Abstract
Survivin plays a key role in regulating the cell cycle and apoptosis, and is highly expressed in the majority of malignant tumors. However, little is known about the roles of survivin in KRAS-mutant lung adenocarcinomas. In the present study, we examined 28 KRAS-mutant lung adenocarcinoma tissues and two KRAS-mutant lung adenocarcinoma cell lines, H358 and H441, in order to elucidate the potential of survivin as a therapeutic target. We found that 19 (68%) of the 28 KRAS-mutant lung adenocarcinomas were differentiated tumors expressing thyroid transcription factor-1 (TTF-1) and E-cadherin. Patients with tumors immunohistochemically positive for survivin (n=18) had poorer outcomes than those with survivin-negative tumors (n=10). In the H358 and H441 cells, which expressed TTF-1 and E-cadherin, survivin knockdown alone induced senescence, not apoptosis. However, in monolayer culture, the H358 cells and H441 cells in which survivin was silenced, underwent significant apoptosis following combined treatment with ABT-263, a Bcl-2 inhibitor, and trametinib, a MEK inhibitor. Importantly, the triple combination of survivin knockdown with ABT-263 and trametinib treatment, clearly induced cell death in a three-dimensional cell culture model and in an in vivo tumor xenograft model. We also observed that the growth of the H358 and H441 cells was slightly, yet significantly suppressed in vitro when TTF-1 was silenced. These findings collectively suggest that the triple combination of survivin knockdown with ABT-263 and trametinib treatment, may be a potential strategy for the treatment of KRAS-mutant lung adenocarcinoma. Furthermore, our findings indicate that the well-differentiated type of KRAS-mutant lung tumors depends, at least in part, on TTF-1 for growth.
Keywords: KRAS-mutant, lung adenocarcinoma, survivin, senescence, thyroid transcription factor 1
Introduction
Lung cancer is one of the most severe forms of cancer, with a 5-year survival rate of only 15% among patients with all stages of the disease (1). Certain subtypes of lung adenocarcinomas are treatable with effective molecular targeted drugs. For example, tyrosine kinase inhibitors against epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) are highly effective against EGFR-mutant and ALK fusion-positive lung adenocarcinomas, respectively (2). Effective therapies for rare lung adenocarcinomas have also been developed. For example, crizotinib for ROS proto-oncogene 1, receptor tyrosine kinase (ROS-1) fusion-positive tumors, and vandetanib for RET fusion-positive tumors (3,4). However, there are still no effective treatment strategies for KRAS-mutant lung adenocarcinomas, which account for ~8.5% and 20–25% of lung adenocarcinoma cases in the Japanese and Caucasian (European and North American) populations, respectively (5–7). As a result, systemic chemotherapy is still preferentially used to treat the majority of patients with KRAS-mutant lung adenocarcinomas. Constitutively activated mutant KRAS protein has been proven to be notoriously difficult to target with small molecule inhibitors, and this has led to the use of trametinib and selumetinib, two MEK (MAPK/ERK kinase) inhibitors, as promising candidates in the treatment of KRAS-mutant cancer (5,6). Clinical trials have been conducted for KRAS-mutant lung cancer using second line treatment with trametinib alone, or combination therapy with selumetinib and docetaxel; however, these treatments have failed to significantly improve progression-free survival (8,9). Other effective therapeutic options are currently available, including treatment with anti-vascular endothelial growth factor (VEGF) antibody (bevacizumab), anti-VEGFR antibody (ramucirumab) and anti-PD1 antibodies (nivolumab and pembrolizumab) (10–13); however, each of these drugs has shown only limited efficacy against KRAS-mutant lung adenocarcinomas.
Survivin, which is encoded by the baculoviral IAP repeat containing 5 (BIRC5) gene, is not expressed in normal adult tissues, apart from the thymus, placenta, CD34-positive hematopoietic stem cells and the colorectal mucosa. However, the protein is clearly expressed in the majority of malignant tumors, and is involved in carcinogenesis and therapeutic resistance (14,15). Survivin is a member of the inhibitor of apoptosis family of proteins, and it functions to suppress the activity of caspase 3/7 (14). Moreover, it plays an important role in chromosomal segregation and cytokinesis, and its expression level increases mostly at the G2/M phase of the cell cycle (15). The overexpression of survivin has been reported to be associated with a poor outcome in brain tumors, colorectal cancers, breast cancers and non-small-cell lung cancers (16–19). Furthermore, its expression levels are particularly high in tumors in which carcinoma cells are known to evade apoptosis, despite treatment with radiation or cytotoxic agents, including taxane and platinum (20–22). Indeed, the inhibition of survivin by treating cells with small-interfering RNAs (siRNAs) or ribozymes has been shown to enhance the sensitivity of cancer cells to these treatments (23). Thus, it has been proposed that inhibiting the expression or function of survivin may be a promising approach for overcoming resistance to therapy. However, to date, to the best of our knowledge, studies on the role(s) of survivin in KRAS-mutant lung adenocarcinomas are limited (24). In this study, through clinicopathological and molecular pathological analyses, we demonstrate that survivin may be a potential therapeutic target in KRAS-mutant lung adenocarcinomas.
The majority of invasive mucinous adenocarcinomas of the lung bear KRAS mutations and are negative for thyroid transcription factor 1 (TTF-1), an essential transcription factor for lung development encoded by the NK2 homeobox 1 (NKX2-1) gene. TTF-1 is expressed and is oncogenic in virtually all EGFR-mutant lung adenocarcinomas (25); however, the expression rate and role(s) of TTF-1 in KRAS-mutant lung adenocarcinomas remain to be elucidated. In the present study, we aimed to determine the role of survivin in KRAS-mutant lung adenocarcinomas, as well as the role of TTF-1. Our data indicate that the majority of KRAS-mutant lung adenocarcinomas analyzed were positive for TTF-1; thus, TTF-1 may play a role in the proliferation of KRAS-mutant cancer cells.
Materials and methods
Primary KRAS-mutant lung adenocarcinoma tissues and immunohistochemistry
All the experimental procedures involving human samples were approved by the Institutional Review Board at Sapporo Medical University. The samples used in this study were obtained from patients (n=208) who were operated on for lung adenocarcinoma at the Sapporo Medical University Hospital, Sapporo, Japan between January, 2005 and December, 2009. From these patients, we selected 28 patients (13%) whose tumors had a KRAS mutation (at codon 12 or 13) detected by the loop-hybrid mobility shift assay (26). These patients included 20 males and 8 females, with a median age of 63 years (range, 31–85 years; Table I). We did not obtain written informed consent from the patients for conducting this retrospective study. Instead, the patients were informed of the outline of this study through the website of Sapporo Medical University so that they could 'opt out' from the study if they wished. All pathological slides were reviewed and evaluated by two of the authors (T.S. and Y.S.). Hematoxylin and eosin (H&E) staining was performed using Tissue-Tek Hematoxylyn 3G (8657; Sakura Finetek Japan, Tokyo, Japan) and Tissue-Tek Eosin (8660; Sakuma FineTek Japan) according to the manufacturer's instructions. Immunohistochemical analysis for the expression of survivin, TTF-1 and E-cadherin was also carried out on formalin-fixed, paraffin-embedded tissue sections of the cancer specimens. Whole-tissue sections were retrieved using Novocastra Epitope Retrieval Solution 1 (pH 6.0) for E-cadherin expression or Solution 2 (pH 9.0) (both from Leica Biosystems, Nussloch, Germany) for the other antigens at 100°C for 20 min. The primary antibodies used were anti-survivin (sc-17779; D-8; 1:200; Santa Cruz Biotechnology, Dallas, TX, USA), anti-TTF-1 (N1635; 8G7G3/1; pre-diluted; Dako Japan, Tokyo, Japan) and anti-E-cadherin (#14472; 4A2; 1:100; Cell Signaling Technology Japan, Tokyo, Japan). Immunohistochemical staining was conducted using the Leica BOND-MAX (Leica Biosystems). In this study, the cancer tissues were judged as positive for survivin and TTF-1 expression when ≥10% and ≥50% of the cancer cells exhibited positive nuclear staining, respectively. As for E-cadherin, the tissues were judged as positive when membranous staining was observed in ≥80% of the cancer cells. In addition, all the cancer tissues were classified as terminal respiratory unit (TRU) type or non-TRU type based on the definition in the literature (27).
Table I.
Clinicopathological findings of the 28 patients with KRAS-mutant lung adenocarcinoma.
| Pt | Age/sex | Smoking history (pack year) | Dominant history | TRU or non-TRU type | Staging | Survivin | TTF-1 | E-cadherin | OS (months) | Deceased or alive | DFS (months) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74/M | 37.5 | Pap | TRU | 1B | + | + | + | 117 | Alive | 117 | − |
| 2 | 68/M | 34.5 | MIA | TRU | 1A | + | + | + | 55 | Alive | 55 | − |
| 3 | 62/M | 82 | MIA | TRU | 1A | − | + | + | 127 | Alive | 127 | − |
| 4 | 81/F | 50 | Pap | TRU | 2A | + | + | + | 25 | Deceased | 25 | − |
| 5 | 60/M | 80 | Pap | TRU | 2A | + | + | + | 120 | Alive | 120 | − |
| 6 | 58/M | 40 | Pap | TRU | 1B | + | − | + | 45 | Deceased | 28 | + |
| 7 | 64/M | 0 | MIA | TRU | 1A | − | + | + | 115 | Alive | 115 | − |
| 8 | 60/M | 69 | AIS | TRU | 1A | − | + | + | 51 | Alive | 51 | − |
| 9 | 49/F | 14 | Pap | Non−TRU | 2A | − | − | + | 97 | Alive | 97 | − |
| 10 | 65/M | 44 | AIS | TRU | 1A | − | + | + | 106 | Alive | 106 | − |
| 11 | 73/F | 50 | Pap | Non−TRU | 2A | + | − | + | 76 | Alive | 76 | − |
| 12 | 31/F | 0 | IMA | Non−TRU | 4 | + | − | + | 7 | Deceased | 1 | + |
| 13 | 73/M | 53 | Pap | Non−TRU | 3A | + | − | + | 11 | Deceased | 11 | + |
| 14 | 33/F | 0 | IMA | Non−TRU | 2B | − | − | + | 71 | Alive | 71 | − |
| 15 | 68/M | 48 | Pap | Non−TRU | 1B | + | − | + | 86 | Alive | 86 | − |
| 16 | 67/M | 0 | Acinar | TRU | 2A | + | + | + | 10 | Deceased | 9 | + |
| 17 | 59/M | 39 | Pap | TRU | 1A | + | + | + | 104 | Alive | 104 | − |
| 18 | 50/M | 40 | IMA | Non−TRU | 2B | + | + | + | 25 | Alive | 25 | − |
| 19 | 61/F | 0 | IMA | Non−TRU | 1A | + | − | + | 82 | Alive | 82 | − |
| 20 | 40/M | 13.5 | AIS | TRU | 1A | − | + | + | 99 | Alive | 99 | − |
| 21 | 62/M | 30 | Pap | TRU | 1A | + | + | + | 96 | Alive | 96 | − |
| 22 | 79/M | 0 | Pap | TRU | 1A | + | + | + | 53 | Deceased | 9 | + |
| 23 | 55/M | 37 | Pap | TRU | 1A | − | + | + | 91 | Alive | 91 | − |
| 24 | 71/F | 0 | Pap | TRU | 1A | − | + | + | 120 | Alive | 120 | − |
| 25 | 59/M | 38 | Solid | Non−TRU | 1A | + | + | + | 42 | Deceased | 43 | − |
| 26 | 85/F | 0 | IMA | Non−TRU | 2B | + | − | + | 26 | Deceased | 14 | + |
| 27 | 73/M | 40 | MIA | TRU | 1A | + | + | + | 57 | Deceased | 57 | − |
| 28 | 78/M | 0 | Pap | TRU | 3A | − | + | + | 64 | Deceased | 64 | − |
Pt, patient; Pap, papillary predominant adenocarcinoma; MIA, minimally invasive adenocarcinoma; AIS, adenocarcinoma in situ; IMA, invasive mucinous adenocarcinoma; acinar, acinar-predominant adenocarcinoma; solid, solid adenocarcinoma; TRU, terminal respiratory unit; TTF-1, thyroid transcription factor 1; OS, overall survival, DFS, disease-free survival.
Kaplan-Meier Plotter database
We used the Kaplan-Meier Plotter online service (http://kmplot.com/analysis/index.php?p=background) to clarify whether a high BIRC5 mRNA expression was associated with an unfavorable outcome in patients with lung adenocarcinomas (28).
Cell culture and drugs used
Two KRAS-mutant lung adenocarcinoma cell lines, NCI-H358 (G12C) and NCI-H441 (G12V), were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained at 37°C in a humidified incubator with 5% CO2. The cells were cultured in RPMI-1640 (Nacalai Tesque, Kyoto, Japan) with 10% fetal bovine serum and antibiotics. The MEK inhibitor, trametinib (AdooQ BioScience, Irvine, CA, USA) and the Bcl-2 inhibitor (also known as a BH3 mimetic drug), ABT-263 (AdooQ BioScience), were also used in this study.
Immunofluorescence staining of the cells
Immunofluorescence staining was conducted as previously described for the expression of survivin (sc-17779; D-8; 1:200; Santa Cruz Biotechnology) and cytokeratin 7 (#4465; D1E4; 1:400; Cell Signaling Technology) in the H441 cells (29,30). Briefly, cells grown on 35-mm glass bottom, collagen-coated dishes (D11134H; Matsunami Glass, Osaka, Japan) were fixed with 4% paraformaldehyde at room temperature for 15 min followed by permeabilization with 0.5% Triton X-100 for 2 min. After the cells were rinsed with tris-buffered saline (TBS), they were blocked using 3% BSA/TBS for 60 min at room temperature. The samples were then incubated overnight at 4°C with the two primary antibodies mentioned above. The samples were subsequently incubated with two secondary antibodies, Alexia Fluor 488-conjugated donkey anti-mouse IgG (A-21202; 1:400; Thermo Fisher Scientific Japan, Yokohama, Japan) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (A-11012; 1:400; Thermo Fisher Scientific). The cells were finally observed under an inverted microscope (IX-71; Olympus, Tokyo, Japan) and photographed (DP80; Olympus).
RNA interference assay
The cells (3×106) were plated in 94-mm culture dishes and transfected with negative control (NC) siRNA duplexes (1027281; Qiagen, Valencia, CA, USA) or siRNA duplexes targeting BIRC5 and NKX2-1 using Lipofectamine RNAiMAX reagent and OPTI-MEM I (Thermo Fisher Scientific), as previously described (28-30). Two types of siRNA duplexes were used for transient survivin (encoded by the BIRC5 gene) or TTF-1 (encoded by the NKX2-1 gene) knockdown: Silencer Select Validated siRNA (Ambion #s1457 and #s1458, termed BIRC5 siRNA #1 and #2, respectively, in this study; Thermo Fisher Scientific) and Silencer Select Pre-designed siRNA (Ambion #s14152 and #s14153, termed NKX2-1 siRNA #1 and #2, respectively; Thermo Fisher Scientific). The final concentration of the siRNA used in each in vitro experiment was 10 nM. The downregulation of the expression of the targeted genes was verified by western blot analysis.
Assessment of cell viability and apoptosis
The number of viable cells was estimated using a CellTiter Glo 3D Cell Viability assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. Apoptosis was assessed by western blot analysis of cleaved poly(ADP-ribose) polymerase 1 (PARP-1) or Caspase-Glo 3/7 assay (Promega), as previously described (30,31). The luminescence of viable or apoptotic cells was measured with the Infinite 200 microplate reader (Tecan Japan, Kawasaki, Japan). All results are presented as the means ± SD.
Cell cycle analysis by flow cytometry
The cells were reverse transfected with NC siRNA or BIRC5 siRNA, and then grown for 48 h. The cells were then harvested and fixed in 75% methanol at 4°C overnight. The cells were washed twice with cold phosphate-buffered saline (PBS) and stained with propidium iodide (PI) (0.5 mg/ml RNase, and 0.1 mg/ml PI in PBS) for 30 min at room temperature. The stained cells were characterized using a flow cytometer (Beckman Coulter Corp., Tokyo, Japan), and the DNA content was analyzed using FlowJo software.
Senescence associated β-galactosidase staining and the counting of multinuclear cells
The cells (5×105 cells) were plated in 60-mm culture dishes before staining. Cellular senescence was detected using the Cellular Senescence kit (OZ Biosciences, San Diego, CA, USA), according to the manufacturer's instructions. Briefly, the cells were washed twice with PBS and then fixed in the fixation buffer provided in the kit at room temperature for 15 min. The cells were then washed twice with PBS, and stained using the β-galactosidase staining solution provided with the kit for 18 h at 37°C in a humidified incubator. The cells were visualized under a light microscope (IX-71; Olympus) and photographed (DP80; Olympus). Granules stained blue within the cytoplasm were considered positive for β-galactosidase staining. We also assessed the proportion of multinuclear cells, which are cells with two or more nuclei in the cytoplasm, by counting the number of multinuclear cells in at least 3 high-power fields under the same microscope mentioned above.
Western blot analysis
Western blot analysis for the expression of E-cadherin, vimentin, TTF-1, PARP-1, γH2AX, Bim, total AKT, phospho-AKT, total ERK1/2, phospho-ERK1/2 and β-actin was performed as previously described (29–31). Additional primary antibodies used in the present study were anti-survivin (#2808; 71G4B7; 1:1,000 dilution; Cell Signaling Technology) and anti-p21 (#2947; 12D1; 1:1,000 dilution; Cell Signaling Technology). The cells were lysed in NuPAGE LDS Sample Buffer (Thermo Fisher Scientific). The cell lysates (15 μg of total protein in each well) were separated by SDS-PAGE (SuperSep Ace, 5–20%, 13 wells; Wako Pure Chemicals, Osaka, Japan) and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked by incubation with 3% non-fat dry milk in TBS for 1 h at room temperature and incubated overnight at 4°C with the primary antibodies mentioned above. The membranes were then washed 3 times and then incubated for 1 h at room temperature with species-specific horseradish peroxidase-conjugated secondary antibodies (NA931 or NA934; GE Healthcare, Buckinghamshire, UK). Blots were visualized using Supersignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Band intensity levels on X-ray films were normalized to β-actin using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Crystal violet staining
The NC siRNA- or BIRC5 siRNA-transfected H358 cells and H441 cells were cultured for 48 h. The cells were then plated at a density of 5×105 cells into the wells of a 6-well plate and treated with ABT-263 (1 μM) alone, trametinib (25 nM) alone, both, or neither for 72 h. The plate was placed on ice, and the cells were washed twice with cold PBS, and then were fixed in ice-cold 100% methanol for 10 min. Following fixation, the cells were moved to room temperature and stained with 0.5% crystal violet solution (Tokyo Chemical Industry, Tokyo, Japan) diluted in 25% methanol for 10 min. Plates containing stained cells were then photographed using a digital camera (D610; Nikon, Tokyo, Japan).
Three-dimensional (3D) 'on-top' culture
The cells were cultured above a thin layer of 100% Matrigel (Corning, Corning, NY, USA) in RPMI-1640 medium, termed '3D on-top culture', as previously described, with minor modifications (32,33). Briefly, the Corning 96-well Flat Clear White Polystyrene TC-Treated Microplates were coated with 30 μl/well of Matrigel and incubated at 37°C for 30 min to allow the Matrigel to solidify. Subsequently, each microspheroid [consisting of 50 cells in suspension for 24 h using PrimeSurface 96U plate (Sumitomo Bakelite, Tokyo, Japan)] was placed on the top of the Matrigel in each well, and grown for 72 h in 100 μl medium.
Mouse tumor xenograft model
All animal experimentation was conducted in accordance with the protocol approved by the Animal Committee at Sapporo Medical University. The experiments were performed using 6-8-week-old female mice (n=15) (KSN/Slc nude mice; Hokudo, Sapporo, Japan). This study used a minimum of 5 mice per group. The mice were kept throughout the experiment in pleasant conditions (temperature, 20-26 °C; humidity, 40–60%) and were able to freely access food and water. The weights of the mice upon purchase and upon sacrifice were 23 g and 20 g on average, respectively. At 6 h following NC siRNA or BIRC5 siRNA #1 transfection, 5×106 H358 cells were collected in 50 μl of RPMI-1640 and then mixed with 50 μl of Matrigel. The cell/Matrigel mixture was subcutaneously injected into the right flanks of mice anesthetized by inhalation of isoflurane (3–5%; Pfizer, New York, NY, USA). Each mouse received a single injection of H358 cells, and thus developed a single tumor nodule later. At 10 days after the injection, we injected AteloGene (Koken, Tokyo, Japan) containing NC siRNA (n=5) or BIRC5 siRNA (n=10) near the tumor nodules to administer the siRNA continuously. Mice that were injected with BIRC5 siRNA-transfected cells (n=10) were administered the vehicle (n=5) or a combination of ATB-263 (50 mg/kg) and trametinib (0.6 mg/kg) (n=5) orally once daily for 22 days (days 10–32 post-injection of the cells) (34–36). ABT-263 was dissolved in 10% ethanol, 30% polyethylene glycol 400, and 60% Phosal 50 PG; trametinib was dissolved in 10% Cremophor EL, 10% PEG400, and 80% dH2O (Nacalai Tesque). The mice were monitored daily for body weight and general condition. Following any sign of deterioration in any mouse during the ABT-263 and trametinib therapy, the administration to the mouse was terminated immediately. Tumors were measured twice weekly using a caliper, and the volume was calculated using the following formula: 0.5 × (width)2 × length. After the observation, mice were anesthetized by inhalation of isoflurane and then sacrificed by cervical dislocation. The tumor nodules were subsequently removed for weight and histology.
Statistical analysis
The Kaplan-Meier method was used to estimate overall survival and disease-free survival of the 28 patients with KRAS-mutant lung adenocarcinoma. Differences in the survival curves between patients with survivin-positive and -negative tumors were compared using the log-rank test. Differences in caspase activity or cell viability between the untreated and treated cells were evaluated by Dunnett's test, a multiple comparison test as were differences in the number of multinuclear cells or senescence-associated β-galactosidase-positive cells between untreated and treated cells. One-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was performed to evaluate the effects of survivin knockdown, and survivin depletion plus combined therapy with ATB-263 and trametinib, on the cells or tumor xenografts. A P-value <0.05 were considered significant. All statistical calculations were performed using JMP software (JMP for Windows version 7, SAS Institute Japan, Tokyo, Japan).
Results
Patients with survivin-positive tumors have poorer outcomes
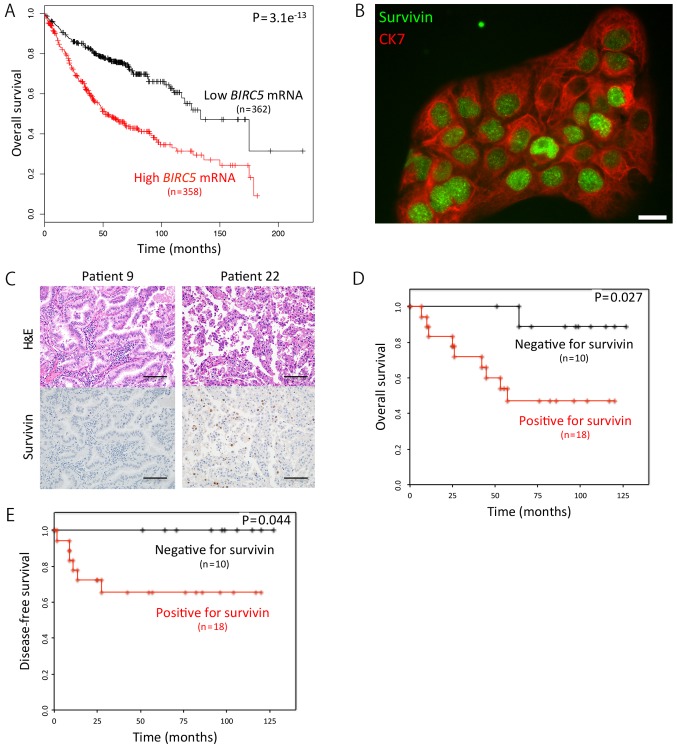
First, we searched the Kaplan-Meier Plotter for an association between the mRNA expression levels of the BIRC5 and the outcome of patients with lung adenocarcinoma. We found that the overall survival (OS) of the patients with a high BIRC5 expression (n=358) was markedly shorter than that of patients with a low BIRC5 expression (n=362) (hazard ratio, 2.41; P=3.1e−13) (Fig. 1A) by utilizing the Kaplan-Meier Plotter (28). We then focused on survivin (BIRC5) protein, not its mRNA, using immunocytochemistry, and found that it was mainly localized to the nuclei of the cells (Fig. 1B). Subsequently, we examined 28 KRAS-mutant lung adenocarcinoma samples for survivin expression using immunohistochemistry (Fig. 1C), and found that the OS rates of the patients with survivin-positive adenocarcinoma (n=18) was significantly shorter than that of patients with survivin-negative tumors (n=10) (P=0.027) (Fig. 1D). As expected, we obtained a similar result in the disease-free survival data analyzed (Fig. 1E).
Figure 1.
Survivin expression affects the survival of KRAS-mutant lung adenocarcinoma patients. (A) Kaplan-Meier curves for overall survival (OS) according to the mRNA expression levels of BIRC5 in patients with lung adenocarcinoma. The data were obtained from the Kaplan-Meier Plotter database (28). (B) Immunofluorescence of H441 cells expressing survivin and cytokeratin 7 (CK7). Survivin was mainly localized to the nuclei. Scale bar, 20 μm. (C) Immunohistochemistry for survivin expression in KRAS-mutant lung adenocarcinoma samples from two patients. Of note, survivin was mainly located in the nuclei of the cancer cells (patient 22). Scale bars, 100 μm. (D) Kaplan-Meier curves for OS according to the immunohistochemical expression levels of survivin in patients with KRAS-mutant lung adenocarcinoma. (E) Kaplan-Meier curves for disease-free survival according to the immunohistochemical expression levels of survivin in patients with KRAS-mutant lung adenocarcinoma.
KRAS-mutant lung adenocarcinomas are differentiated and positive for TTF-1 and E-cadherin
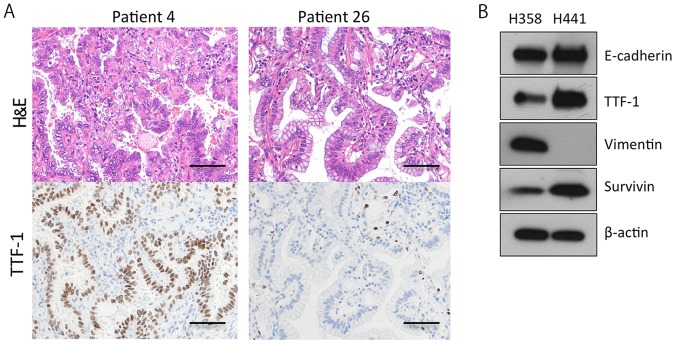
The majority of invasive mucinous adenocarcinomas of the lung bear the KRAS mutation, and do not express TTF-1 (25). Furthermore, more than half of KRAS-mutant lung adenocarcinoma cell lines (41/75, 55%) are negative for E-cadherin and positive for vimentin, indicating that the cell lines are more mesenchymal (36). In this study, however, the KRAS-mutant lung adenocarcinoma tissues were mostly positive for TTF-1 (19/28, 68%) and E-cadherin (28/28, 100%) (Table I). In addition, more than half of the tumors (18/28, 64%) were classified as TRU-type, and 7 out of the 28 (25%) tumors were diagnosed as adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) (Table I; Fig. 2A). These findings suggested that the majority of the operable KRAS-mutant lung adenocarcinomas in Japan are of a relatively well-differentiated type. We further confirmed that 4 out of the 5 invasive mucinous adenocarcinomas stained negative for TTF-1 (Table I; Fig. 2A). Based on these findings, we then sought to analyze two KRAS-mutant lung adenocarcinoma cell lines, H358 cells and H441 cells, expressing TTF-1, E-cadherin and survivin (Fig. 2B).
Figure 2.
There are KRAS-mutant lung adenocarcinomas expressing thyroid transcription factor 1 (TTF-1). (A) Immunohistochemistry for TTF-1 expression in KRAS-mutant lung adenocarcinoma samples from two patients. The tumor cells of invasive mucinous adenocarcinoma (patient 26) were completely negative for TTF-1. Scale bars, 100 μm. (B) Representative western blots of untreated H358 and H441 cells. Both cell lines were positive for TTF-1.
Survivin knockdown impairs cell division, and induces senescence in KRAS-mutant lung adenocarcinoma cells
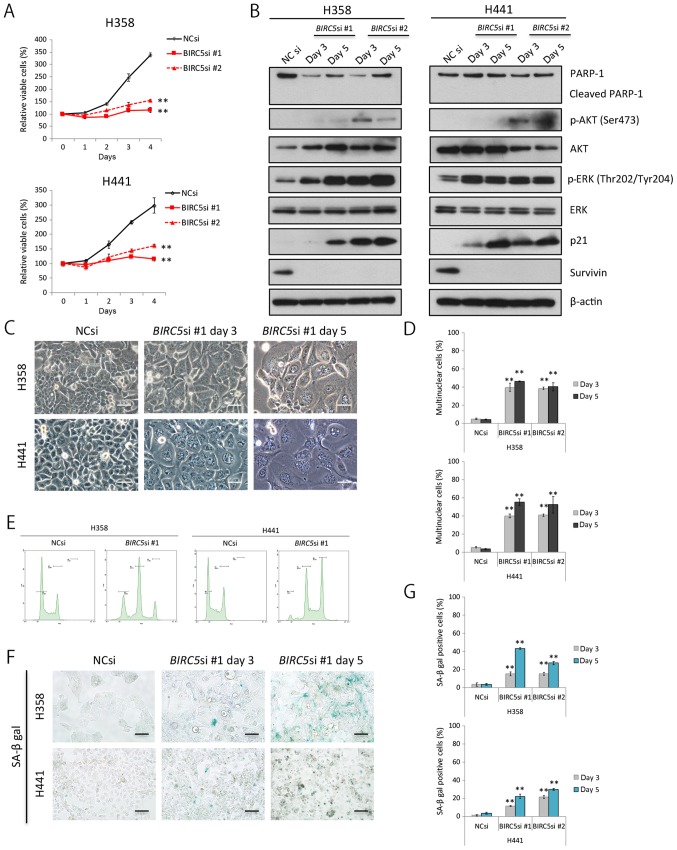
We knocked down survivin expression through RNA interference in the H358 cells and H441 cells in order to elucidate whether and to what degree survivin affects the survival and/or proliferation of these cells. Survivin knockdown clearly decreased the proliferation of the two cell lines examined (Fig. 3A); however, it is unlikely that this was a result of apoptosis in terms of PARP-1 cleavage analyses (Fig. 3B). In addition, survivin knockdown considerably upregulated the phosphorylation of ERK1/2 (the main proliferative signal downstream of KRAS); however, the knockdown of survivin had a lesser effect on the PI3K-AKT pathway (Fig. 3B).
Figure 3.
Survivin knockdown induces senescence, but not apoptosis of KRAS-mutant lung adenocarcinoma cells. (A) Effects of survivin knockdown on proliferation of H358 and H441 cells. Cells were transfected with NC siRNA or BIRC5 siRNA (10 nM each) and cultured for 48 h. Cell viability was then assessed for a further 96 h in triplicate. Results are expressed as the means ± SD; **P<0.01. (B) Western blot analysis of the effects of survivin knockdown in H358 and H441 cells. Cell were transfected with NC siRNA or BIRC5 siRNA and then cultured for 72 or 120 h. (C and D) Induction of multinucleation in cells following survivin knockdown. The NC siRNA- or BIRC5 siRNA (10 nM each)-transfected H358 and H441 cells were cultured for 72 or 120 h, and the number of nuclei in each cell line was then counted. Results are expressed as the means ± SD; Scale bars, 50 μm; **P<0.01. (E) Cell cycle analysis using a flow cytometer. Cells were transfected with NC siRNA or BIRC5 siRNA (10 nM each) and then grown for 48 h. The number of tetraploids and octoploids markedly increased. (F and G) Induction of senescence-associated β-galactosidase-positive cells by survivin knockdown. The NC siRNA- or BIRC5 siRNA (10 nM each)-transfected H358 and H441 cells were cultured for 72 or 120 h, and the number of stained cells was then evaluated. Results are shown as the means ± SD; Scale bars, 50 μm; **P<0.01.
Survivin knockdown significantly increased the ratio of swelled to flattened cells, some of which had several nuclei (Fig. 3C and D). Cell cycle analyses using flow cytometry revealed an increase in the peak of tetraploid cells, reflecting cell cycle arrest and an increase in the number of binuclear cells. Furthermore, we observed a peak in the number of octoploid cells, suggesting that the binuclear cells were unable to divide under these conditions (Fig. 3E). The expression of p21, a senescence-associated protein, increased gradually with time in the cells in which survivin was knocked down (Fig. 3B), and senescence-associated β-galactosidase activity was also elevated (Fig. 3F and G). These findings collectively suggest that survivin knockdown inhibits cell division, and induces senescence in KRAS-mutant lung adenocarcinoma cells.
Combination therapy with survivin knockdown, ABT-263 and trametinib, induces massive apoptosis of KRAS-mutant lung adenocarcinoma cells
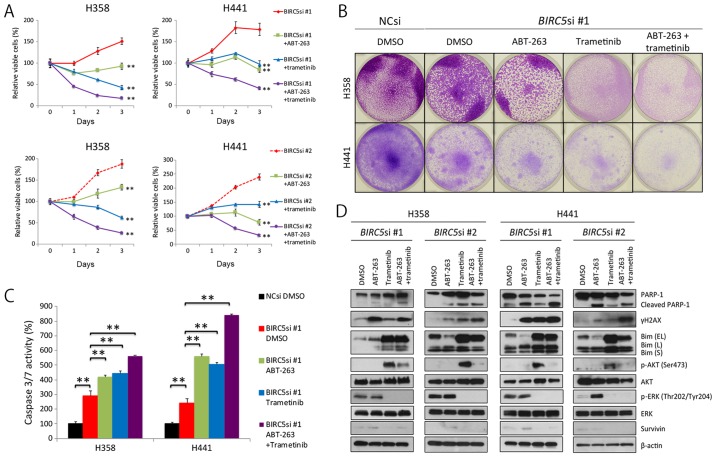
Given that survivin knockdown induced senescence, not apoptosis of KRAS-mutant lung adenocarcinoma cells, we then wished to determine whether ABT-263, a Bcl-2 inhibitor, and trametinib, a MEK inhibitor, induced apoptosis of KRAS-mutant lung cancer cells, as others have demonstrated that ABT-263 preferentially induces apoptosis of senescent cells (37), and MEK inhibitors are particularly effective at inducing apoptosis of KRAS-mutant cells when combined with ABT-263 (38). Thus, in this study, we treated the H358 and H441 cells in which survivin was knocked down with ABT-263 alone, trametinib alone, or a combination of ABT-263 and trametinib (hereafter referred to as AT therapy). As expected, AT therapy clearly decreased cell viability (Fig. 4A and B), markedly elevated caspase 3/7 activation (Fig. 4C), and considerably increased the expression levels of γH2AX and cleaved PARP-1 (Fig. 4D). Of note, selumetinib, another MEK inhibitor, replaced trametinib and had similar results (data not shown). Taken together, these findings suggest that the combination treatment of survivin knockdown, ABT-263 and trametinib, is an efficacious treatment for KRAS-mutant lung adenocarcinomas.
Figure 4.
Combination of survivin knockdown with ABT-263 and trametinib effectively induces apoptosis of KRAS-mutant lung adenocarcinoma cells. (A) Effects of triple combination therapy of survivin knockdown, ABT-263 and trametinib on the viability of H358 and H441 cells. Cells were transfected with BIRC5 siRNA #1 or #2 (10 nM each) and cultured for 48 h. Cells were then treated with the ABT-263 (1 μM) alone, trametinib (25 nM) alone, both, or neither for a further 72 h. Results are shown as the means ± SD; **P<0.01. (B) Crystal violet staining of viable cells. NC siRNA or BIRC5 siRNA #1 (10 nM each)-transfected cells were grown for 48 h. A total of 5×105 cells were then seeded in 6-well plates and treated with ABT-263 (1 μM) alone, trametinib (25 nM) alone, both, or neither for a further 72 h. Cells were then fixed and stained with crystal violet. (C) The effects of triple combination therapy of survivin knockdown, ABT-263 and trametinib on caspase 3/7 activity in H358 and H441 cells. The NC siRNA- or BIRC5 siRNA #1 (10 nM each)-transfected cells were cultured for 48 h, and the cells were then treated with ABT-263 (1 μM) alone, trametinib (25 nM) alone, both, or neither for a further 24 h before evaluating caspase activity. Caspase 3/7 activity was normalized to 100 for the mean of three control (NCsi) dishes. Columns, mean (n=3); bars, SD; **P<0.01. (D) Western blot analysis of the effects of the triple combination therapy on H358 and H441 cells. Cells were treated in the same manner as described in (A) or (B). Of note, Bim (EL) was dephosphorylated by trametinib treatment, and then accumulated in the cell. EL, extra-long; L, long; S, short.
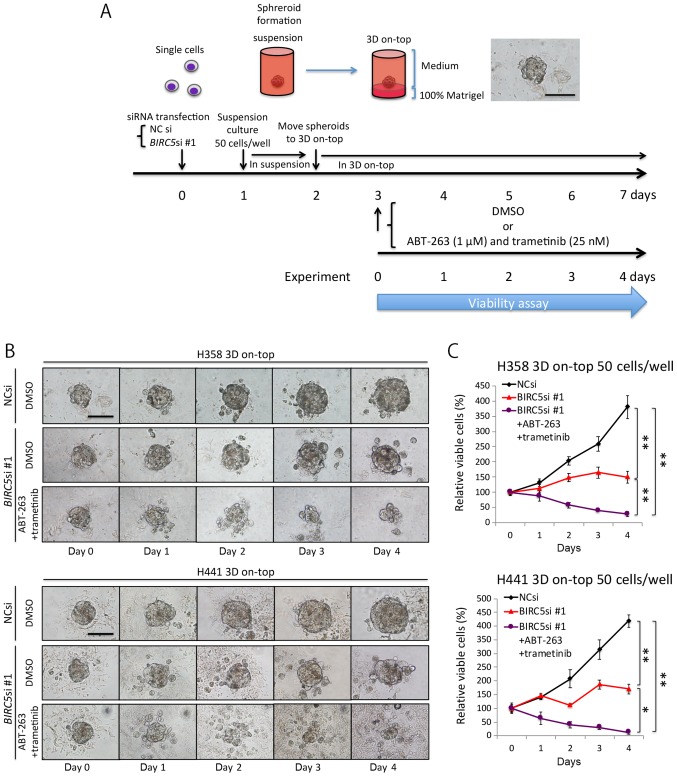
Triple combination therapy is also effective against 3D-cultured KRAS-mutant lung adenocarcinomas cells
Up to this point, our experiments were carried out using conventional, monolayer culture conditions, which do not truly reflect the in vivo environment (32,33). We thus employed a 3D on-top culture condition to recapitulate aspects of the in vivo environment (Fig. 5A), and then analyzed microspheroids comprising ~50 cells (<100 μm in diameter), which we believe mimic the micrometastases of lung adenocarcinoma. The NC siRNA-transfected microspheroids steadily increased in size over time, whereas the BIRC5 siRNA-transfected microspheroids grew at a much slower rate (Fig. 5B). In addition, the microspheroids treated with the triple combination of survivin knockdown and AT therapy were markedly decreased in size (Fig. 5B), and this was confirmed by a viability assay (Fig. 5C). These findings suggest that the triple combination therapy could potentially eradicate the micrometastases of KRAS-mutant lung adenocarcinoma cells in vivo.
Figure 5.
Triple combination therapy comprising of survivin knockdown, ABT-263 and trametinib is also effective against three-dimensionally (3D) cultured KRAS-mutant lung adenocarcinoma cells. (A) Schematic description of the experimental procedure and the representative image of a microspheroid in the 3D 'on-top' culture condition. (B) Representative images of microspheroids of 50 cells in 3D 'on-top' culture condition. The spheroids derived from NC siRNA- or BIRC5 siRNA (10 nM each)-transfected cells were cultured in the absence or presence of ABT-263 (1 μM) plus trametinib (25 nM) for 96 h. (C) Effects of the triple combination therapy on H358 and H441 spheroids. Viability was assessed as described in the schema in (A). Results are shown as the means ± SD; *P<0.05, **P<0.01.
Triple combination therapy is also effective for subcutaneous KRAS-mutant lung adenocarcinomas in mice
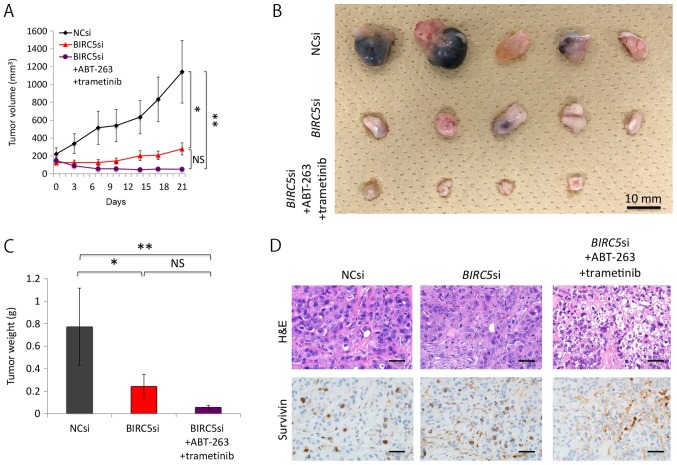
To confirm the in vivo potency of the triple combination therapy, we subcutaneously implanted NC siRNA- or BIRC5 siRNA-transfected H358 cells into mice. Since the efficacy of the pre-treated siRNAs was transient, we used the AteloGene to administer siRNAs to subcutaneous tumors. The NC siRNA-transfected cells grew steadily, whereas the BIRC5 siRNA-transfected cells exhibited minimal proliferation. Moreover, AT therapy further reduced the size and weight of the tumor nodules derived from BIRC5 siRNA-transfected cells, although the effects of the therapy did not reach statistical significance (when comparing the BIRC5 siRNA group to the triple combination treatment group) (Fig. 6A–C). Pathological analyses demonstrated that not only the NC siRNA-transfected tumors, but also the BIRC5 siRNA-transfected nodules thrived in the absence of AT therapy, with a positive survivin expression noted in the cancer cell nuclei of both tumor types (Fig. 6D). We surmised that the BIRC5 siRNA in the AteloGene had already been consumed by day 11, as Fig. 6A indicates that the tumor nodules derived from the BIRC5 siRNA-transfected cells slightly, but steadily increased in size after day 11. However, Fig. 6D demonstrates that the triple combination therapy did suppress the expression of survivin, and induced apoptosis to a certain extent in the cells, indicating the in vivo efficacy of the triple combination therapy.
Figure 6.
Triple combination of survivin knockdown, ABT-263, and trametinib decreases the size of xenografts of H358 cells. (A) Effects of the triple combination therapy on xenografts of H358 cells. Mice with tumor xenografts derived from BIRC5 siRNA-transfected cells were untreated (n=5) or treated (n=5) with ABT-263 (50 mg/kg) and trametinib (0.6 mg/kg) for 22 days. Mice with NC siRNA-transfected xenografts (n=5) were treated with vehicle. Drugs were administered once daily by oral gavage. Tumor volumes (means ± SD) were measured after the initiation of the AT treatment (day 1). 'Day 1' in this figure corresponds to the day 10 after implantation of the cells, when the AteloGene was also administered. Of note, one of the five mice with BIRC5 siRNA-transfected xenografts receiving AT treatment died during the experiment. The death at day 13 was probably due to the side-effects of trametinib used, as only this mouse developed a severe skin rash from day 10, when the administration to the mouse was terminated. As previously shown in clinical practice, the use of trametinib can lead to the development of skin rash (55). *P<0.05, **P<0.01. (B) Effects of the triple combination therapy on xenografts derived from H358 cells. Macroscopic images of the tumors resected from mice are represented. Fourteen tumor nodules presented herein were derived from the 14 mice that survived to the end of the therapy. (C) Effect of the triple combination therapy on xenografts of H358 cells. The weight of each tumor, shown in (B), was measured, and the results are presented as the means ± SD. *P<0.05, **P<0.01. (D) Pathological examination of xenografts. Hematoxylin and eosin (H&E) staining and immunohistochemistry for survivin of xenografts in each treatment condition. Of note, tumors derived from both the NC si-transfected and BIRC5 si-transfected cells without AT therapy expressed survivin in the nuclei, whereas the staining observed in the tumors derived from BIRC5 si-transfected cells with AT therapy were likely to reflect immunoglobulins derived from the mouse; i.e., non-specific staining.
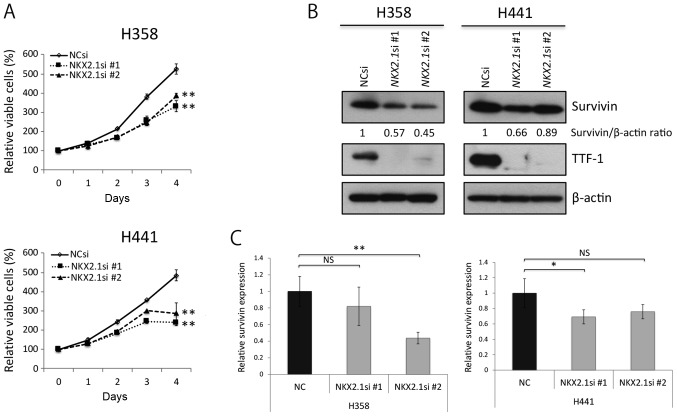
TTF-1 knockdown suppresses the proliferation of KRAS-mutant lung adenocarcinoma cells and induces partial survivin knockdown
TTF-1 knockdown using NKX2-1 siRNA slightly, but significantly suppressed the proliferation of the H358 cells and H441 cells (Fig. 7A). Of note, the expression levels of survivin appeared to decrease slightly when TTF-1 was knocked down (Fig. 7B and C). These observations suggest that TTF-1 probably enhances the growth of TTF-1-positive, differentiated KRAS-mutant lung adenocarcinoma cells, and that the expression levels of survivin are possibly controlled, at least in part, by TTF-1.
Figure 7.
KRAS-mutant lung adenocarcinoma cells are slightly dependent on TTF-1 for proliferation. (A) Effects of TTF-1 knockdown on the proliferation of H358 and H441 cells. Cells were transfected with NC siRNA or NKX2-1 siRNA (10 nM each) and cultured for 48 h. Viable cells were estimated for another 96 h in triplicate. Results are shown as the means ± SD; **P<0.01. (B) Western blot analysis of the effects of TTF-1 knockdown in H358 and H441 cells. Cells were transfected with NC siRNA or NKX2-1 siRNA (10 nM each) and then cultured for 72 h. (C) Quantification of survivin expression, as shown in (B), in NC siRNA- or NKX2-1 siRNA (10 nM each)-transfected H358 and H441 cells. Columns, mean (n=3); bars, ± SD; *P<0.05, **P<0.01.
Discussion
In this study, we demonstrated that in lung adenocarcinoma patients, the OS of patients with tumors expressing high mRNA levels of BIRC5 (survivin) is markedly shorter than that of patients with low expression levels. Furthermore, the OS of patients with KRAS-mutant lung adenocarcinomas expressing survivin protein is also significantly shorter than that of patients with survivin-negative tumors. Since the majority of the KRAS-mutant lung adenocarcinoma tissues we analyzed were well-differentiated and positive for TTF-1 and E-cadherin, we selected and analyzed H358 and H441 cells, two TTF-1-positive, differentiated KRAS-mutant lung adenocarcinoma cell lines, to explore a treatment strategy that would be able to induce extensive apoptosis of these cancer cells. We found that the combination of survivin knockdown along with ABT-263 and trametinib treatment induced massive apoptosis of these cancer cells.
Previous studies have indicated that survivin-positive, small-sized lung adenocarcinomas and non-small cell lung carcinomas are significantly associated with an unfavorable outcome (39–42); however, little information is available as to the roles of survivin in KRAS-mutant lung adenocarcinoma. In this study, we demonstrated that survivin-positive, KRAS-mutant lung adenocarcinomas are more malignant than survivin-negative tumors in terms of survival data. Although the knockdown of survivin in cells reportedly inhibits cell division and causes multinucleation (43,44), we confirmed that survivin knockdown renders the H358 and H441 cells multinucleated owing to a cytokinesis block. Furthermore, we observed a gradual increase in p21 expression and an increase in senescence-associated β-galactosidase activity in the survivin-depleted cells. In other words, survivin knockdown alone was not able to trigger apoptosis of the H358 and H441 cells, but induced cellular senescence. However, the KRAS-mutant cells in which survivin was knocked down in monolayer underwent massive apoptosis following AT therapy, probably as trametinib dephosphorylates Bim, a pro-apoptotic Bcl-2 family member, and dephosphorylated Bim can avoid undergoing degradation through the ubiquitin-proteasome system (37,38). It has also been indicated that KRAS-mutant carcinomas, particularly ones exhibiting an epithelial phenotype, usually depend on Bcl-xL, an anti-apoptotic Bcl-2 family of proteins, for survival (38). Although we inhibited the anti-apoptotic function of Bcl-xL using ABT-263 in this study, a recent study demonstrated that not only Bcl-xL, but also Mcl-1, another anti-apoptotic Bcl-2 family member, plays a key role in the survival of KRAS-mutated lung cancers (45). More importantly, the triple combination of survivin knockdown, ABT-263 and trametinib, substantially decreased the size of the microspheroids of KRAS-mutant cancer cells in 3D on-top culture. Although the 3D 'on-top' culture model exploited in this study cannot fully recapitulate all aspects of the in vivo microenvironment, it does offer more benefits than a monolayer culture (32,33). Distant metastases following surgery in clinical practice are likely to result from the marked growth of pre-existing micrometastatic foci, which cannot be detected using current imaging diagnostics (46). Our triple combination therapy clearly decreased the viability of the cells in the 3D-cultured microspheroids, each of which consisted of only 50 KRAS-mutant cancer cells as a model of micrometastasis. This suggests that the triple combination could eradicate micrometastatic foci, or at least prevent them from growing. We further confirmed the effectiveness of the triple combination therapy for H358 cells in vivo, and also found that the treatment induced extensive apoptosis of the EGFR-mutant H1975 cells in monolayer (data not shown), suggesting that the triple combination could be applicable to lung adenocarcinoma in general.
Almost all EGFR-mutant lung adenocarcinomas express TTF-1, whereas invasive mucinous adenocarcinomas of the lung, most of which harbor the KRAS mutation, do not express TTF-1 (25,27,47). Of note, the loss of TTF-1 expression in pulmonary KRAS-mutated mucinous tumors is probably due to an inactivating mutation and/or hypermethylation of the NKX2-1 gene (48,49). TTF-1 serves as a lineage-specific oncogene in EGFR-mutant lung adenocarcinomas through the induction of ROR1, a tyrosine kinase (50). By contrast, the TTF-1-negative, KRAS-mutant lung adenocarcinoma cell line, A549 cells, are sensitized to cisplatin by forced expression of the NKX2-1 gene, suggesting that KRAS mutation and TTF-1 expression have negative effect(s) on each other (51). However, the majority of the primary KRAS-mutant lung adenocarcinoma tissues analyzed in the present study were relatively differentiated, TTF-1-positive tumors. In addition, the H358 cells and H441 cells, exhibiting a similar phenotype to that of the cancer tissues examined, are partly dependent on TTF-1 for proliferation. This effect of TTF-1 on KRAS-mutant cancer cells may be regulated by, at least in part, survivin. Collectively, these findings raise the possibility that TTF-1 serves as an oncogene in KRAS-mutant, well-differentiated lung adenocarcinomas through survivin induction and other unknown mechanism(s).
More than half of the KRAS-mutated lung adenocarcinomas analyzed in the present study were of the TRU-type, and one fourth of them were classified as AIS or MIA. Although these pathological findings may seem unexpected in that quite a few KRAS-mutant lung adenocarcinomas are less aggressive than might be expected, we would like to stress that the findings mentioned above are consistent with those of previous reports (27,52). This suggests that KRAS-mutant lung adenocarcinomas, at least operable ones, appear more differentiated and less aggressive than might be thought.
This study has several limitations. First, although the immunohistochemical expression of survivin in KRAS-mutant lung adenocarcinomas was linked to an unfavorable outcome, we analyzed the tissue samples from a limited number of patients. We thus need to confirm our findings with a larger selection of KRAS-mutant lung cancer tissue samples. Second, although trametinib is available in clinical practice, ABT-263 is currently undergoing clinical trials (https://clinicaltrials.gov/ct2/show/NCT02079740), and has been reported to cause thrombocytopenia (53,54). Finally, we limited our analysis of KRAS-mutant lung adenocarcinoma cell lines to two types, and therefore we cannot conclude that the majority of KRAS-mutant lung cancers will be sensitive to the triple combination therapy.
In conclusion, KRAS-mutant lung adenocarcinoma tissues examined in this study mostly express TTF-1, and the cancer cells depend, at least in part, on TTF-1 for growth. In addition, survivin-positive KRAS-mutant lung adenocarcinomas are more malignant than survivin-negative adenocarcinomas, and survivin knockdown induces senescence in cancer cells. The triple combination therapy of survivin-depletion, ABT-263, and trametinib can induce substantial apoptosis in KRAS-mutant cancer cells both in vitro and in vivo. It can thus be concluded that survivin is a promising target for the treatment of KRAS-mutant lung adenocarcinomas, with a triple combination therapy potentially a valuable approach for treating such cancers.
Acknowledgments
The authors would like to thank Dr Rebecca Jackson, from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This study was supported in part by Grants-in-Aid for Young Scientists from JSPS, Grant Number 16K19459 (to TS), and funded by support from the Ono Cancer Research Fund (to YS).
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
TS conducted most of the experiments in this study with some assistance from YT and MT. SH, YM, and YS examined the lung cancer tissues for KRAS mutation. MY carried out cell cycle analyses. GY, TH, AW, and HT provided the tumor tissues and analyzed the clinical data. TN, AW and HT provided helpful discussions and critically reviewed the manuscript. YS designed and conceived the study. TS and YS analyzed all the data, and wrote the manuscript. All authors commented on and approved the manuscript.
Ethics approval and consent to participate
All the experimental procedures involving human samples were approved by the Institutional Review Board at Sapporo Medical University. Written informed consent was not obtained from the patients for conducting this retrospective study. Instead, the patients were informed of the outline of this study through the website of Sapporo Medical University so that they could 'opt out' from the study if they wished. All animal experimentation was conducted in accordance with the protocol approved by the Animal Committee at Sapporo Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chalela R, Curull V, Enríquez C, Pijuan L, Bellosillo B, Gea J. Lung adenocarcinoma: From molecular basis to genome-guided therapy and immunotherapy. J Thorac Dis. 2017;9:2142–2158. doi: 10.21037/jtd.2017.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 5.Serizawa M, Koh Y, Kenmotsu H, Isaka M, Murakami H, Akamatsu H, Mori K, Abe M, Hayashi I, Taira T, et al. Assessment of mutational profile of Japanese lung adenocarcinoma patients by multitarget assays: A prospective, single-institute study. Cancer. 2014;120:1471–1481. doi: 10.1002/cncr.28604. [DOI] [PubMed] [Google Scholar]
- 6.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging ras back in the ring. Cancer Cell. 2014;25:272–281. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Jänne PA, van den Heuvel MM, Barlesi F, Cobo M, Mazieres J, Crinò L, Orlov S, Blackhall F, Wolf J, Garrido P, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: The SELECT-1 Randomized Clinical Trial. JAMA. 2017;317:1844–1853. doi: 10.1001/jama.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenschein GR, Jr, Smit EF, Planchard D, Kim DW, Cadranel J, De Pas T, Dunphy F, Udud K, Ahn MJ, Hanna NH, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS- mutant advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2015;26:894–901. doi: 10.1093/annonc/mdv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 11.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 12.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. KEYNOTE-024 Investigators: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 14.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 15.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 16.Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998;351:882–883. doi: 10.1016/S0140-6736(05)70294-4. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–5074. [PubMed] [Google Scholar]
- 18.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- 19.Monzó M, Rosell R, Felip E, Astudillo J, Sánchez JJ, Maestre J, Martín C, Font A, Barnadas A, Abad A. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol. 1999;17:2100–2104. doi: 10.1200/JCO.1999.17.7.2100. [DOI] [PubMed] [Google Scholar]
- 20.Chaopotong P, Kajita S, Hashimura M, Saegusa M. Nuclear survivin is associated with cell proliferative advantage in uterine cervical carcinomas during radiation therapy. J Clin Pathol. 2012;65:424–430. doi: 10.1136/jclinpath-2011-200477. [DOI] [PubMed] [Google Scholar]
- 21.Als AB, Dyrskjøt L, von der Maase H, Koed K, Mansilla F, Toldbod HE, Jensen JL, Ulhøi BP, Sengeløv L, Jensen KM, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 22.Huang W, Mao Y, Zhan Y, Huang J, Wang X, Luo P, Li LI, Mo D, Liu Q, Xu H, et al. Prognostic implications of survivin and lung resistance protein in advanced non-small cell lung cancer treated with platinum-based chemotherapy. Oncol Lett. 2016;11:723–730. doi: 10.3892/ol.2015.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaffaroni N, Daidone MG. Survivin expression and resistance to anticancer treatments: Perspectives for new therapeutic interventions. Drug Resist Updat. 2002;5:65–72. doi: 10.1016/S1368-7646(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz A, Mohamed N, Patterson KA, Tang Y, Shilo K, Villalona-Calero MA, Davis ME, Zhou X, Frankel W, Otterson GA, et al. Increased NQO1 but not c-MET and survivin expression in non-small cell lung carcinoma with KRAS mutations. Int J Environ Res Public Health. 2014;11:9491–9502. doi: 10.3390/ijerph110909491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi T, Hosono Y, Yanagisawa K, Takahashi T. NKX2-1/TTF-1: An enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell. 2013;23:718–723. doi: 10.1016/j.ccr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Matsukuma S, Yoshihara M, Suda T, Shiozawa M, Akaike M, Ishikawa T, Koizume S, Sakuma Y, Miyagi Y. Differential detection of KRAS mutations in codons 12 and 13 with a modified loop-hybrid (LH) mobility shift assay using an insert- type LH-generator. Clin Chim Acta. 2011;412:1874–1878. doi: 10.1016/j.cca.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Yatabe Y, Kosaka T, Takahashi T, Mitsudomi T. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol. 2005;29:633–639. doi: 10.1097/01.pas.0000157935.28066.35. [DOI] [PubMed] [Google Scholar]
- 28.Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8:e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi M, Hirai S, Tanaka Y, Sumi T, Miyajima M, Mishina T, Yamada G, Otsuka M, Hasegawa T, Kojima T, et al. Fibroblastic foci, covered with alveolar epithelia exhibiting epithelial-mesenchymal transition, destroy alveolar septa by disrupting blood flow in idiopathic pulmonary fibrosis. Lab Invest. 2017;97:232–242. doi: 10.1038/labinvest.2016.135. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma Y, Nishikiori H, Hirai S, Yamaguchi M, Yamada G, Watanabe A, Hasegawa T, Kojima T, Niki T, Takahashi H. Prolyl isomerase Pin1 promotes survival in EGFR-mutant lung adenocarcinoma cells with an epithelial-mesenchymal transition phenotype. Lab Invest. 2016;96:391–398. doi: 10.1038/labinvest.2015.155. [DOI] [PubMed] [Google Scholar]
- 31.Sakuma Y, Matsukuma S, Nakamura Y, Yoshihara M, Koizume S, Sekiguchi H, Saito H, Nakayama H, Kameda Y, Yokose T, et al. Enhanced autophagy is required for survival in EGFR-independent EGFR-mutant lung adenocarcinoma cells. Lab Invest. 2013;93:1137–1146. doi: 10.1038/labinvest.2013.102. [DOI] [PubMed] [Google Scholar]
- 32.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi T, Kakefuda R, Tajima N, Sowa Y, Sakai T. Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int J Oncol. 2011;39:23–31. doi: 10.3892/ijo.2011.1015. [DOI] [PubMed] [Google Scholar]
- 36.Kitai H, Ebi H, Tomida S, Floros KV, Kotani H, Adachi Y, Oizumi S, Nishimura M, Faber AC, Yano S. Epithelial-to-mesenchymal transition defines feedback activation of receptor tyrosine kinase signaling induced by MEK inhibition in KRAS-mutant lung cancer. Cancer Discov. 2016;6:754–769. doi: 10.1158/2159-8290.CD-15-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, Greninger P, Brown RD, Godfrey JT, Cohoon TJ, et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121–128. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikehara M, Oshita F, Kameda Y, Ito H, Ohgane N, Suzuki R, Saito H, Yamada K, Noda K, Mitsuda A. Expression of survivin correlated with vessel invasion is a marker of poor prognosis in small adenocarcinoma of the lung. Oncol Rep. 2002;9:835–838. [PubMed] [Google Scholar]
- 40.Wang M, Liu BG, Yang ZY, Hong X, Chen GY. Significance of survivin expression: Prognostic value and survival in stage III non-small cell lung cancer. Exp Ther Med. 2012;3:983–988. doi: 10.3892/etm.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshita F, Ito H, Ikehara M, Ohgane N, Hamanaka N, Nakayama H, Saito H, Yamada K, Noda K, Mitsuda A, et al. Prognostic impact of survivin, cyclin D1, integrin beta1, and VEGF in patients with small adenocarcinoma of stage I lung cancer. Am J Clin Oncol. 2004;27:425–428. doi: 10.1097/01.coc.0000128864.15609.5b. [DOI] [PubMed] [Google Scholar]
- 42.Sun PL, Jin Y, Kim H, Seo AN, Jheon S, Lee CT, Chung JH. Survivin expression is an independent poor prognostic marker in lung adenocarcinoma but not in squamous cell carcinoma. Virchows Arch. 2013;463:427–436. doi: 10.1007/s00428-013-1462-9. [DOI] [PubMed] [Google Scholar]
- 43.Yang D, Welm A, Bishop JM. Cell division and cell survival in the absence of survivin. Proc Natl Acad Sci USA. 2004;101:15100–15105. doi: 10.1073/pnas.0406665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai D, Liang Y, Xie Z, Fu J, Zhang Y, Zhang Z. Survivin deficiency induces apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Oncol Rep. 2012;27:621–627. doi: 10.3892/or.2011.1544. [DOI] [PubMed] [Google Scholar]
- 45.Yan X, Li P, Zhan Y, Qi M, Liu J, An Z, Yang W, Xiao H, Wu H, Qi Y, et al. Dihydroartemisinin suppresses STAT3 signaling and Mcl-1 and Survivin expression to potentiate ABT-263-induced apoptosis in Non-small Cell Lung Cancer cells harboring EGFR or RAS mutation. Biochem Pharmacol. 2018;150:72–85. doi: 10.1016/j.bcp.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 46.Coello MC, Luketich JD, Litle VR, Godfrey TE. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–225. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 47.Sakuma Y. Epithelial-to-mesenchymal transition and its role in EGFR-mutant lung adenocarcinoma and idiopathic pulmonary fibrosis. Pathol Int. 2017;67:379–388. doi: 10.1111/pin.12553. [DOI] [PubMed] [Google Scholar]
- 48.Hwang DH, Sholl LM, Rojas-Rudilla V, Hall DL, Shivdasani P, Garcia EP, MacConaill LE, Vivero M, Hornick JL, Kuo FC, et al. KRAS and NKX2-1 mutations in invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2016;11:496–503. doi: 10.1016/j.jtho.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Matsubara D, Soda M, Yoshimoto T, Amano Y, Sakuma Y, Yamato A, Ueno T, Kojima S, Shibano T, Hosono Y, et al. Inactivating mutations and hypermethylation of the NKX2-1/TTF-1 gene in non-terminal respiratory unit-type lung adenocarcinomas. Cancer Sci. 2017;108:1888–1896. doi: 10.1111/cas.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi T, Yanagisawa K, Sugiyama R, Hosono Y, Shimada Y, Arima C, Kato S, Tomida S, Suzuki M, Osada H, et al. NKX2-1/TITF1/TTF-1-induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21:348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Maeda Y, Tsuchiya T, Hao H, Tompkins DH, Xu Y, Mucenski ML, Du L, Keiser AR, Fukazawa T, Naomoto Y, et al. Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J Clin Invest. 2012;122:4388–4400. doi: 10.1172/JCI64048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Zhang T, Han X, Liu XI, Zhou N, Liu Y. Impact of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of stage IA adenocarcinoma of the lung: Correlation between computed tomography images and EGFR and KRAS gene mutations. Exp Ther Med. 2015;9:2095–2103. doi: 10.3892/etm.2015.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, Chu Q, Giaccone G, Khaira D, Ramalingam SS, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kipps TJ, Eradat H, Grosicki S, Catalano J, Cosolo W, Dyagil IS, Yalamanchili S, Chai A, Sahasranaman S, Punnoose E, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:2826–2833. doi: 10.3109/10428194.2015.1030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.