Abstract
The expression of high mobility group box 1 (HMGB1), breast cancer susceptibility gene 1 (BRCA1) and P62 in ovarian cancer was investigated to explore its association with chemotherapy sensitivity in ovarian cancer patients. Tumor tissues and para-carcinoma normal tissues of 60 ovarian cancer patients hospitalized in Department of Surgery in Dongying Hospital from June, 2012 to June, 2015 were collected. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to detect the mRNA expression levels of HMGB1, BRCA1 and P62 in tumor and para-carcinoma normal tissues. Moreover, immunohistochemistry was used to detect the protein expression of HMGB1, BRCA1 and P62 in tumor tissues and para-carcinoma normal tissues. The cancer tissue specimens were divided into the chemotherapy resistance group and sensitivity group through the in vitro resin droplet experiment to analyze the association of the expression of HMGB1, BRCA1 and P62 in epithelial ovarian cancer with chemotherapy resistance of patients. The RT-qPCR results showed that the expression of HMGB1, BRCA1 and P62 in ovarian cancer tissues at the mRNA level was significantly higher than that in para-carcinoma normal tissues. Immunohistochemical results showed that the positive expression levels of HMGB1, BRCA1 and P62 in ovarian carcinoma tissue were 61.67% (37/60), 76.33% (47/60) and 71.67% (43/60), respectively, while the positive expression levels of HMGB1, BRCA1 and P62 in para-carcinoma normal tissues were 13.33% (8/60), 8.33% (5/60) and 11.67% (7/60), respectively, and the differences were statistically significant (P<0.05). In vitro resin droplet experiment revealed that 38 out of 60 ovarian cancer patients were drug resistant and 22 patients were sensitive to the therapy. The analysis of the association with chemotherapy sensitivity revealed that the positive expression of HMGB1, BRCA1 and P62 was associated with the drug resistance of ovarian cancer patients. The positive expression of HMGB1, BRCA1 and P62 was associated with chemotherapy sensitivity of ovarian cancer patients. Therefore, HMGB1, BRCA1 and P62 may be molecular markers for the prediction of chemotherapy sensitivity of ovarian cancer patients.
Keywords: ovarian cancer, high mobility group box 1, breast cancer susceptibility gene 1, P62, chemotherapy sensitivity
Introduction
Ovarian cancer is a common malignant tumor in women and its morbidity and mortality are high (1). At present, the main therapeutic regime is surgical therapy first, followed by chemotherapy using platinum drugs. Previous findings showed that chemotherapy can prolong the lifetime of patients (2). Multi-drug resistance occurs in approximately 25% of ovarian cancer patients, which leads to the decreased curative effect of chemotherapy, thus reducing the survival rate and quality of life of patients (3).
High mobility group box 1 (HMGB1) is a non-histone nuclear protein that exists in nucleus extensively. At the same time, a small amount of HMGB1 is present in the cytoplasm and cytomembrane (4). HMGB1 is known to play a key role in the occurrence, progression, metastasis and drug resistance of tumors (5). Breast cancer susceptibility gene 1 (BRCA1) is a kind of repair gene for DNA damage, and can lead to the drug resistance of tumor cells by repairing the cell damage caused by chemotherapy drugs (6,7). Clinical studies found that the sensitivity to cisplatin chemotherapy and the prognosis of patients with a specifically high expression of BRCA1 gene are worse than those of patients with a specifically low expression of BRCA1 gene (8). P62, a multi-functional protein, is abnormally expressed in most tumors and play a key role in proliferation, differentiation, anti-apoptosis and induced autophagy (9).
In order to analyze the expression of HMGB1, BRCA1 and P62 in ovarian carcinoma tissues and the association with chemotherapy sensitivity of ovarian cancer patients, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and immunohistochemistry were used to investigate the expression of HMGB1, BRCA1 and P62 in tumor and para-carcinoma normal tissues and analyze the association with chemotherapy sensitivity of cisplatin in order to provide a research basis for clinical rational drug use.
Materials and methods
Materials
In the present study, tumor tissues and the corresponding para-carcinoma normal tissues of 60 ovarian cancer patients admitted into the Department of Surgery in Dongying Hospital (Dongying, China) from June, 2012 to June, 2015, were selected. The patients were definitively diagnosed with ovarian cancer by clinical pathology and did not receive chemotherapy and surgical therapy. Patients were aged 24–78 years, and the median age was 52 years. This study was approved by the Clinical Ethics Committee of Dongying People's Hospital. At the same time, all the patients enrolled signed the informed consent form.
RNA extraction, reverse transcription, and RT-qPCR kit (all from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), HMGB1, BRCA1, P62, GAPDH antibodies (1:800; cat. nos. 10829-1-AP, 22362-1-AP, 18420-1-AP, 10494-1-AP) and HRP-labeled secondary antibodies (1:1000; cat. no. SA00001-2) (all from Proteintech Biotechnology Co., Ltd.; Wuhan Sanying Biotechnology, Wuhan, China); immunohistochemical staining kit SP-9001 (Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd.; OriGene Technologies, Inc., Beijing, China), primer synthesis (Takara Biotechnology Co., Ltd., Dalian, China) were used in the present study.
Detection of the mRNA expression of HMGB1, BRCA1 and P62 in tissue specimens of patients via RT-qPCR
Approximately 100 mg tumor and para-carcinoma normal tissues of patients frozen in liquid nitrogen were taken and the total RNA was extracted according to the instructions of the RNA extraction kit. An ultraviolet and visible spectrophotometer (Hitachi Ltd., Tokyo, Japan) was used to detect the absorbance value of the total RNA extracted at 260 and 280 nm, and the total RNA samples with the A260/A280 value within 1.8–2.0 were selected for the follow-up experiment.
The reverse transcription reaction was carried out according to the instructions of the reverse transcription kit, and the cDNA obtained served as the template. The mRNA expression of HMGB1, BRCA1 and P62 was detected according to the instructions of the PCR kit with GAPDH as the control gene. The primer sequences are shown in Table I. The reaction conditions were: 95°C for 10 min, 95°C for 15 sec, 60°C for 30 sec, 72°C for 30 sec, a total of 42 cycles for amplification; 72°C for 5 min. The Cq value was read, and the 2−ΔCq method was used to calculate the relative expression level. The formula used was: ΔCq (target gene) = Cq (target gene) - Cq (control gene).
Table I.
RT-qPCR primer sequence.
| Genes | Primer sequence |
|---|---|
| HMGB1 | F 5′-TGGACTGCTCAGGAAAC-3′ |
| R 5′-AGGGGCAAACCGTAAT-3′ | |
| BRCA1 | F 5′-CCCATTTTCCTCCCGCA-3′ |
| R 5′-GGACCTTGGTGGTTTCTTCCA-3′ | |
| P62 | F 5′-CCAGCACCAAGAGCACGGACAGCG-3′ |
| R 5′-TGGGGAGAAGAAGGGGACCACGAA-3′ | |
| GAPDH | F 5′-ATGGCACCGTCAAGGCTGAG-3′ |
| R 5′-GCAGTGATGGCATGGACTGT-3′ |
HMGB1, high mobility group box 1; BRCA1, breast cancer susceptibility gene 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; F, forward; R, reverse.
Detection of the protein expression of HMGB1, BRCA1 and P62 in tissue specimens of patients via immunohistochemistry
After the tissues were obtained during surgery, they were fixed using 4% formalin, and then embedded in paraffin, followed by conventional sections. The operation was carried out according to the instructions of the SP-9001 immuno-histochemical kit. The paraffin sections were dewaxed and hydrated for pretreatment, and incubated at 3% H2O2 at room temperature for 10 min. Then they were heated and repaired by citric acid buffer and sealed using 10% goat serum. The primary antibody (dilution 1:100) was added for incubation at 4°C overnight. After the sections were washed with phosphate-buffered saline (PBS), the biotin-labeled secondary antibody was added for incubation at room temperature for 20 min, followed by washing using PBS, color development via diaminobenzidine, hematoxylin staining, dehydration, transparency via xylene, sealing via neutral resin, and microscopic image (TE2000-U; Nikon Instruments Europe BV, Amsterdam, The Netherlands).
Five fields (×400) were selected randomly in each section. The staining results were scored according to the staining intensity and the percentage of positive cells: The number of positive cells were scored as: <5%: 0 point; 5–25%: 1 point; 26–50%: 2 points; >50%: 3 points. The staining intensity was scored as: No staining: 0 point; light yellow: 1 point; brown yellow: 2 points; dark brown: 3 points. Both scores above were added: ≤3 points indicated a negative expression, while >3 points indicated a positive expression, and the statistical results were analyzed.
Detection of patient's drug resistance to cisplatin via in vitro resin droplet experiment
Tumor tissues of the patients were obtained during surgery, immediately digested using the digestive enzyme, filtered and centrifuged to collect cells. RPMI-1640 culture medium containing 10% fetal calf serum was added to prepare the cell suspension. Cell-collagen mixture was prepared in an ice bath, then inoculated onto a 24-well plate and cultured for 24 h. The cells were divided into the administration group (cisplatin) and blank group (no drug) and cultured for 24 h. Then neutral red solution was added and cells were fixed with 10% formaldehyde solution. The in vitro drug sensitivity was evaluated by calculating the number of cells in the administration group/the number of cells in blank group, with <50% indicating drug sensitivity, and ≥50% indicating cell drug resistance (10).
Association of the expression of HMGB1, BRCA1 and P62 with drug resistance of patients
According to the expression status of HMGB1, BRCA1 and P62 in ovarian carcinoma tissue, 60 ovarian cancer patients were divided into the positive and negative expression groups. A Chi-square test was used to analyze the association of the expression of HMGB1, BRCA1 and P62 in ovarian carcinoma tissue with the drug resistance of patients to cisplatin.
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data processing. Measurement data were expressed as mean ± standard deviation. A t-test was used for intergroup comparison, the Chi-square test was used for intergroup comparisons of enumeration data. P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of the mRNA expression of HMGB1, BRCA1 and P62 in tissue specimens via RT-qPCR
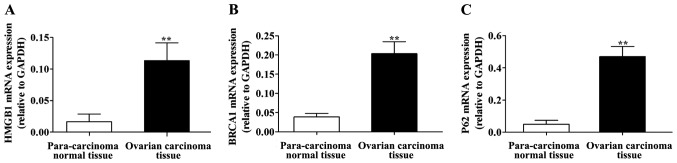
The RT-qPCR detection results are shown in Fig. 1. Compared with those in para-carcinoma normal tissues, the mRNA expression of HMGB1, BRCA1 and P62 was significantly higher in ovarian carcinoma tissues, and the differences were statistically significant (P<0.01).
Figure 1.
Detection of the mRNA expression of HMGB1, BRCA1 and P62 via RT-qPCR. mRNA expression of (A) HMGB1, (B) BRCA1, and (C) P62 in tissue specimens. Compared with those in para-carcinoma normal tissue, the mRNA expression levels of HMGB1, BRCA1 and P62 are significantly higher in ovarian carcinoma tissue, **P<0.01. HMGB1, high mobility group box 1; BRCA1, breast cancer susceptibility gene 1; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Detection of the protein expression of HMGB1, BRCA1 and P62 in tissue specimens by immunohistochemistry
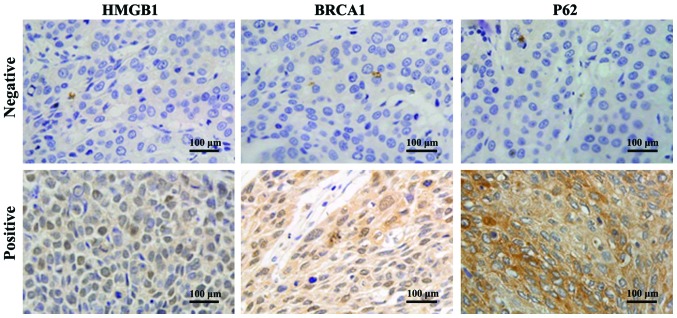
Immunohistochemistry detection results are shown in Fig. 2. The positive immunohistochemical staining of HMGB1, BRCA1 and P62 were all yellow brown, with HMGB1 protein mainly being identified in the cytoplasm, BRCA1 mainly in the cell nucleus, and partially in the cytoplasm; and P62 mainly being identified in the cytoplasm.
Figure 2.
Detection of protein expression of HMGB1, BRCA1 and P62 in tissues of clinical patients (×400) via immunohistochemistry. Positive immunohistochemical staining of HMGB1, BRCA1 and P62 are all yellow brown; HMGB1 protein is mainly present in cytoplasm; BRCA1 mainly exists in cell nucleus, and partially in cytoplasm; and P62 mainly exists in cytoplasm. HMGB1, high mobility group box 1; BRCA1, breast cancer susceptibility gene 1.
The scores of statistical staining are shown in Table II. The positive expression rates of HMGB1 in ovarian carcinoma and para-carcinoma normal tissues were 61.67% (37/60) and 13.33% (8/60), and the difference was statistically significant (P<0.01); the positive expression rates of BRCA1 in ovarian carcinoma and para-carcinoma normal tissues were 78.33% (47/60) and 8.33% (5/60), and the difference was statistically significant (P<0.01). The positive expression rates of P62 in ovarian carcinoma and para-carcinoma normal tissues were 71.67% (43/60) and 11.67% (7/60), and the difference was statistically significant (P<0.01).
Table II.
Protein expression of HMGB1, BRCA1 and P62 in para-carcinoma normal tissues and ovarian carcinoma tissues.
| HMGB1 | BRCA1 | P62 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | No. | Positive | Positive rate | P-value | Positive | Positive rate | P-value | Positive | Positive rate | P-value |
| Ovarian carcinoma tissue | 60 | 37 | 61.67% | <0.01 | 47 | 78.33% | <0.01 | 43 | 71.67% | <0.01 |
| Para-carcinoma normal tissue | 60 | 8 | 13.33% | <0.01 | 5 | 8.33% | <0.01 | 7 | 11.67% | <0.01 |
HMGB1, high mobility group box 1; BRCA1, breast cancer susceptibility gene 1.
Drug resistance to cisplatin of 60 ovarian cancer patients
The results of in vitro resin droplet experiment showed that 38 out of 60 ovarian cancer patients had drug resistance to cisplatin, while 22 cases were sensitive to cisplatin (Table III).
Table III.
Drug resistance to cisplatin of 60 ovarian cancer patients.
| Groups | Case (n) | Ratio (%) |
|---|---|---|
| Drug-resistance | 38 | 63.33 |
| Sensitive | 22 | 36.67 |
Association of protein expression of HMGB1, BRCA1 and P62 with drug resistance to cisplatin in ovarian cancer
The results showed that the positive rates of the protein expression of HMGB1, BRCA1 and P62 in 38 patients in the drug resistance group were 84.21, 94.74 and 92.11%, respectively, while those in the sensitive group were 22.73, 50.00 and 36.36%, respectively. The results of the Chi-square test showed that the positive rates of the protein expression of HMGB1, BRCA1 and P62 in the drug resistance group were significantly higher than those in the sensitive group (P<0.01) (Table IV).
Table IV.
Association of protein expression of HMGB1, BRCA1 and P62 with drug-resistance to cisplatin in ovarian cancer.
| HMGB1 | BRCA1 | P62 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | No. | Positive | Positive rate | P-value | Positive | Positive rate | P-value | Positive | Positive rate | P-value |
| Drug resistance | 38 | 32 | 84.21% | <0.01 | 36 | 94.74% | <0.01 | 35 | 92.11% | <0.01 |
| Sensitive | 22 | 5 | 22.73% | <0.01 | 11 | 50.00% | <0.01 | 8 | 36.36% | <0.01 |
HMGB1, high mobility group box 1; BRCA1, breast cancer susceptibility gene 1.
Discussion
Clinical study statistics found that the 5-year survival rate of patients with advanced ovarian cancer is lower than 20%, and an important reason leading to poor prognosis is the drug resistance of patients to chemotherapy drugs (11). Therefore, identification of the specific indexes to predict the sensitivity of ovarian cancer patients to chemotherapy drugs are issues to be solved, which can provide powerful assistance for clinical doctors in formulating therapeutic regimens and selecting chemotherapy drugs (12).
Cisplatin, a common chemotherapy drug in clinical tumor therapy, is the first-line chemotherapy drug in ovarian cancer treatment, and drug resistance to cisplatin is the main reason affecting the curative effect of chemotherapy. The drug resistance to cisplatin of ovarian cancer patients can be divided into inherent or acquired drug resistance, and the drug resistance mechanism of tumor is very complex. Currently, the molecular mechanism of patient's drug resistance to cisplatin remains unclear (13).
HMBG1 can inhibit cell apoptosis, and when HMBG1 is overexpressed in cells, tumor cell apoptosis is markedly decreased. When siRNA is used to silence HMGB1 expression, the sensitivity of tumor cells to chemotherapy drugs can be elevated (14). BRCA1 gene is located on human chromosome 17q21, and constitutes the susceptibility gene for breast and ovarian cancer (15). BRCA1 has the effect of inhibiting cell growth and plays a key role in gene transcription, DNA damage repair and apoptosis, especially in maintaining the genome stability (16). P62 is a kind of autophagy-induced protein, and cells can relieve the endoplasmic reticulum stress by autophagy, thus decreasing the tumor cell sensitivity to cisplatin. Subsequently, when P62 is highly expressed, intracellular autophagy activity is activated, eventually reducing the drug resistance of ovarian carcinoma cells to cisplatin (17,18).
In order to further explore the expression of HMGB1, BRCA1 and P62 in the tumor tissues of ovarian cancer patients and its effect on the drug resistance of ovarian cancer patients to cisplatin, RT-qPCR was performed to detect the mRNA expression of HMGB1, BRCA1 and P62 in the tumor tissues of ovarian cancer patients. The results showed that the mRNA expression levels of HMGB1, BRCA1 and P62 were significantly higher in ovarian carcinoma tissues compared with those in para-carcinoma normal tissues. In addition, immunohistochemical results showed that the positive protein expression rates of HMGB1, BRCA1 and P62 in ovarian carcinoma tissues were 61.67% (37/60), 76.33% (47/60) and 71.67% (43/60), respectively, which were significantly higher than those in para-carcinoma normal tissues. Moreover, the in vitro resin droplet experiment revealed that 38 out of 60 ovarian cancer patients had drug resistance to cisplatin and 22 cases were sensitive to cisplatin. The positive rates of the protein expression of HMGB1, BRCA1 and P62 in the drug resistance group were 84.21, 94.74 and 92.11%, respectively, while those in the sensitive group were 22.73, 50.00 and 36.36%, respectively. Thus, the positive protein expression rates of HMGB1, BRCA1 and P62 in drug resistance were significantly higher than those in the sensitive group.
Wang et al reported that the higher the intracellular expression level of BRCA is, the lower the cell sensitivity to cisplatin will be (19). Liu et al confirmed that after HMGB1 is added into the cell culture fluid, it can induce cells to develop drug resistance to chemotherapy drugs (14). Yu et al found that P62 protein is overexpressed in tissues of ovarian cancer patients who have drug resistance to cisplatin, and it is involved in the formation mechanism of drug resistance of cells to cisplatin (20). This experiment further confirmed the overexpression of HMGB1, BRCA1 and P62 in tumor tissues of ovarian cancer patients, and it was found that the expression of HMGB1, BRCA1 and P62 was associated with the drug resistance of ovarian cancer patients to cisplatin.
In conclusion, the overexpression of HMGB1, BRCA1 and P62 exists in tumor tissues of ovarian cancer patients. Furthermore, its positive expression is associated with chemotherapy sensitivity of ovarian cancer patients. Thus, HMGB1, BRCA1 and P62 may be molecular markers for the prediction of chemotherapy sensitivity of ovarian cancer patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang Y, Liu P, Qiu L, Sun Y, Zhu M, Gu L, Di W, Duan Y. Toxicity and therapy of cisplatin-loaded EGF modified mPEG-PLGA-PLL nanoparticles for SKOV3 cancer in mice. Biomaterials. 2013;34:4068–4077. doi: 10.1016/j.biomaterials.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 2.Wu YJ, Neuwelt AJ, Muldoon LL, Neuwelt EA. Acetaminophen enhances cisplatin- and paclitaxel-mediated cytotoxicity to SKOV3 human ovarian carcinoma. Anticancer Res. 2013;33:2391–2400. [PMC free article] [PubMed] [Google Scholar]
- 3.Ong PS, Chan SY, Ho PC. Microarray analysis revealed dysregulation of multiple genes associated with chemoresistance to As2O3 and increased tumor aggressiveness in a newly established arsenic-resistant ovarian cancer cell line, OVCAR-3/AsR. Eur J Pharm Sci. 2012;45:367–378. doi: 10.1016/j.ejps.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu D, Ding Y, Wang S, Zhang Q, Liu L. Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol. 2008;216:167–175. doi: 10.1002/path.2391. [DOI] [PubMed] [Google Scholar]
- 6.Beeghly A, Katsaros D, Chen H, Fracchioli S, Zhang Y, Massobrio M, Risch H, Jones B, Yu H. Glutathione S-transferase polymorphisms and ovarian cancer treatment and survival. Gynecol Oncol. 2006;100:330–337. doi: 10.1016/j.ygyno.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Stengel C, Newman SP, Leese MP, Potter BV, Reed MJ, Purohit A. Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br J Cancer. 2010;102:316–324. doi: 10.1038/sj.bjc.6605489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GK, Mullan PB, Johnston PG, Wilson RH, Harkin DP. BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res. 2007;13:7413–7420. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- 9.Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Tanisaka K, Doi O, Kodama K, Higashiyama M, Nakagawa H, Miyake M, Taki T, Hara S, et al. An in vitro chemosensitivity test for solid human tumors using collagen gel droplet embedded cultures. Int J Oncol. 1997;11:449–455. doi: 10.3892/ijo.11.3.449. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto M, Kondo A, Kawasaki K, Goto T, Sakamoto H, Miyake K, Koyamatsu Y, Akiya T, Iwabuchi H, Muroya T, et al. Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cDNA microarray. Hum Cell. 2001;14:305–315. [PubMed] [Google Scholar]
- 13.Takano M, Kudo K, Goto T, Yamamoto K, Kita T, Kikuchi Y. Analyses by comparative genomic hybridization of genes relating with cisplatin-resistance in ovarian cancer. Hum Cell. 2001;14:267–271. [PubMed] [Google Scholar]
- 14.Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Lotze MT, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25:23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- 15.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 16.Garvin AM, Attenhofer-Haner M, Scott RJ. BRCA1 and BRCA2 mutation analysis in 86 early onset breast/ovarian cancer patients. J Med Genet. 1997;34:990–995. doi: 10.1136/jmg.34.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greggio E, Lewis PA, van der Brug MP, Ahmad R, Kaganovich A, Ding J, Beilina A, Baker AK, Cookson MR. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J Neurochem. 2007;102:93–102. doi: 10.1111/j.1471-4159.2007.04523.x. [DOI] [PubMed] [Google Scholar]
- 18.Haugarvoll K, Toft M, Ross OA, White LR, Aasly JO, Farrer MJ. Variants in the LRRK1 gene and susceptibility to Parkinson's disease in Norway. Neurosci Lett. 2007;416:299–301. doi: 10.1016/j.neulet.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Wei J, Qian X, Yin H, Zhao Y, Yu L, Wang T, Liu B. ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel. BMC Cancer. 2008;8:97. doi: 10.1186/1471-2407-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Su J, Xu Y, Kang J, Li H, Zhang L, Yi H, Xiang X, Liu F, Sun L. p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. J Eur Cancer. 2011;47:1585–1594. doi: 10.1016/j.ejca.2011.01.019. [DOI] [PubMed] [Google Scholar]