Abstract
Background
Medications for use as an adjunct to lifestyle modification (LSM) for severe adolescent obesity are limited. Topiramate results in weight reduction in adults with obesity, but has not been studied in adolescents.
Objective
To examine the effect of topiramate plus LSM on BMI reduction in adolescents with severe obesity.
Methods
Data for this retrospective chart review were collected from patients attending a pediatric weight management program who were treated with LSM plus topiramate for 3 months minimum. Mean BMI percent change from baseline was evaluated using t-tests.
Results
Twenty-eight patients (mean age 15.2±2.5 years, mean baseline BMI 46.2±10.3 kg/m2) were identified for inclusion. The 6-month percent change in BMI was −4.9, 95% CI (−7.1, −2.8), p <0.001.
Conclusions
Topiramate with concurrent LSM was associated with clinically-meaningful BMI reduction in adolescents with severe obesity. Randomized controlled clinical trials examining efficacy and safety of topiramate for severe obesity in adolescents are needed.
Keywords: obesity, adolescent, topiramate, pharmacotherapy, medication
Introduction
Severe pediatric obesity, defined as an age- and gender-specific body mass index (BMI) ≥1.2 times the 95th percentile or BMI≥35 kg/m2 is the fastest growing obesity category in children and adolescents, with a reported prevalence of 4–6%.1–5 While obesity in childhood is associated with increased risk of cardiovascular disease (CVD),6,7 type 2 diabetes mellitus (T2DM),8,9 and premature death,10,11 children with severe obesity are at even greater risk than those with moderate obesity.12 Approximately 84% of youth with severe obesity already have at least one CVD risk factor1 and up to 25% of those seeking clinical weight management treatments have impaired glucose tolerance,13 a harbinger of T2DM. Further, severe obesity in early adolescence strongly predicts obesity in adulthood. One study found that severe obesity at age 12 predicted adult obesity 100% of the time, with the majority of youth developing class II obesity in adulthood.1
Not only are the rates of morbidity particularly high in youth with severe obesity, lifestyle modification therapy, the cornerstone of obesity treatment, is relatively ineffective in terms of sustained BMI reduction in this population.14–18 As such, bariatric surgery in adolescents has seen an increase.19 Although bariatric surgery is very effective, it is invasive and carries potentially serious risks;20 it is not widely available; and it is often reserved for only a limited subset of adolescents with obesity who also have the most serious co-morbidities. In contrast, pharmacotherapy may serve as an acceptable, potentially more available and less risky treatment strategy for severe obesity in youth, thus filling the treatment gap between lifestyle modification and bariatric surgery.
Currently, the only FDA approved medication for the treatment of obesity in adolescents is orlistat which has only modest weight loss properties and is coupled by adverse side effects.21 Thus, a significant need exists to identify and evaluate other drug therapies aimed at reducing adiposity and improving CVD and T2DM risk factors in adolescents with severe obesity. One such drug therapy may be topiramate. Because of weight loss observed in individuals treated with topiramate for epilepsy, this medication has been studied as an adjunct therapy to lifestyle modification for the treatment of obesity in adults. However, to our knowledge, it has never been studied as a weight reduction medication in children or adolescents. The objective of this study is to report the BMI outcomes of adolescent patients with severe obesity who were prescribed topiramate plus lifestyle modification therapy in the context of a pediatric medical weight management program.
Methods
Study Design and Clinic Description
This was a retrospective chart review of patients treated at Healthy You! University of Minnesota, Children’s Hospital Weight Management Clinic between June 1, 2010 and April 17, 2013. Healthy You! is a multidisciplinary, tertiary care, university-based pediatric weight management clinic which provides care for children and adolescents up to 18 years of age with obesity. The Healthy You! team includes a pediatric bariatrician who is board certified in obesity medicine, a pediatric endocrinologist, a pediatric nurse practitioner, nurse coordinators, dieticians, a psychologist, and physical therapists. All patients engage in lifestyle modification therapy, including nutrition and exercise counseling supported by behavioral modification strategies.
Patient Population
The patients included in this study were those who, in addition to lifestyle modification therapy, were prescribed topiramate for weight loss for a minimum of three months. Exclusion criteria included: no documented BMI at the time topiramate was initiated; or current use of topiramate for indications other than weight loss.
Medical Record Abstraction
Patient height, weight, BMI, blood pressure, heart rate, and topiramate dose at baseline and all follow-up clinic visits during which the patients were taking topiramate were abstracted from the electronic medical records. The duration and dose of topiramate treatment, documented side effects, other medications taken concurrently with topiramate, and special dietary interventions such as meal replacement plans were also abstracted from the medical records. Passive consents for use of medical records for research were obtained from the study participants’ parents/guardians. This study was approved by the University of Minnesota Institutional Review Board.
Statistical Analysis
Baseline characteristics were summarized with mean and standard deviation for continuous variables and with frequency and percentage for categorical variables. The primary analysis included all patients meeting eligibility criteria who were prescribed topiramate (designated “overall” group), regardless of concomitant usage of other medications (including potentially weight altering medications such as insulin, metformin, serotonin selective reuptake inhibitors (SSRIs), and stimulants) or special dietary interventions, such as meal replacement diets. A secondary analysis included those patients who were only taking topiramate (designated “topiramate only” group), and no other potentially weight altering medications, and did not receive specialized dietary interventions such as meal replacement diets. Changes in BMI over follow-up were characterized as percent change from baseline. Confidence intervals and P-values for the mean were based on a t-distribution and confidence intervals for proportions meeting target thresholds were based on the score test.
Results
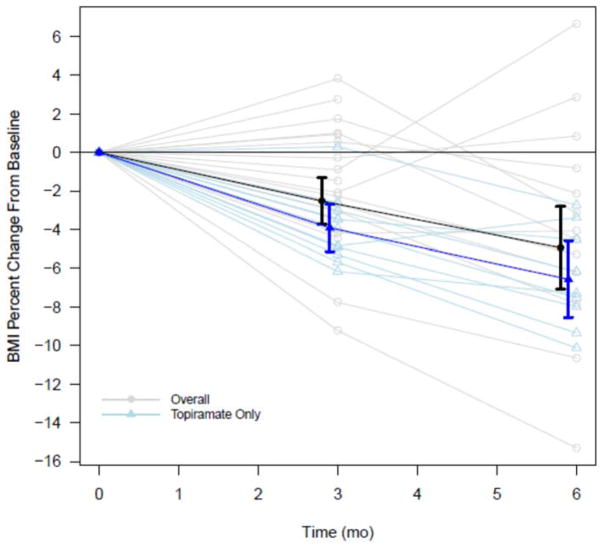
Twenty-eight patients (71.4% girls, mean age 15.2±2.5 years old) were identified for inclusion in this study, 11 of which were in the topiramate only group, i.e. not taking other medications or specialized diets. The baseline mean BMI for the overall group was 46.2±10.3 kg/m2. Most of the patients (82.1%) were prescribed topiramate 75 mg daily (Table 1). Among the other medications used by patients in the overall group were: SSRIs (in 8 patients), stimulants (5), metformin (3), atypical antipsychotic medications (3), other antidepressants (2), and insulin (2). Four in the overall group were prescribed a meal replacement plan. The mean percent change in BMI from baseline to 3-months and baseline to 6-months was statistically significant for both the overall group and the topiramate only group, with greater BMI reductions noted in the topiramate only group (Table 2a, Figure). At 6-months, 50% of the overall group had at least 5% reduction in BMI and 13.6% had 10% reduction or more. Among the topiramate only group, 66.7% and 11.1% had at least 5% and 10% reductions in BMI respectively at 6-months (Table 2b). Two of the 28 total patients experienced paresthesias, one while taking topiramate 75 mg daily, the other while taking 100 mg daily. No other side effects were documented, including cognitive dulling.
Table 1.
Patient descriptive characteristics by treatment group, values presented are mean (SD) or N (%) where indicated.
| Covariate | Overall (N=28) | Topiramate Only (N=11) |
|---|---|---|
| Female | 20 (71.4%) | 8 (72.7%) |
| Age (years) | 15.2 (2.5) | 14.5 (2.8) |
| Height (cm) | 164 (9.6) | 162 (8.3) |
| Weight (kg) | 126 (35.0) | 128 (45.8) |
| BMI (kg/m2) | 46.2 (10.3) | 47.6 (13.8) |
| BMI z-score | 2.68 (0.33) | 2.74 (0.38) |
| Tanner Stage | ||
| - Tanner III | 4 (14.3%) | 1 (9.1%) |
| - Tanner IV | 5 (17.9%) | 2 (18.2%) |
| - Tanner V | 6 (21.4%) | 4 (36.4%) |
| - Missing Tanner | 13 (46.4%) | 4 (36.4%) |
| Topiramate Dose | ||
| - 25 mg | 1 (3.6%) | 0 (0.0%) |
| - 50 mg | 2 (7.1%) | 1 (9.1%) |
| - 75 mg | 23 (82.1%) | 10 (90.9%) |
| - 100 mg | 1 (3.6%) | 0 (0.0%) |
| - 125 mg | 1 (3.6%) | 0 (0.0%) |
| Mean Topiramate Dose (mg) | 74.1 (15.9) | 75.0 (19.8) |
| SBP (mmHg) | 126 (15.6) | 124 (16.4) |
| DBP (mmHg) | 72.6 (13.8) | 74.2 (16.0) |
| HR (bpm) | 86.2 (15.7) | 83.1 (14.2) |
Table 2a.
Mean percent change in BMI at 3-Months and 6-Months.
| Treatment Group | 3-Month | 6-Month | ||
|---|---|---|---|---|
| Mean Percent Change (95% CI) | P-value | Mean Percent Change (95% CI) | P-value | |
| Overall | −2.5 (−3.7, −1.3) n=28 |
<0.001 | −4.9 (−7.1, −2.8) n=22 |
<0.001 |
| Topiramate Only | −3.9 (−5.1, −2.7) n=11 |
<0.001 | −6.6 (−8.5, −4.6) n=9 |
<0.001 |
Figure.
BMI Percent Change from Baseline to 3-Months and 6-Months for the Overall and Topiramate Only Groups. Bold lines present group means with 95% confidence intervals at 3 and 6 months; lighter lines present individual trajectories.
Table 2b.
Percent achieving at least 5% and 10% BMI reduction targets at 3-months and 6-months with 95% confidence intervals.
| 3-Months | 6-Months | |
|---|---|---|
| 5% or more | ||
| Overall | 17.9% (6.8, 37.6) | 50.0% (30.7, 69.3) |
| Topiramate Only | 27.3% (7.3, 60.7) | 66.7% (30.9, 91.0) |
|
| ||
| 10% or more | ||
| Overall | 0% | 13.6% (3.6, 36.0) |
| Topiramate Only | 0% | 11.1% (0.6, 49.3) |
Discussion
This study is the first, to our knowledge, to report on the effect of topiramate for weight reduction in adolescents with obesity. The results demonstrate that topiramate with concurrent lifestyle modification is associated with clinically-significant BMI reduction, on the order of 4–6% over 6 months, and with acceptable tolerability. This level of BMI reduction has been shown to elicit meaningful improvements in risk factors and co-morbidities among adolescents with severe obesity.22 Those patients who were taking other medications concurrently with topiramate, some of which were obesogenic, also reduced their BMI, albeit to a lesser extent.
Presently, orlistat is the only medication that is FDA approved for the indication of weight loss in children ≥12 years of age. Orlistat inhibits gastrointestinal lipase, thus decreasing dietary fat absorption by approximately 30%. The largest of seven studies examining the safety and efficacy of orlistat for weight reduction in children and adolescents found a placebo subtracted BMI reduction of 0.86 kg/m2 (approximately 3%) at 52 weeks in a randomized double blind clinical trial. The most common adverse events were oily stool (50%), oily spotting (29%), and oily evacuation (23%), abdominal pain (22%), and fecal urgency (21%).21
Other medications that have been systematically examined for their weight reduction properties in children and adolescents include: sibutramine, metformin, and exenatide. Sibutramine, a norepinephrine and serotonin reuptake inhibitor, was FDA approved for weight loss in youth ≥16 years of age. However, it was voluntarily removed from the market in 2010 because of a reported greater incidence of cardiovascular events among certain adults who were taking this medication. Metformin is a biguanide whose primary mechanisms of action are reducing hepatic glucose production, increasing insulin sensitivity in peripheral tissues, and decreasing intestinal glucose absorption. Via unclear mechanisms it has also been associated with weight reduction. The largest double blind placebo controlled study assessing the effect of metformin on weight in obese adolescents without diabetes demonstrated a placebo subtracted BMI reduction of 1.1 kg/m2 (approximately 3%) after 48 weeks of metformin XR 2,000 mg daily.23 Finally, exenatide, a glucagon-like peptide receptor agonist primarily used to treat adult type 2 diabetes mellitus has also been associated with weight loss. Mechanisms of action include slowing gastric motility and suppressing appetite via receptors in the hypothalamus. Our group conducted a small randomized controlled study of exenatide for weight reduction in youth with severe obesity and demonstrated a placebo subtracted percent BMI reduction of 2.7% at 3-months. 24
Topiramate has been examined in several large, randomized, placebo controlled clinical trials for weight loss in adults. The weight loss from baseline in these trials ranged from 4.8–9.7% (over 24–60 weeks) and the most common adverse events were neurological in nature, including paresthesias, fatigue, dizziness, and memory difficulty.25–29 Most of these adverse events were dose dependent and were most commonly observed at doses >96 mg daily. Our observations of a 3.9–6.6% reduction in BMI at 6-months with minimal adverse effects are in line with these clinical trials in adults. Further, the mean BMI percent reductions at 3- and 6-months noted in this retrospective chart review exceed what has been reported for almost a year’s duration of treatment with orlistat and metformin in children and adolescents.
The mechanisms by which topiramate induces weight loss are unclear, as this agent has multiple biochemical actions. Mouse studies have demonstrated that topiramate interferes with the efficiency of energy utilization.30, 31 However, in a human study of obese men, topiramate was found to decrease energy intake but not affect energy expenditure.29 This decrease in energy intake may be related to topiramate’s inhibitory action on glutamate neurotransmission, as glutamate stimulation of the lateral hypothalamus increases food intake.32 Topiramate has also been associated with decreased levels of neuropeptide Y in children treated for epilepsy with this drug,33 and neuropeptide Y is a potent appetite stimulating neurohormone.
The retrospective and uncontrolled nature of this study’s design poses notable limitations to the results. Because there was no “blinding” of the patients or health care practitioners to the fact that topiramate was being utilized for the purpose of weight reduction, there is a potential for bias in the outcomes observed. Further, the doses of topiramate varied, as did the patients’ baseline BMIs, and it is recognized that these factors could impact the degree of weight loss.34 Nonetheless, this study describes the “real world” application of a potential pharmacological treatment for severe obesity among adolescents who presented to a tertiary care weight management clinic, most of whom had never before experienced any weight loss with lifestyle modification therapy alone. Of course health care practitioners must weigh the risks and benefits of topiramate, or any other pharmacological agent, for the use of obesity management in children and adolescents. Topiramate has been FDA approved for decades for the indication of seizures in children as young as 2 years, and clearly there are risks associated with this medication, including neurocognitive dulling, short term memory and attention impairment, and the potential for teratogenicity. However, the risks of severe obesity in children and adolescents, including the potential for CVD, type 2 diabetes, non-alcoholic fatty liver disease, musculoskeletal dysfunction, social isolation, lower earning potential, and premature death could arguably outweigh the risks of topiramate. Further it is critical to recognize the chronic nature of severe obesity, which suggests that any medication(s) used will need to be employed long-term.
In conclusion, the current study has shown that topiramate plus lifestyle modification therapy may prove to be an effective and safe treatment for severe obesity in adolescents. Long-term randomized clinical trials are needed to more fully investigate the efficacy and safety of topiramate for this indication.
Acknowledgments
This project was supported in part by NCATS UL1TR000114. CF and AK planned study design. CF and KM collected data. KR and KM performed data analysis. All authors participated in data interpretation and writing of the manuscript.
Contributor Information
Claudia K. Fox, University of Minnesota, Department of Pediatrics, 2450 Riverside Avenue, 6th floor East Building, Minneapolis, MN 55454.
Kara L. Marlatt, University of Minnesota, School of Kinesiology.
Kyle D. Rudser, University of Minnesota, Division of Biostatistics.
Aaron S. Kelly, University of Minnesota, Departments of Pediatrics and Medicine.
References
- 1.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90(5):1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 3.Koebnick C, Smith N, Coleman KJ, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157(1):26–31e2. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen KA, Weedn AE, Crawford PB. Disparities in peaks, plateaus, and declines in prevalence of high BMI among adolescents. Pediatrics. 2010;126(3):434–442. doi: 10.1542/peds.2009-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: The Bogalusa Heart Study. Metabolism. 1996;45(2):235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 8.Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56(12):2964–72. doi: 10.2337/db06-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Bennett PH, Knowler WC. Glucose, insulin concentrations and obesity in childhood and adolescence as predictors of NIDDM. Diabetologia. 1994;37(6):617–23. doi: 10.1007/BF00403382. [DOI] [PubMed] [Google Scholar]
- 10.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 12.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 13.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 14.Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Is family-based behavioral weight control appropriate for severe pediatric obesity? Int J Eat Disord. 2001;30(3):318–328. doi: 10.1002/eat.1091. [DOI] [PubMed] [Google Scholar]
- 15.Kalarchian MA, Levine MD, Arslanian SA, et al. Family-based treatment of severe pediatric obesity: Randomized, controlled trial. Pediatrics. 2009;124(4):1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston CA, Tyler C, Palcic JL, Stansberry SA, Gallagher MR, Foreyt JP. Smaller weight changes in standardized body mass index in response to treatment as weight classification increases. J Pediatr. 2011;158(4):624–627. doi: 10.1016/j.jpeds.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 17.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 18.Knop C, Singer V, Uysal Y, Schaefer A, Wolters B, Reinehr T. Extremely obese children respond better than extremely obese adolescents to lifestyle interventions. Pediatr Obes. 2013 doi: 10.1111/j.2047-6310.2013.00212.x. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher DC, Merrill CT, Cottrell LT, Nadler EP, Burd RS. Recent national trends in the use of adolescent inpatient bariatric surgery: 2000 through 2009. JAMA Pediatr. 2013;167(2):126–132. doi: 10.1001/2013.jamapediatrics.286. [DOI] [PubMed] [Google Scholar]
- 20.Inge TH, Zeller MH, Jenkins TM, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: The Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Study. JAMA Pediatr. 2013 doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: A randomized controlled trial. JAMA. 2005;293(23):2873–2883. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 22.Berkowitz RI, Wadden TA, Gehrman CA, et al. Meal replacements in the treatment of adolescent obesity: A randomized controlled trial. Obesity (Silver Spring) 2011;19(6):1193–1199. doi: 10.1038/oby.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson DM, Abrams SH, Aye T, et al. Metformin extended release treatment of adolescent obesity: A 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164(2):116–123. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptoragonist therapy on body mass index in adolescents with severe obesity: A randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167(4):355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11(6):722–733. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- 26.Astrup A, Caterson I, Zelissen P, et al. Topiramate: Long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obes Res. 2004;12(10):1658–1669. doi: 10.1038/oby.2004.206. [DOI] [PubMed] [Google Scholar]
- 27.Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M OBES-002 Study Group. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28(11):1399–1410. doi: 10.1038/sj.ijo.0802783. [DOI] [PubMed] [Google Scholar]
- 28.Tonstad S, Tykarski A, Weissgarten J, et al. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96(2):243–251. doi: 10.1016/j.amjcard.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay A, Chaput JP, Berube-Parent S, et al. The effect of topiramate on energy balance in obese men: A 6-month double-blind randomized placebo-controlled study with a 6-month open-label extension. Eur J Clin Pharmacol. 2007;63(2):123–134. doi: 10.1007/s00228-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 30.York DA, Singer L, Thomas S, Bray GA. Effect of topiramate on body weight and body composition of Osborne-Mendel rats fed a high-fat diet: Alterations in hormones, neuropeptide, and uncoupling-protein mRNAs. Nutrition. 2000;16(10):967–975. doi: 10.1016/s0899-9007(00)00451-2. [DOI] [PubMed] [Google Scholar]
- 31.Picard F, Deshaies Y, Lalonde J, Samson P, Richard D. Topiramate reduces energy and fat gains in lean (fa/?) and obese (fa/fa) Zucker rats. Obes Res. 2000;8(9):656–663. doi: 10.1038/oby.2000.84. [DOI] [PubMed] [Google Scholar]
- 32.Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011;104(1):40–46. doi: 10.1016/j.physbeh.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 33.Okuyaz C, Kursel O, Komur M, Tamer L. Evaluation of appetite-stimulating hormones in prepubertal children with epilepsy during topiramate treatment. Pediatr Neurol. 2012;47(6):423–426. doi: 10.1016/j.pediatrneurol.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: A review. Epilepsy Res. 2011;95(3):189–199. doi: 10.1016/j.eplepsyres.2011.05.014. [DOI] [PubMed] [Google Scholar]