Abstract
Background
Hartmann’s procedure is a commonly performed operation for complicated left colon diverticulitis or malignancy. The timing for reversal of Hartmann’s is not well defined as it is technically challenging and carries a high complication rate.
Methods
This study is a retrospective audit of all patients who underwent Hartmann’s procedure between 2008 and 2014. Reversal of Hartmann’s rate, timing, American Society of Anesthesiologists grade, length of stay and complications (Clavien–Dindo) including 30-day mortality were recorded.
Results
Hartmann’s procedure (n = 228) indications were complicated diverticular disease 44% (n = 100), malignancy 32% (n = 74) and other causes 24%, (n = 56). Reversal of Hartmann’s rate was 47% (n = 108). Median age of patients was 58 years (range 21–84 years), American Society of Anesthesiologists grade 2 (range 1–4), length of stay was eight days (range 2–42 days). Median time to reversal of Hartmann’s was 11 months (range 4–96 months). The overall complication rate from reversal of Hartmann’s was 21%; 3.7% had a major complication of IIIa or above including three anastomotic leaks and one deep wound dehiscence. Failure of reversal and permanent stoma was less than 1% (n = 2). Thirty-day mortality following Hartmann’s procedure was 7% (n = 15). Where Hartmann’s procedure wass not reversed, for 30% (n = 31) this was the patient’s choice and 70% (n = 74) were either high risk or unfit.
Conclusions
Hartmann’s procedure is reversed less frequently than thought and consented for. Only 46% of Hartmann’s procedures were stoma free at the end of the audit period. The anastomotic complication rate of 1% is also low for reversal of Hartmann’s procedure in this study.
Keywords: Colonic diverticulosis, Colorectal neoplasms, Colorectal surgery, Colostomies
Introduction
Hartmann’s procedure consists of a rectosigmoid resection, closure of the rectal stump and formation of an end colostomy. It was first described by Henri Hartmann in 1921 as a novel solution to high rates of anastomotic dehiscence and mortality following resection and primary anastomosis of obstructing left-sided colonic carcinoma.1 Indications for Hartmann’s procedure have expanded to include a range of pathologies resulting in obstruction or perforation of the left colon including malignancy, diverticulitis, ischemia, volvulus or trauma.
Ideally, Hartmann’s procedure is followed by reversal of Hartmann’s, restoring intestinal continuity. However, this is considered a major operation associated with high morbidity rates of up to 58% and mortality of up to 3.6%.2–14 Hartmann’s procedure is commonly performed in older, comorbid patients and may preclude subsequent reversal. Technical challenges particular to reversal include dense pelvic adhesions, chronic pelvic infection, difficulty in identification of and anastomosis to a short rectal stump. As a result, large numbers of patients are left with a permanent stoma following Hartmann’s procedure, either due to inability to perform reversal or due to anastomotic leak and stoma restoration.15 According to the National Bowel Cancer Audit in 2015, 95% of patients undergoing Hartmann’s still have a stoma at 18 months.16
The identification of factors predicting successful reversal, as well as factors predicting morbidity and mortality following reversal, will allow patient selection, optimisation of risk factors and realistic preoperative counselling before Hartmann’s procedure in those unlikely to proceed reversal.
This study aimed to answer three main questions:
What are the factors that can be used to predict reversal of Hartmann’s procedure?
What are the factors that can predict complications following reversal and did the procedure involve a stapled or handsewn anastomosis?
What were the rates of stoma-free survival following Hartmann’s procedure and the reasons for not proceeding to reversal?
Materials and methods
This was a retrospective observational study. Consecutive patients aged 18 years and above undergoing Hartmann’s procedure with or without reversal from 2008 to 2015 at the Heart of England NHS Trust were identified and included. Cases were retrieved using the Hospital Episode Statistic procedure codes H33.4, (Hartmann’s procedure) and H15.4 (reversal of Hartmann’s procedure). Notes were reviewed and incorrectly coded cases excluded.
Patients were divided into two groups: those undergoing Hartmann’s procedure (group 1) and those proceeding to reversal following Hartmann’s procedure (group 2). The following parameters were collected for all patients: demographic details, smoking status, Charlston comorbidity index and American Society of Anesthesiologists grade (ASA). Pertaining to the initial Hartmann’s procedure, the following were recorded: indication for Hartmann’s procedure (malignant or benign), emergency or elective, 30-day postoperative mortality and length of stay. For group 1, the indication not to proceed to reversal was categorised as patient choice or surgeon recommendation due to advanced age, comorbidity, recurrence of malignancy or anticipated technical difficulties. For group 2, further parameters were collected relating to the reversal including preoperative albumin </> 35g/l, time to reversal, handsewn or stapled anastomosis, technical difficulties, formation of diverting ileostomy, subsequent reversal of ileostomy, and postoperative morbidity (Clavien–Dindo grading).
Definition of parameters
ASA grading is a widely used and accepted tool to assess a patients anaesthetic risk. It is based on five classes (I–V) and is calculated and recorded preoperatively. In this study, statistical analysis an ASA of greater than II was graded as high risk and I–II as low risk.
The Charlston comorbidity index is a method of categorising patient’s comorbidities. Each comorbidity has an associated score of 1–6 based on the adjusted risk of mortality. Scores are summed to give a single comorbidity score. In this studies statistical analysis, a Charlston comorbidity score of 0–1 was regarded as low risk and greater than 1 as high risk.
Clavien–Dindo is a validated method of classifying surgical complications according to the treatment needed from no intervention to reoperation, graded from I to V.
Outcomes
Primary outcome measures were factors predicting reversal, factors predicting complication following reversal. Secondary outcome measures included rates of stoma-free survival, the reasons for non-reversal of Hartmann’s procedure and time to reversal.
Ethics
This study was approved by the Audit and Clinical Governance Committee of the Heart of England NHS Foundation Hospitals Trust.
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS for Windows release 19.0). Univariate analysis was performed using Chi squared for categorical variables and the Mann–Whitney U for continuous variables. Risk factors identified with a significance value of P less than 0.05 on univariate analysis were entered into multivariate logistic regression analysis, and odds ratios (OR) with 95% confidence intervals (CI) are presented.
Results
A total of 228 patients undergoing Hartmann’s procedure during the study period were identified. The group comprised 111 women and 117 men with a median age of 66 years (range 21–91 years). The most common indications for Hartmann’s procedure (Table 1) were complicated diverticular disease, (n = 100, 44%), sigmoid cancer (n = 44, 19%), rectal cancer (n = 24, 10.5%) and stercoral perforation (n = 11, 5%). Patients had numerous comorbidities with a mean Charlston comorbidity score of 2.3. A substantial proportion of patients were ASA grade III–V (n = 92, 40%), One hundred and ninety patients were admitted acutely for Hartmann’s procedure (83%). Fifteen patients died within thirty days of Hartmann’s procedure (7%).
Table 1.
Indications for Hartmann’s procedure in the 228 patients in the study.
| Indication | Incidence | |
| (n) | (%) | |
| Diverticular complication | 100 | 44 |
| Rectal cancer | 24 | 10.5 |
| Sigmoid cancer | 44 | 19 |
| Rectosigmoid cancer | 6 | 2.5 |
| Stercoral perforation | 11 | 5 |
| Anastomotic leak | 9 | 4 |
| Ischaemia | 8 | 3.5 |
| Trauma | 8 | 3.5 |
| Other | 18 | 8 |
Hartmann’s procedure not reversed
One hundred and five of the surviving patients did not proceed to reversal (46%). Seventy-four patients were considered unsuitable for reversal because of recurrent malignancy (n = 26, 35%), high risk comorbidity (n = 41, 55%) or anticipated technical difficulties such as dense pelvic adhesions or a short rectal stump (n = 7, 10%). Thirty-one patients (30%) declined reversal for a mixture of satisfaction with current stoma and not wanting further admission or surgery.
Hartmann’s procedure reversed
Barium enema or sigmoidoscopy was performed in all patients prior to reversal to rule out residual pathology in the proximal colon and to assess the rectal segment. One hundred and eight of the surviving patients underwent a successful reversal (47%). The median time interval to reversal was 11 months (range 4–96 months). Anastomosis was stapled in 85 patients, (79%) and handsewn in 23 (21%). Ten patients had a defunctioning ileostomy, of which 90% had been reversed at a median of five months (range 1–16 months). There were no postoperative mortalities following reversal. Twenty-three patients experienced a postoperative complication (21%), of which wound infection was the most common (n = 9, 8%). Anastomotic leaks occurred in five patients, (5%) of whom 1% (n = 2) were left with permanent ileostomy as they did not want to proceed with reversal following their initial complications. Median length of stay following reversal was eight days (range 2–42 days).
Factors predicting reversal of Hartmann procedure
On univariate analysis (Table 2), patients had an increased likelihood of reversal if they were of younger age (P < 0.0001), ASA less than or equal to 2 (P < 0.0001), emergency Hartmann’s procedure (P = 0.012), benign indication for Hartmann’s procedure (P = 0.003) and a Charlston comorbidity score of less than or equal to 1 (P < 0.001; Table 1). Sex and smoking status were not significantly associated with reversal. On multivariate analysis (Table 3), the following factors retained significance: age (OR 0.95, range 0.92–0.98; P = 0.001), ASA less than or equal to 2 (OR 0.38, range 0.02–0.09; P < 0.001) and emergency Hartmann’s procedure (OR 5.5, range 2.13–14.16, P < 0.001).
Table 2.
Univariate analysis of factors predicting reversal of Hartmann’s procedure.
| Factor | Hartmann’s procedure alone (n = 120) | Hartmann’s procedure + reversal (n = 108) | P-value | ||
| (n) | (%) | (n) | (%) | ||
| Sex: | 0.52 | ||||
| Male | 60 | 50 | 50 | 46 | |
| Female | 60 | 50 | 58 | 54 | |
| Median age (years) | 71a | 58b | < 0.0001 | ||
| ASA score: | < 0.0001 | ||||
| Low risk (I–II) | 36 | 30 | 100 | 93 | |
| High risk (≥ III) | 84 | 70 | 8 | 7 | |
| Smoking status: | 0.15 | ||||
| Non-smoker | 82 | 68 | 83 | 77 | |
| Smoker | 38 | 32 | 25 | 23 | |
| Emergency procedure: | 0.012 | ||||
| Yes | 93 | 78 | 97 | 90 | |
| No | 27 | 22 | 11 | 10 | |
| Malignancy: | 0.003 | ||||
| Malignant | 51 | 43 | 26 | 24 | |
| Benign | 69 | 58 | 82 | 76 | |
| Charlston comorbidity index: | < 0.001 | ||||
| Low risk (0–1) | 30 | 25 | 65 | 60 | |
| High risk (≥ 2) | 90 | 75 | 43 | 40 | |
ASA, American Anesthesiologists Association.
a Range 30–91 years.
b Range 21–84 years.
Table 3.
Multivariate multiple logistic regression of factors predicting reversal of Hartmann’s procedure.
| Factor | Adjusted OR (95% CI) | P-value |
| Age | 0.948 (0.919–0.978) | 0.001 |
| ASA score: | < 0.001 | |
| Low risk (1 or 2) | – | |
| High risk (3 or higher) | 0.038 (0.016–0.092) | |
| Emergency: | < 0.001 | |
| No | – | |
| Yes | 5.496 (2.133–14.164) | |
| Malignancy | Not significant | |
| Charlston comorbidity index | Not significant |
CI, confidence interval; OR, odds ratio.
Factors predicting complication following reversal
On univariate analysis, the following factors were significantly associated with complication following reversal: smoking status (P < 0.001), length of stay over 11 days (P < 0.001). Sex, age, preoperative albumin, benign or malignant indication for Hartmann’s procedure, Charlston comorbidity index, time to reversal and stapled or handsewn anastomosis were not significantly associated with complication following reversal (Table 4). On multivariate analysis (Table 5), the following factors retained significance in predicting complication following reversal: smoking status (OR 6.08, 95% CI 1.77–20.9; P = 0.003) and length of stay (OR 15.45, 95% CI 4.7–50.5; P < 0.001).
Table 4.
Univariate analysis factors predicting complication following reversal of Hartmann’s procedure.
| Factor | No complications (n = 85) | Complications (n = 23) | P-value | ||
| (n) | (%) | (n) | (%) | ||
| Sex: | 0.085 | ||||
| Male | 43 | 86 | 7 | 14 | |
| Female | 42 | 72.4 | 16 | 27.6 | |
| Age (years) | 56a | 63b | 0.348 | ||
| Pre-albumin: | 0.118 | ||||
| ≤ 35 g/l | 73 | 82 | 16 | 18 | |
| > 35 g/l | 12 | 63.2 | 7 | 36.8 | |
| Malignancy: | 0.163 | ||||
| Benign | 62 | 75.6 | 20 | 24.4 | |
| Malignant | 23 | 88.5 | 3 | 11.5 | |
| Smoking status: | < 0.001 | ||||
| Non-smoker | 72 | 86.7 | 11 | 13.3 | |
| Smoker | 13 | 52 | 12 | 48 | |
| Length of stay (days): | < 0.001 | ||||
| < 11 | 74 | 91.4 | 7 | 8.6 | |
| ≥ 11 | 11 | 40.7 | 16) | 59.3 | |
| ASA score: | 0.363 | ||||
| I–II | 80 | 80 | 20 | 20 | |
| ≥ III | 5 | 62.5 | 3 | 37.5 | |
| Charlston comorbidity index: | 0.172 | ||||
| 0–1 | 54 | 83.1 | 11 | 16.9 | |
| ≥ 2 | 31 | 72.1 | 12 | 27.9 | |
| Time to reversal (months): | 0.471 | ||||
| < 12 | 55 | 80.9 | 13 | 19.1 | |
| ≥ 12 | 30 | 75 | 10 | 25 | |
| Anastomoses: | 0.57 | ||||
| Hand-sewn | 17 | 73.9 | 6 | 26.1 | |
| Stapled | 68 | 80 | 17 | 20 | |
ASA, American Anesthesiologists Association.
a Range 4–67 years.
b Range 50–71 years.
Table 5.
Multivariate logistic regression, factors predicting complication following reversal of Hartmann’s procedure.
| Factor | Odds ratio (95% C.I) | P-value |
| Smoking status: | 0.003 | |
| Non-smoker | – | |
| Smoker | 6.08 (1.77–20.9) | |
| Length of stay (days): | < 0.001 | |
| < 11 | – | |
| ≥ 11 | 15.45 (4.7–50.5) |
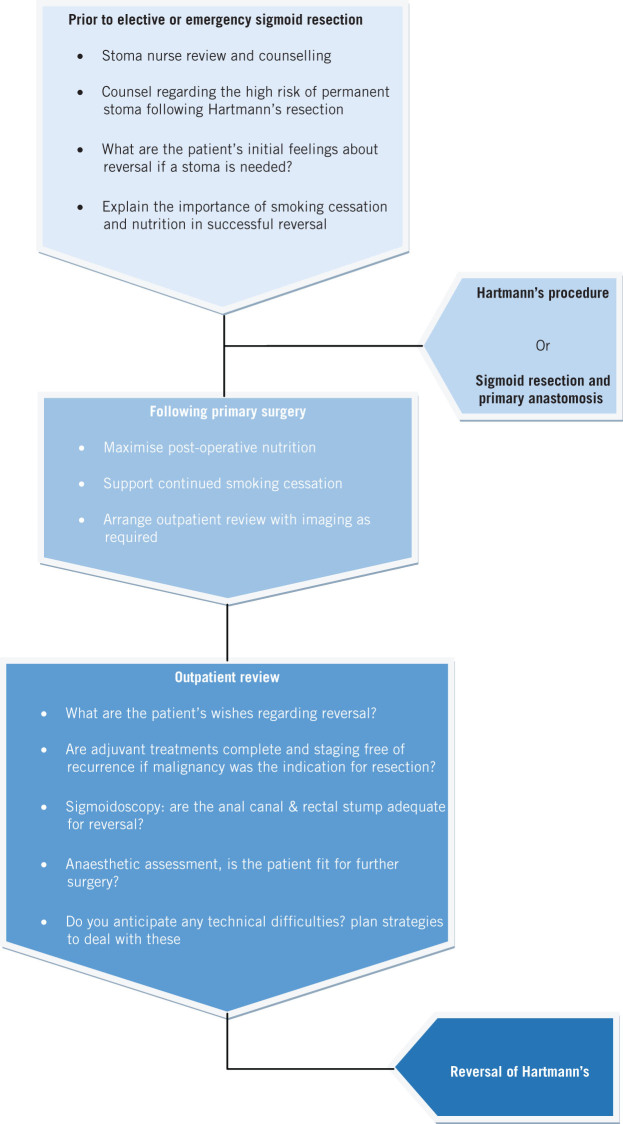
Figure 1.
Flow diagram of decision making for reversal of Hartmann’s procedure
Discussion
To our knowledge, this is the single largest series attempting to identify factors predicting reversal of Hartmann’s procedure. One hundred and eight patients (47%) underwent successful reversal, which compares favourably with rates reported in the literature of 33.5% (range 19.2–69%).2,6–8,11,14,15,17,18
A large proportion of patients in our series declined reversal (30%), which is higher than figures reported in the literature we reviewed (18%).6 Patients declining reversal were older (median age 71 years), had higher ASA grade, increased malignant pathology and underwent elective Hartmann’s procedure more commonly. Patients reasons for refusal of reversal is multifactorial and beyond the scope of our study but review of the notes suggested that patients refusing reversal perceived surgery to be of higher risk and that they expressed higher rates of satisfaction with permanent stoma.
A literature review of a similar study involving quality of life questionnaires for Hartmann’s procedure in perforated diverticular disease showed significantly reduced quality of life, which was strongly related to patient’s stoma and body image. Of note is that the study population was notably younger than our study group (median age 61 years vs. 71 years).19
A further 73 patients were documented as unsuitable for reversal due to recurrent malignancy (35%), high-risk comorbidity (55%) or anticipated technical difficulties following the primary surgery such as dense pelvic adhesions or short rectal stump (10%). Currently, no guidelines exist to help clinicians in selecting patients for reversal following Hartmann’s procedure. This means that clinicians must use a range of measures including disease pathology, performance status, comorbidity, professional experience and patient factors that may affect wound healing such as nutrition and smoking status. With increasing patient autonomy and complaints, all decisions must be clearly documented after carefully assessing the impact of comorbidity and other prohibitory factors and seeking appropriate specialist opinions.
Time to the reversal of Hartmann’s varied widely in this series from 4months to 96 months, occurring at a median of 11 months after Hartmann’s procedure, which is slightly longer than the literature median of 7.63 months (range 5.6–13.3 months). A prolonged time to reversal of 12 months or more, however, was not associated with increased postoperative complication (P = 0.471).
The timing of reversal remains a contentious issue. Some studies 7,17,20,21 report increased complications with longer time to reversal, which they postulate relates to atrophy of the distal stump and hence enhance the difficulty of performing the anastomosis. Selection bias, however, must be considered, since surgeons who experienced a tough Hartmann’s procedure initially may be keen to avoid early reversal and so delay it in the hope that the patient may change their mind. Conversely, several studies support delayed reversal and postulate that the improved clinical and nutritional state of the patient leads to fewer complications.2,20
Postoperative complications occurred in 23 patients (21%). Wound infection was the most common surgical complication (n =9, 8%). Grade III or above Clavien–Dindo complications occurred in five patients (5%) of which all were anastomotic leaks. The complication rate in this study is noted to be lower when compared with those reported in the literature, at 27% (range 5.4–54.8%). 2,6–11,13–15,17,18,21
The lower complication rate might, however, reflect the limitation of this study, which is retrospective in nature and potentially fails to capture the inadequately documented minor grade I–II complications in the notes or which may have been dealt with in the community.
Over the first decade if the 21st century, there has been a change in approach and primary anastomosis is preferred to Hartmann’s procedure with permanent stoma where possible.22 A systematic review by Salem et al. found no difference in the mortality and morbidity rates for patients with perforated diverticulitis, whether aprimary anastamosis or Hartmann’s procedure with stoma is performed.23 A further study looking at obstructed colorectal cancer patients showed improved or equivalent morbidity and mortality for primary anastomosis than Hartmann’s procedure.24 Alternatives include colonic stenting to relieve obstruction as a bridge to definitive surgery.25 The primary anastomosis may be a longer operation than Hartmann’s procedure for those not performing it on a regular basis in the elective setting. For high-risk comorbid patients, therefore, Hartmann’s procedure is still a safe approach and viable option.26
In the UK, emergency surgery is covered by a broad range of subspecialties in general surgery, which include upper gastrointestinal, hepatobiliary and vascular surgeons. Because of this there is a huge difference in the day to day practice adopted for colonic resections, with some surgeons preferring a straightforward procedure such as Hartmann’s procedure over primary anastomosis. A move towards centralisation of services with 24-hour-a-day access to colorectal surgeons may reduce the number of Hartmann’s procedure performed overall, as is suggested from recent data from the USA, which found that colorectal surgeons were more likely to perform primary anastomosis than general surgeons, had shorter operating times and length of stay and equivalent morbidity and mortality to their general surgical colleagues.26
Conclusion
In conclusion, Hartmann’s procedure is still a commonly performed emergency colorectal operation. Reversal of the procedure in our series was a relatively safe operation with a low risk of major morbidity and no mortality. Reversal, however, occurs in less than 50% of patients who undergo Hartmann’s procedure, who must be adequately counselled and consented before the initial procedure. Future NHS changes to the delivery of emergency surgery with the delivery of surgical emergency surgery with centralisation of subspecialty services may lead to a reduction in Hartmann’s procedures and overall improved morbidity and mortality.
References
- 1.Hartmann H. Nouveau procédé d’ablation des cancers de la partie terminale du colon pelvien. Trentieme Congres De Chirurgie; Strasburg, 1921. pp. 411–413. [Google Scholar]
- 2.Banerjee S, Leather AJ, Rennie JA et al. Feasibility and morbidity of reversal of Hartmann’s. 2005; (5): 454–459. [DOI] [PubMed] [Google Scholar]
- 3.Salem L, Anaya DA, Roberts KE, Flum DR. Hartmann’s colectomy and reversal in diverticulitis: a population-level assessment. 2005; (5): 988–995. [DOI] [PubMed] [Google Scholar]
- 4.Aydin HN, Remzi FH, Tekkis PP, Fazio VW. Hartmann’s reversal is associated with high postoperative adverse events. 2005; (11): 2,117–2,126. [DOI] [PubMed] [Google Scholar]
- 5.Lin FL, Boutros M, Da Silva GM et al. Hartmann reversal: obesity adversely impacts outcome. 2013; (1): 83–90. [DOI] [PubMed] [Google Scholar]
- 6.Tokode OM, Akingboye A, Coker O. Factors affecting reversal following Hartmann’s procedure: experience from two district general hospitals in the UK. 2011; (1): 79–83. [DOI] [PubMed] [Google Scholar]
- 7.Roque-Castellano C, Marchena-Gomez J, Hemmersbach-Miller M et al. Analysis of the factors related to the decision of restoring intestinal continuity after Hartmann’s procedure. 2007; (9): 1,091–1,096. [DOI] [PubMed] [Google Scholar]
- 8.Okolica D, Bishawi M, Karas JR et al. Factors influencing postoperative adverse events after Hartmann’s reversal. 2012; (3): 369–373. [DOI] [PubMed] [Google Scholar]
- 9.Antolovic D, Reissfelder C, Ozkan T et al. Restoration of intestinal continuity after Hartmann’s procedure--not a benign operation. Are there predictors for morbidity? 2011; (7): 989–996. [DOI] [PubMed] [Google Scholar]
- 10.Richards CH, Roxburgh CS, Surgical Research Group (SSRG). Surgical outcome in patients undergoing reversal of Hartmann’s procedures: a multicentre study. 2015; (3): 242–249. [DOI] [PubMed] [Google Scholar]
- 11.Cellini C, Deeb AP, Sharma A et al. Association between operative approach and complications in patients undergoing Hartmann’s reversal. 2013; (8): 1,094–1,099. [DOI] [PubMed] [Google Scholar]
- 12.Ramírez JM, Blasco JA, Roig JV et al. Enhanced recovery in colorectal surgery: a multicentre study. 2011; : 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarnescu Vasiliu EC, Zarnescu NO, Costea R et al. Morbidity after reversal of Hartmann operation: retrospective analysis of 56 patients. 2015; (4): 488–491. [PMC free article] [PubMed] [Google Scholar]
- 14.Garber A, Hyman N, Osler T. Complications of Hartmann takedown in a decade of preferred primary anastomosis. 2014; (1): 60–64. [DOI] [PubMed] [Google Scholar]
- 15.Roig JV, Cantos M, Balciscueta Z et al. Hartmann’s operation: how often is it reversed and at what cost? A multicentre study. 2011; (12): e396–e402. [DOI] [PubMed] [Google Scholar]
- 16.National Bowel Cancer Audit Project Team . London: Health and Social Care Information Centre; 2015. [Google Scholar]
- 17.Tan WS, Lim JF, Tang CL, Eu KW. Reversal of Hartmann’s procedure: experience in an Asian population. 2012; (1): 46–51. [PubMed] [Google Scholar]
- 18.Fleming FJ, Gillen P. Reversal of Hartmann’s procedure following acute diverticulitis: is timing everything? 2009; (10): 1,219–1,025. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen J, Gosselink MP, Busschbach JJ, Lange JF. Avoiding or reversing Hartmann’s procedure provides improved quality of life after perforated diverticulitis. 2010; (4): 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce NW, Scott SD, Karran SJ. Timing and method of reversal of Hartmann’s procedure. 1992; (8): 839–841. [DOI] [PubMed] [Google Scholar]
- 21.Schmelzer TM, Mostafa G, Norton HJ et al. Reversal of Hartmann’s procedure: a high-risk operation? 2007; (4): 598–597. [DOI] [PubMed] [Google Scholar]
- 22.Wieghard N, Geltzeiler CB, Tsikitis VL. Trends in the surgical management of diverticulitis. 2015; (1): 25–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Salem L, Flum DR. Primary anastomosis or Hartmann’s procedure for patients with diverticular peritonitis? A systematic review. 2004; (11): 1,953–1,964. [DOI] [PubMed] [Google Scholar]
- 24.Durán Giménez-Rico H, Abril Vega C, Herreros Rodríguez J et al. Hartmann’s procedure for obstructive carcinoma of the left colon and rectum: a comparative study with one-stage surgery. 2005; (7): 306–313. [DOI] [PubMed] [Google Scholar]
- 25.Cirocchi R, Farinella E, Trastulli S et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. 2013; (1): 14–21. [DOI] [PubMed] [Google Scholar]
- 26.Wright GP, Flermoen SL, Robinett DM et al. Surgeon specialization impacts the management but not outcomes of acute complicated diverticulitis. 2016; (6): 1,035–1,040. [DOI] [PubMed] [Google Scholar]