Abstract
Clear cell renal cell carcinoma (ccRCC) is the most common renal malignancy in adults, the incidence of which continues to increase. The lipid droplet protein perilipin 2 (PLIN2), which was originally considered an RNA transcript, is markedly expressed during adipocyte differentiation. In addition, it has been observed to be elevated in numerous types of cancer, including ccRCC; however, its essential function remains unclear in ccRCC. The present study examined the expression of PLIN2 in ccRCC, and aimed to determine the association between PLIN2 expression and patient survival. The present study mined the transcriptional, clinicopathological and survival data of PLIN2 in patients with ccRCC through The Cancer Genome Atlas. The expression levels of PLIN2 were also detected in human ccRCC tissues and cell lines by western blotting and immunohistochemistry, and its biological role was identified by functional analysis. The results demonstrated that PLIN2 was predominantly elevated in RCC tissues and cells. In addition, the expression levels of PLIN2 were significantly associated with various clinicopathological factors, and high PLIN2 expression was associated with a good prognosis. The results of a multivariate analysis demonstrated that high PLIN2 expression was an independent prognostic indicator of overall survival (hazard ratio, 0.586; P=0.001). Furthermore, PLIN2 knockdown promoted proliferation of ccRCC cells, and enhanced cell invasion and migration. These results suggested that PLIN2 may be considered a novel prognostic factor in ccRCC and a specific diagnostic indicator for patients with ccRCC. Furthermore, it could be a potential novel target for the clinical treatment of ccRCC.
Keywords: clear cell renal cell carcinoma, perilipin 2, biomarker, prognosis, therapy
Introduction
Renal cell carcinoma (RCC) is the most common malignancy of the adult kidney, which accounts for ~3% of adult malignant tumors (1,2). The most common subtype of RCC is clear cell RCC (ccRCC), which accounts for ~80% of all diagnosed cases (3). Progress has been made with regards to the diagnosis and treatment of ccRCC; however, its incidence continues to rise and ~30% of newly diagnosed patients, and 20–40% of postoperative patients, will experience metastasis or local recurrence (4–6). Recent advances have been made in ccRCC treatment strategies, including targeted therapies, which have resulted in therapeutic improvements (7); however, the majority of treated patients eventually develop progressive disease due to acquired resistance (8,9). Therefore, it is essential to elucidate the molecular mechanisms underlying ccRCC progression and metastasis, which may contribute to the development of novel strategies for the treatment of ccRCC.
The perilipin (PLIN) family consists of five members, including PLIN1, PLIN2, PLIN3, PLIN4 and PLIN5, which are all characterized as lipid droplet (LD) proteins in adipocytes (10). These proteins are expressed in numerous species, including mammals, Drosophila and Dictyostelium (11–16), in which they are involved in various intracellular activities, such as lipid metabolism and transport, intracellular trafficking and signaling, and cytoskeletal organization (13,17,18). It is well known that ccRCC is characterized by the presence of intracellular LDs, which consist of a neutral lipid core surrounded by a phospholipid monolayer and chimeric LD surface proteins (19). PLIN family members regulate lipid metabolism and are involved in the tumorigenesis and development of various types of cancer. For example, PLIN1, which is primarily expressed in adipose and steroidogenic cells, has been reported to be involved in breast cancer progression and may act as a tumor suppressor gene (20). In cervical cancer, high PLIN3 expression is correlated with advanced tumor stages and poor patient prognosis (21).
PLIN2 was originally considered an RNA transcript, and is markedly involved in adipocyte differentiation (22,23). It is commonly expressed in numerous types of tissue, including the mammary gland, liver and skeletal muscle (24–26). It has previously been reported that PLIN2 abundance is directly associated with the levels of intracellular lipids, and increased expression levels of PLIN2 have been observed in several specific diseases involving fat accumulation (27). Recent studies have demonstrated that numerous types of tumor overexpress PLIN2 (28,29). Notably, the majority of these tumors have clear cell histology (30,31). Two major studies revealed the prognostic value of the transcript and protein levels of PLIN2 in ccRCC (32,33). Furthermore, a recent study provided evidence to suggest that PLIN2 is upregulated in ccRCC and directly proportional to hypoxia-inducible factor (HIF)2α activation (34); however, to the best of our knowledge, a functional analysis of PLIN2 in ccRCC has not yet been performed. Therefore, the present study aimed to investigate whether PLIN2 expression is associated with clinicopathological features of ccRCC and patient survival. In addition, the function of PLIN2 in ccRCC was examined in vitro.
Materials and methods
Renal cancer tissue samples
Between January 2014 and December 2016, 80 pairs of ccRCC and adjacent normal renal tissues and 7 benign renal angiomyolipoma tissues were collected from patients who received partial or radical nephrectomy at the Wuhan Union Hospital (Wuhan, China). Among these, 40 pairs of resected tissues underwent protein expression analysis by western blotting. The remaining tissues were fixed in 10% formalin for 24 h at room temperature and embedded in paraffin for immunohistochemistry (IHC). None of the patients received preoperative or postoperative adjuvant anticancer therapy. The present study and experimental procedures were approved by the Human Research Ethics Committee of Huazhong University of Science and Technology (Wuhan, China). Written informed consent was obtained from the patients/patients' families.
Cell lines and cell culture
The ACHN, 786-O, A-498, OS-RC-2, Caki-1 and HK-2 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin-streptomycin solution. Cells were maintained at 37°C in an incubator containing 5% CO2.
Transient transfection assay
Small interfering (si)RNA sequences specifically targeting PLIN2 (si-PLIN2) and negative control siRNA (si-NC) were chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The off-target effects of all siRNA sequences were detected using the Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For transfection, ccRCC cells were seeded in 6-well plates at 50–70% confluence. Cells (3×105) were transfected with 100 pmol siRNA sequences using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to our previous study (35). A total of 48 h post-transfection, cells were used for subsequent assays. The si-PLIN2 sequence was as follows: 5′-CAGCCAUCAACUCAGAUUGUU-3′.
IHC
ccRCC tissues and adjacent normal tissues were sequentially fixed in formalin, dehydrated and embedded in paraffin. Subsequently, IHC was conducted by incubating tissue sections (4 µm) with a primary rabbit PLIN2 polyclonal antibody (1:100; A6276; ABclonal Biotech Co., Ltd., Wuhan, China) overnight at 4°C. Subsequently, after washing three times with PBS, the sections were incubated with goat anti-rabbit secondary antibody (1:200; GB23303; Servicebio, Inc., Woburn, MA, USA) at room temperature for 2 h.
Cell proliferation analysis
A-498 cells were transfected with si-PLIN2 or si-NC. Subsequently, the cells were added to each well of 96-well plates at a density of 3×103/well. Cell proliferation rate was determined using the Cell Counting kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc, Rockville, MD, USA) every 24 h, according to the manufacturer's protocol. Briefly, 10 µl CCK-8 solution was added to each well. After 4 h, the optical density of each well was measured at 450 nm. Each experiment was conducted in triplicate in three independent experiments.
Migration assay
Boyden Transwell chambers (Corning Incorporated, Corning, NY, USA) containing 8-µm membrane filters were applied to 24-well plates. Cells (1×104) were seeded into the upper chamber in serum-free medium, whereas the bottom chamber was filled with complete medium supplemented with 10% FBS. After 24 h at 37°C, the cells on the upper surface were washed with PBS and cells on the lower surface were fixed with 100% methanol for 10 min and stained with 0.05% crystal violet for 10 min at room temperature. The average number of migrated cells was counted in five randomly selected fields under a microscope (Olympus CX41-32C02; Olympus Corporation, Tokyo, Japan). Experiments were independently repeated in triplicate.
Invasion assays
Invasion assays were performed as previously described (36). Briefly, 24-well Transwell chambers with 8-µm membrane filters (Corning Incorporated) were precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Cells (2×104) were seeded into the upper chamber in serum-free medium, whereas the lower chamber was filled with complete medium containing 10% FBS. Following incubation for 24 h at 37°C, cells on the lower surface were fixed with 100% methanol for 10 min and stained with 0.05% crystal violet for 10 min at room temperature. Five random fields were captured using an optical microscope at 100× magnification (Olympus CX41-32C02; Olympus Corporation). Experiments were independently repeated in triplicate.
Western blotting
Tissues and cells were lysed in radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing a protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN, USA) and 1 mM phenylmethylsulfonyl fluoride. Subsequently, the protein concentrations were measured using a bicinchoninic acid kit (Beyotime Institute of Biotechnology), according to the manufacturer's protocol. Total proteins (30 µg) were separated by 10% SDS-PAGE and were then transferred to polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Bedford, MA, USA) at 90 V for 90 min. The PVDF membranes were blocked in PBS containing 5% nonfat milk for 1 h at room temperature, and were then incubated with primary antibodies against PLIN2 (1:1,000; A6276; ABclonal Biotech Co., Ltd.) and GAPDH (1:3,000; BM3876; Wuhan Boster Biological Technology, Ltd., Wuhan, China) overnight at 4°C. Subsequently, the membranes were incubated with secondary antibodies (1:3,000; GB23303; Servicebio, Inc.) for 2 h at room temperature. Finally, the proteins were visualized using ChemiDoc-XRS+ (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Bioinformatics analysis
PLIN2 mRNA expression and clinical data of The Cancer Genome Atlas (TCGA) ccRCC dataset (TCGA_KIRC) were downloaded from the Xena Functional Genomics Explorer of University of California Santa Cruz (https://xenabrowser.net/heatmap/) (37). To provide understanding of the biological pathways involved in ccRCC pathogenesis via the PLIN2 pathway, a gene set enrichment analysis (GSEA) was conducted to analyze the enrichment of given gene sets (c2.cgp.v6.0.symbols.gmt) in TCGA ccRCC dataset using GSEA software (http://www.broadinstitute.org/gsea) (38). For enriched gene sets, those with a false discovery rate (FDR) <25% and nominal P<0.05 following the performance of 1,000 permutations were considered significantly enriched.
Statistical analysis
All data were analyzed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Numerical data are presented as the means ± standard deviation. Student's t-test was used to assess differences in PLIN2 expression between each ccRCC subgroup. Survival information was evaluated via Kaplan-Meier analysis and was compared using the log-rank test. The correlation between PLIN2 mRNA expression and clinicopathological parameters of patients with ccRCC was evaluated using χ2 test. The significant differences in PLIN2 expression among various T stage groups and grade groups were estimated using one-way analysis of variance followed by Tukey's post hoc test to assess multiple comparisons. Receiver operator characteristic (ROC) curve analysis was used to assess the prognostic value of PLIN2 with regards to various ccRCC clinicopathological factors. Univariate and multivariate analyses were performed using a Cox proportional hazard regression model. P<0.05 was considered to indicate a statistically significant difference.
Results
PLIN2 is strongly upregulated and associated with various types of clinicopathological factors in ccRCC tissues
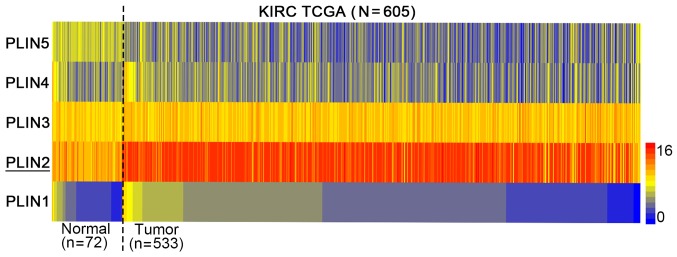
To investigate the role of PLIN2 in ccRCC development, the present study examined the mRNA expression levels of the five PLIN family members (PLIN1-5) in TCGA database. The heat map indicated that among the five PLIN members, PLIN2 exhibited the most obvious difference in expression between normal tissues and ccRCC (Fig. 1). Therefore, PLIN2 was chosen for subsequent investigation.
Figure 1.
Heat map depicting PLIN expression in samples from TCGA combined human clear cell renal cell carcinoma microarray datasets (n=605). Red indicates high expression; yellow indicates medium expression; blue indicates low expression. KIRC, kidney renal clear cell carcinoma; PLIN, perilipin; TCGA, The Cancer Genome Atlas.
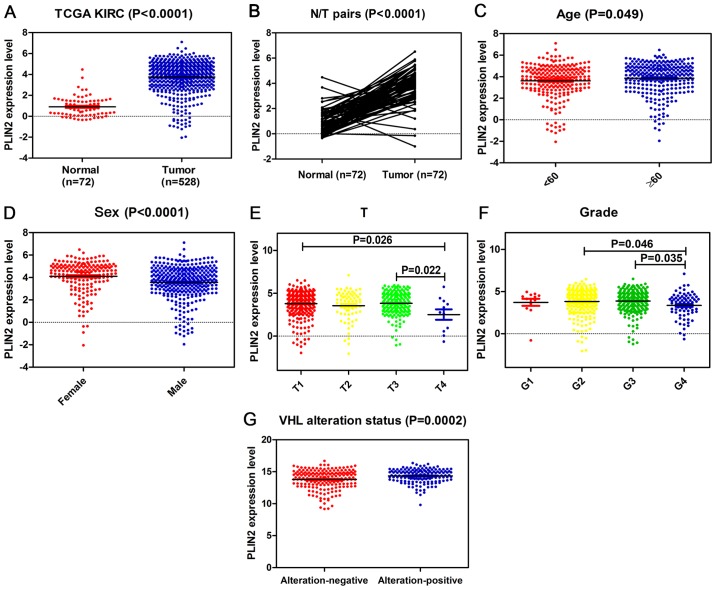
The present study explored the mRNA expression levels of PLIN2 in ccRCC cancer tissues and adjacent normal tissues from TCGA; PLIN2 expression was significantly elevated in tumor tissues compared with in normal tissues (Fig. 2A). Subsequent validation was conducted in 72 pairs of matched ccRCC tissues and adjacent normal tissues. As expected, PLIN2 expression was significantly increased in tumor tissues compared with in paired normal tissues (Fig. 2B). Furthermore, TCGA dataset revealed that PLIN2 expression was increased in patients ≥60 years old compared with in those <60 years old (Fig. 2C). Elevated PLIN2 expression was also significantly associated with sex, T stage and grade in ccRCC (Fig. 2D–F). PLIN2 expression levels tended to decrease with increasing tumor T stage and G grade. Markedly, PLIN2 expression was clearly increased in patients with VHL alteration-positive ccRCC compared with in those with VHL alteration-negative ccRCC (Fig. 2G). Clinicopathological information for the 526 ccRCC tissues in TCGA dataset is listed in Table I. There was a significant association between high PLIN2 expression and age or sex, which is consistent with the aforementioned results. In addition, the χ2 test performed for VHL alteration and PLIN2 expression demonstrated that PLIN2 expression levels were significantly associated with VHL alteration status (P=0.007).
Figure 2.
PLIN2 expression is upregulated in ccRCC, and is associated with various clinicopathological factors in ccRCC tissues. The mRNA expression levels of PLIN2 were obtained from TCGA dataset, which contained 72 adjacent normal tissues and 528 ccRCC tissues. The mRNA expression levels of PLIN2 were compared with respect to various clinicopathological factors: (A) Cancer vs. normal tissues, (B) tumor vs. adjacent normal tissues, (C) age, (D) sex, (E) T stage, (F) grade and (G) VHL alteration status. ccRCC, clear cell renal cell carcinoma; PLIN, perilipin; TCGA, The Cancer Genome Atlas; VHL, von Hippel-Lindau.
Table I.
Association between PLIN2 mRNA expression and clinicopathological parameters of patients with clear cell renal cell carcinoma.
| A, Patient characteristics
| ||||
|---|---|---|---|---|
| Parameter | No. | PLIN2 mRNA expression
|
P-value | |
| Low (n=263) | High) (n=263 | |||
| Age (years) | ||||
| <60 | 243 | 140 | 103 | |
| ≥60 | 283 | 123 | 160 | 0.002 |
| Sex | ||||
| Female | 185 | 64 | 119 | |
| Male | 341 | 199 | 144 | 0.000 |
| T stage | ||||
| T1 or T2 | 337 | 161 | 176 | |
| T3 or T4 | 189 | 102 | 87 | 0.203 |
| N stage | ||||
| N0 or NX | 510 | 252 | 258 | |
| N1 | 16 | 11 | 5 | 0.203 |
| M stage | ||||
| M0 or MX | 448 | 216 | 232 | |
| M1 | 78 | 47 | 31 | 0.065 |
| G grade | ||||
| G1 or G2 | 246 | 119 | 126 | |
| G3 or G4 | 280 | 144 | 137 | 0.541 |
| TNM stage | ||||
| I + II | 319 | 152 | 167 | |
| III + IV | 207 | 111 | 96 | 0.211 |
|
| ||||
| B, VHL status | ||||
|
| ||||
| Parameter | No. | PLIN2 mRNA expression
|
P-value | |
| Low (n=172) | High (n=171) | |||
|
| ||||
| VHL alteration | ||||
| Negative | 190 | 108 | 82 | |
| Positive | 153 | 64 | 89 | 0.007 |
VH, von Hippel-Lindau; PLIN2, perilipin 2.
Association between high PLIN2 expression and good clinical outcome in patients with ccRCC
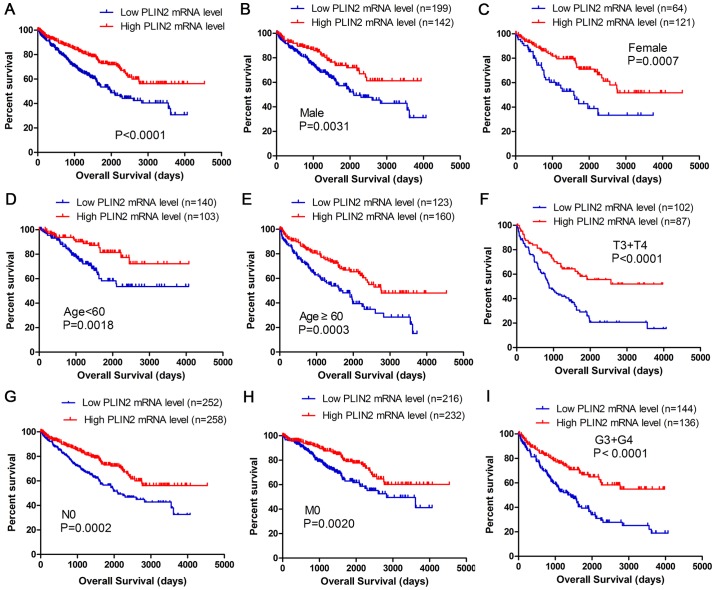
Kaplan-Meier survival analysis was used to determine overall survival (OS) according to PLIN2 expression. Patients with high PLIN2 expression exhibited better OS (P<0.0001) (Fig. 3A). Furthermore, OS analysis with regards to PLIN2 expression was conducted in various subgroups of patients with ccRCC. The results demonstrated that high PLIN2 expression may be considered a useful prognostic indicator for patients with ccRCC with the following characteristics: Male (Fig. 3B) or female (Fig. 3C), aged <60 (Fig. 3D) or ≥60 years old (Fig. 3E), T3 + T4 stage (Fig. 3F), N0 stage (Fig. 3G), M0 stage (Fig. 3H) and G3 + G4 grade (Fig. 3I). The prognostic value of each clinicopathological factor, including PLIN2 expression status, was evaluated for OS (Table II). Univariate Cox proportion hazard ratio (HR) analysis indicated that age (HR, 1.803; P<0.001), T stage (HR, 3.120; P<0.001), N stage (HR, 3.823; P<0.001), M stage (HR, 4.346; P<0.001), G grade (HR, 2.639; P<0.001) and PLIN2 expression status (HR, 0.524; P<0.001) were associated with OS. Further multivariate analysis demonstrated that age (HR, 1.652; P=0.002), T stage (HR, 1.619; P=0.010), N stage (HR, 2.219; P=0.013), M stage (HR, 2.448; P<0.001), G grade (HR, 1.677; P=0.006) and PLIN2 expression (HR, 0.586; P=0.001) could be considered independent prognostic indicators of OS.
Figure 3.
High PLIN2 mRNA expression is associated with good OS in patients with ccRCC. Patient samples from The Cancer Genome Atlas were separated into two groups: Those with low PLIN2 expression and those with high PLIN2 expression. (A) OS of patients with ccRCC was associated with PLIN2 expression. OS subanalysis regarding PLIN2 expression was conducted in subgroups of patients with ccRCC: (B) Male, (C) female, (D) age <60 years, (E) age ≥60 years, (F) T3 + T4 stage, (G) N0 stage, (H) M0 stage and (I) G3 + G4 grade. ccRCC, clear cell renal cell carcinoma; OS, overall survival; PLIN, perilipin.
Table II.
Univariate and multivariate analyses of PLIN2 mRNA expression and patient survival.
| Variable | Univariate analysis
|
Multivariate analysisc
|
||||
|---|---|---|---|---|---|---|
| HRa | 95% CIb | P-value | HR | 95% CI | P-value | |
| Overall survival (n=526) | ||||||
| Age (years) | 1.803 | 1.318–2.468 | <0.001 | 1.652 | 1.199–2.276 | 0.002 |
| Sex | 0.948 | 0.697–1.290 | 0.736 | |||
| T stage | 3.120 | 2.306–4.220 | <0.001 | 1.619 | 1.122–2.337 | 0.010 |
| N stage | 3.823 | 2.070–7.061 | <0.001 | 2.219 | 1.182–4.163 | 0.013 |
| M stage | 4.346 | 3.192–5.918 | <0.001 | 2.448 | 1.702–3.522 | <0.001 |
| G grade | 2.639 | 1.885–3.697 | <0.001 | 1.677 | 1.163–2.420 | 0.006 |
| PLIN2 | 0.524 | 0.386–0.711 | <0.001 | 0.586 | 0.429–0.803 | 0.001 |
HR estimated from Cox proportional hazard regression model;
CI of the estimated HR;
multivariate models were adjusted for T, N, M and G grade classification and age. CI, confidence interval; HR, hazard ratio; PLIN2, perilipin 2.
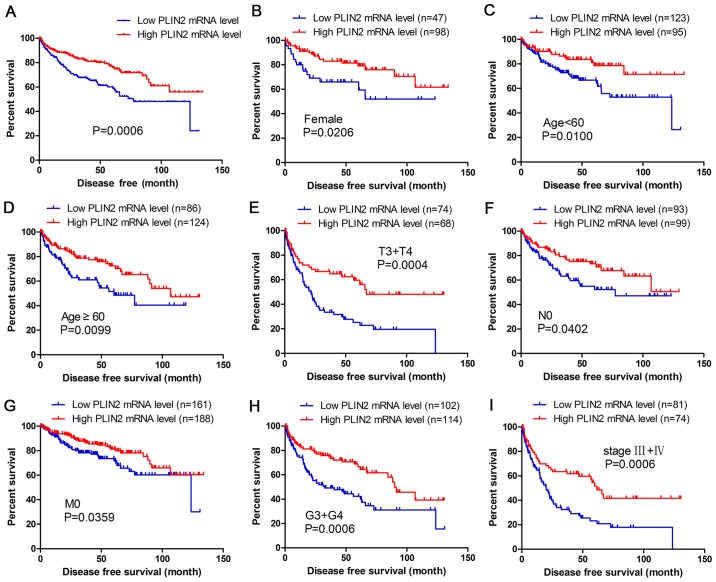
The present study analyzed the association between PLIN2 expression and disease-free survival (DFS) of patients with ccRCC by Kaplan-Meier analysis. The results demonstrated that patients with high PLIN2 expression exhibited better DFS compared with those with low PLIN2 expression (Fig. 4A; P<0.001). Furthermore, DFS analysis with regards to PLIN2 expression was performed in subgroups of patients with ccRCC. As expected, the results demonstrated that high PLIN2 expression was a potential prognostic indicator for patients with ccRCC with the following characteristics: Female (Fig. 4B), aged <60 (Fig. 4C) or ≥60 years (Fig. 4D), T3 + T4 stage (Fig. 4E), N0 stage (Fig. 4F), M0 stage (Fig. 4G), G3 + G4 grade (Fig. 4H) and stage III + IV (Fig. 4I).
Figure 4.
High PLIN2 mRNA expression is associated with good DFS in patients with ccRCC. Patient samples from The Cancer Genome Atlas were separated into two groups: Those with low PLIN2 expression and those with high PLIN2 expression. (A) DFS of patients with ccRCC was associated with PLIN2 expression. DFS subanalysis was conducted in subgroups of patients with ccRCC: (B) Female, (C) age <60 years, (D) age ≥60 years, (E) T3 + T4 stage, (F) N0 stage, (G) M0 stage, (H) G3 + G4 grade and (I) stage III + IV. ccRCC, clear cell renal cell carcinoma; DFS, disease-free survival; PLIN, perilipin.
Association between high PLIN2 expression and diagnostic role in patients with ccRCC
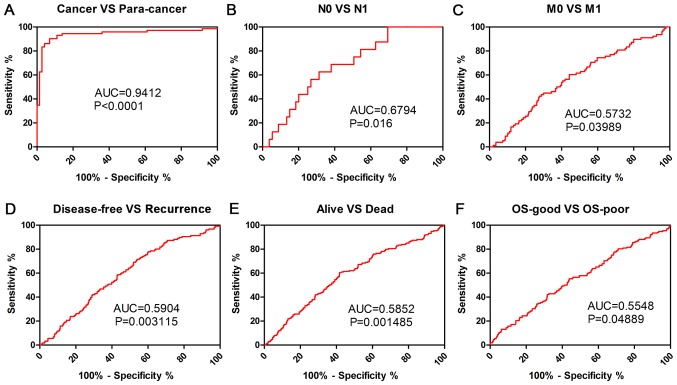
Clinicopathological factors were analyzed using ROC curve to investigate the diagnostic role of PLIN2 in patients with ccRCC. The results demonstrated that PLIN2 could sufficiently discriminate ccRCC from paired normal tissues with an area under the curve (AUC) of 0.9412 (Fig. 5A; P<0.0001). In addition, ROC curve analysis was conducted with regards to PLIN2 expression in various subgroups of patients with ccRCC. The results demonstrated that PLIN2 expression may be an effective diagnostic indicator for patients with ccRCC with the following characteristics: N0 vs. N1 stage (Fig. 5B; AUC=0.6794, P=0.016), M0 vs. M1 stage (Fig. 5C; AUC=0.5732, P=0.03989), disease-free vs. recurrence (Fig. 5D; AUC=0.5904, P=0.003115), alive vs. dead (Fig. 5E; AUC=0.5852, P=0.001485), OS-good vs. OS-poor (Fig. 5F; AUC=0.5548, P=0.04889).
Figure 5.
PLIN2 expression may be a diagnostic biomarker in patients with ccRCC. (A) PLIN2 effectively discriminated between ccRCC and paired normal tissues (AUC 0.9412; P<0.0001). ROC curve subanalysis was performed with respect to the following subgroups of patients with ccRCC: (B) N stage, (C) M stage, (D) DFS status, (E) survival status and (F) OS. AUC, area under the curve; ccRCC, clear cell renal cell carcinoma; DFS, disease-free survival; OS, overall survival; PLIN, perilipin; ROC, receiver operating characteristic.
PLIN2 upregulation is verified in ccRCC cells and tissues, and regulates biological pathways in ccRCC pathogenesis
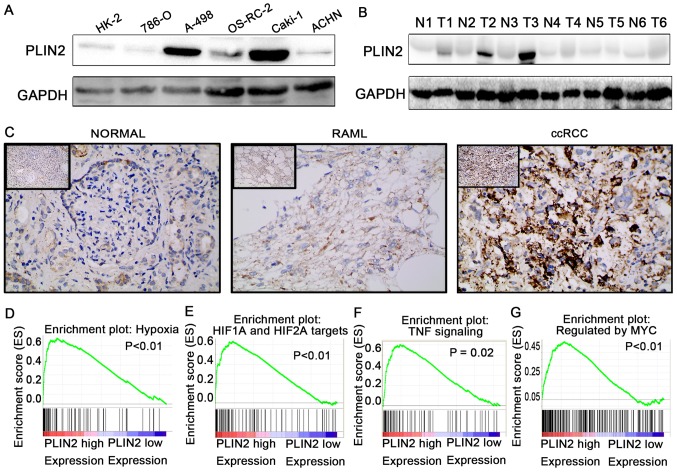
To further validate the results of TCGA dataset, western blotting was conducted to detect the protein expression levels of PLIN2 in ccRCC cells and tissues (Fig. 6A and B). In addition, IHC was conducted in 40 paired ccRCC tumor tissues and adjacent normal tissues (Fig. 6C). The results demonstrated that PLIN2 protein expression was significantly elevated in tumor cells and tissues compared with in immortalized renal epithelial cells and adjacent normal tissues.
Figure 6.
PLIN2 is upregulated in ccRCC cells and tissues, and regulates biological pathways. Western blotting of PLIN2 expression in (A) renal cancer cell lines and (B) ccRCC tissues. (C) Immunohistochemistry of PLIN2 expression in ccRCC tissues, benign RAML tissues and paired normal tissues. Representative images are shown (magnification, ×200 in main images, ×40 in upper left images). Gene set enrichment analysis compared low PLIN2 and high PLIN2 expression groups in The Cancer Genome Atlas database. Enrichment curves are shown for activated gene sets related to (D) hypoxia, (E) HIF1α and HIF2α targets, (F) TNF signaling and (G) regulated by Myc. ccRCC, clear cell renal cell carcinoma; HIF, hypoxia-inducible factor; N, normal; PLIN, perilipin; RAML, renal angiomyolipomas; T, tumor; TNF, tumor necrosis factor.
To elucidate how PLIN2 is involved in ccRCC pathogenesis, GSEA was performed to gain further insight into the biological pathways in TCGA database. GSEA is a computational tool that determines whether a predefined set of genes shows statistically significant, concordant differences between two biological statuses. The GSEA results demonstrated that the gene signatures of hypoxia pathway, HIF1α and HIF2α signaling, tumor necrosis factor signaling and Myc signaling were associated with patients with higher PLIN2 expression compared with those with lower PLIN2 expression (Fig. 6D–G; P<0.05).
Roles of PLIN2 in ccRCC cell lines
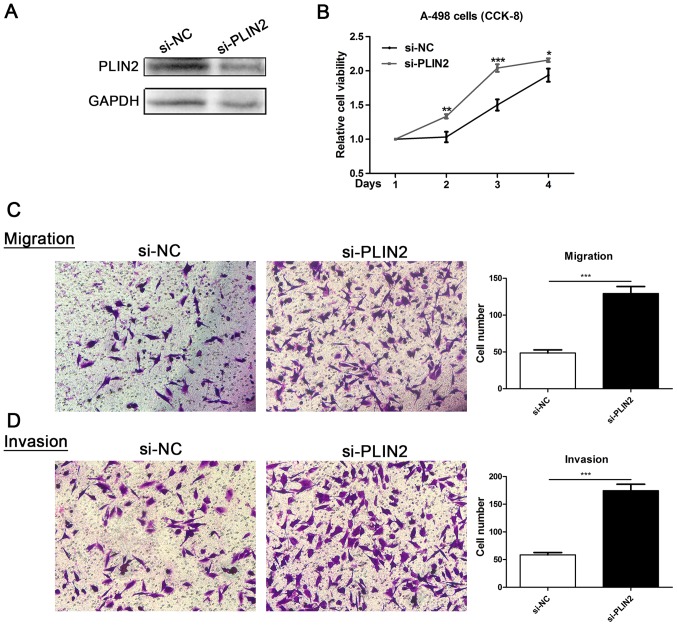
To evaluate the functional role of PLIN2 in ccRCC, ccRCC cell lines with PLIN2 knockdown were generated. Decreased PLIN2 protein expression was observed in A-498 cells post-transfection with si-PLIN2 oligonucleotide sequences (Fig. 7A). Proliferation of A-498 cells transfected with si-PLIN2 was markedly enhanced compared with in cells transfected with si-NC (Fig. 7B). Furthermore, Transwell assays were conducted to assess the migration and invasion of ccRCC cells; downregulation of PLIN2 significantly promoted migration and invasion ability compared with in the si-NC group (Fig. 7C and D). These data revealed that PLIN2 may attenuate the migration and invasion of ccRCC cells.
Figure 7.
Effects of PLIN2 silencing on cell proliferation, migration and invasion. (A) PLIN2 protein expression was successfully knocked down in A-498 cells. (B) Cell counting kit-8 assays detected the effects of PLIN2 knockdown on proliferation of A-498 cells. (C and D) Representative images of migration and invasion assays performed using A-498 cells (magnification, ×100). Data are presented as the means ± standard deviation from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001 vs. si-NC. NC, negative control; PLIN, perilipin; si, small interfering RNA.
Discussion
The present study investigated the expression pattern, clinical significance and biological functions of PLIN2 in ccRCC, including its effects on cell proliferation, migration and invasion. PLIN2 expression was revealed to be elevated in ccRCC tissues compared with in adjacent normal tissues, and was associated with good prognosis of patients with ccRCC. Furthermore, the results demonstrated that knockdown of PLIN2 enhanced proliferation, migration and invasion of ccRCC cells.
PLIN2 is a member of the perilipin protein family, which consists of five members, namely PLIN1-5. PLIN2 serves a vital role in fatty acid uptake and LD formation, and is believed to function in intracellular lipid metabolism (26,28,39). Investigations into PLIN2 in several types of cancer have indicated that it has an important role in tumorigenesis. Numerous studies have reported that PLIN2 levels are markedly increased in specific types of cancer in plasma (29), urine (40) and tumor samples. Several types of cancer, such as Burkitt lymphoma (41), colorectal cancer (29), lung adenocarcinoma (28) and ccRCC (40), exhibit high levels of PLIN2. In addition, PLIN2 is relevant to tumor maintenance in numerous malignancies; high PLIN2 expression has been observed in the majority of malignant melanomas (42), and overexpression of PLIN2 is correlated with a markedly worse prognosis in breast cancer (43). This area of investigation remains superficial, at least for some types of cancer; however, these findings indicated that PLIN2 may serve an important role in tumorigenesis or tumor progression.
Previous studies have provided information regarding the association between PLIN2 expression and ccRCC. A previous study reported that PLIN2 expression was markedly increased in cases of low-stage, low-grade or VHL alteration-positive ccRCC (32), which is consistent with the present findings. These results suggested that PLIN2 levels may represent the tumor differentiation status in ccRCC. By conducting high-density oligonucleotide microarrays on 33 ccRCC tissues and nine normal kidney samples, Yao et al (44) identified 149 significantly differentially expressed genes. When matching them with other microarray data, it was demonstrated that PLIN2 was overexpressed in both datasets. The present study observed increased levels of PLIN2 in clinical ccRCC tissues compared with in paired normal tissues. The mRNA expression levels of PLIN2 were further validated by TCGA database, which revealed significantly increased PLIN2 expression in 72 ccRCC tumor tissues compared with in paired normal tissues. In addition to validation at the protein level, IHC results demonstrated that the expression levels of PLIN2 were significantly higher in ccRCC tissues compared with in normal kidney tissues and benign renal angiomyolipoma tissues. These results demonstrated that PLIN2 may function as an oncogene, serving an important role in the tumorigenesis of ccRCC.
Age is a risk factor for numerous types of cancer, including ccRCC; however, the present study demonstrated that PLIN2 expression was increased in patients ≥60 years group compared with in those <60 years old. These findings indicated that high PLIN2 expression is associated with poor prognosis, which is in contradiction with the follow-up results. Conversely, multivariate regression analysis demonstrated that age and PLIN2 expression levels were independent prognostic factors for ccRCC; age was a risk factor (HR, 1.652; P=0.002) and PLIN2 was a protective factor (HR, 0.586; P=0.001). PLIN2 may therefore be considered a prognostic factor that is not affected by age. Although PLIN2 expression levels may decrease as the tumor progresses, the role of age in tumor progression remains unclear; therefore, there is statistical significance between the two factors, but they do not necessarily have clinical significance.
The present results revealed that high PLIN2 expression was associated with good OS (P<0.001) and DFS (P=0.0006), and may be considered a diagnostic biomarker in patients with ccRCC with various clinicopathological characteristics. Three previous studies also concluded that increased PLIN2 mRNA expression is associated with a satisfactory cancer-specific survival in patients with ccRCC (32,33,44) and metastatic lesions exhibited low expression (32,44). Notably, the present study demonstrated that knockdown of PLIN2 promoted ccRCC cell invasion and migration in vitro. Furthermore, PLIN2 inhibited ccRCC cell proliferation; 4 days following PLIN2 knockdown, the proliferative capacity of A-498 cells was significantly elevated compared with in cells in the control group. In addition, the present study demonstrated that PLIN2 expression levels tended to decrease with increasing tumor grade and T stage. These findings suggested that after PLIN2 is transcriptionally activated, the expression levels of PLIN2 may be gradually downregulated alongside the dedifferentiation processes in the carcinogenic progression of ccRCC. These results indicated that PLIN2 may function as an anti-oncogene, serving an important role in the progression of ccRCC.
Previous studies have elucidated the potential mechanism underlying PLIN2 overexpression. Yao et al (32) revealed that overexpression of PLIN2 may be induced by disruption of the VHL/HIF pathway in ccRCC. Furthermore, it has been reported that HIF2α may enhance PLIN2 expression, thus promoting lipid storage, endoplasmic reticulum homeostasis and cell viability in ccRCC (34). The present study demonstrated that VHL alteration-positive ccRCC was associated with increased PLIN2 expression. Furthermore, GSEA demonstrated that the HIF1α and HIF2α signaling pathways were significantly enriched in response to high PLIN2 expression in patients with ccRCC. It may be hypothesized that inactivation of VHL, which activates downstream HIF expression, contributes to increased expression of PLIN2 in ccRCC. It is well known that PLIN2 is transcriptionally activated by the peroxisome proliferator-activated receptor (PPAR)-mediated pathway (45). Conversely, Qiu et al (34) reported that constitutive HIF2α activity, rather than PPARγ, PPARα or HIF1α, regulates PLIN2 in ccRCC cell lines and primary patient samples. We aim to further explore the molecular mechanisms underlying PLIN2 overexpression and its associated signaling pathways involved in renal cancer in future studies.
To the best of our knowledge, the present study is the first comprehensive study establishing the functional role of PLIN2 in ccRCC tumorigenesis and progression. The results also indicated that PLIN2 may be considered a potential novel biomarker for predicting prognosis of patients with ccRCC. However, one of the potential limitations of our study should be addressed; the exact mechanism by which PLIN2 inhibits ccRCC progression was not investigated. Therefore, further explorations to elucidate the underlying molecular mechanisms are required.
In conclusion, the present results demonstrated that PLIN2 expression was significantly elevated in ccRCC tissues and cells, and was correlated with numerous important clinical factors in patients with ccRCC. Upregulation of PLIN2 expression was associated with good prognosis of ccRCC. In vitro experiments also indicated that PLIN2 knockdown may enhance proliferation, migration and invasion of RCC cells. These findings suggested that PLIN2 may be considered a novel prognostic biomarker in ccRCC and a specific diagnostic indicator for patients with ccRCC. In addition, it could be a potential novel target for the clinical treatment of ccRCC.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant nos. 81672524 and 81672528), the Clinical Research Physician Program of Tongji Medical College, Huazhong University of Science and Technology (grant no. 5001530015) and the Independent innovation foundation of Huazhong University of Science and Technology (grant no. 118530309).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ and HR designed the study. QC, KC, KW, ZS and LB carried out data acquisition and analysis. QC, HR, DL and KC performed the majority of the experiments. QC, TX and HX wrote the manuscript and conducted immunohistochemistry analyses. CW and XM collected the clinical samples and managed the clinical data. GC and JT contributed to bioinformatics analysis. HY and KC were involved in project management, and contributed to preparing and making figures and tables. HY and XZ supervised the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study and experimental procedures were approved by the Human Research Ethics Committee of Huazhong University of Science and Technology (Wuhan, China). Written informed consent was obtained from the patients/patients' families.
Consent for publication
Written informed consent was obtained from the patients/patients' families.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, penile, and testicular Tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Novara G, Ficarra V, Antonelli A, Artibani W, Bertini R, Carini M, Cosciani Cunico S, Imbimbo C, Longo N, Martignoni G, et al. SATURN Project-LUNA Foundation: Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: Are further improvements needed? Eur Urol. 2010;58:588–595. doi: 10.1016/j.eururo.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Gong J, Maia MC, Dizman N, Govindarajan A, Pal SK. Metastasis in renal cell carcinoma: Biology and implications for therapy. Asian J Urol. 2016;3:286–292. doi: 10.1016/j.ajur.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, Pummer K, Zigeuner R. External validation of the Leibovich prognosis score for nonmetastatic clear cell renal cell carcinoma at a single European center applying routine pathology. J Urol. 2011;186:1773–1777. doi: 10.1016/j.juro.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Albiges L, Fay AP, Xie W, Krajewski K, McDermott DF, Heng DY, Dariane C, DeVelasco G, Lester R, Escudier B, et al. Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer. 2015;51:2580–2586. doi: 10.1016/j.ejca.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Oudard S, Vano Y. The role of rechallenge with targeted therapies in metastatic renal-cell carcinoma. Curr Opin Urol. 2015;25:402–410. doi: 10.1097/MOU.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 9.Zanardi E, Verzoni E, Grassi P, Necchi A, Giannatempo P, Raggi D, De Braud F, Procopio G. Clinical experience with temsirolimus in the treatment of advanced renal cell carcinoma. Ther Adv Urol. 2015;7:152–161. doi: 10.1177/1756287215574457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 14.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: The lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;12:741–749. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- 16.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 17.Thiele C, Spandl J. Cell biology of lipid droplets. Curr Opin Cell Biol. 2008;20:378–385. doi: 10.1016/j.ceb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P. A role for lipid droplets in inter-membrane lipid traffic. Proteomics. 2009;9:914–921. doi: 10.1002/pmic.200800584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou C, Wang M, Zhou L, Zhang Y, Liu W, Qin W, He R, Lu Y, Wang Y, Chen XZ, et al. Prognostic significance of PLIN1 expression in human breast cancer. Oncotarget. 2016;7:54488–54502. doi: 10.18632/oncotarget.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szigeti A, Minik O, Hocsak E, Pozsgai E, Boronkai A, Farkas R, Balint A, Bodis J, Sumegi B, Bellyei S. Preliminary study of TIP47 as a possible new biomarker of cervical dysplasia and invasive carcinoma. Anticancer Res. 2009;29:717–724. [PubMed] [Google Scholar]
- 22.Jiang HP, Harris SE, Serrero G. Molecular cloning of a differentiation-related mRNA in the adipogenic cell line 1246. Cell Growth Differ. 1992;3:21–30. [PubMed] [Google Scholar]
- 23.Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci USA. 1992;89:7856–7860. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong BM, Reigan P, Mayle-Combs KD, Orlicky DJ, McManaman JL. Determinants of adipophilin function in milk lipid formation and secretion. Trends Endocrinol Metab. 2011;22:211–217. doi: 10.1016/j.tem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- 26.Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: Relationship to insulin sensitivity. Obes Res. 2005;13:1321–1329. doi: 10.1038/oby.2005.160. [DOI] [PubMed] [Google Scholar]
- 27.Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XD, Li W, Zhang N, Hou YL, Niu ZQ, Zhong YJ, Zhang YP, Yang SY. Identification of adipophilin as a potential diagnostic tumor marker for lung adenocarcinoma. Int J Clin Exp Med. 2014;7:1190–1196. [PMC free article] [PubMed] [Google Scholar]
- 29.Matsubara J, Honda K, Ono M, Sekine S, Tanaka Y, Kobayashi M, Jung G, Sakuma T, Nakamori S, Sata N, et al. Identification of adipophilin as a potential plasma biomarker for colorectal cancer using label-free quantitative mass spectrometry and protein microarray. Cancer Epidemiol Biomarkers Prev. 2011;20:2195–2203. doi: 10.1158/1055-9965.EPI-11-0400. [DOI] [PubMed] [Google Scholar]
- 30.Mentrikoski MJ, Wendroth SM, Wick MR. Immunohistochemical distinction of renal cell carcinoma from other carcinomas with clear-cell histomorphology: Utility of CD10 and CA-125 in addition to PAX-2, PAX-8, RCCma, and adipophilin. Appl Immunohistochem Mol Morphol. 2014;22:635–641. doi: 10.1097/PAI.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 31.Straub BK, Herpel E, Singer S, Zimbelmann R, Breuhahn K, Macher-Goeppinger S, Warth A, Lehmann-Koch J, Longerich T, Heid H, et al. Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Mod Pathol. 2010;23:480–492. doi: 10.1038/modpathol.2009.191. [DOI] [PubMed] [Google Scholar]
- 32.Yao M, Huang Y, Shioi K, Hattori K, Murakami T, Nakaigawa N, Kishida T, Nagashima Y, Kubota Y. Expression of adipose differentiation-related protein: A predictor of cancer-specific survival in clear cell renal carcinoma. Clin Cancer Res. 2007;13:152–160. doi: 10.1158/1078-0432.CCR-06-1877. [DOI] [PubMed] [Google Scholar]
- 33.Tolkach Y, Lüders C, Meller S, Jung K, Stephan C, Kristiansen G. Adipophilin as prognostic biomarker in clear cell renal cell carcinoma. Oncotarget. 2017;8:28672–28682. doi: 10.18632/oncotarget.15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu B, Ackerman D, Sanchez DJ, Li B, Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B, Simon MC. HIF2α-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discov. 2015;5:652–667. doi: 10.1158/2159-8290.CD-14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan H, Li X, Yang H, Song Z, Tong J, Cao Q, Wang K, Xiao W, Xiao H, Chen X, et al. Enhanced expression of caveolin-1 possesses diagnostic and prognostic value and promotes cell migration, invasion and sunitinib resistance in the clear cell renal cell carcinoma. Exp Cell Res. 2017;358:269–278. doi: 10.1016/j.yexcr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Qin X, Ji C, Zeng W, Yang Y, Tan W. Pygopus 2 promotes kidney cancer OS-RC-2 cells proliferation and inva-sionin vitroandin vivo. Asian J Urol. 2015;2:151–157. doi: 10.1016/j.ajur.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cline MS, Craft B, Swatloski T, Goldman M, Ma S, Haussler D, Zhu J. Exploring TCGA pan-cancer data at the UCSC cancer genomics browser. Sci Rep. 2013;3:2652. doi: 10.1038/srep02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targett-Adams P, Chambers D, Gledhill S, Hope RG, Coy JF, Girod A, McLauchlan J. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J Biol Chem. 2003;278:15998–16007. doi: 10.1074/jbc.M211289200. [DOI] [PubMed] [Google Scholar]
- 40.Morrissey JJ, Mobley J, Figenshau RS, Vetter J, Bhayani S, Kharasch ED. Urine aquaporin 1 and perilipin 2 differentiate renal carcinomas from other imaged renal masses and bladder and prostate cancer. Mayo Clin Proc. 2015;90:35–42. doi: 10.1016/j.mayocp.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambrosio MR, Piccaluga PP, Ponzoni M, Rocca BJ, Malagnino V, Onorati M, De Falco G, Calbi V, Ogwang M, Naresh KN, et al. The alteration of lipid metabolism in Burkitt lymphoma identifies a novel marker: Adipophilin. PLoS One. 2012;7:e44315. doi: 10.1371/journal.pone.0044315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujimoto M, Matsuzaki I, Yamamoto Y, Yoshizawa A, Warigaya K, Iwahashi Y, Kojima F, Furukawa F, Murata SI. Adipophilin expression in cutaneous malignant melanoma. J Cutan Pathol. 2017;44:228–236. doi: 10.1111/cup.12868. [DOI] [PubMed] [Google Scholar]
- 43.Lucenay KS, Doostan I, Karakas C, Bui T, Ding Z, Mills GB, Hunt KK, Keyomarsi K. Cyclin E associates with the lipogenic enzyme ATP-citrate lyase to enable malignant growth of breast cancer cells. Cancer Res. 2016;76:2406–2418. doi: 10.1158/0008-5472.CAN-15-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao M, Tabuchi H, Nagashima Y, Baba M, Nakaigawa N, Ishiguro H, Hamada K, Inayama Y, Kishida T, Hattori K, et al. Gene expression analysis of renal carcinoma: Adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J Pathol. 2005;205:377–387. doi: 10.1002/path.1693. [DOI] [PubMed] [Google Scholar]
- 45.Targett-Adams P, McElwee MJ, Ehrenborg E, Gustafsson MC, Palmer CN, McLauchlan J. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim Biophys Acta. 2005;1728:95–104. doi: 10.1016/j.bbaexp.2005.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.