Abstract
Myeloid-derived suppressor cells (MDSCs) serve an immunosuppressive role in human tumors. Human Lin−/low human leukocyte antigen-antigen D related (HLA-DR−) cluster of differentiation (CD)-11b+CD33+ MDSCs are closely linked with tumor staging, progression, clinical therapeutic efficacy and prognosis for various types of tumors. The present study employed multiparametric flow cytometry to measure the proportion of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in the peripheral blood of 105 cervical cancer patients and 50 healthy subjects. The level of MDSC was higher in tumor patients than in the normal control group and this was closely associated with clinical staging. Further analysis of tumor-infiltrating MDSCs was performed in 22 patients. The MDSC proportions in tumor tissue were significantly higher than those in the corresponding adjacent tissue. The phenotypic characteristics of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs were then evaluated and the results revealed that they express high CD13 and CD39, and low CD115, CD117, CD124 and programmed cell death ligand 1; they were also devoid of CD14, CD15 and CD66b. MDSCs and T-cells from peripheral blood were sorted by flow cytometry for co-culture experiments. Lin−/lowHLA-DR−CD11b+CD33+ MDSCs from patients significantly inhibited the proliferation of CD4 and CD8 T-cells. Furthermore, functional analysis verified that MDSCs from cervical cancer patients could inhibit interleukin-2 and interferon-γ production from T-cells. In addition, the associations between peripheral circulating MDSCs and tumor infiltrating MDSCs, and tumor relapse and metastasis were analyzed. The number of peripheral MDSCs and MDSCs in tumor tissue were observed to be associated with relapse-free survival. Thus, MDSCs in the peripheral blood and tumors of cervical cancer patients have a significant immunosuppressive effect, and are associated with cervical cancer staging and metastasis. These results suggest that targeting MDSCs may increase antitumor immunity and increase the efficacy of cervical cancer therapies.
Keywords: cervical cancer, immunosuppressive, myeloid-derived suppressor cells, T cells
Introduction
Cervical cancer, a malignant tumor, is the fourth most common cancer in women. The main cause of cervical cancer is continuous infection with human papilloma virus (HPV) (1–4). Cervical cancer cells rapidly evade the immune system and promote tumor progression by inhibiting antitumor immunity (5–8). Many reports have demonstrated the expansion of various immunosuppressive cells such as regulatory T cells, tumor-associated macrophages, myeloid-derived suppressor cells (MDSCs), and N2 neutrophils in cervical tumors (7–10). Therefore, understanding changes in immunosuppressive cells is important for tumor diagnosis and treatment.
In cancer patients and animal tumor models, there is a significant accumulation of MDSCs, a heterogeneous and diverse population, in the blood, lymph nodes, bone marrow, and cancer tissues, and they can inhibit innate and adaptive immune responses (11,12); this represents an important mechanism of immune evasion for tumor cells. MDSCs have different phenotypes, based on factors secreted during bone marrow differentiation and by tumor cells, which affect cell differentiation (13). In mice, CD11b and Gr-1 are used as specific markers of MDSCs. Further studies divided mouse MDSCs into two major subsets, namely monocytic (Gr-1+Ly6C+) and granulocytic (Gr-1+Ly6G+) (14). In contrast, human MDSCs are not associated with widely recognized specific markers (15,16). However, similarly, they can also be classified as granulocytic (CD15, CD66b, and CD33-expressing) and monocytic (CD14-expressing). In kidney cancer patients, CD14−CD15+CD11b+CD66+ granulocytic MDSCs are immunosuppressive (17). Various MDSC phenotypes in non-small cell lung cancer include Lin−/lowHLA-DR−CD11b+CD33+, Lin−/lowHLA-DR−CD33+, CD14+S100A9+, CD14−CD15+CD11b+CD33+, and CD14+HLA-DR− have been confirmed (18–22). In addition, the immunosuppressive effects of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs have been reported in human malignant gliomas, breast cancers, colon cancers, and kidney cancers (13,23–25), and increased MDSC levels are associated with tumor burden and prognosis in breast and colon cancer patients (19).
In the current study, the level of MDSCs in the peripheral blood of 105 patients with different clinical stages and in tumor tissue and corresponding adjacent tissue of 22 clinical specimens were assessed. Cellular subsets and phenotypic characteristics and function of these cells were analyzed. The accumulated evidence can contribute to understanding the clinical characteristics of peripheral blood and local tumor-infiltrating Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in cervical cancer.
Materials and methods
Ethics statement
All cervical carcinoma patients and healthy donors provided written informed consent prior to blood sampling and/or tumor tissue harvesting. The research protocol was approved by the Medical Ethics Committee of Chinese PLA General Hospital (Beijing, China) and the 307th Hospital of Chinese PLA (Beijing, China).
Patients
Control samples from healthy volunteers (n=50) and cervical cancer patient samples (n=105) were obtained at the gynecology departments of the Chinese PLA general hospital and the 307th Hospital of Chinese PLA. All patients were newly diagnosed and treatment-naive. Table I shows the clinical characteristics of patients included in this study.
Table I.
Patient characteristics.
| Variables | Total n number (%) |
|---|---|
| Number of patients (n) | 105 |
| Age [years; mean (SD)] | 44.3 (7.8) |
| FIGO stage | |
| Stage I | 23 (21.9) |
| Stage II | 28 (26.7) |
| Stage III | 22 (21.0) |
| Stage IV | 32 (30.4) |
| HPV type | |
| 16 | 76 (72.4) |
| 18 | 24 (22.8) |
| Other | 5 (4.8) |
| Histopathology | |
| Squamous | 64 (61.0) |
| Adeno (squamous) | 41 (39.0) |
| Lymph node metastasis | |
| Lymph nodes (+) | 61 (58.1) |
| Lymph nodes (−) | 44 (41.9) |
| Vasoinvasion | |
| No | 31 (29.5) |
| Yes | 74 (70.5) |
| Parametrial involvement | |
| No | 26 (24.8) |
| Yes | 79 (75.2) |
SD, standard deviation; FIGO, International Federation of Gynecology and Obstetrics; HPV, human papillomarvirus.
Flow cytometry
Blood samples for the detection of peripheral circulating MDSCs were collected using EDTA anticoagulant tubes (BD Biosciences, Franklin Lakes, NJ, USA). Monoclonal fluorescent antibodies, CD11b-PECY7 (cat. no. A54822), HLA-DR-ECD (cat. no. IM3636), and CD33-PECY5 (cat. no. IM26 47 U), were from Beckman Coulter, Inc., (Brea, CA, USA). Lineage (CD3, CD14, CD16, CD19, CD20, and CD56-FITC) (cat. no. 340546) antibodies were all from BD Biosciences. Four-color analysis was used to confirm MDSCs. Analysis of tumor-infiltrating MDSCs was performed using anti-human CD45-FITC (cat. no. 304006), CD11b-PECY7 (cat. no. A54822), and CD33-PECY5 (cat. no. IM2647 U; all from BioLegend, Inc., San Diego, CA, USA). For phenotypic characterization of MDSCs, the MDSC population was gated for the analysis of PE expression. Antibodies involved in phenotype analysis included CD13-PE (cat. no. 301703), CD39-PE (cat. no. 328208), CD34-PE (cat. no. 343505), CD73-PE (cat. no. 344004), CD66b-PE (cat. no. 305105), CD115 (CSF-1R)-PE (cat. no. 347303), PD-1 (CD279)-PE (cat. no. 329906), CD124 (IL-4Ra)-PE (cat. no. 355003), PD-L1 (CD274)-PE (cat. no. 329706), and PD-L2 (CD273)-PE (cat. no. 329606). Isotype control antibodies (Mouse IgG1-PE, cat. no. 400114; Mouse IgM-PE, cat. no. 401611; Mouse IgG2a-PE, cat. no. 400214; Mouse IgG2b-PE, cat. no. 401208; Rat IgG1-PE, cat. no. 400408) were used as controls. Beforementioned antibodies were from BioLegend, Inc. For the detection of peripheral blood and tumor-infiltrating cell phenotypes, a standard amount of corresponding antibody was added. Subsequently, 500 µl of OptiLyse C Lysing Solution (cat. no. A11895; Beckman Coulter, Inc.) was added to each blood sample and incubated for 15 min; 500 µl PBS was then added before 500 µl of FACS buffer was added; analysis was performed by flow cytometry. For intracellular cytokine staining, purified MDSCs were added at a ratio of 1:1 to the control group (lymphocytes alone) or to the experimental group. Cell Stimulation cocktail plus protein transport inhibitors (eBioscience; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to each group for 4 h of stimulation. Cell surface marker staining was performed using CD3-ECD (cat. no. A07748), CD4-PC5 (cat. no. IM2636 U), and CD8-PECY7 (cat. no. 6607102) (all from Beckman Coulter, Inc.). The BD Cytofix/Cytoperm™ Plus Fixation/Permeabilization kit (cat. no. 555028) reagent box was used to process cells before intracellular cytokine staining using IL-2-PE (cat. no. 506709) and IFNg-FITC (cat. no. 552887) antibodies and corresponding isotype control antibodies (Rat IgG2a-PE, cat. no. 559317; Mouse IgG1-FITC, cat. no. 556649; all from BD Biosciences). After treatment, cells were analyzed by flow cytometry for the production of T-cell cytokines. Samples were obtained using a flow cytometer FC500-MPL (Beckmam Coulter, Inc.), and data analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). Absolute MDSC counts were calculated using the following formula: [total white blood cell count (cells/ml) percent MDSCs]/100 or [total tumor-infiltrating immune cell count (cells/100 mg tumor) percent MDSCs]/100.
Cell separation
Separation of PBMCs was performed using density gradient centrifugation. Briefly, blood samples with EDTA anticoagulant were carefully separated by Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA) separation media. The PBMC obtained after centrifugation staining was used to determine cell viability by trypan blue before flow cytometry.
To separate tumor-infiltrating immune cells, newly resected tumor tissue (100 mg) and matching surrounding tissue from 22 Stage III or IV cervical cancer patients were cut into pieces and digested using 500 mg/ml Liberase (collagenase) and 200 mg/ml DNase (Roche Applied Science, Penzberg, Germany) for 45 min. The cell suspension was then passed through a 70-µm cell strainer (BD Biosciences). Centrifugation using a density gradient was then performed as described, and the corresponding cell layer was aspirated using a pipette.
In vitro inhibition analysis experiment
Fresh blood samples (20 ml) from three stage IV cervical cancer patients were used for PBMC extraction. CD11b-PECY7, HLA-DR-ECD, CD33-PECY5, Lineage-FITC, and CD3 monoclonal fluorescent antibodies were added to PBMCs before being sorted by the MoFloTM XDP cell sorting system (Beckman Coulter, Inc.). Sorted cells had a purity >95%. For MDSC functional analysis, purified CD3 T-cells were stained with 2 mM CFSE (Invitrogen; Thermo Fisher Scientific, Inc.) and CFSE-stained T-cells were cultured with Lin-/lowHLA-DR-CD11b+ CD33+ MDSCs at ratios of 1:0, 1:0.25, 1:0.5, and 1:1. Soluble anti-CD3 (2 mg/ml) and anti-CD28 (0.5 mg/ml) antibodies were added and cells were incubated for 24 h before being measuring proliferation through flow cytometry.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, United States); unpaired Student's t tests (Mann-Whitney test) and unparametric Spearman tests were used to assess differences and correlations between study groups, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
Increase in the proportion and numbers of peripheral Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in cervical cancer patients
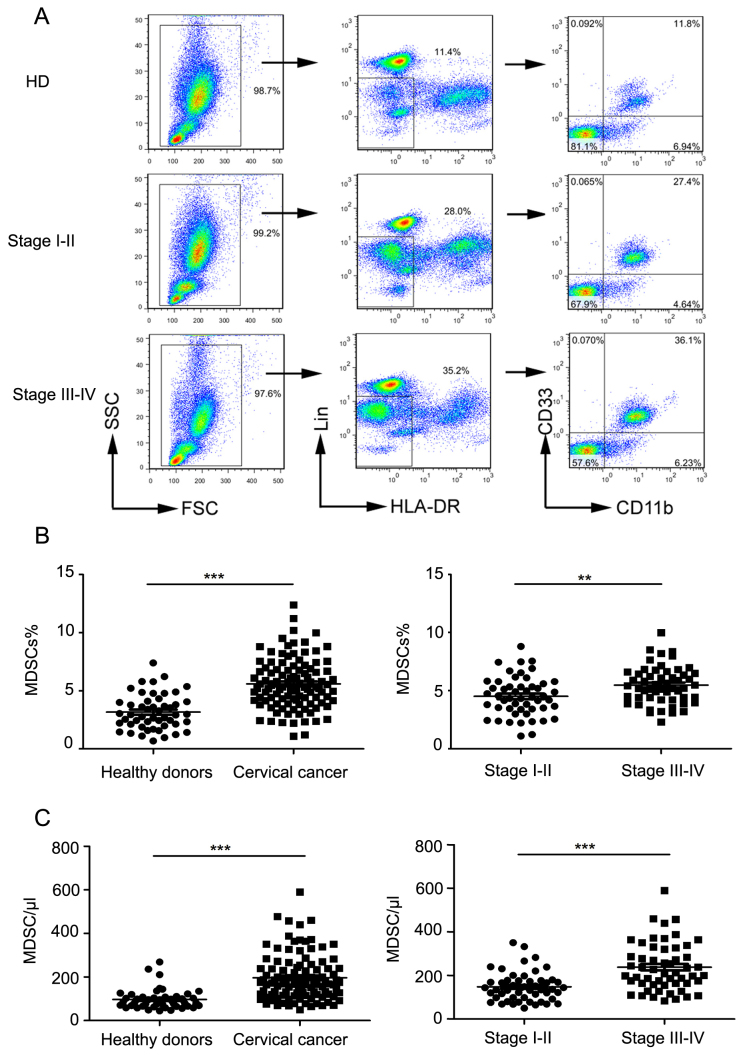
The proportion of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in the peripheral blood of cervical cancer patients of different clinical stages was measured by flow cytometry (Table I shows clinical patients data). The ratio of MDSCs to total leukocytes in healthy volunteers, clinical stage I–II, and Stage III–IV patients was calculated using flow cytometry (as depicted in Fig. 1A). The proportion of MDSCs in cervical cancer patients was significantly higher compared to that in controls (P<0.0001; Fig. 1B). MDSC levels were also significantly increased in the peripheral blood of cervical cancer patients compared to that in controls (P<0.0001; Fig. 1C).
Figure 1.
Levels of peripheral blood Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in normal subjects and cervical cancer patients. (A) Representative flow cytometry and analysis strategy. (B) Comparison of peripheral circulating MDSC proportions in normal controls (n=50) and cervical cancer patients (n=105; ***P<0.0001, as indicated), and comparison of patients in late stage with early stage (**P=0.0014, as indicated). (C) Comparison of absolute MDSC counts in normal controls (n=50) and cervical cancer patients (n=105; ***P<0.0001, as indicated), and comparison of patients in late stage with early stage (***P=0.0001, as indicated). MDSCs, myeloid-derived suppressor cells; HLA-DR, human leukocyte antigen-antigen D related; CD, cluster of differentiation; FSC, forward scatter; SSC, side scatter; HD, healthy donors.
Further, the proportion of MDSCs in clinical stage III–IV patients was significantly higher than that in clinical stage I–II patients (P=0.0014; Fig. 1B). Next, we found that the absolute number of MDSCs in stage III–IV patients was significantly higher than that in stage I–II patients (P<0.0001; Fig. 1C).
Elevation in the proportion and numbers of tumor-infiltrating Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in late stage cervical cancer patients
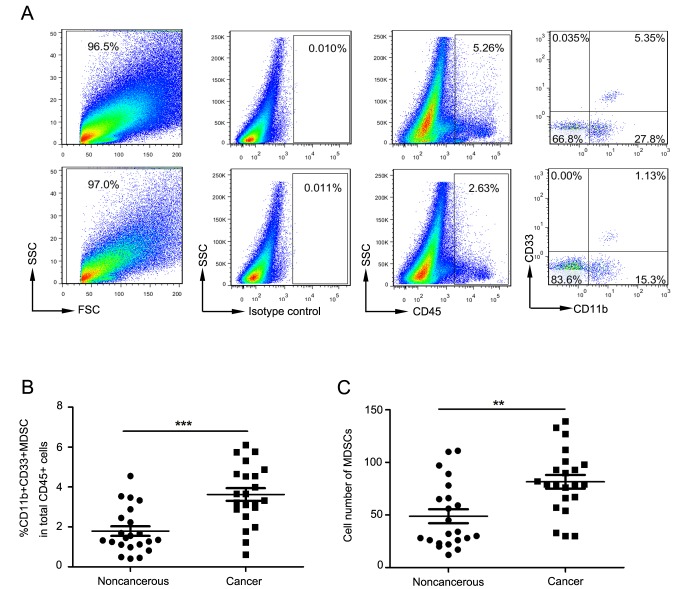
Fig. 2A depicts flow cytometry used to examine tumor-infiltrating MDSCs in cervical cancer patients. For all tumor-infiltrating CD45+ leukocytes, the proportion of MDSCs in cancer tissues was significantly increased compared to that in surrounding non-cancerous tissue (P<0.0001; Fig. 2B). The absolute count of MDSCs in cancer tissues was also significantly increased compared to that in surrounding non-cancerous tissue (P=0.001; Fig. 2C).
Figure 2.
MDSCs increased in the tumor tissues of late stage cervical cancer patients. (A) Representative flow cytometry and analysis strategy. (B) Proportion and (C) absolute counts of MDSCs in tumor tissues and surrounding non-cancerous tissue. **P=0.001 and ***P<0.0001, as indicated. MDSCs, myeloid-derived suppressor cells; FSC, forward scatter; SSC, side scatter; CD, cluster of differentiation.
Functional characteristics of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs
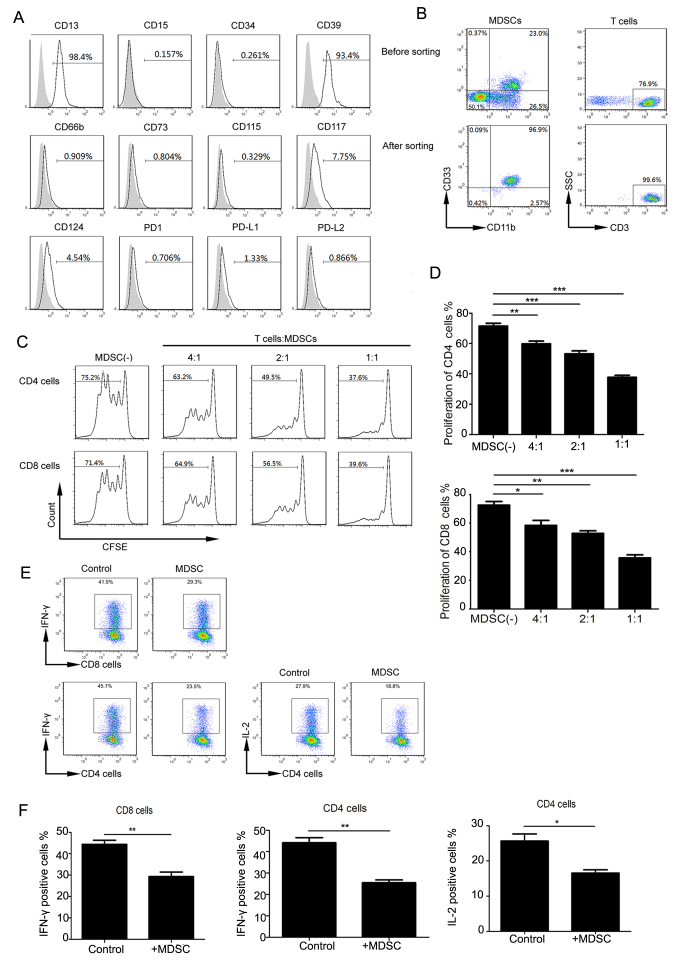
We used flow cytometry to analyze the phenotypic characteristics of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs, including myeloid and lymphoid markers (Fig. 3A). In the peripheral MDSCs of cervical cancer patients, CD13 was highly expressed, CD124 (IL-4Ra), CD115, and CD117 were lowly expressed, and CD66b, CD14, CD15, PDL1, PD1, CD34 were not expressed. These cells expressed high levels of CD39 but did not express CD73. Intracellular staining also failed to detect CD73 expression (results not shown); this was in accordance with a previous study on colorectal cancer (26). Similar to that observed in mouse MDSCs (14), PDL1 expression in Lin−/lowHLA-DR−CD11b+CD33+ MDSCs was low. No significant differences were observed with respect to these markers between normal and cervical cancer samples.
Figure 3.
Functional characteristics of Lin−/lowHLA-DR−CD11b+CD33+ MDSCs. (A) Expression of indicated molecules (gray histograms represent isotype controls). (B) Flow cytometry was used to purify MDSCs and T cells. Representative flow cytometry prior to and following cell sorting. (C) Representative flow cytometry of the inhibitory effect of MDSCs on CD4 and CD8 T cells. (D) Quantitation of MDSC inhibition of CD8 and CD4 cells. *P<0.05, **P<0.01 and ***P<0.001, as indicated. (E) Representative flow cytometry of IL-12 and IFN-γ secretion by CD4 and CD8 cells. (F) The positive percentage of CD8 that secretes IFN-γ, and CD4 that secretes IFN-γ and IL-2, respectively (**P=0.006, **P=0.0024 and *P=0.0372, respectively). MDSCs, myeloid-derived suppressor cells; SSC, side scatter; CFSE, carboxyfluorescein succinimidyl ester; CD, cluster of differentiation; IL, interleukin; PD1, programmed cell death protein 1; PD-L, programmed cell death ligand; IFN, interferon.
Previous studies showed that Lin−/lowHLA-DR−CD11b+CD33+ MDSCs can inhibit T-cell proliferation in other tumor types. We extracted highly pure (> 95%) MDSCs and CD3+ T-cells (Fig. 3B) from peripheral blood. CFSE-labeled CD3+ T cells were co-cultured with MDSCs at different ratios and stimulated with CD3 and CD28 antibodies. Upon analyzing CFSE fluorescence in CD4 and CD8 T cell subsets, we found that with an increasing proportion of MDSCs, CD4 and CD8 T cells were significantly inhibited (Fig. 3C and D).
MDSCs can function through multiple mechanisms, including inhibiting cytokine production in T cells. We tried to verify the effects of MDSCs from cervical cancer patients on CD4 and CD8 cells by analyzing IL-2 and IFN-γ in CD4 T-cells and IFN-γ production in CD8 T-cells. The cytokine production in CD4 and CD8 cells was decreased in the experimental MDSC group compared to that in the control group (no MDSCs) (Fig. 3E). IFN-γ production in CD8, and IFN-γ and IL-2 production in CD4 T cells, respectively, in the control and experimental groups (P=0.006, P=0.0024 and P=0.0372 respectively) (Fig. 3F). These data suggest that cervical cancer-associated MDSCs can inhibit cytokine production in T cells, resulting in decreased proliferation cytotoxicity.
Peripheral and tumor-infiltrating MDSCs is associated with metastasis in late stage cervical cancer
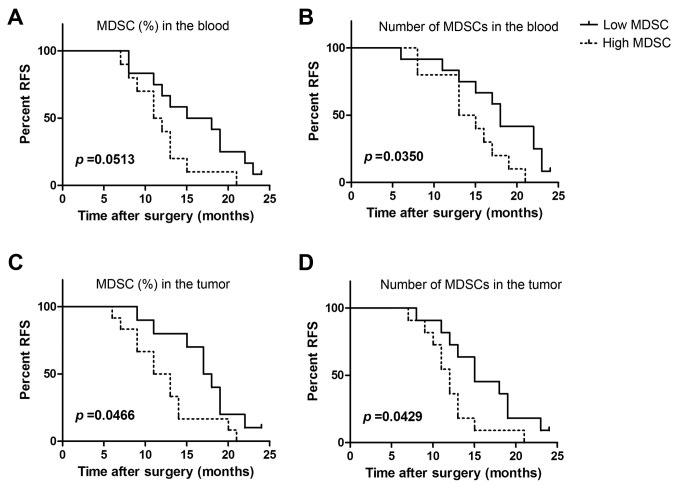
We further analyzed the proportions and absolute numbers of peripheral circulating MDSCs in late stage cervical cancer patients with tumor relapse and metastasis. Peripheral blood MDSCs from 22 patients were divided based on MDSC proportions into the high group (>mean value) and the low group (<mean value). Relapse was found to occur more readily in the high group (Fig. 4A). Although our results did not reach statistical significance (P=0.0531), some correlation was observed. Similarly, levels of peripheral MDSCs in clinical stage IV cervical cancer patients were found to significantly correlate with RFS (P=0.035; Fig. 4B).
Figure 4.
Peripheral blood and tumor-associated MDSCs in cervical cancer are associated with recurrence and progression. The levels of peripheral blood tumor tissue MDSCs from 22 late stage cervical cancer patients were assessed; the follow-up period was 24 months. (A) The associations between RFS and the proportion of peripheral MDSC. Patients were divided into two groups based on the mean value (6.4%), as the high (n=10) and low MDSC groups (n=12). (B) The associations between RFS and the absolute counts of peripheral MDSC. Patients were divided into two groups based on the mean value (300), as the high (n=10) and low MDSC groups (n=12). (C) The associations between RFS and the proportion of peripheral MDSC in tumors. Patients were divided into two groups based on the mean value (3.6%), as the high (n=11) and low MDSC groups (n=11). (D) The associations between RFS and the absolute counts of peripheral MDSC in tumors. Patients were divided into two groups based on the mean value (81), as the high (n=10) and low MDSC groups (n=12). MDSCs, myeloid-derived suppressor cells; RFS, relapse-free survival.
We next analyzed the relationship between MDSC proportion and absolute counts and tumor recurrence and metastasis in 22 tumors from clinical stage IV cervical cancer patients. We observed that a higher proportion of MDSCs was significantly associated with recurrence (P=0.0466; Fig. 4C). MDSC levels in tumors were also found to be significantly associated with tumor recurrence and metastasis (P=0.0429; Fig. 4D). Thus, the proportion of peripheral and tumor-infiltrating MDSCs are related to tumor progression in cervical cancer patients.
Discussion
MDSCs play an important role in tumor immune evasion and tolerance. We examined changes in Lin−/lowHLA-DR−CD11b+CD33+ MDSCs in cervical cancer patients based on clinical stage and obtained results that were consistent with other tumor types.
The samples were all fresh, and conventional sample preparation protocols were used. In addition to examining MDSC phenotypes, we also assessed. In mice, MDSC populations have been verified (14), whereas in humans, three MDSC populations are recognized. A recent review introduced the phenotypes and characteristics of mouse and human MDSCs. Mouse MDSCs are classified as mixed MDSCs (Gr-1+CD11b+), which was further classified, based on Ly6C and Ly6 G expression, as PMN-MDSCs (CD11b+Ly6CloLy6G+) and M-MDSCs (CD11b+Ly6ChiLy6G−). There are three recognized human MDSC subsets, namely PMN-MDSCs (CD14−CD11b+CD15+/CD66b+), M-MDSCs (CD11b+CD14+HLA-DRlow/−CD15−), and E-MDSC (Lin−/lowHLA-DR−CD11b+CD33+). This review also summarized the biological functions of these MDSCs, including inhibiting T lymphocyte proliferation, IL-2 and IFN-γ production, and function (27). Our results were consistent with those previously reported; specifically, we found that MDSCs inhibit T-cell proliferation, IL-2 and IFN-γ production in CD4 T cells, and IFN-γ production in CD8 T cells.
In these other studies, changes in MDSCs were shown to correlate linearly with tumor burden (19,28,29). With increasing clinical stage, circulating MDSCs increase. Late stage cancer patients with higher levels of MDSCs are more prone to recurrence, which in turn affects prognosis (13). Similarly, we showed that peripheral circulating and tumor-infiltrating MDSC levels are associated with RFS. Possible reasons for these are as follows: Circulating tumor cells or tumor cells in the tumor microenvironment could secrete cytokines resulting in the expansion of MDSCs and produce pro-inflammatory and angiogenic cytokines to recruit MDSCs, promoting tumor proliferation and invasion (30).
Previous studies have shown that the proportion of MDSCs is higher in the microenvironment of different tumor types (21,31,32). We also showed that infiltrating MDSC numbers were increased in cervical cancer patients.
We found that Lin−/lowHLA-DR−CD11b+CD33+ MDSCs have a specific phenotypic profile. These cells highly express the myeloid marker CD13, exhibit low expression of CD115, CD124, and CD117, and do not express the monocytic marker CD14 or the granulocytic markers CD15 and CD66b. These phenotypes are generally similar to those previously reported (13). Interestingly, this population of cells highly expresses CD39 but not CD73, which both synergistically promote immunosuppression (33). These two molecules are expressed on human regulatory T cells and mediate an immunosuppressive effect (34), inhibiting the function of Th1, Th2, CTL, and NK cells (33,35). In a mouse study, the expression of CD39 and CD73 increased the immunosuppressive activity of MDSCs (36). We also found that this cell population expresses low levels of PD-L1 and does not express PD-L2 or its receptor PD-1 and B7 family members, but can still regulate the immune response and induce immune tolerance (37).
Consistent with the results of previous studies, circulating MDSCs were shown to inhibit T-cell proliferation. Some reports have confirmed that BM-MDSCs inhibit T-cell proliferation by decreasing their expression of CD3ε and CD3ξ (13). Our in vitro experiments also confirmed that Lin−/lowHLA-DR−CD11b+CD33+ MDSCs can inhibit IL-12 and IFN-γ production in T-cells and reduce T cell performance. BM-MDSCs also express arginase I, which depletes extracellular L-arginine, resulting in downregulation of CD3ε chain and diminished T-cell proliferation (17). Lastly, we confirmed that Lin−/lowHLA-DR−CD11b+CD33+ MDSCs are associated with tumor burden in cervical cancer. Abnormal accumulation of peripheral blood or local MDSCs is an important immunological mechanism of T cell anergy. Our studies could provide the foundation for immunotherapy to treat cervical cancer, and particularly immunotherapy targeting MDSCs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LW performed flow cytometry and was a major contributor in writing the manuscript. HL and HG analyzed and interpreted the patient data for the cervical carcinoma patients, and were major contributors in writing the manuscript. QW and SY performed the separation of PBMC. YQ and LW performed the separation of tumor-infiltrating immune cells. GW and QW performed the in vitro inhibition analysis experiments. LZ and CL performed the collection of patient samples. RZ developed the methodology, performed data analysis and was a major contributor in revising the manuscript. TL and SJ made substantial contributions to the conception and design, analysis and interpretation of data, and fund support.
Ethics approval and consent to participate
The research protocol was approved by the Medical Ethics Committee of Chinese PLA General Hospital (Beijing, China) and the 307th Hospital of Chinese PLA (Beijing, China). All patients provided written informed consent for participation in the present study.
Consent for publication
Written informed consent was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 4.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: Unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 5.Grabowska AK, Riemer AB. The invisible enemy-how human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol J. 2012;6:249–256. doi: 10.2174/1874357901206010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7:79–89. doi: 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008;1:412–420. doi: 10.1038/mi.2008.33. [DOI] [PubMed] [Google Scholar]
- 8.Piersma SJ. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011;4:361–375. doi: 10.1007/s12307-011-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A and CD8+/regulatory T-cell ratio: Which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14:2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 10.De Vos van Steenwijk PJ, Ramwadhdoebe TH, Goedemans R, Doorduijn EM, van Ham JJ, Gorter A, van Hall T, Kuijjer ML, van Poelgeest MI, van der Burg SH, Jordanova ES. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int J Cancer. 2013;133:2884–2894. doi: 10.1002/ijc.28309. [DOI] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: A clinical perspective. J Immunother. 2012;35:107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 16.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients' CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng PH, Lee KY, Chang YL, Chan YF, Kuo LW, Lin TY, Chung FT, Kuo CS, Yu CT, Lin SM, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med. 2012;186:1025–1036. doi: 10.1164/rccm.201204-0636OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR(−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62:1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 24.Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, Yasir S, Jorda M, Garret-Mayer E, Avisar E, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II–IIIc breast cancer. Breast Cancer Res Treat. 2012;132:215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591–599. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 29.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 30.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM, Kusmartsev S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–1119. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 32.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghiringhelli F, Bruchard M, Chalmin F, Rébé C. Production of adenosine by ectonucleotidases: A key factor in tumor immunoescape. J Biomed Biotechnol. 2012;2012:473712. doi: 10.1155/2012/473712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergamin LS, Braganhol E, Zanin RF, Edelweiss MI, Battastini AM. Ectonucleotidases in tumor cells and tumor-associated immune cells: An overview. J Biomed Biotechnol. 2012;2012:959848. doi: 10.1155/2012/959848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.