Abstract
Purpose of review
The crosstalk between the gut and the brain has revealed a complex communication system responsible for maintaining a proper gastrointestinal homeostasis as well as affect emotional mood and cognitive functions. Recent research has revealed that beneficial manipulation of the microbiota by probiotics and prebiotics represent an emerging and novel strategy for the treatment of a large spectrum of diseases ranging from visceral pain to mood disorders. The review critically evaluates current knowledge of the effects exerted by both probiotics and prebiotics in irritable bowel syndrome (IBS) and mood disorders such as anxiety and depression.
Recent findings
Relevant literature was identified through a search of MEDLINE via PubMed using the following words, “probiotics”, “prebiotics”, “microbiota”, and “gut-brain axis” in combination with “stress”, “depression”, “IBS”, and “anxiety”. A number of trials have shown efficacy of probiotics and prebiotics in ameliorating both IBS related symptoms and emotional states. However, limitations have been found especially due to the small number of clinical studies, studies design, patient sample size, and placebo effect.
Summary
Nonetheless, current finding supports the view that beneficial manipulation of the microbiota through both probiotics and prebiotics intake represents a novel attractive strategy to treat gut-brain axis disorders such as IBS and depression.
Keywords: gut-brain axis, IBS, depression, anxiety, probiotics, prebiotics
Introduction
The human gastrointestinal tract harbours trillions of micro-organisms, with numbers recently estimated to be similar to the number of human cells in our bodies, and contains over 100 times more genes than the host genome [1]. Nonetheless, the collective genome of these microorganisms has co-evolved with the host, contributing to its development and functioning [2]. Colonization of the gut commences at birth, during a crucial developmental window for the host, setting the stage for the establishment of a more complex microbiome in adulthood. More than 1000 species and 7000 strains have been identified as constituents of the adult microbiome. This ecosystem is dominated mainly by bacteria, but also contains viruses, protozoa, archaea and fungi. The coexistence of the microbiome with the host establishes a long evolutionary symbiosis that is essential for optimal health throughout life. Indeed, essential processes, such as digestion [3], immune responses [4, 5], absorption of nutrients [3], growth [6], and metabolism [7] are critically influenced by the microbiome. Moreover, an increasing body of evidence has highlighted a mutual influence between the microbiome and the brain [2, 8–11]. The so called gut-brain axis is a bidirectional communication system that enables gut microbes to communicate with the brain and the brain to communicate with the gut [9]. Perturbation of this axis has been shown to exacerbate vulnerability to a range of diseases ranging from visceral pain to mood disorders [12, 2, 10, 13, 14, 11]. Of importance, alterations in the richness and diversity of the microbiota have been observed in both depressed patients and individuals affected by irritable bowel syndrome (IBS) [15, 16]. Moreover, faecal microbiota transplantation from depressed patients into rodents can induce certain features characteristic of depression in the recipient animals, such as anhedonia and anxiety-like behaviors [17]. Given this evidence, scientists have begun to believe that targeting the microbiota may open up novel strategies of prevention and intervention for a large spectrum of diseases that involve impairments both in the gut and in the brain. Of these novel strategies, probiotics, beneficial microorganisms believed to provide health benefits, and prebiotics, dietary soluble fibers that stimulate the growth of beneficial commensal microbiota, have been predominantly studied [18]. The current work aims to offer an updated review on the most recent findings in the field providing an overview of the efficacy of both probiotics and prebiotics in IBS and mood disorders such as anxiety and depression. The review also provides an understanding of the possible mechanisms that identify the routes of communication between the gut and the brain.
Probiotics and prebiotics in IBS
Mono-strain probiotics
A number of clinical trials involving intervention using probiotics have demonstrated improvements in patients with IBS. Perhaps the most compelling evidence demonstrating efficacy in the reduction of IBS symptoms comes from trials involving Bifidobacterium infantis 35624. A single centre (77 IBS patients) and a multicentre trial (362 women with IBS) have both demonstrated efficacy in reducing pain, bloating and improving bowel movements after eight and four weeks of treatment over placebo, respectively [19, 20]. Moreover, such improvements were associated with the normalization of the ratio of the anti-inflammatory IL-10 to the pro-inflammatory IL-12 cytokines in the group receiving the probiotic compared to placebo [19]. Moreover, the fermented dairy product containing B. lactis DN-173010 (Activia, Danone) revealed improvements in accelerating gastrointestinal transit and in ameliorating distention after four weeks of a small single centre (34 IBS patients), randomized controlled trial (RCT) [21]. However, two large multicentre trials (274 and 179 IBS patients, respectively) using the same fermented milk probiotic for a period of six and twelve weeks, respectively, were not able to show improvements in IBS symptoms over placebo [22, 23]. Of notice, Roberts and colleagues observed a 55% of withdrawals (76 out of 179 patients) at week twelve. Therefore, the effect of the probiotic may have been missed due to lack of power.
Lactobacillus-containing probiotics have also been studied clinically. A RCT using Lactobacillus plantarum 299v (DSM 9843) ameliorated both abdominal pain and bloating over placebo after four weeks of treatment in 214 IBS patients fulfilling the Rome III criteria [24]. Moreover, L. plantarum 299v (DSM 9843) treatment could maintain a better overall gastrointestinal function in sixty IBS patients compared to placebo, which was observed twelve months after four weeks of probiotic administration [25] suggesting a long-lasting beneficial impact. In another trial, four weeks of L. acidophilus SDC 2012 and 2013 administration ameliorated abdominal pain in 40 IBS patients who met the Rome III criteria, compared to placebo [26]. A small-sized study of 55 IBS patients treated for 6 months with L. reuteri ATCC 5570 showed improvement in the global symptom score compared to baseline, but failed to demonstrate improvement over placebo due to a high placebo effect [27]. Interestingly, two different studies showed L. rhamnosus GG (LGG)-induced improvements in children with IBS (Rome II criteria). Children (104 and 141 pediatric IBS patients, respectively) were randomly assigned to receive either LGG or placebo for four or eight weeks, respectively. LGG administration reduced frequency of pain [28] and severity of pain [29] over placebo. However, a smaller RCT (55 children with IBS) showed that LGG was not superior to placebo in the treatment of abdominal pain but may help relieve such symptoms as perceived abdominal distention (Bauserman and Michail, 2005).
The Escherichia coli DSM 17252 (Symbioflor 2) has also showed improvements in 298 IBS patients compared to placebo. After eight weeks of treatment both abdominal pain and general pain scores were significantly ameliorated in the IBS group [30].
Multispecies probiotics
Multispecies probiotics, defined as supplementations containing strains belonging to one or more genera have also shown positive effects in patients with IBS (Table 1). Two different RCTs used a combined supplementation of LGG, L. rhamnosus LC705, B. breve Bb99, and Propionibacterium freudenreichii spp shermanii JS on 103 and 86 IBS patients (Rome II criteria), for a period of six and five months, respectively. In both studies the gastrointestinal symptoms (abdominal pain, distension, flatulence and borborygmi) were significantly reduced compared to placebo [31, 32].
Table 1.
The effect of probiotics in irritable bowel syndrome.
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 77 IBS patients. | L. salivarius UCC4331 or B. infantis 35624 1×1010 cfu/ml (malted milk drink), or placebo (malted milk drink alone). | 8 weeks. | IBS symptoms (abdominal pain/discomfort, bloating/distention, and bowel movement difficulty); plasma cytokines levels. | B. infantis 35624 ↓IBS symptoms; and normalized the plasma IL-10/IL-12 ratio. | [19] |
| 362 female IBS patients. | B. Infantis 35624 1×106, 1×108, 1×1010 cfu/ml, or placebo. | 4 weeks. | IBS symptoms (abdominal pain, composite score, bloating, bowel dysfunction, incomplete evacuation, straining, and the passage of gas) scored on a 6-Likert scale. | B. Infantis 35624 1×10(8) cfu/ml ↓ IBS symptoms. | [20] |
| 41 female IBS patients. | B lactis DN-173010 (1.25×1010cfu/pot) or placebo, (fermented milk). | 4 weeks. | IBS symptoms (abdominal girth and gastrointestinal transit). | ↓ IBS symptom severity. | [21] |
| 274 IBS patients. | Activia (Danone) containing: B. animalis DN-173 010 (1.25 × 1010 cfu/pot), S. thermophilus and L. bulgaricus (1.2 × 109 cfu/pot); Placebo (heat treated yoghurt). | 6 weeks. | HRQoL and digestive symptoms (stool frequency, bloating, discomfort). | The HRQoL discomfort score improved; ↓ in bloating and stool frequency ↑. | [22] |
| 179 IBS patients (173 drop off). | B. lactis (1.25×1010 cfu), S. thermophiles and L. bulgaricus(1.2×109 cfu) or placebo. | 12 weeks. | IBS symptoms (pain, bloating, flatulence levels, stool frequency/consistency, ease of bowel movement and quality of life). | By 8 weeks, 46% active and 68% placebo patients showed relief: it did not differ by group. | [23] |
| 214 IBS patients. | One capsule/daily of L. plantarum 299v (1×109cfu) or placebo. | 4 weeks. | Measured frequency and intensity of abdominal pain, bloating and feeling of incomplete rectal emptying weekly. | Pain severity, daily frequency, and IBS symptoms↓. | [24] |
| 60 IBS patients. | 400ml/day of a rose-hip drink with L. plantarum (5×107cfu/ml) and 0.009g/ml oat flour; placebo. | 4 weeks. | Patients recorded their IBS symptoms in the form of a questionnaire at the beginning and end of study. | Flatulence ↓ in the trial and abdominal group, pain was ↓in both groups. 12 months later trial- patients reported an improvement in overall GI health. | [25] |
| 40 IBS patients. | L. acidophilus-SDC 2012, 2013 (2×109 cfu/ml) or placebo. | 4 weeks. | Abdominal pain, discomfort. | ↓ in abdominal pain score by more than 20%in trial group. | [26] |
| 54 IBS patients started the study, 39 ended it. | 1×108 cfu/tablet of L. reuteri twice a day or placebo. | 6 months. | IBS symptoms (abdominal pain, abdominal bloating, feeling of incomplete evacuation, diarrhea, constipation, gases, fatigue) were evaluated monthly by the Francis Severity score. | Treatment and placebo groups both improved significantly, but there was no difference between the two: IBS symptoms showed no significant improvement with probiotic treatments. | [27] |
| 104 children diagnosed with FAPD, IBS or FD. | L. rhamnosus GG (LGG, 3×109) or placebo. | 4 weeks. | Abdominal pain scores. | LGG treatment suggests moderate improvement in abdominal pain. | [28] |
| 141 children with IBS or functional pain. | Children received L. rhamnosus (LGG) or placebo. | 8 weeks. | Measured frequency and severity of abdominal pain and intestinal permeability. | ↓ frequency and severity of abdominal pain. | [29] |
| 50 children that meet Rome II criteria for IBS. | Children received L. GG (1×1010cfu) or placebo. | 6 weeks. | Measured IBS symptoms (abdominal pain, discomfort, diarrhea, constipation, bloating, flatulence) using GSRS. | ↓ incidence of perceived abdominal distention. | [70] |
| 298 patients diagnosed with IBS. | Symbioflor-2, an Escherichia coli product, or placebo. | 8 weeks. | IBS symptoms (abdominal pain and general symptom scores). | probiotic Symbioflor- 2 ↓ IBS symptoms. | [30] |
| 103 patients that met Rome I or Rome II criteria for IBS. | Probiotic mixture containing L. rhamnosus GG, B. breve Bb99 and Propionibacterium freudenreichii ssp. shermanii JS (8- 9×109 cfu/day) or placebo. | 6 months. | Gastrointestinal symptoms (abdominal pain, distension, postmeal distension, distension following sitting, flatulence, and borborygmi) and bowel habits (urgency, straining, feeling of incomplete evacuation, belching, heart burn, naseau, postmeal fullness, vomiting, and mucus or blood in stools). | ↓ abdominal pain, distension, flatulence, and borborygmi. | [31] |
| 86 IBS patients that met Rome II criteria. | Probiotic mixture containing L. rhamnosus GG, L. rhamnosus Lc705, Propionibacterium freudenreichii ssp. shermanii JS and B. animalis ssp. lactis Bb12 (1×107 cfu/ml) or placebo. | 5 months. | Measured IBS symptoms (abdominal pain, distension, flatulence, rumbling), quality of life, microarray-based intestinal microbiota stability, serum cytokines, and sensitive C-reactive protein. | ↓IBS symptoms, notably distension and abdominal pain; stabilization of intestinal microbiota. | [32] |
| 50 D-IBS patients. | Probiotic mixture L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, B. longum, and S. thermophilus 1010 cfu/ml. | 8 weeks. | IBS symptoms (abdominal pain, abdominal discomfort, loose/watery stool, urgency, mucus in stool, bloating, and passage of gas), stool parameters, quality of life, and fecal flora. | ↓ IBS symptoms when compared to placebo; stool consistency improved; stabilization of intestinal microbiota. | [33] |
| 24 diarrhea- predominant IBS patients. | Patients received the probiotic VSL#3 (9×1010 cfu/ml), or placebo. | 8 weeks. | IBS symptoms (pain, bloating, constipation, diarrhea, satiety, global score) using GSRS and quality of life questionnaires, in addition to fecal microbiota composition. | Improvements in GSRS scores; no effect on microbiota composition. | [34] |
| 25 diarrhea- predominant IBS patients. | Probiotic VSL#3 (5×107 cfu/ml) powder, or placebo. | 8 weeks. | Gastrointestinal transit, bowel function and daily symptoms (frequency of bowel function, consistency, ease of passage, abdominal pain, bloating, flatulence, urgency). | ↓ abdominal bloating scores. | [35] |
| 48 Rome II IBS patients. | Probiotic VSL #3 twice daily (450 billion cfu/ml); 31 patients took the doses for 4 weeks, and 17 took it for 8 weeks, or placebo. | 4 weeks and 8 weeks. | Colonic transit, IBS symptoms (stool consistency, ease of passage, sense of complete evacuation), and abdominal bloating. | ↓ flatulence; Colonic transit slowed. | [37] |
| 30 Rome III FC patients; 30 controls. | VSL#3: sachet contained 450 billion lyophilized bacteria: B. longum, B. infantis and B. breve, L. acidophilus, L. casei, L. bulgaricus, and L. plantarum and Streptococcus thermophilus; placebo. | 2 weeks. | Flora analysis performed by quantitative real-time polymerase chain reaction (qRT-PCR); FC relief. | Patients without FC treated with VSL#3 showed an increase in the fold differences in the previously lowered L., B. and Bacteroides species. Complete spontaneous bowel movements/week increased significantly in FC patients after being treated with VSL #3. | [36] |
| 59 children ages 4 to 18 years old with IBS. | VSL#3 (450 billion/sachet), or placebo. | 6 weeks, wash- out, 6 weeks. | IBS symptoms (abdominal pain/discomfort, bloating/gassiness, and family assessment of life disruption and stool pattern). | VSL#3 ↓ IBS symptoms, notably abdominal pain/discomfort, and bloating/gassiness. | [38] |
Abbreviations: IBS, Irritable Bowel Syndrome; FAPD, Functional Abdominal Pain Disorders; FD, Functional Dyspepsia; D-IBS, Diarrhea-dominant IBS; HRQoL, Health-Related Quality of Life; GSRS, Gastrointestinal Symptom Rating Scale; FC, Functional Constipation.
Similarly, fifty IBS patients were randomized into placebo or a multispecies probiotic product containing a mixture of L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, B. longum, and Streptococcus for a period of eight weeks [33]. Although the probiotic mixture did not ameliorate abdominal pain, it significantly improved adequate relief of IBS symptoms when compared to placebo over time.
VSL#3, a probiotic supplementation made of B. longum, B. infantis, B. breve, L. acidophilus, L. casei, L. bulgaricus, L. plantarum, and Streptococcus salivarius subspecies thermophiles has been largely investigated in several RCTs involving IBS patients. Eight weeks of VSL#3 improved bowel functions, including stool consistency, as assessed by the gastrointestinal symptom rating scale for IBS patients (GSRS-IBS) over placebo in a total of 23 IBS patients that fulfilled the Rome III criteria. However, the gut microbiota composition was not modified following treatment [34]. Kim and colleagues conducted three different studies in patients diagnosed with IBS according to the Rome II criteria. Eight weeks of VSL#3 treatment showed improvements in gastrointestinal symptoms over placebo, reducing flatulence score, abdominal pain, abdominal bloating and slowing colonic transit without altering bowel function [35–37]. Furthermore, fifty-nine children (age 4–15 years), selected in accordance to the Rome II criteria, reported gastrointestinal benefits after six weeks of VSL#3 treatment. Specifically, VSL#3 treatment effectively reduced abdominal pain/discomfort, abdominal bloating/gassiness, and family assessment of life disruption over placebo [38].
Prebiotics
Few studies have evaluated the efficacy of prebiotics in reversing IBS symptoms in a clinical setting (Table 2). One study showed the effects of fructoligosaccharides (FOS) in patients affected by minor functional bowel disorders (FBD; Rome II criteria). 105 IBS patients were randomized into two groups to receive either 5 g FOS or 5 g placebo over a six week period. At the end of the treatment period, the FOS group showed reduced incidence and intensity of gastrointestinal symptoms over placebo [39]. A smaller RCT investigated the effects of galactooligosaccharide (GOS) in 44 IBS patients fulfilling the Rome II criteria. Patients randomly received either 7 g/day placebo, 3.5 g/day GOS and 3.5 g/day placebo or 7 g/day GOS for 6 weeks. The two prebiotic concentrations differentially improved IBS symptoms. Specifically, GOS 3.5 g/day improved flatulence, bloating, the composite score of symptoms, as well as subjective global assessment (SGA). GOS 7 g/day significantly improved SGA and anxiety symptoms as assessed by hospital anxiety and depression scale (HADS) [40].
Table 2.
The effect of prebiotics in irritable bowel syndrome.
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 105 patients with minor FBD. | 5g of sc-FOS or 5g of placebo (sucrose and maltodextrins). | 6 weeks | Incidence and intensity of digestive disorders; quality of life. | sc-FOS ↓ intensity of digestive disorder symptoms ↑ quality of life, ↑ discomfort scores. | [39] |
| 44 patients with Rome II positive IBS. | 3.5g/d of GOS or placebo; 7g/d of GOS or placebo. | 12 weeks. | Faecal microflora, stool frequency, SGA form, anxiety, abdominal pain/bloating, rumbling, transit disorders, pain, flatulence and depression. | GOS 3.5 g/day improved flatulence, bloating, the composite score of symptoms, as well as SGA. GOS 7 g/day significantly improved SGA and anxiety symptoms as assessed by HADS. | [40] |
| 96 patients with IBS symptoms. | 20g FOS powder or placebo. | 12 weeks | IBS symptoms (abdominal distension, abdominal rumbling, abnormal flatulence, and abdominal pain). | Initially developed worse symptoms, but over time, the condition stabilized. | [71] |
Abbreviations: FBD, Functional Bowel Disorders; sc-FOS, short-chain Fructo-oligosaccharides; SGA, Subjective Global Assessment; GOS, Galacto-oligosaccharide; HADS, Hospital Anxiety and Depression Scale; qRT-PCR, quantitative Real-Time Polymerase Chain Reaction.
One of the complaints of prebiotic administration involves unwanted side effects associated with higher doses. For instance, daily treatment of 20 g/day FOS has been shown to worsen IBS symptoms (abdominal distension, abdominal rumbling, abnormal flatulence, and abdominal pain) compared to placebo during the first six weeks of treatment. However, continuous treatment for 12 weeks resulted in adaptation and no difference was found between groups [41].
Overall, the clinical evidence accumulated to date characterizing the improvements of gastrointestinal and extra-intestinal symptoms in patients with IBS following treatment with probiotics or prebiotics is limited. This is in part due to the small number of clinical studies, small sample size of the studies performed and placebo effect. Specifically, subjective psychological symptoms in addition to the use of subjective measures of gastrointestinal function in IBS patients may further impact the observed placebo effect. Nonetheless, the data presented suggests possible benefits of specific probiotics and prebiotics in patients with IBS and further studies are highly recommended.
Probiotics and prebiotics in mood disorders
Increasing evidence suggests that the microbiota is altered in patients with mood disorders, highlighting a role for the gut-brain axis in disease. With respect to treatment with either probiotics or prebiotics to modulate the microbiota, there is still a paucity of information that exists describing their effects on anxiety and depression (Table 3). One RCT investigated the effects of multispecies probiotic intervention on cognitive reactivity to sad mood in 40 healthy young students. Twenty participants were assigned to B. bifidum, B. lactis, L. acidophilus, L. brevis, L. casei, L. salivarius, and Lactococcus lactis multispecies probiotic supplementation, whereas 20 control participants received an inert placebo. After four weeks of treatment, the probiotic group showed reduced cognitive reactivity to sad mood (reduced aggressive thoughts and rumination) as assessed by the Leiden Index of Depression Sensitivity - Revised (LEIDS-R). Of note, eating habits were not reported in the study. Hence, it should be taken into account that the potential consumption of other probiotics or fermented foods may have affected the final outcomes of the study [42]. Another RCT (66 healthy volunteers) showed that thirty days of L. helveticus R0052 and B. longum R0175 were able to mitigate psychological distress over placebo. Indeed, the probiotic group scored significantly better in the Hopkins Symptom Checklist (HSCL-90), the Hospital Anxiety and Depression Scale (HADS), the Coping Checklist (CCL). Moreover, cortisol levels, used as an index of stress response, were also reduced, supporting subjects resilience to stress and improved emotional responses induced by probiotics [43, 44]. Similarly with prebiotics, a decrease in the neuroendocrine stress response in healthy subjects was observed after three weeks of Bimuno (B)-GOS treatment compared to placebo. Participants also demonstrated improved emotional attention as assessed by the attentional dot-probe task [45].
Table 3.
Probiotics and prebiotics in mood disorders.
| Participants | Treatment | Length of Trial | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| 40 healthy participants without current mood disorders. | Probiotic food-supplement containing B. bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, and Lactococcus lactis (W19 and W58) (2.5×109CFU/g); placebo. | 4 weeks. | Cognitive reactivity to sad mood using the LEIDS-R. | ↓ overall cognitive reactivity to sad mood by minimizing aggressive thoughts. | [42] |
| 25 subjects with UFC levels less than 50ng/ml. | L. helveticus R0052 and B. longum R0175 (PF). | 30 days. | HADS, and perceived stress. | ↑ HADS score, ↓ anger, depression and anxiety in the UFC patients | [44] |
| 45 healthy patients. | 5.5g of FOS, or B- GOS, or placebo (maldextrin). | 3 weeks. | Salivary cortisol awakening response before and after treatments; processing of emotionally salient information. | Salivary cortisol awakening response ↓ after treatment with B-GOS when compared to placebo. | [45] |
| 25 healthy women. | FMPP containing species B. animalis subsp Lactis, Streptococcus thermophiles, L. bulgaricus, and Lactococcus lactis subsp Lactis (1.2×109 cfu/cup) or a non- fermented milk product twice daily. | 4 weeks. | Brain response to an emotional faces attention task and resting brain activity. | ↓ task-related response of a distributed functional network containing affective, viscerosensory, and somatosensory cortices. FMPP treated women also showed changes in midbrain connectivity. | [46] |
| 172 students undergoing a day medical school exam. | Stressed medical students received LcS-fermented milk or placebo daily. | 8 weeks prior to exam. | Subjective anxiety scores, salivary cortisol levels, and the presence of physical symptoms during the intervention. | ↓ salivary cortisol levels; ↓ rate of physical symptoms. | [47] |
| 40 MDD patients. | Probiotic cocktail (L. acidophilus (2 × 109 CFU/g), L. casei (2 × 109 CFU/g), and B. bifidum (2 × 109CFU/g)); placebo. | 8 weeks. | Dietary intake, depression symptoms, insulin resistance, and glutathione concentrations. | ↓ BDI total scores, ↓ insulin levels and insulin resistance, and ↑ plasma total glutathione levels. | [48] |
| 39 CFS patients. | 24 × 1010 cfu/g of L. casei strain Shirota (LcS); placebo daily. | 2 months. | Flora in stool samples, BDI and BAI scores. | LcS ↑ in both L. and B. and ↓ anxiety symptoms. | [50] |
| 710 young adults (445 females). | Questionnaire on food consumption. | 30 days. | Patients completed self- report measures of fermented food consumption, neuroticism, and social anxiety. | Fermented food consumption correlates with reduced social anxiety and neuroticism. | [51] |
| 66 healthy individuals with <13 HADS-A, and <21 HADS-D scores. | Probiotic mixture containing L. helveticus R0052 and B. longum R0175 (PF). | 30 days | HSCL-90, HADS, PSS, CCL and 24 h UFC level. | Treatment with PF alleviated psychological distress, depression, anger, somatization, and anxiety. | [43] |
Abbreviations: LEID-S, Leiden index of depression sensitivity scale; UFC, Urinary Free Cortisol; HADS, Hospital Anxiety and Depression Scale; FOS, Fructooligosaccharide; B_GOS, Bimuno®-Galactooligosaccharides; FMPP, Fermented Milk Product with Probiotic; MDD, Major Depressive Disorder; BDI, Beck Depression Inventory; CFS, Chronic Fatigue Syndrome; BAI, Beck Anxiety inventory; HSCL-90, Hopkins Symptom Checklist; PSS, Perceived Stress Scale; CCL, Coping Checklist; HADS-A, HADS-anxiety subscale; HADS-D, HADS-depression subscale.
Probiotics have also been shown to beneficially modulate brain activity in female healthy subjects. Brain activity was evaluated in 23 women which following consumption of a fermented milk product containing B. animalis lactis, Streptococcus thermophiles, L. bulgaricus, and Lactococcus lactis (n = 12) or a non-fermented milk product (n = 11) for four weeks. Negative emotional faces, expressing fear and anger, were shown to the participants while brain activity was recorded by functional magnetic resonance imaging (fMRI). The activity of specific brain regions responsible for control of emotional processing, including the somatosensory cortex, the insula, and the periaqueductal gray, were markedly reduced by probiotic intake. The evidence sheds light on the ability of probiotics to modulate reactions to emotional stimuli in healthy individuals [46].
L. casei Shirota, in the form of fermented milk, was evaluated for stress resilience in a double blind placebo-controlled trial. 137 healthy medical students underwent daily treatment of probiotics over eight weeks prior to academic examination. Salivary cortisol levels, physical symptoms and a self-reported anxiety score were analysed. Interestingly, L. casei Shirota attenuated the stress-induced increases in cortisol levels one day before examination and reduced the presence of physical symptoms along the last week of treatment [47].
In a study of major depressive disorder (MDD), 40 patients, as classified by the diagnostic and statistical manual of mental disorders (DSM)-IV criteria, were randomly assigned to eight weeks of either L. acidophilus, L. casei, and B. bifidum probiotics supplementation or placebo. Interestingly, probiotic intervention significantly ameliorated the severity of depression as assessed by the Beck Depression Inventory (BDI) over placebo [48]. Similarly, the probiotic L. casei Shirota was evaluated in patients with chronic fatigue syndrome (CFS). CFS patients often manifest symptoms of depression and mood disorder [49]. CFS patients (n=39) were randomly assigned to eight weeks of probiotic treatment or placebo. Anxiety, but not depressive symptoms, was significantly reduced by L. casei intake as assessed by the Beck Anxiety Inventory (BAI) and BDI, respectively [50].
One recent study has assessed the effects of consumption of probiotics in subjects genetically predisposed to develop social anxiety. Fermented food consumption, neuroticism and social anxiety were self-reported by 715 participants (445 female). Social anxiety was significantly and independently predicted by exercise frequency, neuroticism, and fermented food consumption. Specifically, social anxiety symptoms were reduced by the consumption of probiotics in subjects at higher genetic risk of social anxiety, as indexed by high neuroticism traits [51].
Despite these promising findings to date, there is still limited evidence for the efficacy of probiotics and prebiotics intervention in patients with mood disorders. Additional clinical trials are critical prior to making any conclusions on the efficacy of probiotics and prebiotics in mental health.
Probiotics and prebiotics: gut-brain interaction
The crosstalk between the gut and brain has revealed a complex communication system, which is responsible for maintaining a proper gastrointestinal homeostasis as well as affecting emotional mood and cognitive functions. Although the gut-brain axis has been one of the main focuses of research in the last decades, the understanding of the role of the microbiota in modulating this signaling pathway is still at its infancy, (Table 4). One of the most prominent studies in the field, conducted by Bravo and colleagues, revealed the vagus nerve as one of the principal routes of communication between the two systems. Indeed, the observed benefits exerted by the chronic administration of L. rhamnosus JB-1 in reducing anxiety- and depressive-like behaviors were vanished after vagotomy in mice. At the brain level, alterations in the expression of GABA receptors by probiotics in specific areas involved in the pathogenesis of anxiety, and depression disappeared in vagotomized mice [52]. Similarly, the administration of B. longum in an animal model of chronic colitis induced by dextran-sodium sulfate (DSS) failed to reduce anxiety-like behavior in those mice that underwent vagotomy prior to induction of colitis [53].
Table 4.
The effect of probiotics and prebiotics in animal behaviour and neurochemistry.
| Animals | Treatment | Length of treatment | Measurements | Outcomes | References |
|---|---|---|---|---|---|
| BALB/c mice. | L. rhamnosus (JB- 1) 109 cfu, and vagotomy. | 28 days. | GABA (B1b and Aα2) mRNA expression in cortical regions, hippocampus, amygdala, and locus coeruleus. SIH, EPM, FST behavioural tests. | ↓ GABAB1b in the Hippocampus, locus coeruleus, and amygdala; ↓ GABAAα2 in prefrontal cortex and amygdala, ↑ in hippocampus; ↓ stress-induced corticosterone and anxiety and depression related behavior in a manner that is dependent on the vagus nerve. | [52] |
| C57BL/6 mice. | Probiotic mixture containing L. rhamnosus and L. helveticus (1010cfu/ml) one week prior to DSS treatment. | DSS 5 days; probiotics mixture 15 days. | Light/dark box, recognition memory, microbiota composition. | Probiotics reversed DSS-induced ↓ in recognition memory, ↑ anxiety-like behavior, and disrupts microbiota composition. | [14] |
| Sprague- Dawley rat pups. | MS rat pups received L. helveticus and L. rhamnosus (108cfu). | PND 4 to PND 19. | Corticosterone plasma levels, colonic function (ion transport (ISC), bacterial adherence/penetration, and macromolecular permeability (HRP)) and host defence. | MS ↑ colon ISC and HRP flux, ↑ adhesion/penetration of total bacteria and ↓ of L. species. Probiotic ↓ gut function abnormalities and bacterial adhesion/penetration and ↓ corticosterone levels in the MS group. | [56] |
| Female Wistar rats. | L. farciminis for 2 weeks, then were administered ML- 7 (myosin light chain kinase inhibitor), followed by antibiotic administration in drinking water. | 26 days. | ACTH and corticosterone plasma levels, hypothalamic CRF, pro- inflammatory cytokine mRNA expression, and CPP. | L. farciminis and ML-7 ↓ stress-induced hyperpermeability, endotoxemia and prevented HPA axis stress response and neuroinflammation. | [55] |
| B and T cell deficient Rag1 (−/−) mice. | Probiotic mix containing L. rhamnosus and L. helveticus (6×109cfu/ml). | 4 weeks. | NOR, light/dark box tests, hippocampal c-Fos expression, corticosterone plasma levels, intestinal physiology, intestinal secretory state. | Probiotic reversed Rag1 (−/−)-induced cognitive impairment, anxiety-like behaviour, overactive HPA axis, ↑ secretory state, dysbiosis, and ↓ hippocampal c-Fos expression. | [57] |
| C57BL/6 mice. | L. rhamnosus CNCM I-3690 or L. paracasei, and DiNitroBenzene Sulfonic (DNBS). | 10 days. | Barrier permeability, NF- kB expression, and IL-6, IL-4, IFN-y levels. | L. rhamnosus ↓ gut permeability, ↓ NF-kB expression, and ↓ cytokine production induced by DNBS. | [54] |
| BALB/c mice. | L. casei, 108cfu/m4 + acute stress (restrain stress). | 11 days | CD4+ and CD8+ T lymphocytes, CD11b+ macrophages, CD11c+ dendritic cells, IgA+ B lymphocytes, IgA and interferon gamma levels. | L. casei ↑ IgA producing cells, CD4+ cells in the lamina propria of the small intestine, and S-IgA in the lumen. | [58] |
| CD1 mice. | B-GOS + LPS injection. | 3 weeks. | 5HT2A, 5HT1A, NMDA receptors and IL-1B cortical expression levels. Marble burying, light- dark box. | B-GOS ↓ anxiety-like behavior, altered cortical 5HT2A levels. | [60] |
| C57BL/6J mice. | FOS, GOS, or the combination of the two. | 3 weeks. | Plasma corticosterone, microbiota composition, cSCFA. FUST, FST, tail suspension, 3-chambered social approach task, resident-intruder test. | GOS+FOS induced antidepressant and anxiolytic effects. GOS, and GOS+FOS ↓ stress- induced corticosterone release. GOS+FOS altered hippocampal Gabbr1, Gabbr2, BDNF, Grin2a mRNA levels, ↑ cecal acetate and propionate, and ↓ isobutyrate concentrations. | [59] |
| Sprague- Dawley rats. | MS Rats were treated with B. infantis or citalopram. | ~40 days. | FST, cytokine concentrations, monoamine levels in the brain, and HPA axis measures. | Probiotic reversed MS- induced depressive- like behavior, ↓ NA in the brain stem, ↑ plasmatic IL-6 and CRF mRNA in the amygdala. | [61] |
| Balb/c mice, Sprague- Dawley rats. | L. acidophilus NCFM. | 15 days. | Analgesic receptors: mu-, kappa-, and delta-opiod receptors, and CB2A receptors. | L. acidophilus alters expression of μ-opioid and cannabinoid receptors in intestinal epithelial cells. | [62] |
| F344 rats. | L. casei strain Shirota (LcS) (109cfu/ml) + WAS | 2 weeks. | Plasma corticosterone, cFos and CRF expressions in the paraventricular nucleus (PVN). | Lcs reversed WAS- induced ↑ corticosterone and CRF in the PVN. | [47] |
Abbreviations: DSS, Dextran Sodium Sulfate; SIH, Stress-induced Hyperthermia; EPM, Elevated Plus Maze; OF, Open Field; SP, Sucrose Preference; FST, Forced Swim Test; WAS, Water Avoidance Stress; MS, Maternal Separation; PND, Post Natal Day; ACTH, Adrenocorticotropic Hormone; CRF, Corticotropin Releasing Factor; CPP, Colonic Paracellular Permeability; LPS, lipopolysaccharide; FUST, Female Urine Sniffing Test; cSCFA, cecal Short-Chain Fatty Acids.
Another potential route through which the gut and the brain can interface is the immune system. Stress and bacterial antigens can increase the immune response to the luminal environment, inducing cytokine release in the blood stream. Although it is not clear yet whether cytokines can cross the blood brain barrier (BBB) under these conditions, increasing evidence indicates that cytokines can influence brain areas, such as the hypothalamus, where the BBB is deficient. This is considered by most a plausible explanation of the observed activation of the hypothalamic-pituitary-adrenal (HPA) axis induced by pro-inflammatory cytokines and consequently corticosterone release, which is the most potent activator of the stress system. A number of probiotics belonging to the lactobacillus and bifidobacterium families have demonstrated the capability to restore corticosterone and adrenocorticotropic hormone (ACTH) plasma concentration as well as reduce hypothalamic corticotropin-releasing factor (CRF), interleukin (IL) -6 and tumour necrosis factor (TNF) -α levels, which were altered by both chronic stress and following increases in colonic permeability [54–57]. One study showed that L. casei reinforced the immune system of adult mice subjected to chronic stress, increasing IgA producing cells, CD4+ cells in the lamina propria of the small intestine, and secreted IgA in the lumen as well as reducing the levels of IFN-γ [58]. Similarly, administration of the prebiotics FOS and GOS demonstrated attenuation of stress-induced corticosterone levels, reduction of plasma proinflammatory cytokines levels and decreased anxiolytic-like behaviour in rodents [59, 60].
In addition, evidence exists suggesting that probiotics can interact with the brain-gut axis through the regulation of neurotransmitters signalling. Specifically, B. infantis has been shown to regulate the serotonin (5-HT) system through the elevation of plasma tryptophan, a precursor of 5-HT [61]. It was also demonstrated that L. acidophilus may modulate intestinal dysfunction by inducing the expression of μ-opioid and cannabinoid receptors in intestinal epithelial cells [62]. Several bacterial species can also directly produce neurotransmitters such as 5-HT, γ-aminobutyric acid (GABA), dopamine and acetylcholine in the lumen of the intestine [63]. However, it is believed that these neurotransmitters may have an indirect influence on brain function acting on the enteric nervous system, rather than crossing the BBB, which is highly unlikely [2].
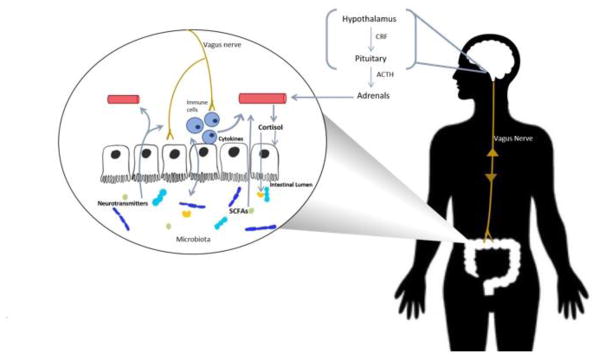
Bacterial metabolites represent another pathway of communication between the gut and the brain. One of the key product of bacterial metabolism is short-chain fatty acids (SCFAs) including butyric acid, propionic acid and acetic acid. SCFAs have been shown to have a central effect, mostly influencing memory and learning processes through histone deacetylases modulation in rodents [64, 65]. SCFAs can also control body energy expenditure and maintain metabolic homeostasis binding specific G-protein-coupled receptors such as, GPR41 [66]. Probiotics have been shown to modify SCFAs levels in both healthy adults [67] and patients affected by ulcerative colitis [68]. However, to our knowledge there is a lack of evidence supporting the assumption of a strong correlation between probiotics and prebiotics, and the levels of SCFAs in either IBS or mood disorders [69]. Despite all the advances described above, our understanding of the mechanisms that outline the gut-brain communication urges to be further developed. A schematic representing the routes of communications between the gut and the brain is shown in Figure 1.
Figure 1. Gut-brain axis: routes of communication.
Schematic representing how probiotics and prebiotics orchestrate the gut-brain axis crosstalk.
Conclusions
The impact of the gut microbiome on health, including mental health, is now a frontier research area that has caught the attention of the scientific community in a degree that has been rarely seen in other areas of science. The enthusiasm generated by a considerable number of preclinical data and supported by a growing clinical literature makes the microbiota an appealing therapeutic target for the prevention and treatment of various human diseases. In this review, we have provided evidence of the impact of probiotics and prebiotics treatments in both IBS and mood disorders such as, anxiety and depression. Moreover, we have attempted to highlight the currently known routes of communication of the gut-brain axis. The evidence compiled in this review indicates that treatments with probiotics and prebiotics can ameliorate some of the symptomatology that characterize both IBS and mood disorders such as, anxiety and depression. Despite that, the evidence base is still limited, such as the small number of clinical studies, study design, size of the studies, and placebo effect have been highlighted throughout this work. Further clinical studies are required to shed light on the possible therapeutic effect exerted by both probiotics and prebiotics in health and mental health.
Acknowledgments
This work was supported by NIH 1R01AT009365-01 (MGG), 5R21MH108154-01 (MGG).
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Matteo Pusceddu, Kaitlin Murray, and Melanie Gareau declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1••.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164(3):337–40. doi: 10.1016/j.cell.2016.01.013. This study provides the most updated description of the ratio between the number of bacteria and human cells in our bodies. [DOI] [PubMed] [Google Scholar]
- 2.Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 4.Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 5.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 7.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 8.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–12. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–42. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 10.Gareau MG. Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol. 2014;817:357–71. doi: 10.1007/978-1-4939-0897-4_16. [DOI] [PubMed] [Google Scholar]
- 11.Gareau MG. Cognitive Function and the Microbiome. Int Rev Neurobiol. 2016;131:227–46. doi: 10.1016/bs.irn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Moloney RD, Johnson AC, O’Mahony SM, et al. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci Ther. 2016;22(2):102–17. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pusceddu MM, El Aidy S, Crispie F, et al. N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS One. 2015;10(10):e0139721. doi: 10.1371/journal.pone.0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emge JR, Huynh K, Miller EN, et al. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G989–98. doi: 10.1152/ajpgi.00086.2016. [DOI] [PubMed] [Google Scholar]
- 15.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–14. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 17••.Kelly JR, Borre Y, COB, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. doi: 10.1016/j.jpsychires.2016.07.019. This study shows that faecal microbiota transplantation from depressed patients into rodents can induce certain features characteristic of depression in the recipient animals. [DOI] [PubMed] [Google Scholar]
- 18.Singh VP, Sharma J, Babu S, et al. Role of probiotics in health and disease: a review. J Pak Med Assoc. 2013;63(2):253–7. [PubMed] [Google Scholar]
- 19.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 20.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. The American journal of gastroenterology. 2006;101(7):1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal A, Houghton LA, Morris J, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Alimentary pharmacology & therapeutics. 2009;29(1):104–14. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 22.Guyonnet D, Chassany O, Ducrotte P, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Alimentary pharmacology & therapeutics. 2007;26(3):475–86. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC gastroenterology. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World journal of gastroenterology. 2012;18(30):4012–8. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nobaek S, Johansson ML, Molin G, et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. The American journal of gastroenterology. 2000;95(5):1231–8. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 26.Sinn DH, Song JH, Kim HJ, et al. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Digestive diseases and sciences. 2008;53(10):2714–8. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 27.Niv E, Naftali T, Hallak R, et al. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clinical nutrition. 2005;24(6):925–31. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Gawronska A, Dziechciarz P, Horvath A, et al. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Alimentary pharmacology & therapeutics. 2007;25(2):177–84. doi: 10.1111/j.1365-2036.2006.03175.x. [DOI] [PubMed] [Google Scholar]
- 29.Francavilla R, Miniello V, Magista AM, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126(6):e1445–52. doi: 10.1542/peds.2010-0467. [DOI] [PubMed] [Google Scholar]
- 30.Enck P, Zimmermann K, Menke G, et al. Randomized Controlled Treatment Trial of Irritable Bowel Syndrome with a Probiotic E.-coli Preparation (DSM17252) Compared to Placebo. Zeitschrift fur Gastroenterologie. 2014;52(1):64. doi: 10.1055/s-0034-1366796. [DOI] [PubMed] [Google Scholar]
- 31.Kajander K, Hatakka K, Poussa T, et al. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Alimentary pharmacology & therapeutics. 2005;22(5):387–94. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 32.Kajander K, Myllyluoma E, Rajilic-Stojanovic M, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Alimentary pharmacology & therapeutics. 2008;27(1):48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 33.Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Journal of clinical gastroenterology. 2012;46(3):220–7. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 34.Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double-blind, Placebo-controlled Trial of VSL#3 in Diarrhea-predominant Irritable Bowel Syndrome. Probiotics and antimicrobial proteins. 2011;3(1):1–7. doi: 10.1007/s12602-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2003;17(7):895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim SE, Choi SC, Park KS, et al. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients With Functional Constipation. Journal of neurogastroenterology and motility. 2015;21(1):111–20. doi: 10.5056/jnm14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2005;17(5):687–96. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 38.Guandalini S, Magazzu G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. Journal of pediatric gastroenterology and nutrition. 2010;51(1):24–30. doi: 10.1097/MPG.0b013e3181ca4d95. [DOI] [PubMed] [Google Scholar]
- 39.Paineau D, Payen F, Panserieu S, et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. The British journal of nutrition. 2008;99(2):311–8. doi: 10.1017/S000711450779894X. [DOI] [PubMed] [Google Scholar]
- 40.Silk DB, Davis A, Vulevic J, et al. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2009;29(5):508–18. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 41.Olesen M, Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. The American journal of clinical nutrition. 2000;72(6):1570–5. doi: 10.1093/ajcn/72.6.1570. [DOI] [PubMed] [Google Scholar]
- 42.Steenbergen L, Sellaro R, van Hemert S, et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. 2011;105(5):755–64. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 44.Messaoudi M, Violle N, Bisson JF, et al. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–61. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt K, Cowen PJ, Harmer CJ, et al. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 2015;232(10):1793–801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–401. 401 e1–4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Takada M, Nishida K, Kataoka-Kato A, et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2016;28(7):1027–36. doi: 10.1111/nmo.12804. This study shows that probiotics play a pivotal role in the regulation of the stress response both in rats and humans. [DOI] [PubMed] [Google Scholar]
- 48.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–20. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Griffith JP, Zarrouf FA. A systematic review of chronic fatigue syndrome: don’t assume it’s depression. Prim Care Companion J Clin Psychiatry. 2008;10(2):120–8. doi: 10.4088/pcc.v10n0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilimire MR, DeVylder JE, Forestell CA. Fermented foods, neuroticism, and social anxiety: An interaction model. Psychiatry Res. 2015;228(2):203–8. doi: 10.1016/j.psychres.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 52.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23(12):1132–9. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laval L, Martin R, Natividad JN, et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. 2015;6(1):1–9. doi: 10.4161/19490976.2014.990784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–95. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Gareau MG, Jury J, MacQueen G, et al. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–8. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CJ, Emge JR, Berzins K, et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2014;307(8):G793–802. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palomar MM, Maldonado Galdeano C, Perdigon G. Influence of a probiotic lactobacillus strain on the intestinal ecosystem in a stress model mouse. Brain Behav Immun. 2014;35:77–85. doi: 10.1016/j.bbi.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Burokas A, Arboleya S, Moloney RD, et al. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 60.Savignac HM, Couch Y, Stratford M, et al. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-beta levels in male mice. Brain Behav Immun. 2016;52:120–31. doi: 10.1016/j.bbi.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desbonnet L, Garrett L, Clarke G, et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–88. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature medicine. 2007;13(1):35–7. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 63.Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:3–24. doi: 10.1007/978-1-4939-0897-4_1. [DOI] [PubMed] [Google Scholar]
- 64.Levenson JM, O’Riordan KJ, Brown KD, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 65.Ferrante RJ, Kubilus JK, Lee J, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23(28):9418–27. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108(19):8030–5. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrario C, Taverniti V, Milani C, et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144(11):1787–96. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 68.Kato K, Mizuno S, Umesaki Y, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Alimentary pharmacology & therapeutics. 2004;20(10):1133–41. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 69.Distrutti E, Monaldi L, Ricci P, et al. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World journal of gastroenterology. 2016;22(7):2219–41. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. The Journal of pediatrics. 2005;147(2):197–201. doi: 10.1016/j.jpeds.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 71.Segal HE, Gresso WE, Thiemanun W. Longitudinal malaria studies in rural Northeast Thailand. Chloroquine treatment of falciparum malaria infections. Trop Geogr Med. 1975;27(2):160–4. [PubMed] [Google Scholar]