Abstract
The present study was undertaken to investigate the mechanism behind the anti-obesity effect of the 50% ethanol extract of Chrysanthemum indicum L. flowers (CIEE) in a mouse model of high-fat diet (HFD)-induced obesity. Male C57BL/6J mice (six mice in each group) were administered CIEE (8, 40 and 200 mg/kg) for 6 weeks while being fed with a HFD. Garcinia cambogia (GC) was used as the positive control and was administered in the same manner as CIEE. Results demonstrated that oral administration of CIEE significantly reduced body weight, epididymal white adipose tissue (EWAT), liver weight and serum levels of total cholesterol and triglyceride (P<0.05). In addition, CIEE reduced serum leptin and increased adiponectin levels. CIEE significantly downregulated peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding protein-α and fatty acid synthase expression levels in EWAT, and upregulated the protein expression of PPARα in liver tissue of HFD-fed obese mice (P<0.05). These results suggested that Chrysanthemum indicum L. flowers may be a potentially effective therapeutic agent for obesity and its associated complications.
Keywords: Chrysanthemum indicum, anti-obesity, high-fat diet, PPARγ, C/EBPα
Introduction
Obesity is a serious public health issue in the world related with increase in morbidity, mortality and decrease lifespan (1). It associated with metabolic disorders, such as, hypertension, type 2 diabetes, nonalcoholic fatty liver diseases, atherosclerosis and cardiovascular diseases (2,3). Obesity is related to an imbalance in intake of energy, lack of exercise (4) and the excessive accumulation of adipose tissues (3). The development of obesity is characterized by adipogenesis, differentiation of preadipocytes to adipocytes and increased triglycerides deposition (5,6). Adipogenesis is regulated by transcription factors, such as, peroxisome proliferation-activated receptor-gamma (PPAR-γ) and CCAAT/enhancer-binding protein-α (C/CBPα) (6). In addition, increased expressions of PPARγ and C/CBPα have been shown to induce adipogenesis by activating adipocyte-specific proteins, like leptin, adiponectin (7,8). As a result of these findings, several studies have targeted adipogenesis as a means of controlling obesity. Liver is responsible for lipid synthesis and fatty acid oxidation, and thus, plays an important role in lipid metabolism (9). Lipid synthesis in liver is regulated by sterol regulatory element-binding proteins (SREBP), which control several downstream genes, such as acetyl-CoA carboxylase (ACC), stearoyl-CoA destaturase (SCD) and fatty acid synthesis (FAS) (10,11).
Several anti-obesity drugs, like orlistat, topiramate, sibutraine are commercially available, however their intolerable side effects such as, insomnia, anorexia, dry mouth and gastrointestinal indisposition (12) have limited their uses. This has called scientists to search for new, effective and more tolerated anti-obesity agents from natural products (13).
Chrysanthemum indicum (CI) is herb that is widely used in China and Korea to treat various immune-related disorders, such as, the symptoms of hypertension and several infectious diseases (13,14). Previous studies have reported CI possesses various bioactivities such as, anti-oxidant, anti-nociceptive, anti-bacterial, anti-viral and anti-inflammatory effects (14–17). Recently, it was reported that an extract of CI has anti-oxidant and anti-adipogenic effects in 3T3-L1 cells (18). However, to our knowledge the inhibitory effect of CIEE in overweight and obesity-related metabolic diseases have not been studied. Therefore, the present study investigated the effect of ethanol extract of Chrysanthemum indicum L. flowers (CIEE) on a mouse model of obesity induced by high-fat diet (HFD) and elucidated its underlying mechanism.
Materials and methods
Materials and reagents
Trizol reagent and SuperScript III kit were obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The protein assay kit (RIPA buffer) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The primary antibodies, mouse anti-(PPARγ (sc-7273), CEBPα (sc-166258), β-actin (sc-47778) and rabbit anti-PPARα (sc-9000) were purchased from Santa Cruz Biotechnology, Inc., and rabbit anti-FAS (C20G5) from Cell signaling Technology, Inc. (Danvers, MA, USA). Primary antibodies at 1:1,000 and secondary antibodies at 1:5,000 dilutions were used. For rabbit primary antibodies: Goat-anti-rabbit IgG HRP (sc-2030; Santa Cruz Biotechnology, Inc.) and for mouse primary antibodies: Goat-anti-mouse IgG HRP (sc-2030; Santa Cruz Biotechnology, Inc.) were used.
Preparation of the ethanol extract of CI (CIEE)
CI was obtained from Gungangbogam (Jechon, Korea). The dried and powdered fruit of CI (500 g) were extracted with 50% ethanol for 2 h using mantle-reflux at 80°C. After filtration, CIEE was obtained, as a powder, by removing solvent on a rotary vacuum evaporator (N-000; Eyela, Tokyo, Japan) (19). The amount of CIEE obtained using this procedure was 83.5 g (16.7%) with respect to the starting material. CIEE was stored at 4°C for further use.
Animal treatment
Male C57BL/6 mice (5 weeks old) were purchased from Samtako Bio Korea (Samtako Bio Korea, Osan, South Korea). The experimental procedures were conducted using a protocol approved by the Institutional Animal Care Committee of Chonbuk National University. Mice were housed at 22±2°C and 50±5% RH and provided a normal diet. After one week of acclimation, mice were divided into six groups containing six mice in each group (n=6), as follows. i) ND, mice fed with normal diet; ii) HFD, mice fed with high-fat diet; iii) HFD+CIEE (8), mice fed with HFD and treated with CIEE (8 mg/kg); iv) HFD+CIEE (40), mice fed with HFD plus CIEE (40 mg/kg) treatment; v) HFD+CIEE (200), mice fed with HFD plus CIEE (200 mg/kg) treatment; and vi) HFD+GC, mice fed with HFD plus GC treatment. Garcinia cambogia (GC) at dose of 200 mg/kg was used as a positive control and was administered in its powdered form via oral gavage (p.o). The treatment of CIEE (8, 40 or 200 mg/kg) or GC was administered once a day for 6 weeks (p.o). Body weight and food consumption were measured weekly.
Determination of abdominal fat volume by micro-computed tomography (micro-CT)
Mice were starved for 6 h prior to sacrifice and anesthetized by intraperitoneal injection of ketamine (75 mg/kg) and rompun (xylazine) (15 mg/kg) (20). To measure the sign of consciousness, we used a physical stimulus method, toes were pinched with atraumatic forceps and the reflex was observed. Images were acquired using a Skyscan-1076 micro-CT scanner (Skyscan, Aartselaar, Belgium). CT was performed using a pixel size of 35 µm, a source voltage of 50 kVp, and a source current of 200 µA. The X-ray detector contained a 12-bit, water-cooled CCD (charge-coupled device) camera with 4,000 × 2,300 resolutions and scintillator. The images were acquired with 0.6 degree. The exposure time was 0.46 sec and a 1 mm aluminum energy filter was used. Abdominal fat volume was measured using an Olympus SP-500 UZ camera (Olympus America, Inc., Center Valley, PA, USA). Following micro-CT, under anesthesia, mice were euthanized through intracardiac puncture. Blood was collected (0.8–0.9 ml) via cardiac puncture followed by removal of organs such as liver, kidney and spleen. Following exsanguination, the death was confirmed by incising the heart and making sure about no respiratory movement of mice for at least 3 min.
Histological analysis
Liver and abdominal fat tissues were fixed with 10% neutral buffered formalin, embedded in paraffin wax, and cut serially into 10 µm-thick sections. Sections were stained with hematoxylin and eosin (H&E) and histological alterations were observed under a microscope and photographed (magnification, ×100; Olympus CX21; Olympus America, Inc.) (21).
Serum lipid profile
Serum samples were analyzed for levels of total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDLc) using a commercially available kit (Asian Pharmaceutical, Hwaseong-Si, Korea).
Enzyme-linked immunosorbent assay (ELISA)
Serum leptin and adiponectin levels were measured by leptin mouse ELISA kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA) and adiponectin mouse ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), respectively, according to the manufacturer's instructions.
Western blot analysis
Epididymal white adipose tissue (EWAT) and liver tissues were lysed in ice-cold RIPA buffer for 40 min and centrifuged (12,000 × g) for 20 min at 4°C (22,23). Tissue lysates were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Amersham; GE Healthcare, Chicago, IL, USA), which were then blocked with 5% skimmed milk in tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature. Membranes were probed with primary antibodies at 4°C overnight, washed with TBST for 4 times, incubated with horseradish peroxidase-conjugated secondary antibody for 45 min at RT, and rewashed with TBST 3 times. Proteins were visualized using an enhanced chemiluminescence detection kit (EMD Millipore, Billerica, MA, USA).
Ultra pressure liqid chromatography (UPLC)
UPLC was performed using ACQUITY UPLC® BEH C18 equipped with (2.1×50 mm, 1.7 µm) column and a photodiode array detector (Waters Corp., Milford, MA, USA) (19). The elution was performed at flow rate of 0.15 ml/min, using distilled water (DW) containing 0.1% formic acid (solvent A) and ACN containing 0.1% formic acid (solvent B) in gradient mode (B 5% from 0 to 1 min, B 5 to 95% from 1 to 16 min, B 95 to 100% from 16 to 18 min, and B 100% from 18 to 26 min; flow rate was 0.15 ml/min, detection by UV at 330 nm, and temperature at 25°C.
Statistical analysis
Results are expressed as means ± standard error of mean (SEM) and analyzed using Graph Pad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of variance (ANOVA) was used to measure the significant difference followed by Tukey's post hoc test for comparison of mean. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of CIEE on body weight, food consumption and efficiency in HFD-fed mice
To investigate the anti-obesity effect of CIEE, mice were fed HFD with or without CIEE (8, 40 and 200 mg/kg, oral gavage daily) or GC (200 mg/kg, oral gavage daily). No significant difference in dietary intake was observed between the HFD-fed control and the CIEE treated groups. HFD induced weight gain as compared to mice fed the normal diet, whereas CIEE administration significantly and dose-dependently decreased weight gain (Table I).
Table I.
Effect of CIEE on body weight gain, food intake and efficiency (%) in HFD fed mice.
| CIEE (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| Groups | ND | HFD | 8 | 40 | 200 | GC (mg/kg) 200 |
| Initial body weight (g) | 21.68±1.10 | 22.32±1.16 | 21.14±0.86 | 22.07±1.30 | 21.96±1.07 | 21.43±0.97 |
| Final body weight (g) | 26.97±١.٧٥ | 36.73±3.89a | 32.33±2.48 | 31.47±1.84b | 30.11±2.08b | 28.55±1.61b |
| Gain weight (g) | 5.29±1.65 | 14.41±3.64a | 11.20±2.29 | 9.40±1.68b | 8.15±1.93b | 7.10±0.53b |
| Food intake | 2.67±0.33 | 2.14±0.27 | 2.12±0.21 | 2.08±0.19 | 2.05±0.24 | 1.98±0.22 |
| Food efficiency (%) | 4.47 | 16.03 | 12.57 | 10.76 | 9.46 | 8.53 |
Results are means ± SEM (n=6). aP<0.05 vs. ND group, bP<0.05 vs. HFD group. ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia.
Effect of CIEE on fat deposition (mass of abdominal WAT, weight of EWAT, and liver tissue) in HFD-fed mice
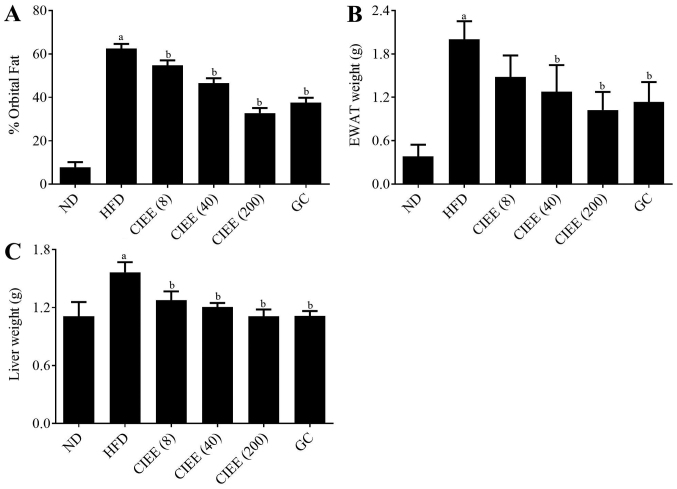
To assess the effect of CIEE on adipose tissue accumulation, body and abdominal fat weight gains were measured in the study. CIEE dose-dependently diminished orbital fat volume (%) as determined by micro-CT as compared with the HFD-fed group as shown in Fig. 1A. CIEE also significantly reduced EWAT weight in HFD-fed obese mice to a level below than in GC fed controls (Fig. 1B). Also, CIEE induced significant reductions in liver tissue weight (Fig. 1C; P<0.05).
Figure 1.
Effect of CIEE on fat deposition in HFD fed mice. (A) Percentages of abdominal fat deposition determined by micro-CT analysis, (B) EWAT weight and (C) liver weight. Results are means ± SEM (n=6). aP<0.05 vs. ND group, bP<0.05 vs. HFD group. ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia: CT, computed tomography; EWAT; epididymal white adipose tissue;
Effect of CIEE on histological observations in HFD-fed mice
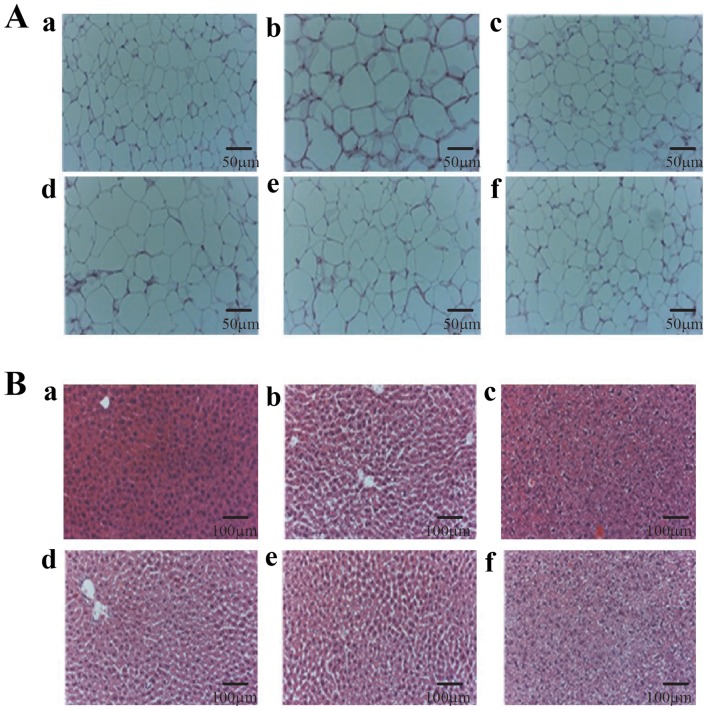
Histological analysis of EWAT and liver tissue were performed by using H&E staining. Results showed enlarged size of EWAT in the HFD group whereas; CIEE groups reduced the size of EWAT (Fig. 2A). Also, data revealed that CIEE significantly reduced vacuolization and lipid droplet numbers in liver tissue (Fig. 2B; P<0.05).
Figure 2.
Effect of CIEE on histopathological examination of EWAT and liver tissues in experimental groups. Photograph showing haematoxylin and eosin staining for (A) EWAT and (B) liver tissue of HFD-fed mice. Representative images of a, treatment naïve control (ND); b, HFD control; c, HFD+GC; d, HFD+CIEE (8 mg/kg); e, HFD+CIEE (40 mg/kg) and f, HFD+CIEE (200 mg/kg); ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia; EWAT; epididymal white adipose tissue.
Effect of CIEE on the lipid profiles of HFD-fed mice
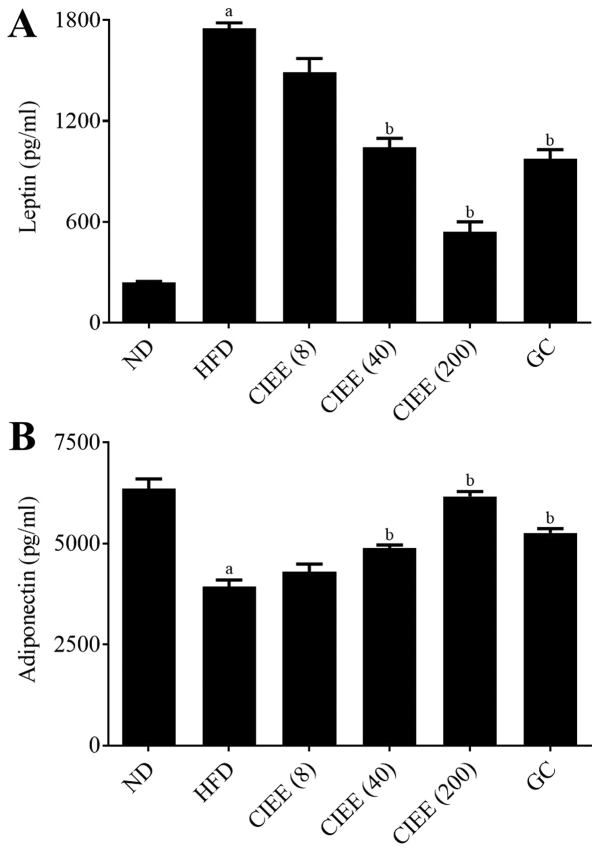
HFD-fed mice had higher serum levels HFD-fed mice had higher serum levels of TC, low-density lipoprotein cholesterol (LDLc) and TG, and lower levels of HDLc than mice in the normal group (P<0.05). CIEE significantly inhibited TC, LDLc and TG increases, but dose-dependently increased vs. HFD-fed mice (Table II). Furthermore, CIEE dose-dependently inhibited leptin levels (Fig. 3A) and increased adiponectin levels as compared with HFD-fed mice (Fig. 3B).
Table II.
Effect of CIEE on serum lipid profiles in HFD fed mice.
| CIEE (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| Groups | ND | HFD | 8 | 40 | 200 | GC (mg/kg) 200 |
| Total cholesterol (TC, mg/dl) | 125.5±19.6 | 233.1±13.4a | 212.1±14.5b | 183.4±20.9b | 160.8±12.5b | 190.7±38.9b |
| Triglyceride (TG, mg/dl) | 85.7±8.9 | 142.7±6.5a | 133.7±11.3 | 124.4±18.1 | 109.1±31.4b | 106.3±9.4b |
| LDL-cholesterol (LDLc, mg/dl) | 54.56±11.7 | 126.4±29.7;a | 111.1±1.6 | 89.8±15.7 | 55.1±16.3b | 86.9±43.2 |
| HDL-cholesterol (HDLc, mg/dl) | 53.7±11.1 | 78.1±16.7a | 74.3±14.3 | 68.7±18.7 | 87.8±12.4b | 82.6±12.4 |
Results are means ± SEM (n=6). aP<0.05 vs. ND group, bP<0.05 vs. HFD group. ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia.
Figure 3.
Effect of CIEE on serum adipokines levels in HFD fed mice. ELISA was used to measure serum (A) leptin levels and (B) adiponectin levels. Results are means ± SEM (n=6). aP<0.05 vs. ND group, bP<0.05 vs. HFD group. ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia.
Effect of CIEE on transcription factors of adipogenesis and FAS
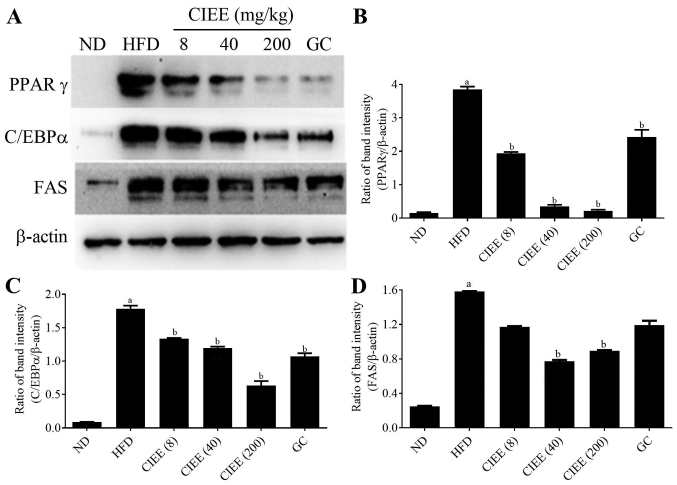
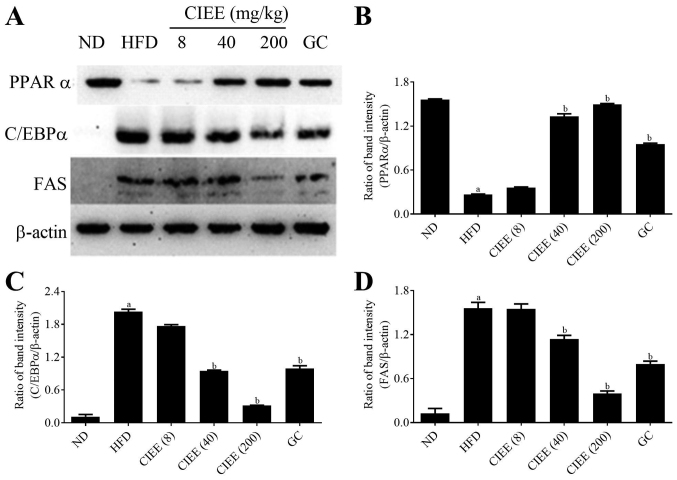
Western blotting was used to determine whether the observed increases of EWAT and liver weight in obese mice were accompanied by changes in the expressions of regulator transcription factors, PPARα/γ and C/EBPα. HFD-fed mice showed a significant increase in PPARγ and C/EBPα protein expression in EWAT. CIEE significantly and dose-dependently inhibited HFD-induced PPARγ and C/EBPα in EWAT (Fig. 4) (P<0.05). Furthermore, CIEE dose dependently enhanced the protein expression of PPARα but decreased C/EBPα in liver tissue vs. HFD-fed mice (Fig. 5A-C).
Figure 4.
Effect of CIEE on protein expressions of PPARγ, C/EBPα and FAS in EWAT of HFD fed mice. Protein levels of regulator transcription factors of adipogenesis and FAS in EWAT were assessed by western blot. (A) Immunoblot analysis and bar diagram of the relative band intensities of (B) PPARγ (C) C/EBPα (D) FAS in EWAT of HFD-fed obese mice. Results are means ± SEM (n=6). aP<0.05 vs. ND group, bP<0.05 vs. HFD group. ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia; EWAT; epididymal white adipose tissue; PPAR, proliferator-activated receptor; C/EBPα, CCAAT/enhancer-binding protein-α; FAS, fatty acid synthesis.
Figure 5.
Effect of CIEE on protein expressions of PPARα, C/EBPα and FAS in liver tissue of HFD fed mice. Protein levels of regulator transcription factors of adipogenesis and FAS in liver tissues were assessed by western blot. (A) Immunoblot analysis and bar diagram of the relative band intensities of (B) PPARα (C) C/EBPα (D) FAS in liver tissue of HFD-fed obese mice. Results are means ± SEM (n=6). aP<0.05 vs. ND group, bP<0.05 vs. HFD group. ND, normal diet group; HFD, high-fat diet group; CIEE, ethanol extract of Chrysanthemum indicum L.; GC, Garcinia cambogia; PPAR, proliferator-activated receptor; C/EBPα, CCAAT/enhancer-binding protein-α; FAS, fatty acid synthesis.
PPARα/γ regulates FAS protein during adipogenesis, and thus we examined the effect of CIEE on FAS protein level in both EWAT and liver tissues by western blotting. Our results revealed HFD-fed mice had elevated FAS protein levels and that CIEE significantly and dose-dependently inhibited this increase in EWAT (Fig. 4A and D) and liver tissue (Fig. 5A and D).
Chemical components of CIEE by UPLC
UPLC showed that quercitrin was the main component of CIEE (Fig. 6). However, other peaks observed were unidentified and further experiments are necessary to illustrate the other components of CIEE.
Figure 6.
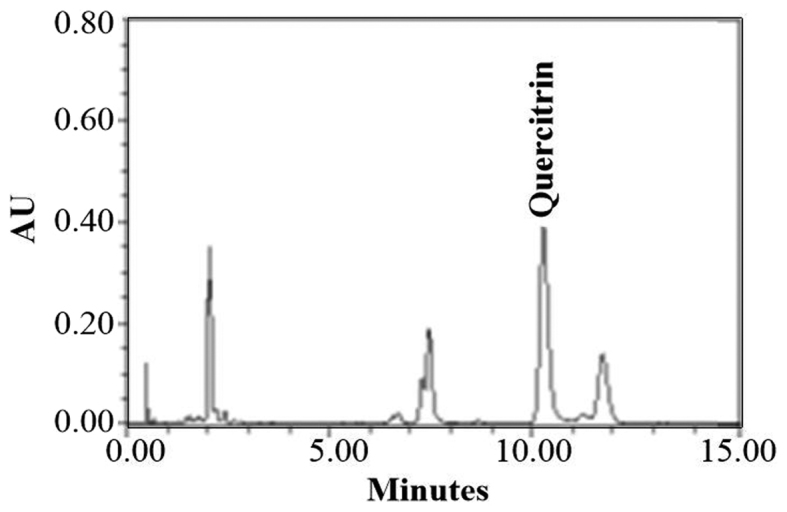
UPLC profile of CIEE. Chromatogram for the analysis of compounds isolated from CIEE. UPLC, Ultra pressure liqid chromatography; CIEE, ethanol extract of Chrysanthemum indicum L.
Discussion
Obesity is a serious risk factor for hypertension, cardiovascular disease, type-2 diabetes, and cancers (2–6). Though anti-obesity drugs, like orlistat and sibutramine, are commercially available, their adverse effects are abundant, such as, diarrhea, stomach pain, irregular menstruation, steatorrhea and hypertension (24,25). Therefore, the identification of effective natural products with lesser side effects is a topic of considerable research (26). Several studies have described the anti-inflammatory and anti-antioxidant effects of CI. However, the effect of CIEE on HFD-induced obesity has not been previously studied. In the present study, we investigated whether CIEE could inhibit obesity in HFD-induced obese C57BL/6 mice and explored its underlying mechanisms.
Reductions in body weight and fat deposition can prevent obesity (27), and increased fat cell numbers and/or sizes are characteristic of obesity (28). In the present study, food intakes were similar in the study groups, but efficiency percentage (body weight gain per unit of food consumption) was reduced by CIEE treatment in HFD-fed mice. Furthermore, CIEE significantly decreased body weight gains, EWAT and liver weights, volumes and lipid accumulation in EWAT and liver tissues in HFD-fed mice, and reduced HFD-induced increases in serum lipid profiles, that is, TC, LDLc and TG, but increased serum levels of HDLc. These improved lipid profiles induced by CIEE may have been associated reduced fatty liver and hyperlipidemia in HFD-fed mice. Furthermore, these findings concur with those of a previous study, which showed Triticumaestivum sprouts had an anti-obesity effect in HFD-fed mice (19).
The adipokines, leptin and adiponectin secreted by adipose tissue and regulate lipid metabolism. Leptin plays an important role in weight control by suppressing food intake and increasing energy expenditure (29), and adiponectin is involved in fatty acid oxidation and glucose regulation in liver (30,31). We found CIEE significantly reduced serum leptin levels and increased adiponectin levels in HFD-induced obese mice. Similarly, a previous study reported Poncritus trifoliate leaf extracts had an anti-obesity effect in HFD-fed mice (32).
PPARα/γ is ligand-activated transcription factor, which plays role in glucose and protein metabolism and regulates preadipocytes proliferation and differentiation (33). C/EBPs are proteins involved in adipogenesis, and C/EBPα and PPARγ control the biosynthesis of fatty acids, such as acetyl CoA carboxylase and FAS, are key regulators of lipogenesis (34). Also, PPARs regulates lipid metabolism, lipid synthesis, and fatty acid oxidation in liver tissue. In the present study, CIEE significantly down-regulated PPARγ and C/EBPα in EWAT but up-regulated PPARα in liver in HFD fed mice. Moreover, FAS induction by HFD was significantly reduced by CIEE on both EWAT and liver tissues. In line with our results was previously reported anti-obesity effect of germinated brown rice extract through down-regulation of PPARγ and C/EBPα in HFD fed obese mice (35).
Chemical analysis by UPLC in our study showed the detection of quercitrin in CIEE. Recent study reported that quercitrin containing extract of Chrysobalanusicoco leaves inhibited fat accumulation in HFD-fed mice (36). Also, quercitrin isolated from Rhododendron oldhamii leaf extract improves fatty liver syndrome via increase of lipid oxidation and decrease of lipogenesis (37). Thus, quercitrin may be one of the compounds that contribute to the anti-obesity effect of CIEE. The present study demonstrates for the first time CIEE treatment effectively inhibits adipogenesis, and contains anti-adipogenic molecules that down-regulate transcription factors for adipogenesis, such as C/EBPα and PPARα/γ. These results suggest CIEE possesses anti-obesity effects is a promising agent for the treatment of obesity and obesity-associated metabolic syndrome.
Acknowledgements
This study was supported by the Ministry of Trade, Industry and Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region (R0004885).
References
- 1.World Health Organization (WHO): Obesity: Preventing and managing the global epidemic Series 894. WHO; Geneva: 2000. p. 252. [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Kwon TH, Wu YX, Kim JS, Woo JH, Park KT, Kwon OJ, Seo HJ, Kim T, Park NH. 6,6′-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J Sci Food Agric. 2015;95:1830–1837. doi: 10.1002/jsfa.6881. [DOI] [PubMed] [Google Scholar]
- 4.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: Human and rodent perspectives. J Exp Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poudel B, Nepali S, Xin M, Ki HH, Kim YH, Kim DK, Lee YM. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol Med Rep. 2015;12:3139–3145. doi: 10.3892/mmr.2015.3700. [DOI] [PubMed] [Google Scholar]
- 6.Poudel B, Lim SW, Ki HH, Nepali S, Lee YM, Kim DK. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int J Mole Med. 2014;34:1401–1408. doi: 10.3892/ijmm.2014.1921. [DOI] [PubMed] [Google Scholar]
- 7.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46:1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 8.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton JD, Goldstein JL, Brown MS. SREBPs: Transcriptional mediators of lipid homeostasis. Cold Spring Harb Symo Quant Biol. 2002;67:491–498. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- 12.Derosa G, Cicero AF, Murdolo G, Piccinni MN, Fogari E, Bertone G, Ciccarelli L, Fogari R. Efficacy and safety comparative evualation of orlistat and sibutramine treatment in hypertensive obese patients. Diabeted Obes Metab. 2005;7:47–55. doi: 10.1111/j.1463-1326.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 13.Montagut G, Bladé C, Blay M, Fernández-Larrea J, Pujadas G, Salvadό MJ, Arola L, Pinent M, Ardévol A. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J Nutr Biochem. 2010;21:961–967. doi: 10.1016/j.jnutbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J Ethnopharmacol. 2005;101:334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96:151–158. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73:199–207. doi: 10.1016/S0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- 17.Shi GB, Zhao MH, Zhao QC, Huang Y, Chen YF. Mechanisms involved in the antinociception of petroleum ether fraction from the EtOH extract of Chrysanthemum indicum in mice. Phytomedicien. 2011;7:609–616. doi: 10.1016/j.phymed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim RH, Song JH, Shon MS, Chun KS, Choi SU, Kim GN. Evaluation of water extract prepared from Chrysanthemim indicum Linne as Nurti-cosmetic and cosmetic material in vitro model. Asian J beauty Cosmetol. 2016;14:78–88. doi: 10.20402/ajbc.2016.0032. [DOI] [Google Scholar]
- 19.Im JY, Ki HH, Xin M, Kwon SU, Kim YH, Kim DK, Hong SP, Jin JS, Lee YM. Anti-obesity effect of Triticum aestivum sprouts extract in high-fat-diet-induced obese mice. Biosci Biotechnol Biochem. 2015;79:1133–1140. doi: 10.1080/09168451.2015.1006567. [DOI] [PubMed] [Google Scholar]
- 20.Histing T, Andonyan A, Klein M, Scheuer C, Stenger D, Holstein JH, Veith NT, Pohlemann T, Menger MD. Obesity does not affect the healing of femur fractures in mice. Injury. 2016;47:1435–1444. doi: 10.1016/j.injury.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Nepali S, Ki HH, Lee JH, Cha JY, Lee YM, Kim DK. Triticum aestivum sprout-derived polysaccharide exerts hepatoprotective effects against ethanol-induced liver damage by enhancing the antioxidat system in mice. Int J Mole Med. 2017;40:1243–1252. doi: 10.3892/ijmm.2017.3095. [DOI] [PubMed] [Google Scholar]
- 22.Nepali S, Son JS, Poudel B, Lee JH, Lee YM, Kim DK. Luteolin is a bioflavonoid that attenuates adipocyte-derived inflammatory responses via suppression of nuclear factor-κB/mitogen-activated protein kinases pathway. Pharmacogn Mag. 2015;11:627–635. doi: 10.4103/0973-1296.160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poudel B, Ki HH, Luyen BT, Lee YM, Kim YH, Kim DK. Triticumoside induces apoptosis via caspase-dependent mitochondrial pathway and inhibits migration through downregulation of MMP2/9 in human lung cancer cells. Acta Biochim Biophys Sin (Shanghai) 2016;48:153–160. doi: 10.1093/abbs/gmv124. [DOI] [PubMed] [Google Scholar]
- 24.Chaput JP, St-Pierre S, Tremblay A. Currently available drugs for the treatment of obesity: Sibutramine and orlistat. Mini Rev Med Chem. 2007;1:3–10. doi: 10.2174/138955707779317849. [DOI] [PubMed] [Google Scholar]
- 25.Siebenhofer A, Jeitler K, Horvath K, Berghold A, Posch N, Meschik J, Semlitsch T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev. 2016;3:CD007654. doi: 10.1002/14651858.CD007654.pub4. [DOI] [PubMed] [Google Scholar]
- 26.Choudhary M, Grover K. Development of functional food products in relation to obesity. Funct Foods Health Dis. 2012;2:188–197. [Google Scholar]
- 27.Hue JJ, Lee KN, Jeong JH, Lee SH, Lee YH, Jeong SW, Nam SY, Yun YW, Lee BJ. Anti-obesity activity of diglyceride containing conjugated linoleic acid in C57BL/6J ob/ob mice. J Vet Sci. 2009;10:189–195. doi: 10.4142/jvs.2009.10.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han LK, Kimura Y, Okuda H. Reduction in fat storage during chitin-chitosan treatment in mice fed a high-fat diet. Int J Obes Relat Metab Disord. 1999;23:174–179. doi: 10.1038/sj.ijo.0800806. [DOI] [PubMed] [Google Scholar]
- 29.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kin S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 30.Combs TP, Berg AH, Obici W, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pajvani UB, Scherer PE. Adiponectin: Systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–213. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 32.Jia S, Gao Z, Yan S, Chen Y, Sun C, Li X, Chen K. Anti-obesity and hypoglycemic effects of poncirus tridoliata L. Extracts in high-fat diet C57BL/6 mice. Molecules. 2016;21:543. doi: 10.3390/molecules21040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christodoulides C, Vidal-Puig A. PPARs and adipocyte function. Mol Cell Endocrinol. 2010;318:61–68. doi: 10.1016/j.mce.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;27:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 35.Ho JN, Son ME, Lim WC, Lim ST, Cho HY. Anti-obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice. Biosci Biotechnol Biochem. 2012;76:1068–1074. doi: 10.1271/bbb.110666. [DOI] [PubMed] [Google Scholar]
- 36.White PA, Cercato LM, Batista VS, Camargo EA, De Lucca W, Jr, Oliveira AS, Silva FT, Goes TC, Olivera ER, Moraes VR, et al. Aqueous extract of Chrysobalanus icaco leaves, in lower doses, prevent fat gain in obese high-fat fed mice. J Ethnopharmacol. 2016;179:92–100. doi: 10.1016/j.jep.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Liu YL, Lin LC, Tung YT, Ho ST, Chen YL, Lin CC, Wu JH. Rhododendron oldhamii leaf extract improves fatty liver syndrome by increasing lipid oxidation and decreasing the lipogenesis pathway in mice. Int J med Sci. 2017;9:862–870. doi: 10.7150/ijms.19553. [DOI] [PMC free article] [PubMed] [Google Scholar]