Abstract
Purpose
Summarize and critically evaluate the effects of Tai Chi and Qigong (TCQ) mind-body exercises on symptoms and quality of life (QOL) in cancer survivors.
Methods
A systematic search in 4 electronic databases targeted randomized and non-randomized clinical studies evaluating TCQ for fatigue, sleep difficulty, depression, pain, and quality of life (QOL) in cancer patients, published through August 2016. Meta-analysis was used to estimate effect sizes (ES, Hedges’ g) and publication bias for randomized controlled trials (RCTs). Methodological bias in RCTs was assessed.
Results
Our search identified 22 studies, including 15 RCTs that evaluated 1283 participants in total, 75% women. RCTs evaluated breast (n=7), prostate (n=2), lymphoma (n=1), lung (n=1), or combined (n=4) cancers. RCT comparison groups included active intervention (n=7), usual care (n=5), or both (n=3). Duration of TCQ training ranged from 3 to 12 weeks. Methodological bias was low in 12 studies and high in 3 studies. TCQ was associated with significant improvement in fatigue [ES=−0.53, p<.001], sleep difficulty [ES=−0.49, p=.018], depression [ES=−0.27, p=.001], and overall QOL [ES=0.33, p=.004]; a statistically non-significant trend was observed for pain [ES=−0.38, p=.136]. Random effects models were used for meta-analysis based on Q-test and I-squared criteria. Funnel plots suggest some degree of publication bias. Findings in non-randomized studies largely paralleled meta-analysis results.
Conclusions
Larger and methodologically sound trials with longer follow-up periods and appropriate comparison groups are needed before definitive conclusions can be drawn, and cancer- and symptom-specific recommendations can be made.
Implications for Cancer Survivors
TCQ shows promise in addressing cancer-related symptoms and QOL in cancer survivors.
Keywords: Tai Chi, Qigong, Meta-analysis, Cancer, Fatigue, Quality of life
INTRODUCTION
Improvements in the detection and treatment of cancer have resulted in an increasing number of cancer survivors, with recent estimates predicting there will be over 20 million cancer survivors living in the Unites States by the year 2026.[1] However, many cancer survivors are left with long-term physical and psychosocial morbidities resulting from their cancer, its treatment, and concerns about its possible recurrence.[2] Consequently, strategies and treatment options for common cancer-related sequelae, such as fatigue, sleep disturbance, mood, pain, and QOL are essential.
Like many other diseases, the burden of cancer is increasingly appreciated as a complex biopsychosocial condition.[2–4] The biopsychosocial framework emphasizes that physical, psychological, and social dimensions of health, and their optimal care, are often highly interdependent. This perspective supports a potentially unique role for holistic mind-body therapeutic approaches that target multiple physical and psychosocial aspects of cancer symptoms, and that may offer the patient a flexible toolset for addressing their experience of the disease.[5–8]
Tai Chi and Qigong are two increasingly popular mind-body interventions that have the potential to address a range of biopsychosocial factors that are part of supportive cancer care.[7, 9] Tai Chi and Qigong share a common history, which integrates elements of traditional Chinese medicine, martial arts conditioning, and lifestyle philosophy. Both incorporate elements of slow, gentle movement, awareness and regulation of breathing, as well as intentional direction of thoughts, attention, imagery, and sensation.[7] For these reasons, Tai Chi and Qigong are grouped together for this review and considered equivalent interventions, paralleling other recent reviews, and subsequently referred to as TCQ.[7, 9, 10] With respect to addressing complex constellations of symptoms, it has been hypothesized that the multi-component nature of TCQ may possess a unique potential to target and impact multiple physiological and psychological processes, thus affording an advantage over conventional single-component therapies.[11–14]
The goal of this paper is to systematically review and quantitatively synthesize the state of evidence for TCQ as an intervention in supportive cancer care, to identify strengths and gaps in evidence of TCQ for cancer care, and to suggest directions for future research. Our study builds upon and extends a number of prior reviews[9, 15–17], by including a significant number of recently published clinical trials not considered in prior studies[18–23], and by specifically focusing on five clinical outcomes of key concern to cancer survivors: fatigue, sleep difficulty, mood, pain and quality life.
METHODS
Literature search
Search Methods
Electronic literature searches were performed using PubMed, CINAHL, Web of Science, and Embase from inception until Jan 30th, 2017. The search terms included multiple variations of the spelling and transliteration of Tai Chi and Qigong, and multiple cancer-related key words. Search strategies in Pubmed, for example, were as follows: [cancer AND (“tai chi” or “tai chi chuan” or “tai ji quan” or “tai-ji” or “taiji” or “taijiquan”) or qigong] and the database interpretation [“neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “cancer”[All Fields] AND (“tai chi”[All Fields] OR “tai chi chuan”[All Fields] OR “tai ji quan”[All Fields] OR “tai-ji”[All Fields] OR “taiji”[All Fields] OR “taijiquan”[All Fields]) OR (“qigong”[MeSH Terms] OR “qigong”[All Fields])]. Hand searches were performed using the bibliographies of all retrieved articles for additional references.
Eligibility Criteria
Randomized controlled trials (RCTs), prospective non-randomized controlled studies, and prospective non-controlled studies published in English, in which cancer was the primary disease and Tai Chi and/or Qigong were the primary interventions were included. To strike a balance between minimizing bias and being comprehensive, meta-analyses were limited to RCTs, and tables and narrative methods were used to report on additional studies, as well as the degree to which they support or broaden findings from RCTs. Inclusion and exclusion of studies were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[24]
Assessment of risk of bias in the included studies
Three authors (ML, JN, RS) assessed the risk of bias using the Cochrane Collaboration Risk of Bias Tool updated in 2009.[25] The tool utilizes 10 items to evaluate various sources of bias including: random sequence generation and allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other possible sources of bias (timing of outcome assessment, similarity in randomized groups at baseline, protocol compliance, and appropriate rationale for control group). Additionally, dropout rates greater than 20% for short-term, and 30% for long-term follow-up studies were used to determine high risk of attrition bias.[25] All evaluated individual domains were endorsed with a ‘Low’ (low risk of bias), “High” (high risk of bias), or Unclear (insufficient information provided to assess bias), following guideline criteria. Studies were rated as having an overall “low risk of bias” when at least 50% of criteria were designated as low bias. Studies with more than 50% of the individual criteria endorsed with a high bias or “unclear” were designated with an overall “high risk of bias” status.[25] All disagreements among independent bias assessors for a given study item were resolved by team discussion.
Safety monitoring
Safety monitoring in studies was evaluated with respect to explicit mention of formal protocols for systematically monitoring adverse events, and numbers and types of adverse events reported in the study associated with the intervention.[26]
Data extraction and synthesis
Data were extracted independently by two reviewers (MS, RS) in a standardized manner using an EXCEL spreadsheet with predefined data fields. Data relevant to study design, duration, subject population, interventions, outcomes measured and results were extracted.
Meta-analytical methods using random effects models were used to synthesize outcomes reported in 15 RCTs identified. For each outcome, data extracted included the mean and standard deviation (SD) of the pre-test and post-test values for each group, mean and SD of change scores in each group, t score or p-value within group, and sample size in each group. When these data were not available, data in the form of standard errors, confidence intervals, or medians with ranges were converted into mean and SD format using previously validated statistical formulas.[27, 28] Comprehensive Meta-Analysis (CMA V 3.0) was used to estimate effect size (ES, Hedges’ g) with 95% CI and publication bias (visual analysis of funnel plots). Heterogeneity was assessed by inspecting the forest plots and I2 tests for quantifying inconsistencies among the included studies. An I2 value higher than 50% indicates substantial heterogeneity.[29] For such outcomes, random effects models were employed. Subgroup and sensitivity analyses were also performed to examine possible sources of heterogeneity. Effect sizes based on random effects models were calculated for fatigue, mood (depression), sleep difficulty, pain, and quality of life, pooling findings from studies of patients with various types of cancer. The pooled effect size in our study was interpreted as small (ES=0.2–0.49), medium (ES=0.5–0.79), or large (ES=≥0.8) according to Cohen’s rule of thumb for effect sizes.[27]
Missing data
Only data that could be extracted from final, published articles were used in this systematic review and meta-analysis. No additional data were obtained from the authors.
RESULTS
Literature search
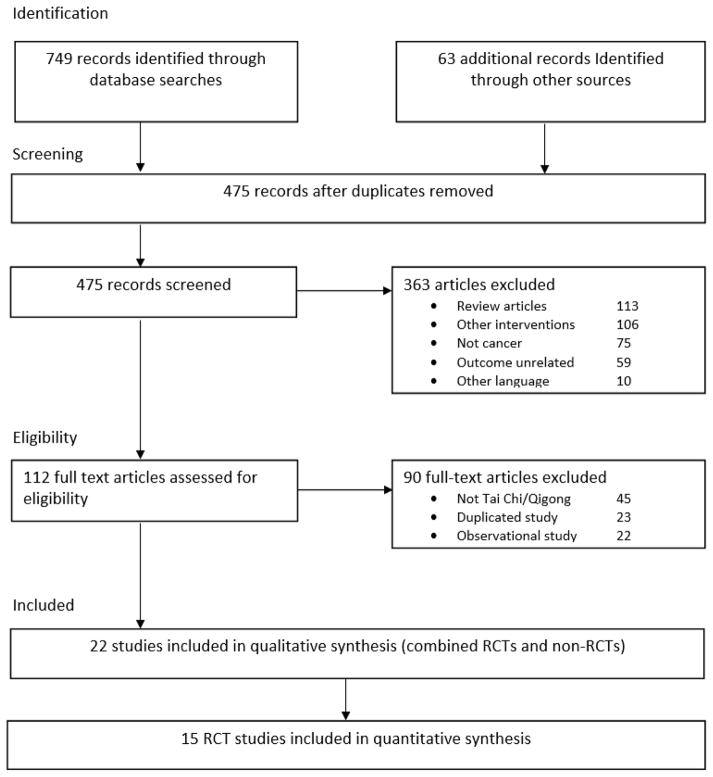
Figure 1 summarizes the flow of the literature search and publication selection process following (PRISMA) guidelines. The search returned 749 results from the four databases and 63 additional records through manual search. After removing duplicates, there were 475 unique papers. Titles and abstracts were then reviewed to determine whether papers met inclusion criteria, which resulted in 112 articles for in-depth, full-text review. Full text reports were then reviewed to further specify whether publications met inclusion criteria, and a final list of 22 publications was established and subjected to the qualitative analysis, including 15 RCTs[18–23, 30–38] and 7 studies with non-randomized or no control group.[39–45] Studies by Larkey et al.[19, 33] and Fong et al.[39, 45] were represented by 2 papers from the same trial with separate outcomes in each publication, and 3 studies[20, 34, 38] compared Tai Chi/Qigong with both an active control and a no treatment control for which each comparison was separately evaluated in analyses. A total of 15 RCTs were included in the quantitative analysis.
Figure 1.
Summary of the flow of our literature search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Study designs
Our search identified 22 studies that examined the role of TCQ in cancer care, including 15 RCTs and 7 studies with non-randomized or no control design. Key features of these studies including participant cancer type, gender, and age, and study interventions and outcomes, are summarized in Table 1.
Table 1.
Summary of Tai Chi/Qigong studies for cancer survivors
| Study | Country | Design | Type of cancer | N (Female) |
Age | Intervention | Timing of intervention |
Duration (weeks) |
Length, Freq (# of sessions per week) |
Control | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mustian 2008 | USA | RCT | Breast | 31 (31) | 52 | Tai Chi | Tx completed | 12 | 1h, 3x | PST | FACIT-F* |
| Oh 2008 | Australia | RCT | Breast/Ovary/Lung | 30 (24) | 54 | Qigong | Outpatient | 5 | 1h, 1–2x | UC | EORTC-QLQ |
| Oh 2010 | Australia | RCT | Breast/Lung/Prostate | 162 (93) | 60 | Qigong | Outpatient | 10 | 1.5h, 2x | UC | FACTG*, POMS*, FACT-F* |
| Campo 2013 | USA | RCT | Breast/Gynecological/Colorectal/Others | 63 (63) | 66 | Tai chi | Outpatient | 12 | 1h, 3x | HE | SF36 |
| Chen 2013 | China | RCT | Breast | 96 (96) | 46 | Qigong | During RT | 5–6 | 1h, 5x | NT | FACT-G*, CES-D*, BFI*, PSQI |
| Robins 213 | USA | RCT | Breast | 145 (145) | 50 | Tai Chi | During chemo | 10 | 1.5h, 1x | SG/NT | FACT-B, CES-D |
| Campo 2014 | USA | RCT | Prostate | 40 (0) | 72 | Qigong | Outpatient | 12 | 1h, 2x | SE | BSI*, FACIT-F* |
| Loh 2014 | Malaysia | RCT | Breast | 197 (197) | NR | Qigong | Tx completed | 8 | 1h, 1x | Ex/UC | FACT-B*, DASS, FACT-F |
| Larkey 2015 | USA | RCT | Breast | 101 (101) | 59 | TCQ | Outpatient | 12 | 1h, 2x | SQ | BDI, FSI*, PSQI |
| Thongteratham 2015 | Thailand | RCT | Breast | 30 (30) | NR | TCQ | Tx completed | 12 | 1h, 4x | UC | FACT-B*, FSI* |
| McQuade 2016 | USA | RCT | Prostate | 76 (0) | 62 | TCQ | During RT | 6–8 | 1h, 3x | LE/WC | EPIC, BFI, PSQI* |
| Zharg 2016 | China | RCT | Lung | 96 (23) | 63 | Tai Chi | During chemo | 12 | 1h, 3x | Ex | MFSI-SF* |
| Chuang 2017 | Taiwan | RCT | NH Lymphoma | 96 (41) | 60 | Qigong | During chemo | 3 | 1h, 7x | UC | EORTC-QLQ*, BFI*, VSHSS* |
| Vanderbyl 2017 | Canada | RCT | Lung/Gastrointestinal | 19 (11) | 65 | Qigong | During chemo | 6 | 1h, 3x | SE | FACT-G, HADS, pain |
| Larkey 2016 | USA | RCT | Breast | 101 (101 | 59 | TCQ | Outpatient | 12 | 1h, 2x | SQ | SF36 |
| Lee 2006 | Taiwan | Non-RCT | Breast | 67 (67) | 47 | Qigong | During chemo | 3 | 1h, 7x | UC | SDS, pain* |
| Lee 2010 | Korea | NC | Gastric | 33 (11) | 60 | Tai Chi | Outpatient | 12 | 1h, 1x | NC | FACT-G, CES-D |
| Reid-Arndt 2012 | USA | NC | Breast/Gynecological | 29 (29) | 62 | Tai Chi | Tx completed | 10 | 1h, 2x | NC | POMS |
| Galantino 2013 | USA | NC | Breast | 12 (12) | 59 | Tai Chi | Tx completed | 8 | 1h, 2x | NC | FACT-B, FACT-F, HADS, BPI |
| Fong 2014/2015 | Hong Kong | Non-RCT | Nasopharyngeal | 52 (24) | 57 | Qigong | Tx completed | 24 | 1.5h, 3x | UC | EORTC-QLQ, MOS-sleep* |
| Huang 2016 | Taiwan | Non-RCT | Breast | 95 (95) | 41 | Qigong | During chemo | 12 | 1h, 3x | Ex | SF36 |
Note. RCT, randomized controlled trial; NC, no control group; NH lymphoma, non-hodgkin lymphoma; NR, not reported; TCQ, Tai Chi plus Qigong; Tx, treatment; RT, radiotherapy; PST, psychosocial support; UC, usual care; HE, health education; NT, no treatment; SG, spiritual growth; SE, stretching exercise; Ex, exercise; LE, light exercise; WC, waiting control; SQ, sham qigong; FACIT-F, functional assessment for chronic illness therapy; EORTC-QLQ, European organization for research and treatment of cancer-quality of life questionnaire; FACT-G, functional assessment for cancer therapy-general; POMS, profile of mood state; FACT-F, functional assessment for cancer therapy-fatigue; SF36, short form 36; CES-D, center for epidemiologic studies depression scale; BFI, brief fatigue inventory; PSQI, Pittsburgh sleep quality index; BSI, brief symptom inventory; FACT-B, functional assessment for cancer therapy-breast; FSI, fatigue symptom inventory; EPIC, expanded prostate cancer index composite; DASS, depression and anxiety stress scale; MFSI-SF, multidimensional fatigue symptom inventory-short form; VSHSS, Verran and Snyder-Halpern sleep scale; HADS, hospital anxiety and depression scale; SDS, symptom distress scale; BPI, brief pain inventory, MOS-sleep, medical outcome study sleep scale.
significant between-group differences.
Participant characteristics and study setting
A total of 1571 cancer patients from the 22 studies (1194 women and 377 men) were included for systematic review (Table 1). Identified studies evaluated a variety of cancer types. The majority of studies recruited patients with a single specific cancer type, mostly solid tumor with the exception of non-Hodgkin’s lymphoma.[18] Breast cancer was the most prevalent cancer studied, evaluated in 10 studies (47%), followed by pooled types of breast or gynecological cancer (n=4, 18%), lung or gastrointestinal (n=4, 18%), prostate (n=3, 13.6%), nasopharyngeal cancer (n=1, 4%), and lymphoma (n=1, 4%).
Intervention and control group characteristics
TCQ interventions varied in their content, dosage, duration, and intensity. Of the 22 studies, Tai Chi was applied in 7 studies, Qigong in 10 studies, and Tai Chi and Qigong were explicitly combined in 4 studies. Individual TCQ sessions varied from 30 minutes to 120 minutes, with session frequency ranging from 1 to 7 times per week. Lengths of the overall training programs ranged from 3 to 24 weeks. Among the 15 RCTs, 7 studies used active control groups for comparison,[19, 22, 23, 30, 31, 33, 35] including 5 studies evaluating alternative exercise.[19, 22, 23, 30, 33] Five studies utilized a waitlist control or no-treatment control for comparison,[18, 21, 32, 36, 37] and 3 studies used a 3-arm study design with both an active control and a no-treatment control.[20, 34, 38]
Risk of bias assessment in RCTs
Quality of RCTs indicated an overall low risk of bias for 12 studies[18, 19, 21–23, 31–37] and an overall high risk of bias for 3 studies[20, 30, 38] (see Table 2). Among 15 RCTs, 12 studies (80%) reported a specific random sequence,[18–20, 22, 23, 31–34, 36–38] but only 2 studies (13%)[18, 35] mentioned the allocation concealment process. Blinding of participants was not possible in most studies due to the features of TCQ, but two studies from one trial applied sham Qigong as an active control comparison.[19, 33] Blinding of outcome assessment was either not possible or not mentioned in 12 studies (80%).[18, 20, 21, 23, 30–32, 34–38] No study employed intention-to-treat procedures, but 7 studies had no or low numbers of dropouts,[18, 19, 21, 23, 31–33] while 2 studies reported more than 40% dropouts at the completion of the program.[36, 38] All studies reported the timing of outcome assessments, but 3 studies did not report a rationale for the choice of control group.[18, 21, 31] Three studies reported significant differences at baseline on some of the study variables.[18, 20, 38]
Table 2.
Quality assessment of the included studies
| Selection Bias |
Performance bias |
Attrition bias |
Reporting bias |
Other bias |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Random sequence |
Allocation concealment |
Blinding of participants |
Blinding of outcome assessment |
Incomplete outcome data (ITT) |
Selective reporting |
timing of outcome assessment |
Similar at baseline |
compliance acceptable in all groups |
Rational for control group |
| Mustian 2008 | H | L | H | U | H | H | L | L | L | L |
| Oh 2008 | L | U | H | U | H | L | L | L | U | L |
| Oh 2010 | L | U | H | U | H | L | L | L | U | L |
| Campo 2013 | L | U | H | U | L | L | L | L | L | H |
| Chen 2013 | L | U | H | U | L | L | L | L | L | L |
| Robins 2013 | L | H | H | U | H | H | L | H | U | L |
| Campo 2014 | U | U | H | U | H | H | L | L | L | L |
| Loh 2014 | L | U | H | U | H | L | L | L | U | L |
| Larkey 2015 | L | U | L | L | L | H | L | L | L | L |
| Thongteratham 2015 | H | U | H | U | L | L | L | L | L | H |
| McQuade 2016 | L | U | H | U | H | H | L | H | L | L |
| Zhang 2016 | L | U | H | H | L | L | L | L | U | L |
| Chuang 2017 | L | L | H | H | L | L | L | H | L | H |
| Vanderbyl 2017 | L | U | H | L | H | L | L | L | L | L |
| Larkey 2016 | L | U | L | L | L | H | L | L | L | L |
Note. Bias was assessed as L ‘low’, H ‘high’ or U ‘unclear’.
Attrition bias scored High for those > 20% dropouts (Furlan 2009)
Outcome measures
Clinical symptoms evaluated included fatigue, sleep difficulty, depression, pain and quality of life. These measures were based on multiple specific instruments and subscales described below. Many instruments, such as those that seek to assess quality of life, had been validated to measure multiple types of outcomes based on subscale scoring. Below we focus our remaining discussion of results on meta-analyses of RCTs organized by cancer symptoms. When relevant, additional evidence from non-RCTs or uncontrolled studies are mentioned.
Fatigue
Cancer related fatigue was assessed in 12 studies, including 10 RCTs.[18, 20, 21, 23, 30, 32–34, 36, 37] Fatigue was assessed using the following questionnaires: Brief Fatigue Inventory (BFI),[18, 20, 32] Fatigue Symptom Inventory (FSI),[21, 33] Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F),[30, 34] Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF),[23] Functional Assessment of Cancer Therapy-Fatigue (FACT-F),[36, 46] and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ) subscale for fatigue.[36, 45]
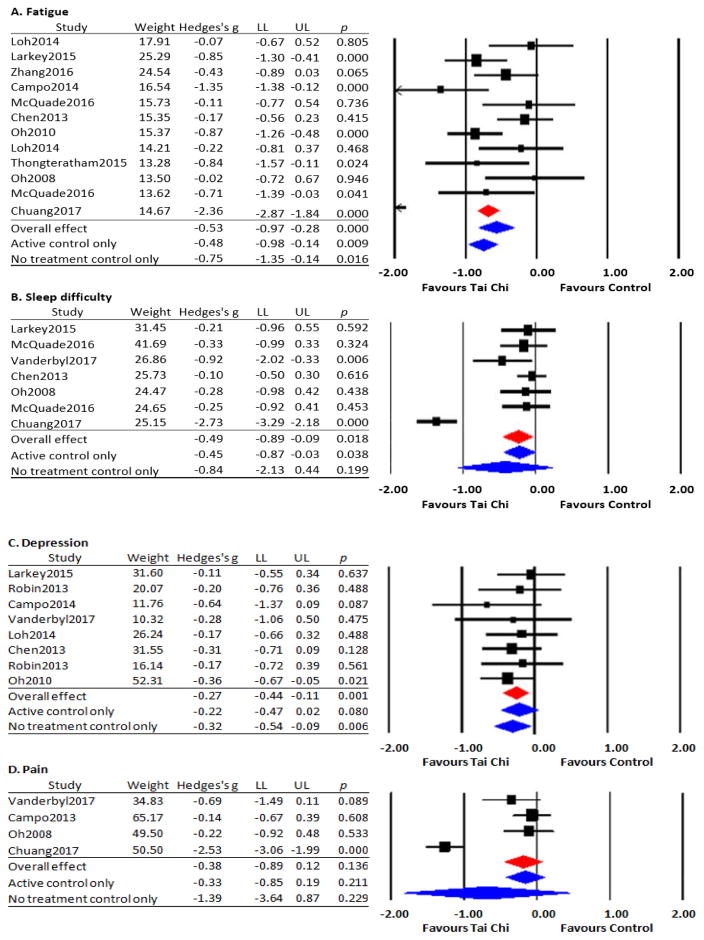
Data from 10 studies were pooled for analysis; two studies[20, 34] utilized both active and no-treatment control groups. The Q-value (p<.001) suggests substantial heterogeneity with I2 of 82%. The overall effect size based on a random effects model indicates a beneficial effect of TCQ on fatigue in cancer patients (Hedges’ g=−0.53, 95% CI −0.97 to −0.28, p<.001). A subgroup meta-analysis with a random effects model on 5 RCTs restricted to active control comparison groups also indicates a beneficial effect of TCQ on fatigue (Hedges’ g=−0.48, 95 % CI −0.98 to −0.14, p=.009), as did a subgroup analysis limited to 7 RCTs with no-treatment control group (Hedges’ g=−0.75, 95% CI −1.35 to −0.14, p=.016) (Figure 2A). One non-controlled study also reported a beneficial effect of an 8-week Tai Chi program targeting fatigue in breast cancer survivors.[46]
Figure 2.
Effects of Tai Chi/Qigong on cancer-related symptoms and quality of life
Note. Data presented included weighted contribution, effect size (Hedges g) and confidence interval of LL (lower limit) to UL (upper limit) of each study.
Sleep difficulty
Cancer related sleep difficulty was assessed in 7 studies, including 6 RCTs.[18, 20, 22, 32, 33, 36] Sleep difficulty was evaluated using the following questionnaires: Pittsburgh Sleep Quality Index (PSQI),[20, 32, 33] Verran and Snyder-Halpern Sleep Scale (VSHSS),[18] EORTC-QLQ Subscale for Symptom,[36, 45] and Symptom Questionnaire.[22]
Data from 6 studies were pooled for analysis; one study[20] utilized both an active and no-treatment control group. The Q-value (p<.001) suggests substantial heterogeneity with I2 of 92%. The overall effect size based on a random effects model indicates a beneficial effect of TCQ on sleep difficulty in cancer patients (Hedges’ g=−0.49, 95% CI −0.89 to −0.09, p=.018). A subgroup meta-analysis with a random effects model limited to the 3 RCTs with an active control group also support a beneficial effect of TCQ on sleep difficulty (Hedges’ g=−0.45, 95 % CI −0.87 to −0.03, p=.038). A subgroup limited to the 4 RCTs using a no-treatment control group showed a statistically non-significant trend in favor of Tai Chi (Hedges’ g=−0.84, 95 % CI −2.13 to 0.44, p=.199) (Figure 2B). One additional non-randomized study reported that sleep difficulty score was significantly decreased in nasopharyngeal cancer survivors following a 6-month TCQ program.[45]
Mood (Depression)
Cancer related depression was assessed in 11 studies, including 7 RCTs.[22, 30, 32–34, 37, 38] Depression was evaluated using the following questionnaires: Beck Depression Inventory (BDI),[33] Brief Symptom Inventory (BSI-18),[30] Center for Epidemiological Studies-Depression (CES-D),[32, 38, 42] Depression and Anxiety Stress Scale (DASS-21),[34] Hospital Anxiety and Depression Scale (HADS),[22, 46] Profile of Mood States (POMS),[37, 44] and Symptom Distress Scale (SDS).[43]
Data from 7 RCTs were pooled for meta-analysis; two studies[34, 38] utilized both an active control and a no-treatment control group. The Q-value (p=.40) suggests non-significant heterogeneity with I2 of 4%. Although the Q-value confirms consistency on depression among included studies, we decided to apply random effect model to be consistent with other variables that showed statistical heterogeneity.[47] The overall effect size based on a random effects model favors TCQ on depression in cancer patients (Hedges’ g=−0.27, 95% CI −0.44 to −0.11, p=.001). A subgroup meta-analysis limited to 5 RCTs using an active control group showed a statistically non-significant trend towards TCQ improving depression (Hedges’ g=−0.22, 95 % CI −0.47 to 0.02, p=.080). A subgroup meta-analysis limited to the 3 RCTs with a no-treatment control group showed a statistically positive effect of TCQ (Hedges’ g=−0.32, 95 % CI −0.54 to −0.09, p=.006) (Figure 2C). One additional non-controlled study[46] also reported a beneficial effect of TCQ on anxiety and depression in breast cancer survivors following an 8-week Tai Chi program.
Pain
Cancer related pain was assessed in 7 studies, including 4 RCTs.[18, 22, 31, 36] Pain was evaluated using the following questionnaires: EORTC-QLQ Subscale for Symptom,[18, 36, 45] Medical Outcomes Study Short-Form Health Survey (SF-36) Subscale for Pain,[31] Brief Pain Inventory (BPI),[46] and Symptom Questionnaire/Checklist.[22, 43]
Data from 4 RCTs were pooled for analysis. The Q-value (p<.001) suggests substantial heterogeneity with I2 of 94%. The overall effect size based on a random effects model indicated a statistically non-significant trend in favor of TCQ on cancer related pain (Hedges’ g=−0.38, 95% CI −0.89 to 0.12, p=.136). Subgroup meta-analyses limited to RCTs using active control groups (Hedges’ g=−0.33, 95 % CI −0.85 to 0.19, p=.211) and no-treatment control groups (Hedges’ g=−1.39, 95 % CI −3.64 to 0.87, p=.229) showed similar trends. Since the number of studies included for cancer related pain was too small to conduct random effect model (less than 5),[47] we conducted fixed effects model after excluding a single outlier study.[18] This sensitivity analysis based on a fixed effects model revealed a similar non-significant trend in favor of TCQ (Hedges’ g=−0.28, 95 % CI −0.66 to 0.09, p=.137, I2=0) (Figure 2D). Two additional non-controlled studies in breast cancer also reported positive effects of TCQ on pain.[43] [46]
Quality of Life
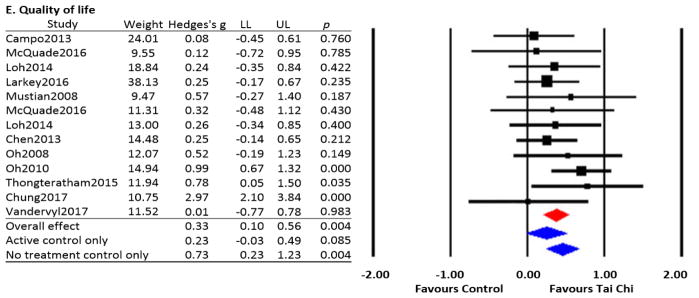
Quality of life (QOL) was assessed in 16 studies, including 12 RCTs.[18–22, 31, 32, 34–38] QOL was evaluated using the following questionnaires: EORTC-QLQ,[18, 37, 45] FACIT-F,[35] FACT-General,[22, 32, 37, 42] FACT-Breast,[21, 34, 38, 46] SF-36,[19, 31, 41] and Expanded Prostate Cancer Index Composite (EPIC).[20]
Data from 11 RCTs were pooled for analysis; two studies[20, 34] utilized both an active and a no-treatment control group. The Q-value (p<.001) suggests substantial heterogeneity with I2 of 73%. The overall effect size based on random effects model favors TCQ on QOL in cancer patients (Hedges’ g=0.33, 95% CI 0.10 to 0.56, p=.004). A subgroup analysis limited to the 5 RCTs with an active control group indicated a statistically non-significant trend in favor of TCQ on QOL (Hedges’ g=0.23, 95 % CI −0.03 to 0.49, p=.085).[19, 20, 31, 34, 35] A subgroup analysis limited to the 8 RCTs utilizing a no-treatment control indicated a statistically significant benefit of TCQ (Hedges’ g=0.73, 95 % CI 0.23 to 1.23, p=.004) (Figure 2E).[18, 20–22, 32, 34, 36, 37] Non-controlled studies add mixed evidence, with one study reporting improvements in QOL in breast cancer survivors,[46] but a second study reporting no improvement in QOL in nasopharyngeal cancer survivors.[39]
Reports of Safety and Adverse Events
Of the 14 randomized trials (15 papers), only one study[22] explicitly reported use of a formal protocol for monitoring adverse events. Four trials[18, 22, 32, 33] reported that there were no intervention-related adverse events. The remaining 10 trials did not include any mention of adverse events.
DISCUSSION
Tai Chi and Qigong for Cancer Care
Exercise is increasingly recommended for cancer survivors,[48] but optimal forms and regimens for addressing different symptoms across different cancer populations have yet to be identified. Tai Chi and Qigong, which both integrate musculoskeletal conditioning along with training in multiple cognitive skills and breath regulation, and which are typically delivered in groups that provide psychosocial support, show great promise for addressing the constellation of physical and psychological morbidities faced by cancer survivors. However, few evidence-based syntheses are available to inform TCQ’s integration into cancer care. Extending prior meta-analyses and systematic reviews by including 6 RCTs[18–23] not considered in earlier reviews, our findings support that TCQ may be effective in reducing multiple symptoms commonly experienced by cancer survivors. Statistically significant and clinically meaningful medium effect sizes in favor of TCQ were observed for symptoms of fatigue and sleep difficulty. Smaller but statistically significant effect sizes were also observed for quality of life and depression, and a non-significant trend in favor of TCQ was observed for pain. Of note, for fatigue and sleep difficulty, significant effect sizes persisted even when comparisons were limited to active controls, a much more rigorous test of effectiveness than simply usual care or waitlist. This finding suggests that the benefits of TCQ are likely not solely due to attention or psychosocial support factors, but instead, are the result of mind-body exercise-specific activities. Finally, although the evaluation of TCQ’s safety within the majority of trials included was not systematically assessed or reported, there were no serious adverse events cited in any study. This finding, along with other more comprehensive reviews of adverse events reported in clinical trials, suggests that TCQ is likely to be safe for cancer survivors.[26]
The overall conclusions of our study largely parallel conclusions reported in other recent reviews, but in addition to including updated evidence, the methods employed in our study differed in important ways. First, a number of prior reviews have been limited to qualitative synthesis and have not also included quantitative meta-analytical methods.[7, 11, 49–51] For studies that did include meta-analysis, two studies published in 2014 focused only on breast cancer and included outcomes not evaluated in our study.[15, 16] Pan and colleagues[16] included 9 published trials (4 based on the same data set) and concluded there is no significant effects of Tai Chi on the symptoms we evaluated, but this review did not include 3 more recently published studies of breast cancer.[19, 21, 52] However, this study reported improvements in outcomes of grip strength and upper extremity function which were not evaluated in our study. Yan and colleagues’ meta-analyses[15] were limited to 5 Tai Chi trials published as of 2012 and reported improvements in emotional well-being, but not in other domains of quality of life. Zeng and colleagues’ meta-analyses[9] included 13 RCTs evaluating both Tai Chi and Qigong, only 5 of which overlapped with those included on our study. They also reported clinically meaningful and statistically significant benefits to cancer-specific quality of life following TCQ training, with smaller effects on depression and anxiety. Finally, one 2016 review[17] including meta-analytic methods employed broader inclusion criteria, synthesizing results from multiple mind-body modalities, including acupuncture, massage, and music therapy, in addition to Tai Chi and Qigong. All previous meta-analyses[9, 16, 49, 51] shared common methodological concerns––including the small number of RCTs on specific cancer-related outcomes, the moderate to high risk of bias, and the heterogeneity of outcome measures––that limit conclusions that could be drawn.
It has been suggested that the broad, multi-symptom benefits of TCQ may result from its multi-component mind-body approach.[12, 13, 53] Klein and colleagues[7] outline multiple potentially therapeutic training components commonly delivered in TCQ programs targeting cancer patients––including low impact exercise, breath regulation, mindfulness and meditation, self-massage, relaxation techniques, and ‘energy cultivation’ practices based on principles of traditional Chinese medicine––each of which could potentially impact multiple cancer-relevant outcomes. In fact, indirect evidence from controlled trials evaluating individual therapeutic elements of TCQ such as moderate physical exercise,[19, 41] mindfulness meditation,[54–56] breath regulation[57], imagery/visualization,[58] and psychosocial support,[59, 60] demonstrates that each of these elements individually can all impact relevant clinical outcomes. Mechanistic exploration of how multi-component practices like TCQ, in comparison to more unimodal interventions (e.g. exercise, meditation) impact individual as well as constellations of symptoms[11] represents a rich area for further research.
Our study which was limited to the effects of TCQ on cancer-related symptom management did not address two important and related questions: Does TCQ impact biological processes that may impact prevention or progression of cancer, and is there any evidence that long-term practice of TCQ changes risks of cancer-related death? Regarding the first question, a growing number of experimental and clinical studies suggest that TCQ and related mind-body training may possibly lead to a down regulation of inflammatory processes (e.g. nuclear factor kappa B pathway) that have been associated with cancer progression, but studies to date are too limited in number and quality to draw strong conclusions.[61] Regarding longer-term effects on cancer-related mortality, no randomized trials have evaluated this question. However, data from the Shanghai Men’s Health Study which includes mortality rates for 61,477 men followed from 2002–2009, reported that men who reported practicing Tai Chi or Qigong had a Hazard Ratio of 0.78 (CI’s .066 to 0.91) for cancer related death, compared to sedentary men.[62] This finding parallels the general observation that higher physical activity is associated with reduced cancer-related mortality rates.[55]
An important methodological decision in this study was to use random-effects models for meta-analyses. Our decision was based on a number of factors and follow established guidelines.[29, 47] First, we began with the statistical assumption that the sample of studies we evaluated showed heterogeneity since study populations across studies varied with respect to different types of cancer. This assumption is also supported by I2 and Q statistics that indicated more than 50% between-study heterogeneity in effect size. Second, it has been suggested that random-models can be used when > 5 studies are included in analyses. As this was not the case in some subgroup analyses, we chose a fixed-effect model approach when I2 was less than 50%. While not reported in our results, exploratory analyses of effect sizes for all outcomes based on random models were found to be either equal to or larger than the fixed-effect results we reported, with only minor qualitative differences in statistical significance.
Limitations and Suggestions for Future Research
There are a number of limitations to this study. First, the analysis was limited to papers published in the English language. Including studies in other languages may better represent the evidence and could make the conclusions drawn more generalizable to other cultures. Second, because of the heterogeneity of outcomes reported in these studies, the meta-analysis was by necessity limited to the most common outcome measures. Until there are more studies that use the same outcomes, it will be difficult to robustly evaluate TCQ and the various domains that it can impact. In addition to better reporting of key design features (e.g., blinding, randomization, adverse event reporting) future studies should report features specifically relevant to TCQ studies (e.g. details, rationale, and validity of training protocols). This will enable future reviews to better evaluate protocol-specific effects. Third, our analyses pooled results from studies with a wide range of training exposure, with intervention spanning 3 to 24 weeks with additional variations related to frequency and length of weekly classes. Future individual studies and pooled meta-analyses should evaluate the impact of dosing and explore optimal durations, frequencies, and intensities of protocol delivery. Fourth, the pooling of studies that was done for meta-analysis does not capture the diversity and complexity of participants across studies, and thus limits the inferences that can be drawn regarding the benefits of TCQ to specific populations. That being said, the largest group by cancer type was breast cancer, while most other randomized controlled trials included a variety of cancer types. Future reviews should further stratify results based on type of cancer, age, or other demographic groupings of interest. Lastly, because TCQ is a multi-component intervention, outcomes that can explore the biopsychosocial model of human health and healing may be appropriate. Measures of biological markers (e.g. inflammatory markers), complex physiological processes (e.g. aerobic capacity, bone metabolism, motor control), and behavioral measures (e.g. stress, mood, body awareness, perceived social support), should be evaluated and explored as mechanisms or mediators of the effects of TCQ.
CONCLUSION
TCQ shows promise in addressing cancer-related symptoms and QOL of cancer survivors. Larger and methodologically sound trials with longer follow-up periods are needed before definitive conclusions can be drawn, and cancer- and symptom- specific recommendations can be made.
Acknowledgments
Funding: This study was supported by grants to Peter Wayne from the National Center for Complementary and Integrative Health/National Institutes of Health (K24AT009282) and the Osher Center for Integrative Medicine. Myeong Soo Lee was supported by a grant from Korea Institute of Oriental Medicine (K17111). Linda Carlson holds the Enbridge Research Chair in Psychosocial Oncology, co-funded by the Canadian Cancer Society Alberta/NWT Division and the Alberta Cancer Foundation. Rhayun Song was supported by a grant from the National Research Foundation of Korea’s Ministry of Education (2013R-1A-1A-2065536).
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: For this type of study formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. Only data that could be extracted from final, published articles, cited in the text, were used in this systematic review and meta-analysis.
References
- 1.Society AC. Book Cancer treatment and survivorship facts and figures 2016–2017. City: American Cancer Society; 2016. Cancer treatment and survivorship facts and figures 2016–2017. [Google Scholar]
- 2.Loscalzo M, Clark K, Pal S, Pirl WF. Role of biopsychosocial screening in cancer care. Cancer J. 2013;19:414–420. doi: 10.1097/PPO.0b013e3182a5bce2. [DOI] [PubMed] [Google Scholar]
- 3.Novy DM, Aigner CJ. The biopsychosocial model in cancer pain. Curr Opin Support Palliat Care. 2014;8:117–123. doi: 10.1097/SPC.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 4.Richtig E, Trapp M, Kapfhammer HP, Jenull B, Richtig G, Trapp EM. The Importance of a Biopsychosocial Approach in Melanoma ResearchExperiences from a Single-center Multidisciplinary Melanoma Working Group in Middle-Europe. Acta Derm Venereol. 2016;96:51–54. doi: 10.2340/00015555-2426. [DOI] [PubMed] [Google Scholar]
- 5.Chaoul A, Milbury K, Sood AK, Prinsloo S, Cohen L. Mind-body practices in cancer care. Curr Oncol Rep. 2014;16:417. doi: 10.1007/s11912-014-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danhauer SC, Addington EL, Sohl SJ, Chaoul A, Cohen L. Review of yoga therapy during cancer treatment. Support Care Cancer. 2017;25:1357–1372. doi: 10.1007/s00520-016-3556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein PJ, Schneider R, Rhoads CJ. Qigong in cancer care: a systematic review and construct analysis of effective Qigong therapy. Support Care Cancer. 2016;24:3209–3222. doi: 10.1007/s00520-016-3201-7. [DOI] [PubMed] [Google Scholar]
- 8.Fulop NJ, Ramsay AI, Vindrola-Padros C, Aitchison M, Boaden RJ, Brinton V, Clarke CS, Hines J, Hunter RM, Levermore C, et al. Reorganising specialist cancer surgery for the twenty-first century: a mixed methods evaluation (RESPECT-21) Implement Sci. 2016;11:155. doi: 10.1186/s13012-016-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Y, Luo T, Xie H, Huang M, Cheng AS. Health benefits of qigong or tai chi for cancer patients: a systematic review and meta-analyses. Complement Ther Med. 2014;22:173–186. doi: 10.1016/j.ctim.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Song R, Grabowska W, Park M, Osypiuk K, Vergara-Diaz GP, Bonato P, Hausdorff JM, Fox M, Sudarsky LR, Macklin E, Wayne PM. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. 2017 doi: 10.1016/j.parkreldis.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage. 2010;39:126–138. doi: 10.1016/j.jpainsymman.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne PM, Kaptchuk TJ. Challenges inherent to t’ai chi research: part II-defining the intervention and optimal study design. J Altern Complement Med. 2008;14:191–197. doi: 10.1089/acm.2007.7170b. [DOI] [PubMed] [Google Scholar]
- 13.Wayne PM, Kaptchuk TJ. Challenges inherent to t’ai chi research: part I--t’ai chi as a complex multicomponent intervention. J Altern Complement Med. 2008;14:95–102. doi: 10.1089/acm.2007.7170a. [DOI] [PubMed] [Google Scholar]
- 14.Payne P, Fiering S, Leiter JC, Zava DT, Crane-Godreau MA. Effectiveness of a Novel Qigong Meditative Movement Practice for Impaired Health in Flight Attendants Exposed to SecondHand Cigarette Smoke. Front Hum Neurosci. 2017;11:67. doi: 10.3389/fnhum.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan JH, Pan L, Zhang XM, Sun CX, Cui GH. Lack of efficacy of Tai Chi in improving quality of life in breast cancer survivors: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:3715–3720. doi: 10.7314/apjcp.2014.15.8.3715. [DOI] [PubMed] [Google Scholar]
- 16.Pan Y, Yang K, Shi X, Liang H, Zhang F, Lv Q. Tai chi chuan exercise for patients with breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:535237. doi: 10.1155/2015/535237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao WW, Jiang H, Tao XM, Jiang P, Sha LY, Sun XC. Effects of Acupuncture, Tuina, Tai Chi, Qigong, and Traditional Chinese Medicine Five-Element Music Therapy on Symptom Management and Quality of Life for Cancer Patients: A Meta-Analysis. J Pain Symptom Manage. 2016;51:728–747. doi: 10.1016/j.jpainsymman.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Chuang TY, Yeh ML, Chung YC. A nurse facilitated mind-body interactive exercise (Chan-Chuang qigong) improves the health status of non-Hodgkin lymphoma patients receiving chemotherapy: Randomised controlled trial. Int J Nurs Stud. 2017;69:25–33. doi: 10.1016/j.ijnurstu.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Larkey LK, Roe DJ, Smith L, Millstine D. Exploratory outcome assessment of Qigong/Tai Chi Easy on breast cancer survivors. Complement Ther Med. 2016;29:196–203. doi: 10.1016/j.ctim.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuade JL, Prinsloo S, Chang DZ, Spelman A, Wei Q, Basen-Engquist K, Harrison C, Zhang Z, Kuban D, Lee A, Cohen L. Qigong/tai chi for sleep and fatigue in prostate cancer patients undergoing radiotherapy: a randomized controlled trial. Psychooncology. 2016 doi: 10.1002/pon.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thongteratham N, Kanaungnit P, Olson K, Adune R, Dechavudh N, Doungrut W. Effectiveness of Tai Chi Qi Qong Program for Thai Women with Breast Cancer: A Randomized Control Trial. Pacific Rim International Journal of Nursing Research. 2015;19:280–294. 215. [Google Scholar]
- 22.Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, Kasymjanova G, Jagoe RT. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer. 2017;25:1749–1758. doi: 10.1007/s00520-017-3579-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LL, Wang SZ, Chen HL, Yuan AZ. Tai Chi Exercise for Cancer-Related Fatigue in Patients With Lung Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. J Pain Symptom Manage. 2016;51:504–511. doi: 10.1016/j.jpainsymman.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board CBRG. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34:1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 26.Wayne PM, Berkowitz DL, Litrownik DE, Buring JE, Yeh GY. What do we really know about the safety of tai chi?: A systematic review of adverse event reports in randomized trials. Arch Phys Med Rehabil. 2014;95:2470–2483. doi: 10.1016/j.apmr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cochrane handbook for systematic reviews of interventions. Book Cochrane handbook for systematic reviews of interventions. City: 2011. [Google Scholar]
- 28.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campo RA, Agarwal N, LaStayo PC, O’Connor K, Pappas L, Boucher KM, Gardner J, Smith S, Light KC, Kinney AY. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv. 2014;8:60–69. doi: 10.1007/s11764-013-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campo RA, O’Connor K, Light KC, Nakamura Y, Lipschitz DL, Lastayo PC, Pappas L, Boucher K, Irwin MR, Agarwal N, Kinney AY. Feasibility and Acceptability of a Tai Chi Chih Randomized Controlled Trial in Senior Female Cancer Survivors. Integr Cancer Ther. 2013 doi: 10.1177/1534735413485418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Meng Z, Milbury K, Bei W, Zhang Y, Thornton B, Liao Z, Wei Q, Chen J, Guo X, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer: results of a randomized controlled trial. Cancer. 2013;119:1690–1698. doi: 10.1002/cncr.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larkey LK, Roe DJ, Weihs KL, Jahnke R, Lopez AM, Rogers CE, Oh B, Guillen-Rodriguez J. Randomized Controlled Trial of Qigong/Tai Chi Easy on Cancer-Related Fatigue in Breast Cancer Survivors. Ann Behav Med. 2015 doi: 10.1007/s12160-014-9645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loh SY, Lee SY, Murray L. The Kuala Lumpur Qigong trial for women in the cancer survivorship phase-efficacy of a three-arm RCT to improve QOL. Asian Pac J Cancer Prev. 2014;15:8127–8134. doi: 10.7314/apjcp.2014.15.19.8127. [DOI] [PubMed] [Google Scholar]
- 35.Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi Chuan for breast cancer survivors. Med Sport Sci. 2008;52:209–217. doi: 10.1159/000134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh B, Butow P, Mullan B, Clarke S. Medical Qigong for cancer patients: pilot study of impact on quality of life, side effects of treatment and inflammation. Am J Chin Med. 2008;36:459–472. doi: 10.1142/S0192415X08005904. [DOI] [PubMed] [Google Scholar]
- 37.Oh B, Butow P, Mullan B, Clarke S, Beale P, Pavlakis N, Kothe E, Lam L, Rosenthal D. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann Oncol. 2010;21:608–614. doi: 10.1093/annonc/mdp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robins JL, McCain NL, Elswick RK, Jr, Walter JM, Gray DP, Tuck I. Psychoneuroimmunology-Based Stress Management during Adjuvant Chemotherapy for Early Breast Cancer. Evid Based Complement Alternat Med. 2013;2013:372908. doi: 10.1155/2013/372908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong SS, Ng SS, Luk WS, Chung LM, Wong JY, Chung JW. Effects of qigong training on health-related quality of life, functioning, and cancer-related symptoms in survivors of nasopharyngeal cancer: a pilot study. Evid Based Complement Alternat Med. 2014;2014:495274. doi: 10.1155/2014/495274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galantino ML, Capito L, Kane RJ, Ottey N, Swizer S, LP The effects of tai chi and walking on fatigue and body mass index in women living with breast cancer: a pilot study. Rehabil Oncol. 2003;21:17–21. [Google Scholar]
- 41.Huang SM, Tseng LM, Chien LY, Tai CJ, Chen PH, Hung CT, Hsiung Y. Effects of non-sporting and sporting qigong on frailty and quality of life among breast cancer patients receiving chemotherapy. Eur J Oncol Nurs. 2016;21:257–265. doi: 10.1016/j.ejon.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Lee EO, Chae YR, Song R, Eom A, Lam P, Heitkemper M. Feasibility and effects of a tai chi self-help education program for Korean gastric cancer survivors. Oncol Nurs Forum. 2010;37:E1–6. doi: 10.1188/10.ONF.E1-E6. [DOI] [PubMed] [Google Scholar]
- 43.Lee TI, Chen HH, Yeh ML. Effects of chan-chuang qigong on improving symptom and psychological distress in chemotherapy patients. Am J Chin Med. 2006;34:37–46. doi: 10.1142/S0192415X06003618. [DOI] [PubMed] [Google Scholar]
- 44.Reid-Arndt SA, Matsuda S, Cox CR. Tai Chi effects on neuropsychological, emotional, and physical functioning following cancer treatment: a pilot study. Complement Ther Clin Pract. 2012;18:26–30. doi: 10.1016/j.ctcp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Fong SS, Ng SS, Lee HW, Pang MY, Luk WS, Chung JW, Wong JY, Masters RS. The effects of a 6-month Tai Chi Qigong training program on temporomandibular, cervical, and shoulder joint mobility and sleep problems in nasopharyngeal cancer survivors. Integr Cancer Ther. 2015;14:16–25. doi: 10.1177/1534735414556508. [DOI] [PubMed] [Google Scholar]
- 46.Galantino ML, Callens ML, Cardena GJ, Piela NL, Mao JJ. Tai chi for well-being of breast cancer survivors with aromatase inhibitor-associated arthralgias: a feasibility study. Altern Ther Health Med. 2013;19:38–44. [PubMed] [Google Scholar]
- 47.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13:196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 48.Hilfiker R, Meichtry A, Eicher M, Nilsson BL, Knols RH, Verra ML, Taeymans J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2017 doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan CL, Wang CW, Ho RT, Ng SM, Chan JS, Ziea ET, Wong VC. A systematic review of the effectiveness of qigong exercise in supportive cancer care. Support Care Cancer. 2012;20:1121–1133. doi: 10.1007/s00520-011-1378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandwani KD, Ryan JL, Peppone LJ, Janelsins MM, Sprod LK, Devine K, Trevino L, Gewandter J, Morrow GR, Mustian KM. Cancer-related stress and complementary and alternative medicine: a review. Evid Based Complement Alternat Med. 2012;2012:979213. doi: 10.1155/2012/979213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee MS, Chen KW, Sancier KM, Ernst E. Qigong for cancer treatment: a systematic review of controlled clinical trials. Acta Oncol. 2007;46:717–722. doi: 10.1080/02841860701261584. [DOI] [PubMed] [Google Scholar]
- 52.Larkey LK, Roe DJ, Weihs KL, Jahnke R, Lopez AM, Rogers CE, Oh B, Guillen-Rodriguez J. Randomized Controlled Trial of Qigong/Tai Chi Easy on Cancer-Related Fatigue in Breast Cancer Survivors. Ann Behav Med. 2014 doi: 10.1007/s12160-014-9645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wayne PM, Manor B, Novak V, Costa MD, Hausdorff JM, Goldberger AL, Ahn AC, Yeh GY, Peng CK, Lough M, et al. A systems biology approach to studying Tai Chi, physiological complexity and healthy aging: design and rationale of a pragmatic randomized controlled trial. Contemp Clin Trials. 2013;34:21–34. doi: 10.1016/j.cct.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 55.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. doi: 10.1002/14651858.CD005001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson LE, Tamagawa R, Stephen J, Drysdale E, Zhong L, Speca M. Randomized-controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy among distressed breast cancer survivors (MINDSET): long-term follow-up results. Psychooncology. 2016;25:750–759. doi: 10.1002/pon.4150. [DOI] [PubMed] [Google Scholar]
- 57.Shahriari M, Dehghan M, Pahlavanzadeh S, Hazini A. Effects of progressive muscle relaxation, guided imagery and deep diaphragmatic breathing on quality of life in elderly with breast or prostate cancer. J Educ Health Promot. 2017;6:1. doi: 10.4103/jehp.jehp_147_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charalambous A, Giannakopoulou M, Bozas E, Marcou Y, Kitsios P, Paikousis L. Guided Imagery And Progressive Muscle Relaxation as a Cluster of Symptoms Management Intervention in Patients Receiving Chemotherapy: A Randomized Control Trial. PLoS One. 2016;11:e0156911. doi: 10.1371/journal.pone.0156911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunes DF, Rodriguez AL, da Silva Hoffmann F, Luz C, Braga Filho AP, Muller MC, Bauer ME. Relaxation and guided imagery program in patients with breast cancer undergoing radiotherapy is not associated with neuroimmunomodulatory effects. J Psychosom Res. 2007;63:647–655. doi: 10.1016/j.jpsychores.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Cameron LD, Booth RJ, Schlatter M, Ziginskas D, Harman JE. Changes in emotion regulation and psychological adjustment following use of a group psychosocial support program for women recently diagnosed with breast cancer. Psychooncology. 2007;16:171–180. doi: 10.1002/pon.1050. [DOI] [PubMed] [Google Scholar]
- 61.Bower JE, Irwin MR. Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang N, Zhang X, Xiang YB, Li H, Yang G, Gao J, Zheng W, Shu XO. Associations of Tai Chi, walking, and jogging with mortality in Chinese men. Am J Epidemiol. 2013;178:791–796. doi: 10.1093/aje/kwt050. [DOI] [PMC free article] [PubMed] [Google Scholar]