Abstract
Background
In animal models of continuous alcohol self-administration, in which physical dependence does not constitute the major factor of ethanol intake, two factors likely contribute to the perpetuation of alcohol self-administration: (i) the rewarding effects of ethanol and (ii) the contextual conditioning cues that exist along with the process of self-administration. Present studies are aimed at understanding the relative contribution of these factors on the perpetuation of heavy alcohol self-administration, as an indication of relapse.
Methods
Wistar derived UChB high ethanol drinker rats were allowed access to 10% ethanol and water on a 24-hour basis. In initial studies, an anticatalase shRNA gene coding lentiviral vector aimed at inhibiting acetaldehyde generation was administered into the ventral tegmental area (VTA) of the animals prior to ethanol access. In subsequent studies the lentiviral vector was administered to animals, which had consumed ethanol on a 24-hour basis, or a one-hour basis, after the animals had reached high levels of ethanol intake for 60–80 days. In final studies, quinine (0.01%) was added to the ethanol solution to alter the conditioning taste/smell cues of alcohol that animals had chronically ingested.
Results
Data indicate that the administration into the VTA of an anti-catalase vector to naïve animals blocked reward and alcohol self-administration in naive animals while it was, nevertheless, inactive in inhibiting alcohol self-administration in rats that had been conditioned to ingest ethanol for over 2 months. The lack of inhibitory effect on ethanol intake of the anti-catalase vector in animals that had chronically self-administered ethanol was fully reversed when the contextual conditioning cues of the alcohol solution were changed.
Conclusions
Data highlight the importance of conditioning factors in relapse and suggest that only abolishing or blunting it, along with appropriate pharmacological treatments to reduce ethanol reward, may have protracted effects in reducing alcohol self-administration.
Keywords: Reward, Relapse, Lentiviral vector, Catalase, Acetaldehyde
It is well established that without psychotherapeutic or pharmacological treatment, and often both, alcohol and drug dependent individuals will generally fail to interrupt the use of the drug on which they are dependent. Classically two main elements are recognized as involved in the perpetuation of the addictive behavior (Koob and LeMoal, 1997): (i) the fact that the drug is rewarding and (ii) the relief of the drug withdrawal reaction. Volkow and Li (2005) have pointed out that physical dependence refers to adaptations that result in withdrawal symptoms when the drug is discontinued; which should be distinguished from addiction, which refers to the loss of control over the intense urge to take the drug. The latter state is prompted by mechanisms associated to environmental or contextual cues (including visual or olfactory cues) that may either reproduce the rewarding effects of the drug or may trigger a protracted withdrawal reaction. This additional conditioned component, referred to as craving in humans, is accepted as a third major constituent leading to relapse (Buccafalo and Shuster 2009; Cunningham. and Noble, 1992; Wikler, 1959).
The above three components have been partially dissociated in different animal models of alcohol self administration suggesting that they have different neurobiological mechanisms. Drugs that are known to block alcohol self administration in animal models that aim at generating physical dependence do not block the self administration of ethanol when available on a continued basis (see Simms et el 2008). Alternatively, these drugs are either significantly less active (Gilpin and Koob, 2010) or may lack specificity (see Steensland et al 2007). Conversely, low concentrations of a bitter substance such as quinine, added to the ethanol solutions, block self-administration when ethanol is freely available, but do not influence the self administration of ethanol in rat models aimed at generating a major withdrawal reaction (likely involving physical dependence), or high concentrations of quinine are needed (Hopf et al 2010; see also Spanagel et al 1996). These results suggest that in animals allowed a continuous access to alcohol (which have blood ethanol levels generally low and minimal physical dependence) the motivation to drink alcohol is due to the rewarding effects of ethanol and the contextual cues. When animals remain in their home cage the contextual cues that are likely associated to the rewarding effects are the ethanol taste and smell. Taste has been shown to be highly associated to alcohol preference in animal studies (Blednov et al 2010), and adulteration by addition of another taste (e.g quinine) likely affects the contextual cues. Odor is also an important component in lower mammals, in which the olfactory cortex is comparatively large (Herrick, 1933). The fact that lower animals can smell ethanol present at low concentrations in fermented fruits in the wild has been proposed to have an evolutionary advantage (Dudley. 2000).
In most relevant recent studies in humans, Volkow et al (2011) addressed the neurophysiological correlates of drug reward, withdrawal and conditioned relapse. Cocaine is used as a model of a variety of addictive drugs, which include alcohol. The drug initially increases the levels of dopamine in the striatum (including the nucleus accumbens) while these increases in dopamine, as well as the rewarding effects perceived, are blunted in the addict. Most importantly, upon presentation of drug-conditioned cues, addicted subjects show marked increases in dopamine in the striatum. The authors postulate that the conditioned effects (implied as the expectation of drug effects) along with the pharmacological effects are aimed at achieving the expected reward, thus leading to relapse and maintaining drug self administration. The question arises as to which of these two mechanisms is most relevant to relapse. Such was the aim of the present report, which utilized a recent methodology that allows to fully blocking the rewarding effects of alcohol.
Work by Karahanian and associates (2011) showed that a single injection into the VTA of an anti-catalase shRNA coding lentiviral vector, designed to inhibit the metabolism of alcohol into acetaldehyde, a highly rewarding metabolite in the CNS (see Deitrich, 2011), fully blocks (a) the self administration of ethanol by rats bred for their high preference for alcohol, and (b) the alcohol induced release of dopamine in the nucleus accumbens
The present study investigates whether the full abolition of the rewarding effects of ethanol shown in naïve animals by administration of an anti-catalase vector (Karahanian et al, 2011) also reduced alcohol intake in animals that have become dependent on ethanol following 60 days of alcohol self-administration (Ocaranza et al 2008). The effect of the anti-catalase viral vector was examined in two experimental conditions (i) following the continuous self administration of ethanol (10%) and water on a 24-hour basis in their home cage (i.e. no withdrawal period); and (ii) upon alcohol self administration, when ethanol 10% was available for only one hour/day in their home cage (i.e. a 23-hour withdrawal period daily). Keeping constant the physical environment reduced the number of possible contextual variables.
Our hypothesis that reward was the most important element in eliciting relapse proved wrong. Rather, data strongly indicate that relapse has neurobiological bases that are fully independent of the original rewarding effects that led to dependence, and suggest that other mechanisms, such as cued conditioning (or a conditioned withdrawal reaction, as different from a non-cued withdrawal reaction) likely play the most important role in alcohol relapse in this animal model. Only when these cues are blunted, reward plays again a major role in alcohol self administration. Overall, the study suggests that in dependent animals under continuous access to alcohol, self administration is abolished only when both behavioral and pharmacological modifications are combined.
MATERIALS AND METHODS
Generation of lentiviral vectors
A lentiviral vector expressing a rat catalase-targeting shRNA driven by the human U6 promoter (The RNAi Consortium, Broad Institute of MIT and Harvard TRCN0000120679) was packaged with the pPack Lentivector Packaging System (System Biosciences, Mountain View, CA) in HEK 293T cells, according to the manufacturer protocols. The control viruses were generated from the same vector but containing no shRNA sequences. The methods followed were those described by Karahanian et al (2011).
Intracerebral administration of lentiviral vectors
The studies were performed in Wistar-derived rats (UChB) bred for over 80 generations to ingest ethanol solutions in preference to water (Mardones and Segovia-Riquelme, 1983; Quintanilla et al., 2006). Rats (approximately 200–250 g) were anesthetized with a mixture of air and isoflurane administered by a mask fitted over the nose of the animal, and placed in a Kopf stereotaxic frame with the skull oriented according to the atlas of Paxinos and Watson (1986). The skull was exposed and a 2μl Hamilton syringe with a conic tip (diameter at the insertion tip less than 0.2 mm), filled with lentiviral vectors expressing a rat anticatalase shRNA or control vectors, was inserted into the ventral tegmental area (VTA) (coordinates: B-5.2; L-0.8; V-7.2, from the dura mater). Two minutes after syringe implantation, 1 μl of the corresponding solution (anticatalase-Lenti-shRNA 8×104 virus/μl, or the corresponding controls) was infused at the rate of 0.4μl/min. The syringe was kept in place for an additional 2min period, before removing it slowly. The skin was then sutured, and the rat left to recover in the surgery station before being transferred to individual cages at the animal station.
Preparation of VTA homogenates
Thirty days after a single injection of control- or anticatalase-lentiviral vectors, alcohol-naïve UChB rats were killed and their brains rapidly dissected out and placed onto a glass plate on ice. Coronal sections (200–300um) were cut on a cryostat. The VTA was identified according to Paxinos and Watson atlas (1986), and collected with a 1 mm diameter punch (Stille Kirurgiska Instrument, Solna, Sweden), according to Chen et al. (1997). These were rinsed with saline and blotted dry, the VTAs were weighed and 0.8% homogenates were prepared in ice-cold 0.1 M potassium phosphate buffer (pH 7.4) containing 0.1% Triton. The samples were homogenized using a Potter-Elvehjem homogenizer with a glass mortar and Teflon pestle.
Determination of acetaldehyde levels after incubation of VTA homogenates with ethanol
The level of acetaldehyde in VTA homogenates after incubation with 50 mM ethanol was measured by gas chromatography as described previously (Zimatkin et al., 2001, Tampier et al., 1988). An aliquot equivalent to 5 mg of wet tissue was incubated at 37°C in a Dubnoff shaker, using flask stopped with Mininert® valves. The incubation medium contained ethanol, 50 mM; glucose, 10 mM; potassium phosphate buffer (pH 7.4) 90 mM; final volume 1.0 ml. After 30, 60, 90 and 120 minutes of incubation, 1 ml of the gas phase was removed with a Hamilton gas syringe and analized by head space gas chromatography (Perkin Elmer SRI 8616) in order to measure acetaldehyde concentration. Nitrogen was used as the carrier gas at 65 ml/min through a stainless steel column packed with 5% Carbowax 20 M on 60/80 Carbopack at an oven temperature of 65°C and detected by flame ionization. Incubation of VTA homogenate with 10 mM 3-Amino-1,2,4-triazole, a catalase inhibitor inhibited (80%) the formation of acetaldehyde. The residual 20% formation is likely due to acetaldehyde generation by CYP2E1 (Zimatkin et al, 2006). Thus, this residual value was subtracted and the difference in activity is referred to as VTA catalase activity. It is noted that no allowance is made for the possible oxidation of acetaldehyde into acetate, which is unlikely affected differentially by an anticatalase shRNA lentiviral vector versus a control vector.
Experiment 1: Effect of an anticatalase vector injection into the VTA of alcohol-naïve rats on ethanol intake
Four days after a single injection of control- or anticatalase-lentiviral vectors into the VTA of alcohol-naïve rats (n= 12 rats per group), animals were allowed 24 hours continuous access to ethanol solution (10% v/v) and water for 59 days. Ethanol consumption was determined on a daily basis and expressed as g ethanol/kg body weight/day.
Experiment 2: Inhibition of catalase activity in the VTA following the administration of an anti-catalase vector
Twenty four adult (2 month-old) female alcohol naïve UChB rats were divided into two groups (n = 12 rats per group), anesthetized and injected with 1 μl of anticatalase-Lenti-shRNA 8×104 virus/μl, or the corresponding controls, as described previously. Rats were returned to their homecage with food and water ad libitum. After 30 – 35 days rats were killed and the VTA was dissected for catalase activity determination as described.
Experiment 3: Effect of anticatalase vector injection into the VTA and of alcohol deprivation on ethanol intake of dependent animals
Following two months of 24-hour continuous access to ethanol solution (10% v/v) and water, rats were deprived of alcohol for seventeen hours prior to injecting the control- or anticatalase-lentiviral vectors in the VTA (n=10 rats per group). Thereafter, they were transferred to their home cage and allowed 24 hours of continuous access to ethanol solution (10% v/v) and water. Following 18 days of continuous voluntary ethanol intake rats were deprived of alcohol for 4 weeks. After this deprivation phase all rats were re-exposed to the continuous ethanol intake paradigm for 18 days.
Experiment 4: Effect of an anti-catalase vector injection into the VTA on ethanol self-administration in dependent animals before and after adulteration of the ethanol solution with quinine
Following two months of continuous 24-hour access to ethanol solution (10% v/v) and water, rats were deprived of alcohol for seventeen hours before injecting the control- or anticatalase-lentiviral vectors into the VTA (n=10 rats per group). Thereafter, they were transferred to their home cage and allowed continuous 24-hour access to ethanol solution (10% v/v) and water for 18 days. After this time, the rats were deprived of alcohol for 3 days and then ethanol access was restricted to one hour every day (from 14:00 to 15:00 hours), while food and water were freely available. After eleven days of limited access, quinine hydrochloride (Sigma-Aldrich, Atlanta, GA) (0.01%) was added to the ethanol solution, but not to the water, and the ethanol consumption under the one-hour limited access was registered every day for 17 days.
Statistical analyses
Data are expressed as means ± SEM. Statistical differences were analysed by Student’s t test or ANOVA for repeated measures for the time factor, with a post hoc test (Student-Newman-Keuls) when required. A level of P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
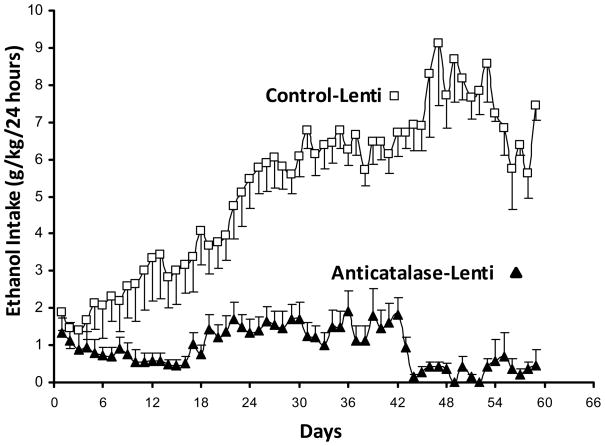
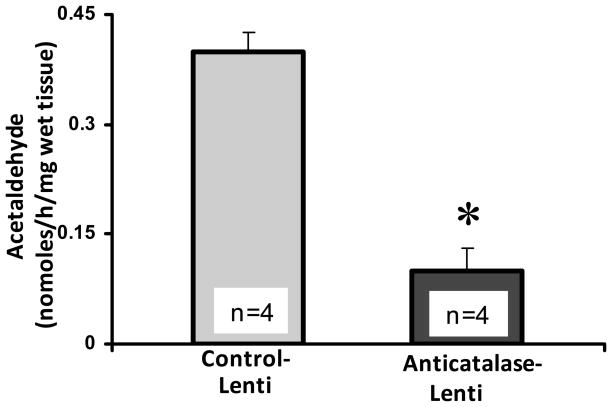
Figure 1 shows the robust effect of the anti-catalase vector in inhibiting alcohol self administration of naïve rats bred for their high ethanol preference ethanol. The control and the active anti-catalase viral vectors were injected into the VTA four days prior to placing the animals in individual cages and offering 10% ethanol and water (shown as day zero in Fig 1). A maximal inhibition of ethanol intake (about 95%) was found after 7 weeks of the active viral vector administration, while the effect at intermediate times was of the order of 70 to 75%. As suggested earlier (Karahanian et al 2011), the lesser inhibitory effect seen at the intermediate times may relate to the fact that for lentiviral vectors the initial gene expression occurs in the episomal state, generated by reverse transcription, while the subsequently long-term expression is due to its permanent insertion into the genome, with a likely period of reduced expression between the two processes (see Haas et al 2000). The inhibitory effect of the lentiviral vector anti-catalase administration on VTA catalase activity determined 30–35 days following viral administration (a time chosen from earlier studies; Karahanian et al 2011) was of the order of 70 to 80% (Figure 2).
Figure 1. Anticatalase-Lentiviral vector administration into the ventral tegmental area reduces voluntary alcohol intake in alcohol-naïve UChB rats.
Alcohol-naïve UChB rats significantly [ANOVA; F (1,117) = 221.23, P < 0.001] reduced their alcohol intake when injected a lentiviral vector coding for a shRNA against catalase (Anticatalase-Lenti) into the VTA, compared to alcohol-naïve rats that received an injection of an empty lentiviral vector (control-Lenti). Animals were allowed free availability of 10% (v/v) ethanol and water four days after the administration a single dose (8×104 virus) of anticatalase-Lenti-shRNA vector (n=12) or an empty lentiviral vector (control-Lenti) (n=12). See Experiment 1 in Methods. Abscissa: days of ethanol availability. Data shown are means± S.E.M.
Figure 2. Anticatalase-Lentiviral vector injection into the ventral tegmental area (VTA) inhibited acetaldehyde generation by VTA homogenates.
VTA homogenates from UChB rats pretreated with a single dose (8×104 virus) of anticatalase-Lenti-shRNA vector injected locally into the VTA, 30 days before the experiment, produced a significantly (p<0.001) lower concentration (−75%) of acetaldehyde via ethanol oxidation than VTA homogenates from rats pretreated with empty lentiviral vector (control-Lenti). Incubations were carried out for 60 min at 37° C in the presence of 50 mM ethanol 10 mM glucose and VTA homogenate (5 mg wet tissue). At 10 mM, 3-amino-1,2,4-triazole (AT), a catalase inhibitor, blocked by 80% the formation of acetaldehyde by the homogenates. Acetaldehyde generated was therefore adjusted to reflect only that due to catalase activity. See Experiment 2 in Methods. Data shown are means ± S.E.M.
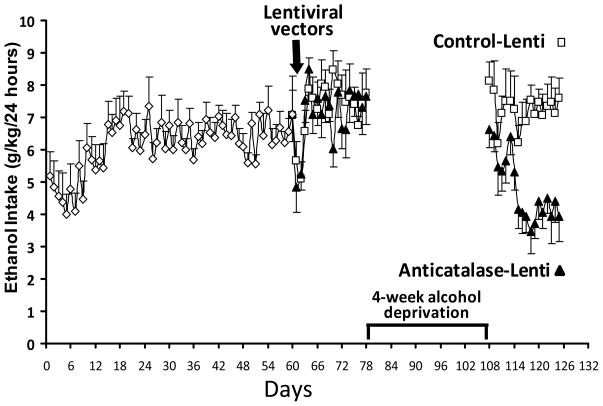
Figure 3 shows the alcohol intake of naïve rats allowed 10% alcohol and water for 60 days, which attained alcohol consumption levels similar to those obtained earlier (7 to 8 g ethanol/kg/day). At such time animals were divided in two groups, matched according to their average ethanol intake, and the anti-catalase or control vectors were administered. As can be observed, except for the first day after the viral vector injection under anesthesia, no reduction in ethanol intake was observed in the groups following 18 days. Thus, the anti-catalase vector was fully inactive in preventing alcohol self-administration in chronically alcohol consuming animals. These data fully contrasts with the powerful inhibitory effects on ethanol consumption observed in naïve animals (see for example 12 to 18 days in Figure 1) following the administration of the anti-catalase vector. For these animals conditioning cues of ethanol smell and/or taste may be relevant to the continuation of intake, as given the continued presence of ethanol a withdrawal reaction can be ruled out. Thereafter, the experiment proceeded with a 4-week period of ethanol deprivation aimed at blunting the mechanisms that prompted animals to continue consuming ethanol, after which the choice of ethanol 10% (water was always available throughout the study) was again freely available on a 24-hour basis. Initially, both the control and the active viral vector groups consumed high levels of ethanol, but the animals that had received the anti-catalase vector started reducing their consumption in time while stabilizing only at one half of consumption levels of the control animals. This time-dependent limited reduction of alcohol self administration after the 4-week of ethanol deprivation vector is likely due to the lack of reward in animals administered the anti-catalase vector following a limited extinction of the conditioning to the ethanol smell/taste. This reduction was clearly not seen in animals administered the control viral vector in which the rewarding mechanisms were intact. Thus, rewarding mechanisms appear necessary to maintain the reconsolidation of conditioning following a short period of extinction.
Figure 3. A period of alcohol deprivation is required to reduce voluntary alcohol intake following the Anticatalase-Lentiviral vector administration into the ventral tegmental area.
UChB rats (n=10) allowed 10% ethanol and water access on a 24-hour basis for 2 months did not change their voluntary alcohol intake when these were subsequently injected into the VTA a single dose of a lentiviral vector coding for a shRNA against catalase (Anticatalase-Lenti), but significantly [ANOVA; F(1,33) = 111.54, P <0.001] reduced (50%) their alcohol intake following 4 weeks of alcohol deprivation when compared to the ethanol intake of animals (n=10) that received a control lentiviral vector (control-Lenti). After the alcohol deprivation period the animals were returned to a free access of 10% ethanol and water on a 24-hour basis. See Experiment 3 in Methods. Abscissa: days of ethanol availability. Data shown are means ± S.E.M.
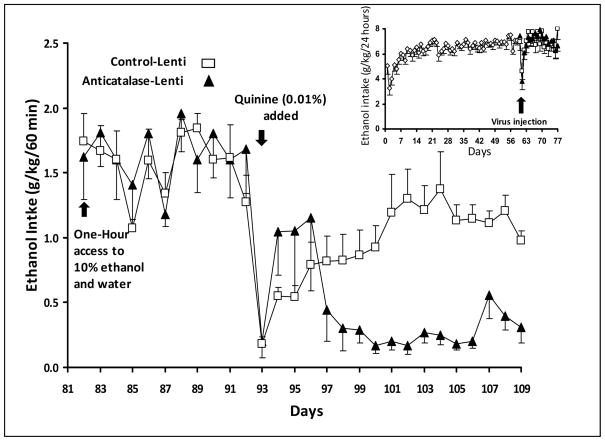
In a subsequent experiment (Figure 4), rats were allowed access to 10% ethanol and water for 60 days time at which time the animals were divided in two groups that were administered into the VTA either the anti-catalase or the control vector. Again, under a continued 24-hour ethanol access, alcohol self-administration was not altered by the anti-catalase vector for the 18 days post vector tested (Figure 4 inset). Thereafter, alcohol was removed for three days and animals were allowed access to 10% ethanol (and water) for only one hour while deprived of ethanol for 23 hours each day. Under this condition, alcohol intake was very high in both groups, independently of the pretreatment with anti-catalase or the control viral vector, reaching consumption levels of 1.6 to 1.8 g ethanol/kg/hour (i.e. some 5 fold higher that the average hourly consumption in 24 hours). This oral consumption would reach blood alcohol levels of the order of 150 mg/dl (0.15%), levels never observed in animals allowed access to 10% ethanol and water on a 24 hour basis (unpublished data). Under this condition, consumption upon re-administration may be driven by a (i) a non-conditioned withdrawal reaction, resulting in relief by negative reinforcement, or (ii) a conditioned (alcohol taste/smell) reaction, as it has been proposed, might also occur in animals and humans experiencing the contextual cues of the drug to which they have been chronically exposed (Volkow et al 2011; Buccafusco and Shuster, 2009). To test if the contextual cues were an important factor leading to a high alcohol self-administration, a low concentration of quinine (0.01%) was permanently added to adulterate the 10% ethanol solution while the one-hour daily administration of 10% ethanol (and 23-hour deprivation) was maintained. Data show that upon adulteration of ethanol with quinine both groups greatly reduced their alcohol intake suggesting that a contextual taste cue was important for the drinking in both groups, whether having received the control viral vector or the anti-catalase vector. The quinine adulteration was maintained until the end of the experiment, such that a new cue for ethanol would be constantly present. It is noteworthy that animals treated with the control vector returned to a high one-hour consumption of ethanol, suggesting that in these animals reward continued to play a role in alcohol-self administration. In contrast, animals treated with the anti-catalase vector remained at a low consumption level. After ethanol adulteration, the overall inhibition of ethanol-quinine intake exerted by the anti-catalase viral vector was 80–85%, compared to the intake of animals that received the control lentiviral vector.
Figure 4. Anticatalase-Lentiviral vector administration into the ventral tegmental area plus adulteration of ethanol solution with quinine reduces voluntary alcohol intake in long-term alcohol-drinking rats.
Alcohol (10%) and water pre-exposed UChB rats for 2 months under a 24-hour access schedule were injected into the VTA a single dose of lentiviral vector coding for a shRNA against catalase (n=10) or a control lentiviral vector (n=10). Thereafter, animals were transferred to their home cage and returned to continuous 24-hour access to a 10% ethanol and water for 18 days (Fig 4 Inset). Following this time rats were deprived of alcohol for 3 days and ethanol (10%) access was restricted to one hour every day (from 14:00 to 15:00 hours on a normal circadian light-dark cycle) in their home cage. After eleven days of limited alcohol access, quinine hydrochloride (Sigma-Aldrich, Atlanta, GA) (0.01%) was added to the 10% ethanol solution and ethanol consumption under the one-hour limited access was registered every day for 17 days. Following the quinine addition, animals that received the anticatalase viral vector significantly reduced their alcohol intake [ANOVA; F(1,53) = 6.94, P < 0.01] versus controls injected with empty lentiviral vector (control-Lenti). See Experiment 4 in Methods. Data shown are means ± S.E.M.
On the basis of long-term studies in animal models of acute ethanol intoxication and repeated withdrawal, where physical dependence is likely generated, in which quinine does not greatly affect alcohol self administration (Hopf et al, 2010; Spanagel et al 1996), the present findings suggest that contextual cues (the taste/odor of ethanol) rather than physical dependence play a more important role in the continued alcohol consumption model. For the animals treated with the control virus, addition of quinine to the ethanol solution appears akin to resetting the contextual drug cues vs. drug reward. For these animals, in which the mechanism of reward is intact, a new association (conditioning) between the novel cues (ethanol-quinine) and reward is likely, while this was clearly not observed in animals given the anti-catalase vector, in which the rewarding effects of ethanol were blocked.
Overall, data suggest that abolishing or blunting of conditioning, added to appropriate pharmacological means to reduce ethanol reward, appear to have optimum effects in reducing relapse. The studies may have relevance to the treatment of alcoholics.
Acknowledgments
Studies presented were supported by grants from Fondecyt #1095021, #1080447; the Millennium Scientific Initiative (P05-001-F; P09-015-F) and NIAAA R01 AA 015421. We appreciate the skilful technical help of Mr. Juan Santibáñez.
References
- Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice genetic models of high alcohol consumption. Behav Genet. 2101;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Shuster L. In: Methods of Behavior Analysis in Neuroscience Chapter 10. Buscafusco JJ, editor. CRC Press Boca Raton; 2009. pp. 1–13. [PubMed] [Google Scholar]
- Deitrich R. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. A commentary. Alcohol Clin Exp Res. 2011;35:581–583. doi: 10.1111/j.1530-0277.2011.01454.x. [DOI] [PubMed] [Google Scholar]
- Dudley R. Evolutionary origins of human alcoholism in primate frugivory. Q Rev Biol. 2000;75:3–15. doi: 10.1086/393255. [DOI] [PubMed] [Google Scholar]
- Chen Y, Engidawork KE, Loidl F, Dell’Anna E, Goiny M, Lubec G, Andersson K, Herrera-Marschitz M. Short- and long-term effects of perinatal asphyxia on monoamine amino acid and glycolysis product levels measured in the basal ganglia of the rat. Dev Brain Res. 1997;104:19–20. doi: 10.1016/s0165-3806(97)00131-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: Role of conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effect of β-adenoreceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology. 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick CJ. The Functions of the Olfactory Parts of the Cerebral Cortex. Proc Nat Acad Sci USA. 1933;19:7–74. doi: 10.1073/pnas.19.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang S-J, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistance to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol: Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, Gonzalez Lira V, Morales P, Herrera-Marschitz M, Israel Y. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol Clin Exp Res. 2011;35:606–612. doi: 10.1111/j.1530-0277.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug Abuse: hedonic homeostatic dysregulation. Science. 1977;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Ocaranza P, Quintanilla ME, Tampier L, Karahanian E, Sapag A, Israel Y. Gene Therapy reduces etanol intake in an animal model of alcohol dependence. Alcohol Clin Exp Res. 2008;32:52–57. doi: 10.1111/j.1530-0277.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1986. [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler JL, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol: Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM, Allingham K, Landgraf R, Zeiglgaänsberger W. Acamprosate and alcohol: I Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Jemma J, Richards K, Selena E, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Nat Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampier L, Quintanilla ME, Letelier C, Mardones J. Effect of 3-amino-1,2,4-triazole on narcosis time and lethality of ethanol in UChA rats. Alcohol. 1988;5:5–8. doi: 10.1016/0741-8329(88)90035-3. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li T-K. The Neuroscience of Addiction. Nat Neurosci. 2005;11:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proc Nat Acad Sci USA ePub March. 2011;14:2011. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Interaction of physical dependence and classical operant conditioning in the genesis of relapse. In: Wikler A, editor. The Addictive States. Williams and Wilkins; Baltimore: 1968. pp. 280–287. [PubMed] [Google Scholar]
- Zimatkin SM, Liopo AV, Slychenkov VS, Deitrich RA. Relationship of brain ethanol metabolism to the hypnotic effect of ethanol. I: Studies in outbred animals. Alcohol Clin Exp Res. 2001;25:976–981. [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]