ml. After treatments, cells were incubated with 20 µl MTT to each well and then incubated at 37°C for 4 h. Following the incubation, MTT solution was removed and added 200 µl DMSO into each well to dissolve the formazan crystals. The plates were shook for 5 min and subjected to absorbance reading at 570 nm. Percentage of cell survival was determined as [(Mean OD of test wells-Mean OD of blank group)/(Mean OD of negative group-Mean OD of blank group)]x100%. The results were obtained from 3 independent experiments, each reactions were performed in triplicate.
Western blot analysis
For western blot analysis, cells were lysed with RIPA lysis buffer supplemented with cOmplete Protease Inhibitor EASYpacks EDTA-Free (Roche). The lysate were subject to centrifugation at 12,000 g for 10 min at 4°C and remove the cell debris. Then, supernatant concentrations were measured by the BCA Assay kit (Beyotime Biotech, Jiangsu, China) according to the standard protocol. Sample (50 µg/lane) were separated by 10% SDS-PAGE. The separated proteins were transferred to PVDF western blot analysis membrane (Roche) and probed with rabbit anti-SphK1 (1:1,500; Abcam, Cambridge, UK), SphK2 (1:2,000; Abcam), Akt (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Phospho-Akt (Ser473) (1:1,000; Santa Cruz), GSK3β (1:1,000; CST, Beverly, MA, USA), Phospho-GSK3β (1:1,000; CST) and β-actin (1:1,000; CST) primary antibodies. Target protein bands visualized were detected with HRP-linked goat anti-rabbit secondary antibodies (1:2,000; Abcam).
Clonogenic survival assay
Log-phase cells were plated overnight and treated with 60 µg/ml oxaliplatin for 48 h. After treatment, cells were harvested, and 500 cells/well were plated in triplicate into 60 mm dishes. After two weeks, cells were fixed using 4% parafor-maldehyde and stained with crystal violet. The number of colonies per plate was assessed and then expressed as percent survival relative to untreated cells.
Statistical analysis
Data analyses were performed using SPSS 15.0. All data are presented as means ± SEM of a minimum of three or more replicates. P<0.05 was considered to indicate a statistically significant difference..
Results
Upregulation of SphK1 correlates with poor prognosis in HCC
To investigate the role of SphK in HCC, we collected tumor tissues and adjacent non-tumors tissues from 21 HCC patients (Table I), and the transcriptional levels of SphK1 and SphK2 were analyzed by qRT-PCR. Our results demonstrated that the transcriptional levels of SphK1, but not SphK2, were significantly upregulated in HCC tissues compared with that in adjacent non-tumors tissues (Fig. 1A-B). Additionally, Kaplan-Meier survival analysis was used to further evaluate the correlations between the SphK1 transcriptional levels and prognosis in patients with HCC. The 21 patients with HCC were classified into a high expression group (n=11) and a low expression group (n=10). The result showed that patients with high SphK1 expression had significantly shorter overall survival than those with low SphK1 expression (Fig. 1C).
Table I.
Association between Sphk1 expression and clinicopathological features of 21 hepatocellular carcinoma patients.
| Characteristics | Cases (21) | Sphk1 mRNA expression | P-value |
|---|---|---|---|
| Gender | 0.612 | ||
| Male | 11 | 15 | |
| Female | 10 | 8 | |
| Age, years | 0.463 | ||
| <50 | 15 | 14 | |
| ≥50 | 6 | 9 | |
| Tumor stage | 0.048 | ||
| I/II | 12 | 5 | |
| III/IV | 9 | 11 | |
| Tumor size, cm | 0.023 | ||
| <5 | 25 | 9 | |
| ≥5 | 22 | 14 | |
| Lymph node metastasis | 0.135 | ||
| Negative | 18 | 15 | |
| Positive | 3 | 9 |
Sphk1, sphingosine kinase 1.
Figure 1.
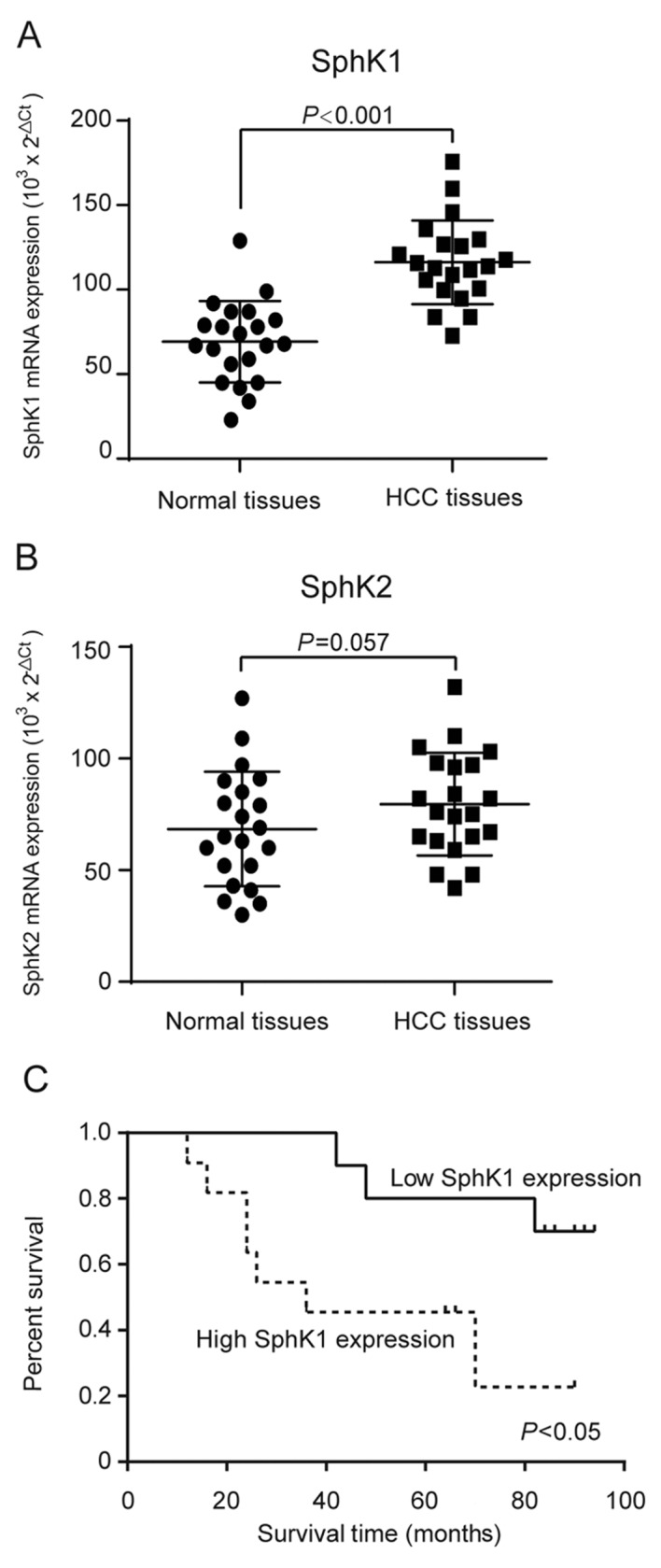
Upregulation of SphK1 correlates with poor prognosis in HCC. qRT-PCR analysis of relative (A) SphK1 and (B) SphK2 expression levels in 21 HCC tissues and the adjacent non-tumors tissues. Data were normalized to the expression of 18S rRNA. Each sample was analyzed in triplicate. (C) Kaplan-Meier survival curve for HCC patients with high SphK1 expression (n=11) and low SphK1 mRNA expression (n=10). The survival data were compared using the log-rank test. SphK, Sphingosine kinase; HCC, hepatocellular carcinoma.
SphK1 is overactivated in the oxaliplatin-resistant HCC cell lines
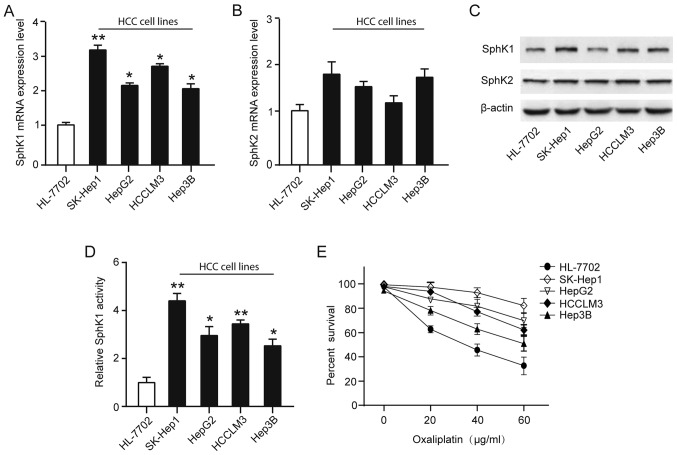
On the basis of the suggested correlation between SphK and resistance to anticancer-targeted agents, we analyzed SphK1 and SphK2 expression and activity in four human HCC cell lines (Hep3B, HepG2, HCCLM3 and SK-Hep1) and normal liver cell line (HL-7702). Although both SphK1 and SphK2 isoforms are responsible for total cellular SphK activity, we found the transcriptional levels of SphK1, but not SphK2, were significantly upregulated in HCC cell lines compared with that in normal liver cell line (Fig. 2A-B). Similarly, SphK1 protein expression levels were significantly upregulated in HCC cell lines, especially in SK-Hep1, a high invasiveness and metastasis cell line (Fig. 2C). To explore whether HCC cell lines resistance toward the oxaliplatin correlates with a dysregulation of the SphK1, a cell survival assay using increasing concentrations of oxaliplatin (0–60 µg/ml) for 48 h showed a greater resistance to oxaliplatin of HCC cell lines, and found the activity of SphK1 were positively associated with resistance to oxaliplatin (Fig. 2D-E). Taken together, these observations suggest that the elevated levels in SphK1 expression and activity in HCC cells may associate with the oxaliplatin-resistant phenotype.
Figure 2.
SphK1 is overactivated in the oxaliplatin-resistant HCC cell lines. qRT-PCR analysis of relative (A) SphK1 and (B) SphK2 mRNA expression levels in four HCC cell lines and normal liver cell line (HL-7702). (C) Western blot analysis of relative SphK1 and SphK2 protein expression levels in four HCC cell lines and normal liver cell line (HL-7702). (D) SphK1 activity was measured by using SphK1 Activity Assay kit. (E) Percentage of survival of cells treated with increasing doses of oxaliplatin (0–60 µg/ml) after 48 h, as measured by the MTT assay. *P<0.05; **P<0.01. SphK, Sphingosine kinase; HCC, hepatocellular carcinoma.
Downregulation of SphK1 decrease resistant to oxaliplatin in HCC cells
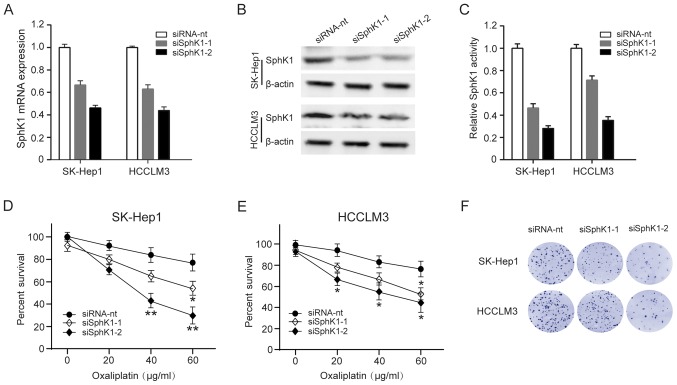
To investigate whether downregulating SphK1 activity in HCC cells might decrease resistant to oxaliplatin, we used 2 siRNAs to deplete SphK1 in SK-Hep1 and HCCLM3 cells, and the effectiveness was analyzed by qRT-PCR and western blot analysis (Fig. 3A-B). At the same time, SphK1 activity was also decrease in SK-Hep1 and HCCLM3 cells (Fig. 3C). We then explored whether decreasing SphK1 activity affects SK-Hep1 and HCCLM3 cells survival and sensitivity to oxaliplatin. Compared with mock-transfected cells, both SK-Hep1 and HCCLM3 cells were less resistant to oxaliplatin by a cell survival assay analysis (Fig. 3D-E). Similarly, clonogenic survival of SK-Hep1 and HCCLM3 cells was also significantly compromised in SphK1-depleting cells (Fig. 3F). These results indicate that downregulation of SphK1 make HCC cells sensitive to oxaliplatin-induced cell death.
Figure 3.
Downregulation of SphK1 decrease resistant to oxaliplatin in HCC cells. SK-Hep1 and HCCLM3 cells were transfected with a non-targeting siRNA (siRNA-nt) and 2 SphK1-targeting siRNA (siSphK1-1, −2). Three days after transfection, cells were collected for (A) qRT-PCR and (B) western blot analysis. (C) SphK1 activity was measured in cells transfected with a non-targeting siRNA (siRNA-nt) and 2 SphK1-targeting siRNA (siSphK1-1, −2) by using SphK1 Activity Assay kit. percentage of survival of (D) SK-Hep1 and (E) HCCLM3 cells treated with increasing doses of oxaliplatin (0–60 µg/ml), as measured by the MTT assay. (F) Clonogenic assay of cells were treated with 60 µg/ml oxaliplatin for 48 h, the number of colonies per plate was assessed after two weeks. *P<0.05; **P<0.01. SphK, Sphingosine kinase; HCC, hepatocellular carcinoma; siRNA, small interferring RNA.
SphK1 promotes HCC cells survival through activating Akt/GSK3β signaling pathway
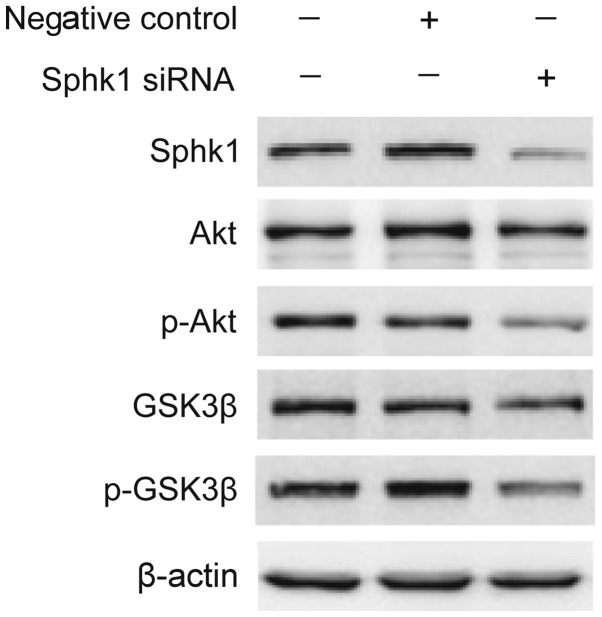
High levels of SphK1 have been related to chemotherapy drug resistance in certain cancers, which are closely related with maintaining cancer signaling pathways (17,18). Previous studies have shown that inhibition of SphK1 contributes to apoptosis by Akt signaling pathways (19,20). Therefore, we speculated whether SphK1 affect HCC cells survival in oxaliplatin through Akt signaling pathways. As shown in Fig. 4, knockdown of SphK1 markedly suppressed the phosphorylations of Akt and GSK3β in SK-Hep1 cells, while there was no signicantly reduction in the level of Akt and GSK3β protein. Therefore, we speculate that SphK1 promotes HCC cells survival in oxaliplatin through activating Akt/GSK3β signaling pathway.
Figure 4.
SphK1 promotes HCC cells survival through activating Akt/GSK3β signaling pathway. SK-Hep1 cells were transfected with the SphK1-targeting siRNA. Three days after transfection, the p-Akt, Akt, p-GSK3β and GSK3β protein expression levels were determined by western blot analysis. β-actin was used as internal control for normalization in western blot analysis. SphK, Sphingosine kinase; HCC, hepatocellular carcinoma; siRNA, small interferring RNA..
Discussion
HCC is characterized as a highly chemoresistant cancer with no effective systemic therapy. Although patients have been given surgical or locoregional therapies, they often have poor prognosis because of high tumor recurrence or tumor progression. Therefore, identifying biological markers associated with the resistance of HCC is important for providing the scientific rationale for the development of new therapeutic targets.
Sphingolipid metabolites are ceramide, sphingosine, ceramide-1-phosphate (C1P), and sphingosine-1-phosphate (S1P) (9). These lipid mediators involved in cells life as bioactive signaling molecules, for example, ceramide and sphingosine can active the apoptosis pathways whereas S1P and C1P primarily exert mitogenic effects (21,22). Therefore, the balance between lipid mediators has been considered as a converter determining the biological processes of cells (21,22). SphK1, as one of the key enzymes, regulates the ceramide/S1P conversion that contributes to determining whether a cell undergoes apoptosis or proliferates (17,23). Previous studies found that SphK1 is elevated in various types of cancers, functioning as an oncogene in tumorigenesis, however, little is known about the role of SphK1 in HCC progression. In the present study, we found that SphK1 is upregulated in HCC and that high expression of SphK1 significantly associated with poorer overall survival in HCC patients.
Overexpression of SphK1 may confer the development of resistance to chemotherapeutics, whereas disruption of SphK1/S1P signaling may restore or improve chemotherapy sensitivity (24). For example, raised SphK1 has been associated with chronic myeloid leukemia resistance to imatinib and pancreatic cancer cell resistance to gemcitabine (16,25). Conversely, SphK1 inhibition can sensitize CML and pancreatic cancer cells to the proapoptotic effects of gemcitabine and imatinib, respectively, apparently by increasing the ceramide/S1P ratio and thereby enabling C18-ceramide-dependent apoptosis to proceed (16,25). Moreover, blockade of the SphK1 signaling pathway may reprogram cellular responsiveness to tamoxifen and abrogate antiestrogen resistance in human breast cancer cells (14). Consistent with previous studies, our results found that knockdown of SphK1 by siRNA markedly surpressed the ability of resistant oxaliplatin to in HCC cells.
Although our evidence has highlighted the oxaliplatin resistence effect of SphK1 in HCC, the molecular mechanism is still unclear. To explore the mechanisms underlying the resistence effects of SphK1, we tested the effect of SphK1 on the AKT/GSK3β pathways, which have been demonstrated to be involved in tumor progression (24). We have demonstrated in the present study that p-Akt and p-GSK3β was downregulated in SphK1-knocked down HCC cells, suggested Akt/GSK3β activation through SphK1 signaling is associated with oxaliplatin resistance in HCC cells. However, the molecular mechanism underlying SphK1-mediated activation of Akt/GSK3β pathways, as well as its biological outcome, needs to be further delineated. In addition, several other issues also remain to be addressed. For example, it would be very meaningful to know whether other pathways are also involved in mediating the resistant effect of SphK1 in HCC cells, and what other malignant phenotypes of HCC cells could also be modulated by upregulated SphK1. These issues are under further investigation in the laboratory. Nevertheless, understanding the role of SphK1 in HCC progression will not only advance our knowledge of the mechanisms underlying HCC survival, but also will help establish SPHK1 as a potential therapeutic target for the treatment of HCC.
References
- 1.Stewart B, Wild CP. World cancer report 2014. IARC Press; Lyon: 2003. World Health Organization. [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 5.Thomas MB, O'Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: Cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15:1008–1014. doi: 10.1245/s10434-007-9705-0. [DOI] [PubMed] [Google Scholar]
- 6.Uzma A, Tim M. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol. 2012;56:686–695. doi: 10.1016/j.jhep.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Alcindor T, Beauger N. Oxaliplatin: A review in the era of molecularly targeted therapy. Curr Oncol. 2011;18:18–25. doi: 10.3747/co.v18i1.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du H, Yang W, Chen L, Shi M, Seewoo V, Wang J, Lin A, Liu Z, Qiu W. Role of autophagy in resistance to oxaliplatin in hepatocellular carcinoma cells. Oncol Rep. 2012;27:143–150. doi: 10.3892/or.2011.1464. [DOI] [PubMed] [Google Scholar]
- 9.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu, Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 10.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 11.Heffernan-Stroud LA, Obeid LM. Sphingosine kinase 1 in cancer. Adv Cancer Res. 2012;117:201–235. doi: 10.1016/B978-0-12-394274-6.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: Roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 13.Ruckhäberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grösch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- 14.Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–4492. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 15.Rosa R, Marciano R, Malapelle U, Formisano L, Nappi L, D'Amato C, D'Amato V, Damiano V, Marfè G, Del Vecchio S, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2012;19:138–147. doi: 10.1158/1078-0432.CCR-12-1050. [DOI] [PubMed] [Google Scholar]
- 16.Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, Delisle MB, Cuvillier O, Susini C, Bousquet C. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther. 2009;8:809–820. doi: 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- 17.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song L, Xiong H, Li J, Liao W, Wang L, Wu J, Li M. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin Cancer Res. 2011;17:1839–1849. doi: 10.1158/1078-0432.CCR-10-0720. [DOI] [PubMed] [Google Scholar]
- 20.Ji F, Mao L, Liu Y, Cao X, Xie Y, Wang S, Fei H. K6PC-5, a novel sphingosine kinase 1 (SphK1) activator, alleviates dexamethasone-induced damages to osteoblasts through activating SphK1-Akt signaling. Biochem Biophys Res Commun. 2015;458:568–575. doi: 10.1016/j.bbrc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, Salas A, Ogretmen B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–1624. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50:104–114. doi: 10.1016/j.plipres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 24.Ader I, Malavaud B, Cuvillier O. When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: New targets for cancer therapy. Cancer Res. 2009;69:3723–3726. doi: 10.1158/0008-5472.CAN-09-0389. [DOI] [PubMed] [Google Scholar]
- 25.Marfe G, Di Stefano C, Gambacurta A, Ottone T, Martini V, Abruzzese E, Mologni L, Sinibaldi-Salimei P, de Fabritis P, Gambacorti-Passerini C, et al. Sphingosine kinase 1 overexpression is regulated by signaling through PI3K, AKT2, and mTOR in imatinib-resistant chronic myeloid leukemia cells. Exp Hematol. 2011;39:653–665.e6. doi: 10.1016/j.exphem.2011.02.013. [DOI] [PubMed] [Google Scholar]