Abstract
Universal screening for Lynch syndrome (LS) among all cases of colorectal cancer (CRC) could increase the diagnosis of LS and reduce morbidity and mortality of LS-associated cancers. Given universal screening includes all patients, irrespective of high risk factors such early age at onset or family history of CRC, it is important to understand perspectives of all patients and not just those at high risk. As part of a study to assess the feasibility and implementation of universal screening, 189 patients newly diagnosed with CRC were surveyed about their interest in screening for LS and communication of results with at-risk family members. Overall, participants responded positively regarding screening for LS, with most wanting to know their genetic risks in general (86%) and risk of hereditary CRC (93%). Prior to receiving screening results, most participants stated they intended to share their screening results with parents (89%), siblings (96%), and children (96%). Of the 28 participants who received a positive LS screening result, 26 (93%) reported sharing their result with at least one first-degree family member. Interest in screening for LS and communication of screening results with family members was not associated with high risk factors. This study indicates that patients are interested in being screened for LS and that sharing information on the risk of LS with at-risk family members is not a significant barrier. These findings provide novel insight into patient perspectives about screening for LS and can guide successful implementation of universal screening programs.
Keywords: Lynch syndrome, HNPCC, colorectal cancer, genetic screening, genetic testing, microsatellite instability
INTRODUCTION
Lynch syndrome is an autosomal dominant cancer disorder that accounts for roughly 3% of colorectal cancer (CRC) cases and 2% of endometrial cancer cases.[1] Lynch syndrome is due to germline pathogenic variants in the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6, or PMS2.[2, 3] Deletions in EPCAM, which result in methylation of MSH2, are also associated with Lynch syndrome.[4] Patients with Lynch syndrome have an estimated lifetime risk of CRC of 30–75% for men and 25–50% for women; women also have an estimated 30–40% lifetime risk of endometrial cancer.[5] In addition, patients with Lynch syndrome are at an increased risk for other cancers, including those of the stomach, ovary, small bowel, and urinary tract.[6] The morbidity and mortality rates of Lynch syndrome-associated cancers can be moderated by more frequent cancer surveillance protocols and risk-reducing surgery.[5, 7] However, implementing such interventions relies on adequate diagnosis of individuals and families with Lynch syndrome.
Typically, assessment for Lynch syndrome involves an initial screen of tumor tissue for the presence of MMR deficiency, either via testing for microsatellite instability (MSI) or immunohistochemistry (IHC) to detect the absence of one or more of the MMR proteins.[1] Patients with an MMR-deficient tumor in the absence of a somatic cause (e.g., BRAF mutation or MLH1 promoter hypermethylation) can then undergo genetic counseling and germline testing to confirm a diagnosis of Lynch syndrome.[1]
Selective criteria based on factors such as a family history or early age of onset (prior to age 50) of CRC or other Lynch syndrome-associated cancers have been used previously to limit Lynch syndrome screening only to high risk groups.[8–11] Risk assessment algorithms such as PREMM1,2,6, MMRpro, and MMRpredict are tools to support selective screening programs.[12–14] However, selective screening will miss cases of Lynch syndrome for reasons such as limited or unreliable family history information or because not all individuals with Lynch syndrome meet high risk criteria.[15, 16] Moreover, not all individuals who meet high risk criteria undergo evaluation for Lynch syndrome.[17–21]
Alternatively, universal screening of all newly diagnosed cases of CRC, regardless of age of onset and family history, is an approach recommended by multiple expert guidelines to increase the identification of Lynch syndrome cases.[22–26] Some guidelines also recommend screening all newly diagnosed cases of endometrial cancer.[27] However, the effectiveness of any screening protocol relies on patient response to receiving screening information, including following-up with genetic counseling and testing to confirm a diagnosis of Lynch syndrome, and, for those diagnosed with Lynch syndrome, sharing this information with at-risk relatives so they too can undergo genetic testing.
This study assessed perspectives on screening for Lynch syndrome among a population of CRC cases not selected based on high-risk factors. Surveys were used to assess patients’ interest in obtaining information on genetic risk and screening for Lynch syndrome. In addition, patients were asked regarding their intent to share their MSI screening results with at-risk relatives and motivations and potential barriers to this communication. Though the ultimate goal of universal screening is to confirm cases of Lynch syndrome among those that screen positive and communicate those results to at-risk relatives, understanding communication with at-risk relatives among all newly diagnosed patients with CRC, regardless of screening result, provides a general sense of whether family communication will likely pose as a potential barrier among an unselected population. Understanding patient perspectives on screening and potential barriers in family communication, as well as patient characteristics that might be associated with these outcomes (e.g., sex/gender or age), will guide the successful implementation of universal screening programs.
MATERIALS AND METHODS
Recruitment
Kaiser Permanente Northwest (KPNW) is an integrated health care system that covers roughly 550,000 members in northwest Oregon and southwest Washington. Study recruitment has been described previously.[28] Briefly, study participants were KPNW members newly diagnosed with CRC who had been scheduled for surgical treatment from January 2012 through December 2015. Inclusion criteria were an age of 18 years or older, English-speaking, no known cognitive impairment (e.g., Alzheimer disease), not in hospice, no prior contact with the genetics department for screening for hereditary CRC screening, and no prior screening for or diagnosis of Lynch syndrome. Eligible patients who consented to participate were enrolled and a tumor sample was submitted for a screening test for MSI. Tumors with high MSI (MSI-H) were submitted for IHC testing for the absence of MMR protein expression and, when relevant, MLH1 hypermethylation and BRAF variant analysis to rule out sporadic cases. Germline mutation analysis of genes lacking expression based on IHC results was performed in cases lacking MLH1 hypermethylation and the BRAF V600E variant. Participants also completed questionnaires regarding MSI screening and Lynch syndrome. The protocols and consent forms for recruitment were approved by the Institutional Review Board at KPNW.
Surveys and Genetic Counseling
Two surveys were administered to assess participants’ attitudes and perspectives on screening for Lynch syndrome. In addition, participants with a screening result of high MSI (MSI-H) had a session with a genetic counselor.
Survey 1 assessed patients’ attitudes towards MSI screening for Lynch syndrome prior to receiving their MSI results. Patients were asked about their knowledge of screening for Lynch syndrome prior to enrollment in the study and to choose between three responses: “almost nothing,” “a fair amount,” or “a lot.” Participants were then asked to respond to a variety of questions regarding their attitudes on genetic screening and testing, interest in understanding why they developed CRC, perceived barriers and benefits to Lynch syndrome screening, and perceived susceptibility to Lynch syndrome. Responses to these questions were coded using a 5-point Likert-type scale (“strongly disagree,” “disagree,” “neither agree nor disagree,” “agree,” and “strongly agree”). For analyses, these responses were consolidated to a 3-point scale, combining “strongly disagree” with “disagree” and “strongly agree” with “agree.” Participants were also asked to indicate whether they planned to share their results with family members. The responses were coded as a 5-point Likert-type scale (“not at all likely,” “neither likely nor unlikely,” “somewhat likely,” “very likely,” and “not applicable”). For analyses, these responses were consolidated to a 3-point scale, combining “somewhat likely” with “very likely.” At this time, participants were also administered the Genetic Risk Easy Assessment Tool (GREAT) to obtain information from patients regarding cancer types and age of diagnosis of themselves and relatives and create an extended pedigree.[29]
All participants with a result of MSI-H were contacted by a genetic counselor to discuss the screening result, provide information on Lynch syndrome, and offer additional testing to confirm or exclude a diagnosis of Lynch syndrome. Participants were also told that if they did have Lynch syndrome, their first-degree family members would have a 50% risk of also having the syndrome and could also receive genetic counseling and testing through their healthcare provider. Participants who received an MSI-H result were also offered additional screening by genetic counselors to confirm a diagnosis of Lynch syndrome. Those who underwent germline genetic testing and were found to have a pathogenic variant associated with Lynch syndrome had genetic counseling sessions in the Genetics department to discuss the results and to facilitate the communication of these results with their at-risk relatives.
Survey 2, given to all participants who received an MSI-H result, was administered 4–6 months after the return of screening results. The goal was to assess patients’ perspectives and communication with family members in response to receiving a positive screening result for Lynch syndrome. Participants were asked whether they shared their screening results with at-risk relatives, including parents, siblings, and children. Those who did were asked if they are aware of specific actions that their relatives had taken (“saw a medical provider,” “made family planning decisions,” “got colon cancer screening,” “got other cancer screening,” and “sought genetic counseling”). Patients were also asked how important certain reasons were for wanting (or not wanting) to share their screening results with family members; responses to these questions were coded on a 5-point Likert-type scale (“not at all important,” “slightly important,” “somewhat important,” “important,” or “very important”). For analyses, these responses were consolidated to a 3-point scale, combining “very important” with “important” and combining “slightly important” with “somewhat important.”
The surveys were based on existing questions in the literature and were pilot tested in the beginning of the study among a small number of patients who had been scheduled for a colonoscopy procedure in order to obtain the perspectives of potential participants. Revisions to the wording and order of the questions were made based on feedback during this pilot testing.
Statistical Analysis
In primary analyses, we assessed whether patient demographic characteristics (age at the time of survey, self-reported sex, annual household income, level of education, and self-reported race/ethnicity) were predictive of survey responses. Age was applied as a continuous variable; the remaining predictors were categorical. Survey responses with dichotomous responses were modeled using logistic regression. For surveys with three possible responses (e.g., “strongly agree” or “agree”, “strongly disagree” or “disagree”, and “neither agree nor disagree”) ordered logistic regression was applied. If the odds were not proportional across all levels of responses, a multinomial logistic regression was modeled. To account for multiple testing, a p-value of 0.01 was used as the threshold to indicate statistical significance. Thus, the results of these models are presented as odds ratios (ORs) and 99% confidence intervals (CIs).
In secondary analyses, we explored whether patient clinical characteristics and family history were predictors of survey responses. These predictors were added individually to the models described above. Clinical characteristics included the American Joint Committee on Cancer (AJCC) stage of the current CRC occurrence and any prior cancer diagnosis (CRC or any other cancer, not including non-melanoma skin cancer). Prior cancer diagnoses were obtained from both self-report by participants and diagnoses recorded by the KPNW tumor registry. Family-specific variables included a family history of CRC (defined as the number of first-, second-, and third-degree relatives diagnosed with CRC at any age as reported by the participant), the number of living children, and the number of all living first-degree relatives. We also considered additional factors such as perceived risk of Lynch syndrome and number of benefits and barriers to screening for Lynch syndrome endorsed by participants, as described previously.[28] Briefly, 85% of participants endorsed 6 or more of 8 potential benefits (e.g., “I want to know that I have a possible risk of hereditary CRC,” “I would like my doctor to have access to the most up to date technology,” and “I would like to learn new information that could benefit my family”), while 89% endorsed 4 or fewer of 9 potential barriers (“I do not trust modern medicine,” “I am worried about losing my health insurance,” and “I do not have a family history of CRC,”) of screening for Lynch syndrome. SAS V9.1 was used for all statistical analyses.
RESULTS
Study Population
The study population in the current analysis consisted of 189 participants who consented to participate and completed Survey 1 (Figure 1; Table 1). Of those with a definitive MSI result, 76.7% (n=125) had a result of low or stable MSI and 23.3% (n=38) had a result of MSI-H. The remaining 26 patients did not have a tumor sample sufficient for an MSI screening test. Of the 38 patients with a result of MSI-H, 2 died before Survey 2 could be administered, 4 could not remember receiving their MSI screening results and therefore could not complete Survey 2 and 4 were lost to follow-up. Thus, we obtained Survey 2 data for 28 participants with a result of MSI-H.
Figure 1.
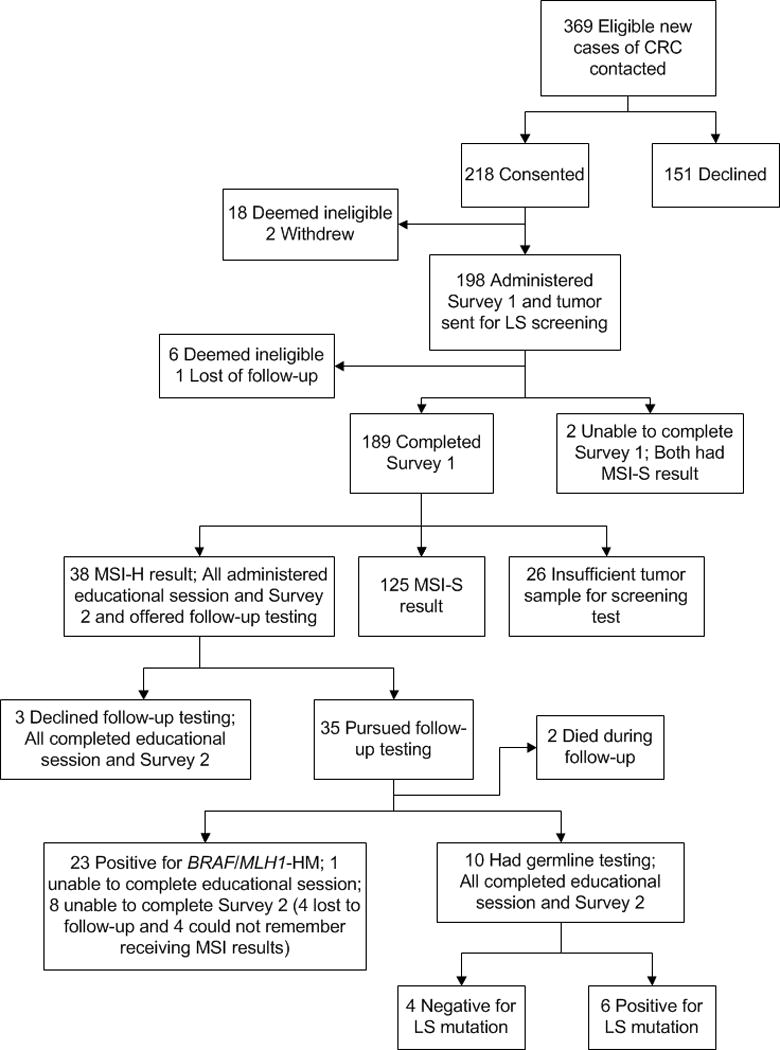
Study workflow
CRC=Colorectal cancer; MSI-H=MSI high result; MSI-S=MSI stable result; MLH1-HM=MLH1 hypermethylation; LS=Lynch syndrome
Table 1.
Demographic information of study participants (N=189)
| Variable | Categories | N (%) |
|---|---|---|
|
| ||
| Sex/Gender | Male | 112 (59) |
| Female | 77 (41) | |
|
| ||
| Age at Survey | Mean (SD) | 66 (12) |
| Range | 25–90 | |
|
| ||
| Race/Ethnicity | White, Non-Hispanic | 155 (82) |
| Other | 34 (18) | |
|
| ||
| Household Incomea | < $40,000 | 60 (33) |
| $40,000-$59,999 | 38 (21) | |
| $60,000-$79,999 | 24 (13) | |
| >$80,000 | 58 (32) | |
|
| ||
| Level of Educationb | Less than high school | 5 (3) |
| High school degree or GED | 39 (21) | |
| Some college | 67 (36) | |
| College degree | 38 (20) | |
| Masters or doctorate degree | 38 (20) | |
Information on household income was missing for 9 participants: 4 who did not know their income and 5 who refused to respond
Information on level of education as missing for 2 participants
Most participants (n=112, 61.6%) had a diagnosis of Stage I or II CRC cancer, 38 (20.2%) had a prior diagnosis of any cancer, and 3 (1.6%) had a prior diagnosis of CRC specifically. Due to the low number of prior CRC diagnoses, this variable was not used as a predictor in subsequent statistical models.
Prior Knowledge of MSI Screening
Five participants (2.7%) had any prior knowledge regarding an MSI screening test for Lynch syndrome. Prior knowledge was not statistically significantly associated with any demographic, clinical, or family history variables.
Attitudes about Genetic Screening and Screening for Lynch Syndrome
Overall, participants responded positively to receiving information on genetic risk (Figure 2). Specifically, most wanted to be screened for genetic conditions (n=138, 73.8%) and learn their genetic risks (n=161, 85.6%). In addition, most participants wanted know if they were at risk of hereditary CRC (n=175, 92.6%) and to understand why they developed CRC (n=174, 93.0%).
Figure 2.
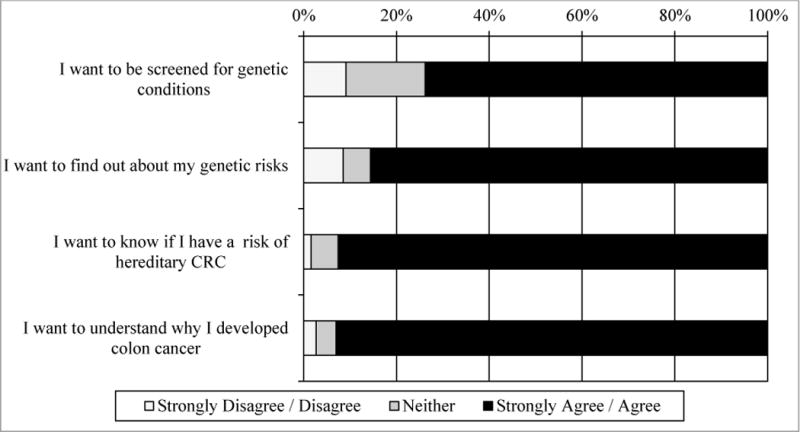
Patient attitudes on genetic screening
Attitudes regarding screening were associated with perceived risk for Lynch syndrome. Participants who worried that they carried a mutation for Lynch syndrome were more likely to want to be screened for genetic conditions (OR=1.94, 1.08–3.49). Not surprisingly, participants who endorsed more benefits of Lynch syndrome screening were more likely to want to be screened for genetic conditions (OR=4.40, 2.56–7.57), find out about their genetic risks (OR=4.18, 2.16–8.09), know their risk of hereditary CRC (OR=5.37, 2.02–14.31), and understand why they developed CRC (OR=2.25, 1.33–3.82). Conversely, participants who endorsed more barriers to Lynch syndrome screening were less likely to want to be screened for genetic conditions (OR=0.53, 0.37–0.77), find out about their genetic risks (OR=0.44, 0.27–0.73), and know their risk of hereditary CRC (OR=0.49, 0.27–0.89). Interest in screening was not significantly associated with age, family history, or other demographic or clinical variables (results not shown).
Sharing Results with At-Risk Relatives
Prior to receiving their MSI screening results, participants were asked in Survey 1 to select the likelihood that they would share their results with family members (Figure 3). Of the respondents who reported having at least one living parent, sibling, child, or other relative, 89.1%, 95.5%, 95.8%, and 83.7%, respectively, indicated they were somewhat or very likely to share their results. All participants who had a spouse indicated that they would share their results with their spouse. Participants who endorsed more barriers to screening for Lynch syndrome were less likely than other participants to indicate they would share their results with their children (OR=0.35, 0.13–0.94). Demographic, clinical and family history variables were not significant predictors of the intent to share MSI screening results (results not shown).
Figure 3.
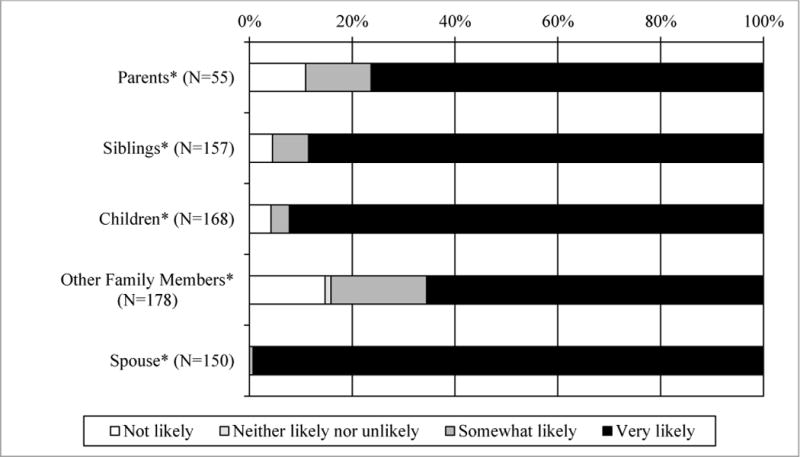
Patients’ intention to share results with family members
*When applicable
Participants who received a result of MSI-H and completed Survey 2 (n=28) were asked whether they actually shared their results with family members (Figure 4). For the most part, participants were consistent about whether they expected to share results and actually did. Two participants had not shared their results with any family member; one had reported three living children but had not shared their results at the time of Survey 2, and one reported not having any living parents, siblings, or children. We also provided potential reasons for sharing the screening result with family members and asked how important each reason was (Figure 5). Most participants agreed it was important or somewhat important to share the results so their relatives could do something to reduce their risk for cancer (n=22, 78.6%), that they had a responsibility to let their relatives know they may be at higher risk of CRC (n=25, 89.3%), and so that their relatives could make family-planning decisions (n=20, 71.4%). We also provided potential reasons for participants not wanting to share screening results with family members and asked how important these reasons were (Figure 6). Few were concerned that relatives would worry about getting colon cancer (n=3, 10.7%) or that talking about CRC would hurt their relationship (n=1, 3.6%). None of the participants indicated that having to talk to a relative that they preferred not to talk to or not feeling emotionally close to their relatives were barriers to sharing results.
Figure 4.
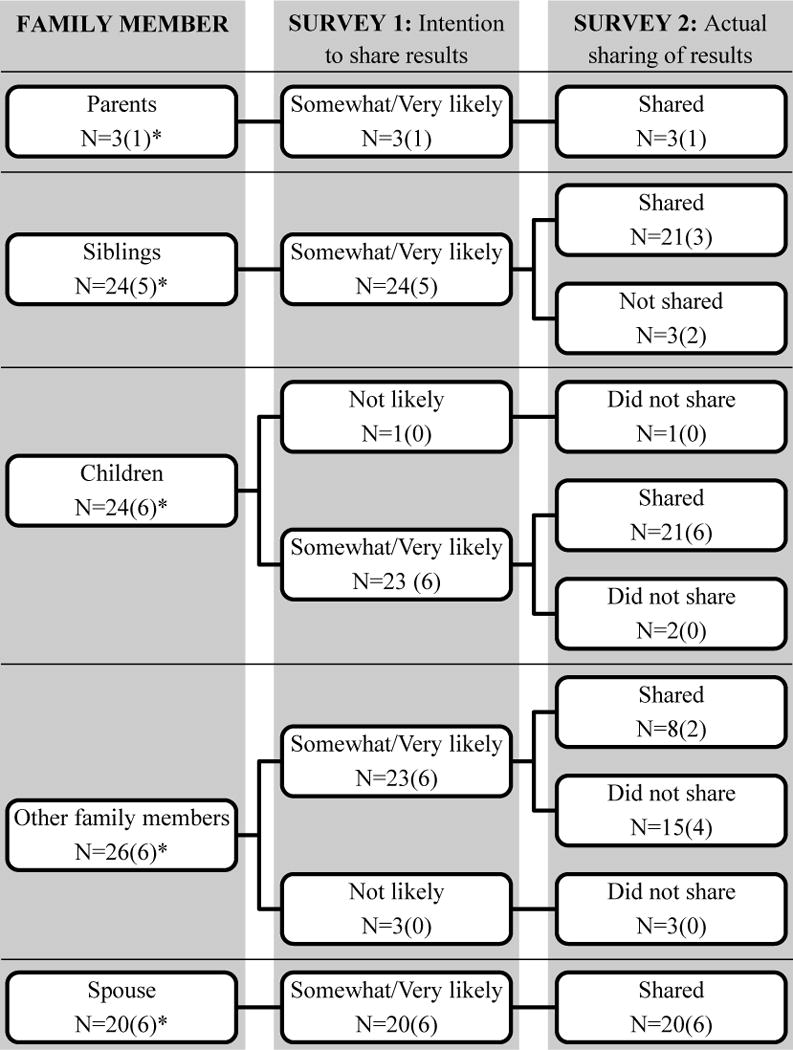
Sharing results with family members by participants with an MSI-H result with responses for the 6 cases of LS indicated in parentheses
*When applicable
Figure 5.
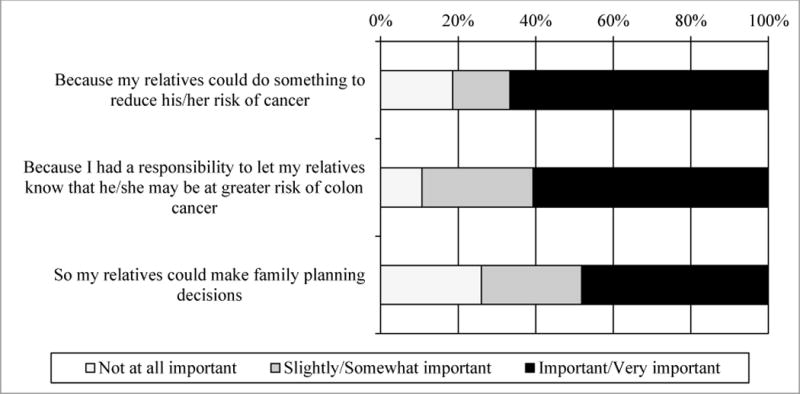
Reasons for sharing results with family members
Figure 6.
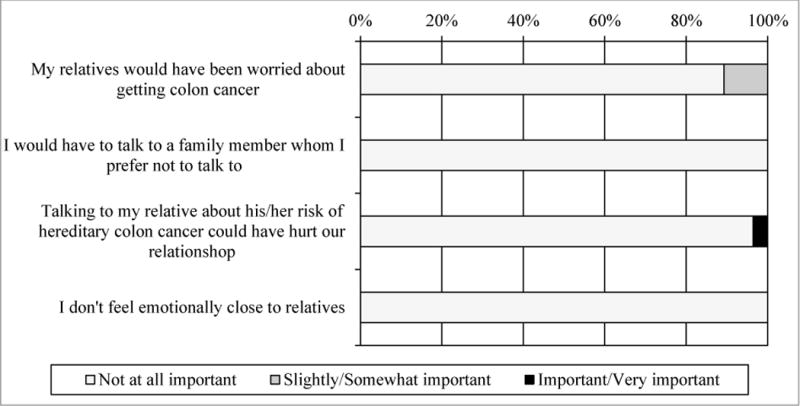
Reasons for not sharing results with family members
Actions Taken by At-Risk Relatives
Participants who received an MSI-H result and indicated they had shared this screening result with relatives were asked on Survey 2 if they knew what actions their relatives had taken. Most participants were not aware of what actions relatives had taken. Of the 21 participants who reported sharing their results with siblings, three (one of which received a diagnosis of Lynch syndrome, see Case 2 below) had at least one sibling who underwent CRC screening (e.g., colonoscopy) and two (one of which received a diagnosis of Lynch syndrome, see Case 3 below) had at least one sibling who saw a health care provider to discuss the information. Of the 21 participants who shared their results with their child(ren), two indicated that their child(ren) underwent CRC screening and two others saw a health care provider to discuss the information. One participant, who received a diagnosis of Lynch syndrome (see Case 3 below), indicated that their child(ren) planned to get genetic testing at a later time, while another indicated that their child(ren) were delaying CRC screening. Actions taken by other relatives were unknown.
Participants with Confirmed Diagnoses of Lynch Syndrome
Of the 38 participants who received an MSI-H result, 35 were interested in additional testing to confirm a diagnosis of Lynch syndrome. Of these, 2 passed away before follow-up could be completed and 23 were found to be positive for a BRAF variant and/or MLH1 hypermethylation, indicative of sporadic CRC. The remaining 10 participants were referred for genetic testing, and pathogenic variants associated with Lynch syndrome were confirmed in 6 with results returned by a genetic counselor who facilitated the participant sharing of this diagnosis with at-risk relatives. Case 1 had no living siblings to inform and it is unknown if the participant contacted the single living parent; however, the only living child of the participant was present during the genetic counseling session and intended to pursue genetic testing. Case 2, who had no living parents, followed up with the genetic counselor after receiving results to share that both siblings and both children had been informed of the diagnosis of Lynch syndrome. At least one sibling underwent a colonoscopy soon after being told about the diagnosis in the participant. Both parents of Case 3 underwent genetic testing as did the two oldest children; however, the remaining 3 children were too young for genetic testing at the time. The participant informed both sisters with at least one sister planning on undergoing genetic testing. It is unknown if Case 4, who had no living parents, shared their diagnosis with their sibling, but the participant did indicate that all 4 children were informed. Case 5 (who had no living parents) had two children who were both informed, but opted not to get genetic testing at the time. One sister was also informed, but not information was available on whether she had genetic testing or whether other two siblings were informed. No information was available on whether Case 6 shared diagnosis with their 3 siblings or 2 children.
DISCUSSION
This study assessed patients’ attitudes regarding screening for Lynch syndrome and the communication of screening results with at-risk relatives. Among the 189 patients newly diagnosed with CRC ascertained through the KPNW integrated healthcare system, most responded positively to genetic screening: they wanted to know their risk for hereditary CRC and generally any genetic risks they might have and, even before receiving their screening results, most expressed an intention to share their results with at-risk relatives (e.g., children, siblings, and parents). In addition, most patients who did have a positive screening result shared the finding with at least one first-degree relative, indicating consistency between intention and actual sharing of information. Lastly, most participants endorsed potential reasons for sharing their screening results with family members and did not endorse potential reasons for not sharing the results.
Our findings suggest that patients identified through a universal Lynch syndrome screening program are not less likely to share information with their family members compared with patients identified through selective screening of high risk populations that have a high burden of cancer in their family. Leenan et al. recently reported perspectives on sharing diagnosis information with family members among a cohort of 40 families with a history of Lynch syndrome in the Netherlands.[30] Most respondents (73%) indicated that they were satisfied with the approach of relying on index cases to share Lynch syndrome diagnosis information with biological relatives, and 82% agreed that it was the personal duty of the mutation carrier to inform family members. In addition, 92% of respondents indicated that family relations stayed the same or improved following the sharing of diagnosis information. Altogether, these findings suggests that sharing screening results with at-risk relatives will likely not be a significant barrier to cascade testing in families of patients with a diagnosis of Lynch syndrome. However, these results are in contrast with past studies among families with a history of Lynch syndrome which cited barriers to sharing Lynch syndrome diagnosis information with relatives due to lack of closeness, concern that relatives would worry, and concern that the relative would not understand the meaning of the results [31] as well as studies that have indicated that family history impacts family communication about risk.[32]
We were not able to obtain information on what actions these relatives took in response to receiving the screening information. Nevertheless, the 189 participants in this study reported a total of 894 first-degree relatives, 384 of which were their children. These numbers illustrate that a universal screening programs will have broader impact beyond the index cases who are screened as first-degree relatives have a 50% chance of also carrying an MMR mutation. The willingness of these index patients to share their screening results will maximize the impact of such screening programs.
Prior studies have indicated high rates of interest in receiving information on genetic risk among patients with CRC, family members of patients with CRC, and the general population [33–37]. Our study confirms these findings in the target population for universal screening for Lynch syndrome: patients who have just been diagnosed with CRC and are unselected for risk factors such as family history and age. These results indicate that the timing of screening and the absence of personal or family risk factors are likely not barriers to interest in undergoing screening for Lynch syndrome and the implementation of a universal screening program.
It is important to note that the consent process for this study included providing patients with information on Lynch syndrome, specifically that it is a common inherited cause of CRC, it is associated with an increased risk of CRC and other cancers at a younger age, and screening for Lynch syndrome can inform the future risk of cancer among patients and their family members. Providing this information might have influenced patients’ perspective on the importance of undergoing screening and the likelihood of sharing the results with at-risk relatives. Given that a prior study has indicated a lack of understanding of the clinical utility of genetic assessment [38], universal screening programs should provide educational material to patients that explain the clinical implications of screening and diagnosis.
The survey responses presented in this study may not represent the patient perspectives and experiences of patients newly diagnosed with CRC in general. First, assessment was limited to participants who consented to participate in the study activities including surveys and screening for Lynch syndrome and thus generate a bias towards participants interested in receiving this information. Second, all participants were insured members of KPNW, an integrated healthcare system with access to comprehensive medical services, including genetic counseling. Third, limited information was obtained from participants regarding any clinical actions taken by their family members after sharing their MSI screening results, which may have been improved with additional follow-up time. Lastly, the generalizability of these conclusions may be limited as the perspectives of those who received an MSI-H screening result may be different from those who receive a definitive diagnosis of Lynch syndrome and by the small sample size of participants who received an MSI-H screening result and completed the second survey.
Future studies should focus on the perspectives and family communication among cases with a confirmed diagnosis of Lynch syndrome. The current study assessed these outcomes among cases of CRC unselected for age and family history, irrespective of Lynch syndrome screening results and without confirmation of a Lynch syndrome diagnosis among those who received an MSI-H screening result. In addition, while we assessed communication among first degree relatives, future studies could focus on more distant relatives who may be less likely to be contacted to ensure that all at-risk relatives are informed.[30, 31] In addition, while we attempted to collect information on specific actions that relatives took, our efforts based on report of the index case were not successful. Future studies may need to employ alternative strategies to collect this information in order to assess the specific actions that relatives took in response to receiving the Lynch syndrome diagnosis information from the index patient.
Universal screening for Lynch syndrome among patients newly diagnosed with CRC and endometrial cancer is being increasingly implemented across healthcare systems. Our study provides important information on patients’ interest in obtaining information on genetic risk and their willingness to share this information with their family members who might also be at risk. The results of our study indicate a general interest in obtaining information on genetic risk for themselves and their family members, rather than being limited to those with certain patient-specific factors such the the presence of a personal of family history of cancer, number of at-risk relatives (e.g., children), CRC cancer stage, age, or sex.
Table 2.
Participant clinical and family history information (N=189)
| Variable | Categories | N (%) |
|---|---|---|
|
| ||
| CRC Stagea | I | 68 (37) |
| II | 44 (24) | |
| III | 53 (29) | |
| IV | 17 (9) | |
|
| ||
| Previous CRC Diagnosisb | No | 185 (98) |
| Yes | 3 (2) | |
|
| ||
| Previous Diagnosis of Any Cancerb | No | 150 (80) |
| Yes | 38 (20) | |
|
| ||
| Number of Relatives with CRCc | 0 | 140 (75) |
| 1 | 34 (18) | |
| 2 | 12 (6) | |
| 3 | 1 (1) | |
|
| ||
| Number of Living Children | 0 | 33 (18) |
| 1 | 34 (18) | |
| 2 | 57 (30) | |
| 3 | 35 (19) | |
| 4 | 18 (10) | |
| 5 | 8 (4) | |
| 6 | 2 (1) | |
| 7 | 1 (1) | |
|
| ||
| Number of Living First-Degree Relativesd | Mean (SD) | 5 (3) |
| Range | 0–15 | |
|
| ||
| Number of Living First-Degree Relativesd | 0 | 3 (2) |
| 1 | 10 (5) | |
| 2 | 22 (12) | |
| 3 | 28 (15) | |
| 4 | 29 (15) | |
| 5 | 34 (18) | |
| 6 | 25 (13) | |
| 7 | 14 (8) | |
| 8 | 8 (4) | |
| 9 | 6 (3) | |
| 10 | 3 (2) | |
| 11 | 2 (1) | |
| 12 | 2 (1) | |
| 13 | 1 (1) | |
| 15 | 1 (1) | |
|
| ||
| MSI Result | Insufficient sample | 26 |
| Low/Stable | 125 | |
| High | 38 | |
CRC stage is based on the current CRC diagnosis and is coded using the American Joint Committee on Cancer (AJCC) classification system and was missing for 7 participants
Participant history of cancer, CRC and any cancer, was based on self-report and was missing for 1 participant
Defined as the presence of a first degree relative with a prior CRC diagnosis and information was missing for 2 participants
Includes parents, children, full siblings, and half siblings and information was missing for 1 participant
Acknowledgments
The authors would like to thank Elizabeth Hess for her editorial support as well as all participants who made this work possible.
FUNDING
This work was supported through a grant by the National Institute of Health: 5R01CA140377 (Goddard).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in this study.
References
- 1.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–8. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21(6):1174–9. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 3.Peltomaki P, Vasen H. Mutations associated with HNPCC predisposition – Update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2004;20(4–5):269–76. doi: 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempers MJ, Kuiper RP, Ockeloen CW, et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol. 2011;12(1):49–55. doi: 10.1016/S1470-2045(10)70265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrow E, Hill J, Evans DG. Cancer risk in Lynch Syndrome. Fam Cancer. 2013;12(2):229–40. doi: 10.1007/s10689-013-9615-1. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HF. Clinical description of the Lynch syndrome [hereditary nonpolyposis colorectal cancer (HNPCC)] Fam Cancer. 2005;4(3):219–25. doi: 10.1007/s10689-004-3906-5. [DOI] [PubMed] [Google Scholar]
- 7.Jarvinen HJ, Mecklin JP, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 1995;108(5):1405–11. doi: 10.1016/0016-5085(95)90688-6. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89(23):1758–62. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 9.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34(5):424–5. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 11.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 12.Kastrinos F, Steyerberg EW, Mercado R, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology. 2011;140(1):73–81. doi: 10.1053/j.gastro.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Wang W, Lee S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296(12):1479–87. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354(26):2751–63. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129(2):415–21. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137(5):1621–7. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15(12):933–40. doi: 10.1038/gim.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352(18):1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 19.Julie C, Tresallet C, Brouquet A, et al. Identification in daily practice of patients with Lynch syndrome (hereditary nonpolyposis colorectal cancer): revised Bethesda guidelines-based approach versus molecular screening. Am J Gastroenterol. 2008;103(11):2825–35. doi: 10.1111/j.1572-0241.2008.02084.x. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Carbonell L, Ruiz-Ponte C, Guarinos C, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 2012;61(6):865–72. doi: 10.1136/gutjnl-2011-300041. [DOI] [PubMed] [Google Scholar]
- 21.Van Lier MG, De Wilt JH, Wagemakers JJ, et al. Underutilization of microsatellite instability analysis in colorectal cancer patients at high risk for Lynch syndrome. Scand J Gastroenterol. 2009;44(5):600–4. doi: 10.1080/00365520802706008. [DOI] [PubMed] [Google Scholar]
- 22.Group EW. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzale D, Jasperson K, Ahnen DJ, et al. Genetic/Familial High-Risk Assessment: Colorectal 2014 [Google Scholar]
- 25.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–62. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014;109(8):1159–79. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- 27.Bulletins-Gynecology CoP, Oncology SoG. ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet Gynecol. 2014;124(5):1042–54. doi: 10.1097/01.AOG.0000456325.50739.72. [DOI] [PubMed] [Google Scholar]
- 28.Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015 doi: 10.1002/cncr.29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acheson LS, Zyzanski SJ, Stange KC, Deptowicz A, Wiesner GL. Validation of a self-administered, computerized tool for collecting and displaying the family history of cancer. J Clin Oncol. 2006;24(34):5395–402. doi: 10.1200/JCO.2006.07.2462. [DOI] [PubMed] [Google Scholar]
- 30.Leenen CH, Heijer M, van der Meer C, Kuipers EJ, van Leerdam ME, Wagner A. Genetic testing for Lynch syndrome: family communication and motivation. Fam Cancer. 2016;15(1):63–73. doi: 10.1007/s10689-015-9842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoffel EM, Ford B, Mercado RC, et al. Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6(3):333–8. doi: 10.1016/j.cgh.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCann S, MacAuley D, Barnett Y, et al. Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: a qualitative study. Psychooncology. 2009;18(11):1208–15. doi: 10.1002/pon.1487. [DOI] [PubMed] [Google Scholar]
- 33.Leventhal KG, Tuong W, Peshkin BN, et al. “Is it really worth it to get tested?”: primary care patients’ impressions of predictive SNP testing for colon cancer. J Genet Couns. 2013;22(1):138–51. doi: 10.1007/s10897-012-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croyle RT, Lerman C. Interest in genetic testing for colon cancer susceptibility: cognitive and emotional correlates. Prev Med. 1993;22(2):284–92. doi: 10.1006/pmed.1993.1023. [DOI] [PubMed] [Google Scholar]
- 35.Hadley DW, Jenkins J, Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163(5):573–82. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- 36.Kinney AY, Choi YA, DeVellis B, Millikan R, Kobetz E, Sandler RS. Attitudes toward genetic testing in patients with colorectal cancer. Cancer Pract. 2000;8(4):178–86. doi: 10.1046/j.1523-5394.2000.84008.x. [DOI] [PubMed] [Google Scholar]
- 37.Petersen GM, Larkin E, Codori AM, et al. Attitudes toward colon cancer gene testing: survey of relatives of colon cancer patients. Cancer Epidemiol Biomarkers Prev. 1999;8(4 Pt 2):337–44. [PubMed] [Google Scholar]
- 38.Tomiak E, Samson A, Spector N, et al. Reflex testing for Lynch syndrome: if we build it, will they come? Lessons learned from the uptake of clinical genetics services by individuals with newly diagnosed colorectal cancer (CRC) Fam Cancer. 2014;13(1):75–82. doi: 10.1007/s10689-013-9677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


