Abstract
The effect of utilizing a pure cis-α-dimethoxy carbonyl fulleropyrrolidine C70 (DMEC70) isomer as the electron transporting material (ETM) in inverted perovskite solar cells (PSCs) was evaluated. The as-prepared C70 mono-adduct products are mixtures of regioisomers and the interest was to evaluate them independently as ETMs. Three different cis-DMEC70 isomers (α, β-endo and β-exo) (mix-DMEC70) were synthesized and purified by HPLC. It was found that PSCs based on the pure α-DMEC70 exhibit a substantially enhanced maximum power conversion efficiency (PCE) of 18.6% as compared to devices based on the mixed-DMEC70 isomers that yielded a PCE of 16.4%. A maximum PCE of 15.7% was observed for devices based on [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM). This work points out the importance of using pure fullerene derivative isomers as ETMs to reduce the intrinsic energy disorder, which enhances the overall device performance.
Introduction
Organic–inorganic halide PSCs offer great potential for cost-effective, solution-processable, and large-area fabrication of solar energy conversion technologies.1,2 PSCs can be either fabricated using conventional n–i–p or inverted p–i–n configurations, and in both cases the perovskite layer is in contact with a hole transporting layer (HTL) and an electron transporting layer (ETL) on opposite sides. These selective interlayers not only improve the charge extraction from the perovskite layer but also determine the polarity of the device.3,4 To date, PSCs present the fastest growth in terms of PCE with certified efficiencies rising from 3.8% in 2009 to 22.1% in 2016 for small-area devices (0.1 cm2),5,6 whereas a PCE of 19.6% has been reported for large-area devices (1 cm2).7 It is worth mentioning that higher PCEs and long-term device stability have been achieved after the first report of an all-solid-state PSC, introduced by Kim et al. in 2009.8
In inverted PSCs, the HTL, typically PEDOT:PSS, modified PEDOT:PSS, or NiOx,9–14 is in contact with the anode (ITO or FTO), while the ETL, typically a fullerene derivative, is in contact with the back electrode (Al or Ag). The standard fullerenes used in PSCs are [6,6]-phenyl-C61/71-butyric acid methyl esters (PC61/71BM), mainly due to their efficient electron transporting and solution processable properties.15,16 Inverted PSCs based on ITO/doped-poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA)/MA0.60FA0.40PI3/PC71BM/C60/2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP)/Ag have achieved a maximum PCE of 20.2%.17
Zhu18 and Huang's19 groups have reported that charge trap states at the surfaces and grain boundaries of perovskite crystals act as non-radiative recombination centers, which drastically affect the optoelectronic properties of PSCs. Snaith20 and co-workers proposed that non-coordinated halide ions on the surface of perovskite crystals can be passivated using iodopenta fluorobenzene. The same group separately reported that crystal surface vacancies can be significantly passivated using organic Lewis bases such as thiophene and pyridine.21 To mitigate the effects on grain boundaries and trap states, the concept of perovskite/fullerene (P/F) heterojunctions has attracted considerable attention.22–25 Huang et al.26 demonstrated that the trap states on the surface and grain boundaries are the origin of photocurrent hysteresis and that fullerene layers deposited on perovskites can effectively passivate these charge trap states and eliminate the undesirable photocurrent hysteresis.26
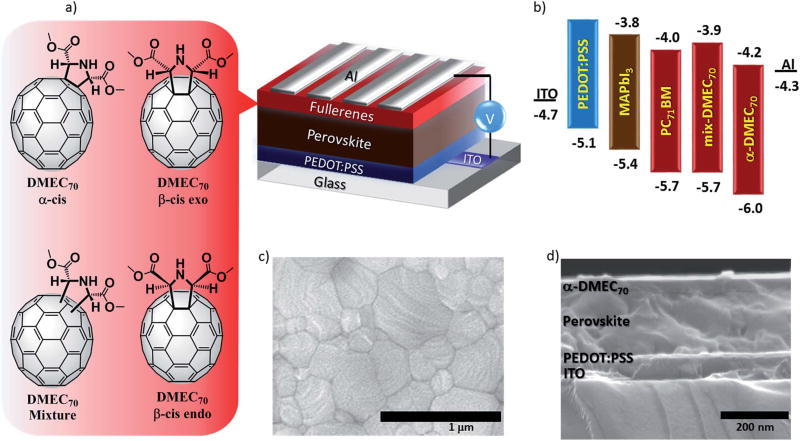
We have recently reported that PSCs based on an isomeric mixture (mix-DMEC70) exhibited both higher PCE and higher device-stability than devices based on PC71BM. These results were attributed to the ability of the mix-DMEC70 to extract electrons efficiently, likely due to specific interactions between the pyrrolidine groups (carbonyls and amino groups) and the perovskite crystals at the interfaces.27 There are three regioisomers in mix-DMEC70 (cis-α, cis-β-endo and cis-β-exo) as shown in Fig. 1a. It should be noted that the as-prepared mix-DMEC70 was used as the ETL in PSCs without further purification. The effect of using pure fullerene C60 bis-adduct isomers as opposed to isomeric mixtures in bulk heterojunction solar cells (BHJ-SCs) was first studied by Imahori I28 Pure isomers have been shown to have a smaller energy disorder and a higher electron mobility than their corresponding regioisomeric mixtures, thus leading to higher performance solar cells.29–36 Imahori et al.37 reported photophysical properties and remarkable photovoltaic performance improvement when using isomerically pure C70 mono-adducts in BHJ-SCs. Recently Huang et al.38 reported the use of pure C60 bis-adducts in PSCs to match the energy levels to obtain higher photovoltaic performance.
Fig. 1.
(a) Device structure of the inverted PSC using α-DMEC70, mix-DMEC70, or PC71BM as the ETM. (b) Schematic illustration of the estimated HOMO and LUMO energy levels, calculated from CV and UV-vis. (c) Top-view SEM image of CH3NH3PbI3 deposited on PEDOT:PSS. (d) Cross-sectional SEM image of an inverted PSC.
Shao et al.26 reported a simple solvent annealing method to mitigate the energy disorder in the PC61BM layer in PSCs, resulting in an improved open circuit voltage (Voc). Grätzel et al.39 recently reported that improved device performance and stability in PSCs can be obtained when using a pure bis-adduct of PC61BM as an additive in an antisolvent chlorobenzene solution. Since the packing of the C70 fullerene derivatives in the solid state should have an impact on their charge separation and transporting properties in PSCs, the three obtained isomers were purified and characterized in this work, but only the pure α-DMEC70 was evaluated as the ETM in inverted PSCs since this compound was the major regioisomer obtained.
Results and discussion
By changing the reaction conditions from sonication at room temperature to reflux, the formation ratio of the α-DMEC70 with respect to the other two isomers (Fig. 1a) was improved from 82% to 90% (Scheme S1†). The detailed synthetic procedures and characterization are described in the ESI.† The pure cis-DMEC70 isomers were purified by HPLC from the as-obtained mix-DMEC70 using a 5PYE column. As shown in Fig. S1,† three peaks that correspond to the three DMEC70 isomers were observed. The three different isomers were fully characterized by means of MALDI-TOF, 1D and 2D 1H and 13C-NMR, and UV-vis absorption (Fig. S2–S7†).
Fig. S5 and S6† show the UV-vis absorption profiles of the three DMEC70 isomers in chloroform and the structures of the isomers were unambiguously assigned by comparing their UV-vis profiles with those of known C70 fullerene analogs.40
The electrochemical properties of the three DMEC70 isomers were measured using cyclic voltammetry (CV) (Fig. S8†). The DMEC70 isomers exhibit three reduction waves in the negative potential range from 0 to −2.0 V vs. Fc/Fc+. The lowest unoccupied molecular orbital (LUMO) energy levels were estimated from their onset reduction potentials ,41 and the corresponding values are listed in Table 1. The LUMO energy levels of cis-α, cis-β-endo and cis-β-exo were estimated to be −4.24 eV, −3.96 eV and −3.93 eV, respectively. These results show that the electrochemical properties of C70 fullerene mono-adducts are affected by changing the position of the addend. The energylevel diagram of α-DMEC70 and mix-DMEC70 is shown in Fig. 1b.
Table 1.
Electrochemical and photophysical data of α-DMEC70, β-endo-DMEC70 and β-exo-DMEC70
| Compound | λabs (nm) | Eg (eV) |
|
LUMO (eV) | HOMO (eV) | |
|---|---|---|---|---|---|---|
| α-DMEC70 | 683 | 1.81 | −0.78 | −4.24 | −6.05 | |
| β-endo-DMEC70 | 705 | 1.76 | −1.06 | −3.96 | −5.72 | |
| β-exo-DMEC70 | 705 | 1.76 | −1.09 | −3.93 | −5.69 |
The electron mobilities of α-DMEC70, mix-DMEC70 and PC71BM based on electron-only devices (ITO/Al/fullerene/Al) were calculated by the space-charge limited current (SCLC) method and the Mott–Gurney law,42–45 and values of 9.98 × 10−4, 8.01 × 10−4 and 9.20 × 10−4 cm2 V−1 s−1 were obtained, respectively.
As expected, the smaller energy disorder for the pure isomer leads to a higher electron mobility compared to the regioisomeric mixture (Fig. S9†).
To further characterize the effect of pure α-DMEC70 in PSCs, inverted planar PSCs using this compound as the ETM with a configuration of ITO/PEDOT:PSS/CH3NH3PbI3/α-DMEC70/Al were fabricated (see the Experimental section). Scanning electron microscopy (SEM) (Fig. 1c) of the perovskite surface shows a uniform microscale grain structure (~300 nm) with no apparent pinholes. Additionally, the cross section shown in Fig. 1d shows all of the different layers. The perovskite layer was also analyzed by X-ray diffraction (XRD). Fig. S10† shows diffraction peaks for a pure perovskite material with no evidence of PbI2 or CH3NH2I.
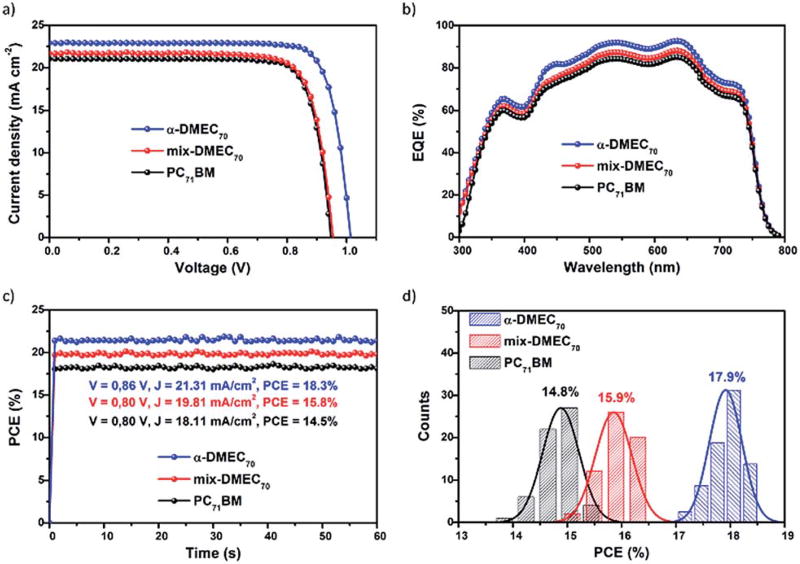
The current density–voltage (J–V) curves of the inverted planar structures based on α-DMEC70, mix-DMEC70 and PC71BM are shown in Fig. 2a. The photovoltaic parameters are listed in Table 2. As shown in Fig. 2a and in Table 2, the pure α-DMEC70 regioisomer based devices exhibit higher PCEs (18.6%) than those of mix-DMEC70 and PC71BM (16.4% and 15.6%, respectively).
Fig. 2.
(a) J–V curves under 1 sun illumination. (b) EQE spectra of perovskite solar cells fabricated using α-DMEC70, mix-DMEC70 or PC71BM as the ETM. (c) Power output under maximum power point tracking for 60 s. (d) The PCE histograms measured for 20 independent devices.
Table 2.
Summary of the performance of devices based on α-DMEC70, mix-DMEC70, or PC71BM as the ETM
| ETM |
Jsc (mA cm−2)a |
Jsc (mA cm−2) |
Voc (V) |
FF (%) |
PCE (%) |
|---|---|---|---|---|---|
| α-DMEC70 | 22.88 | 22.90 | 1.02 | 0.80 | 17.9 ± 0.7 (18.6) |
| mix-DMEC70 | 21.90 | 21.92 | 0.95 | 0.77 | 15.9 ± 0.5 (16.4) |
| PC71BM | 21.07 | 21.09 | 0.95 | 0.78 | 14.8 ± 0.8 (15.6) |
Calculated current; () highest PCE. The average was calculated from 20 devices.
As shown in Table 2, the Voc values obtained from the α-DMEC70 based devices are higher than those obtained from the mix-DMEC70 based devices. These results may seem counterintuitive solely based on HOMO–LUMO values, but recent results have demonstrated that the ETM layer order can affect and even reverse the expected results based on energy level considerations.26 EQE spectra in Fig. 2b show that the charge extraction efficiency of the α-DMEC70-based devices is higher than those the mix-DMEC70 and PC71BM-based devices.
The photocurrent density (Jsc) calculated from the EQE spectra agrees with the measured Jsc from the J–V curves, con-firming the accuracy of the device efficiency. The higher Jsc and FF obtained for the α-DMEC70-based devices can be associated with the higher electron mobility of α-DMEC70. The maximum steady-state photocurrent and PCE outputs were monitored by applying a voltage close to the maximum power and plotted as a function of time.
Fig. 2c shows that for devices based on α-DMEC70, mix-DMEC70 and PC71BM steady-state photocurrents of 21.31 mA cm−2, 19.81 mA cm−2, and 18.11 mA cm−2 and PCEs of 18.3%, 15.8%, and 14.5% were obtained, respectively. The stabilized power outputs were calculated by multiplying the photocurrent and the applied voltage.
The statistical histograms of PCEs for 20 individual devices are shown in Fig. 2d. The distributions show average efficiencies of 17.9 ± 0.7%, 15.9 ± 0.5% and 14.8 ± 0.8% for α-DMEC70, mix-DMEC70 and PC71BM based devices, respectively. To investigate the hysteresis behavior, the devices were tested in both forward and reverse scan directions.
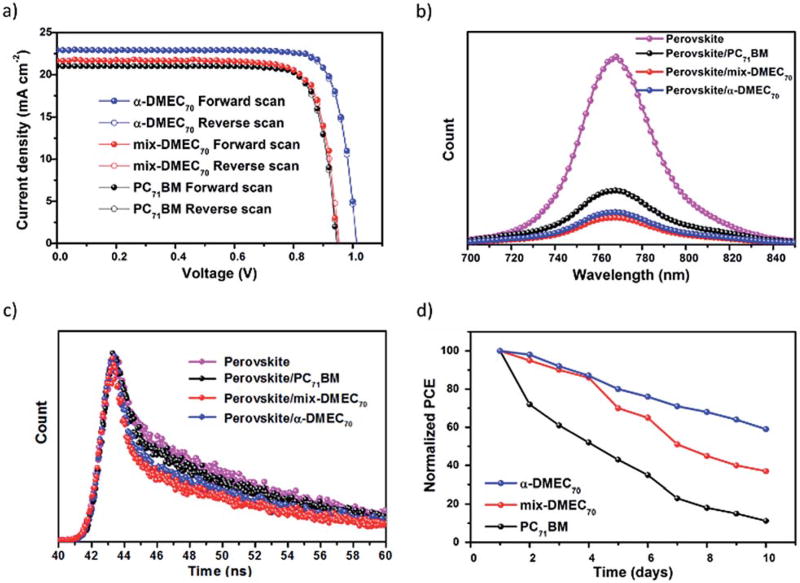
As shown in Fig. 3a, all the devices displayed relatively consistent J–V curves regardless of the scan direction, indicating negligible hysteretic effects in the PSCs.
Fig. 3.
(a) J–V curves under 1 sun illumination in forward and reverse voltage scans. (b) Steady-state PL spectra of the perovskite and perovskite/ETM films. (c) TR-PL spectra of the perovskite and perovskite/ETM films. (d) Normalized PCE of PSCs measured as a function of time at ~20% humidity at room temperature.
To study the charge carrier recombination of the devices based on DMEC70 (pure and mixture) and PC71BM as the ETMs, steady-state photoluminescence (PL) (Fig. 3b) and time-resolved PL decay measurements (Fig. 3c) were recorded for perovskite, perovskite/DMEC70 (pure and mixture), and perovskite/PC71BM films. As shown in Fig. 3b the emission peak at 778 nm is attributed to the recombination process in the nanocrystalline perovskite.
A significant PL quenching effect was observed within the perovskite/ETM thin films, which indicates that surface charge carrier recombination was suppressed.46 Moreover, a more pronounced PL quenching effect was observed within the perovskite/α-DMEC70 thin film than for the perovskite/mix-DMEC70 or perovskite/PC71BM thin films, which resulted in an enhanced Jsc and FF, and thus in a higher PCE. Fig. 3c shows shorter PL decay times for perovskite/ETMs (0.10, 0.04 and 0.15 ns for perovskite/α-DMEC70, perovskite/mix-DMEC70, and perovskite/PC71BM, respectively) than for simple perovskite (0.21 ns) thin films.
The shortened decay lifetimes of the perovskite with any of the ETMs leads to faster transport of charge carriers and more efficient charge carrier collection, which suppresses electron–hole recombination and thus increases the Jsc and the FF.47
Air-stability is one of the major concerns for the commercialization of PSCs.48,49 As shown in Fig. 3d, the stability of unencapsulated devices based on α-DMEC70, mix-DMEC70, and PC71BM as the ETMs was monitored for 10 days at room temperature with a relative humidity of ~20%. High stability was observed for the devices based on α-DMEC70, which retained more than 60% of their initial PCE. In contrast, the mix-DMEC70-based devices only retained 35% of their initial PCE, whereas the PC71BM-based devices retained less than 10% of their initial PCE after 10 days. To understand the reasons for these differences, the contact angles of water droplets on perovskite/α-DMEC70, perovskite/mix-DMEC70 and perovskite/PC71BM were measured and the values were 92°, 86° and 82°, respectively (Fig. S11†). These small but significant differences clearly show that the improved stability of the α-DMEC70-based devices can be attributed to the better packing of the pure isomer, which leads to a more hydrophobic and compact layer and thus reduces water intrusion into the perovskite layer.
The surface morphologies of perovskite, perovskite/mix-DMEC70 and perovskite/α-DMEC70 films were characterized by atomic force microscopy (AFM). As shown in Fig. S12a,† the perovskite film exhibits a crystalline structure with a calculated root-mean-square (RMS) roughness of around 48 nm. The perovskite/mix-DMEC70 film shows a morphology with a reduced RMS roughness of around 15.9 nm (Fig. S12b†), whereas a smoother morphology with a smaller RMS roughness of around 8.5 nm was observed for the perovskite/α-DMEC70 film (Fig. S12c†). The pure-isomer film leads to a smoother ETL, likely due to better molecular packing.
Conclusions
By changing the reaction conditions, we improved the yield of the α-DMEC70 isomer. Using this pure isomer as the ETM layer in PSCs resulted in considerably improved device performance and long-term stabilities. These improvements are likely the result of structural and energy advantages of using the single isomer and of improved hydrophobicity. To our knowledge, this is the first example of such an improvement observed by using a pure C70-fullerene isomer, and the stabilized PCE of 18.3% is among the highest for this simple inverted PSC architecture. Our results highlight the importance of developing synthetic approaches that yield pure C70-fullerene isomers without the need for HPLC purification.
Experimental section
Synthesis of DMEC70
By changing the reaction conditions from sonication at room temperature to reflux, the formation ratio of the α-DMEC70 with respect to the other two isomers (Fig. 1a) was improved from 82% to 90% (Scheme S1†). C70 (84 mg, 0.10 mmol) was dissolved in chlorobenzene (30 mL) under sonication for 5 min, and then DIB (200 mg, 0.62mmol), glycine methyl ester hydrochloride (100 mg, 0.79 mmol) and sodium carbonate decahydrate (100 mg, 0.35 mmol) were added. The flask was wrapped with aluminum foil and refluxed for 30 min. The solution was directly poured onto a silica gel column and CS2 was used to separate the unreacted C70, and DMEC70 was purified using a toluene/ethyl acetate (9 : 1) mixture. The regioisomeric yield was 85%. The ratio of the mono-adducts in the mixture was 90 : 5 : 5 (α : β-endo : β-exo) as determined by 1H NMR and the molecular mass was determined by matrix assisted laser desorption/ionization-time-of-light-mass spectrometry (MALDI-TOF-MS) (Fig. S1†).
Device fabrication
Methylammonium iodide (CH3NH3I) was prepared using a previously reported procedure.50 PSCs with a configuration of ITO/PEDOT:PSS/perovskite/fullerene derivative/Al were fabricated on ITO-coated glass substrates with a resistivity of 10 Ω cm−2. The patterned ITO glass substrates were cleaned sequentially with detergent, deionized water, isopropyl alcohol and acetone, each step for 30 min, and then dried with nitrogen gas and finally treated in a UV-ozone oven for 30 min. After passing through a 0.45 µm PVDF filter, the PEDOT:PSS solution (Baytron P VP AI 4083) was spin-coated onto the treated ITO substrates at 5000 rpm for 30 s, and heated at 150 °C for 15 min in air. Then the substrates were transferred to a N2-filled glovebox where CH3-NH3PbI3 (1 M solution in DMF) was spincoated on top of the PEDOT:PSS coated substrates at 800 rpm for 10 s and at 4000 rpm for 25 s, 80 µL of toluene were added 5 s after the second step and then the devices were annealed at 70 °C for 60 min and the fullerene derivatives dissolved in chlorobenzene (20 mg mL−1) were spin-coated onto the CH3-NH3PbI3 layer at 5000 rpm for 30 s. Finally, aluminum electrodes (100 nm) were deposited by thermal evaporation under a pressure of 1 × 10−6 Torr through a shadow mask. The active area of the fabricated devices was 6 mm2. The top aluminum electrodes were encapsulated with a UV-curable epoxy resin and a glass slide before testing.
Supplementary Material
Acknowledgments
The authors thank the US National Science Foundation (NSF) for generous support of this work under the NSF-PREM program (DMR 1205302), CHE-1408865 (to L.E.) and 1401188 (to F. D.). The Robert A. Welch Foundation is also gratefully acknowledged for an endowed chair to L. E. (Grant AH-0033). The National Institute of General medical Sciences of the National Institutes of Health under linked Award Numbers RL5GM118969, TL4GM118971, and UL1GM118970, is also acknowledged.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ta06338e
Conflicts of interest
Authors declare no conflict of interest.
References
- 1.Zhang H, Cheng J, Li D, Lin F, Mao J, Liang C, Jen AKY, Grätzel M, Choy WCH. Adv. Mater. 2017:1604695. doi: 10.1002/adma.201604695. [DOI] [PubMed] [Google Scholar]
- 2.Shin SS, Yeom EJ, Yang WS, Hur S, Kim MG, Im J, Seo J, Noh JH, Seok SI. Science. 2017;356:167–171. doi: 10.1126/science.aam6620. [DOI] [PubMed] [Google Scholar]
- 3.Ratcliff EL, Zacher B, Armstrong NR. J Phys. Chem. Lett. 2011;2:1337–1350. doi: 10.1021/jz2002259. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Fu W, Zhang Z, Chen H, Li C-Z. J Mater. Chem. A. 2017;5:11462–11482. [Google Scholar]
- 5.Kojima A, Teshima K, Shirai Y, Miyasaka T. J Am. Chem. Soc. 2009;131:6050–6051. doi: 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- 6.NREL. [accessed June 2017];Best Research-Cell Efficiencies. 2017 Jun; http://www.nrel.gov/pv/assets/images/efficiency_chart.jpg.
- 7.Li X, Bi D, Yi C, Décoppet J-D, Luo J, Zakeeruddin SM, Hagfeldt A, Grätzel M. Science. 2016;353:58–62. doi: 10.1126/science.aaf8060. [DOI] [PubMed] [Google Scholar]
- 8.Kim H-S, Lee C-R, Im J-H, Lee K-B, Moehl T, Marchioro A, Moon S-J, Humphry-Baker R, Yum J-H, Moser JE, Grätzel M, Park N-G. Sci. Rep. 2012;2:591. doi: 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docampo P, Ball JM, Darwich M, Eperon GE, Snaith HJ. Nat. Commun. 2013;4:2761. doi: 10.1038/ncomms3761. [DOI] [PubMed] [Google Scholar]
- 10.Jeng J-Y, Chen K-C, Chiang T-Y, Lin P-Y, Tsai T-D, Chang Y-C, Guo T-F, Chen P, Wen T-C, Hsu Y-J. Adv. Mater. 2014;26:4107–4113. doi: 10.1002/adma.201306217. [DOI] [PubMed] [Google Scholar]
- 11.Wang J-M, Wang Z-K, Li M, Hu K-H, Yang Y-G, Hu Y, Gao X-Y, Liao L-S. ACS Appl. Mater. Interfaces. 2017;9:13240–13246. doi: 10.1021/acsami.7b02223. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z-K, Gong X, Li M, Hu Y, Wang J-M, Ma H, Liao L-S. ACS Nano. 2016;10:5479–5489. doi: 10.1021/acsnano.6b01904. [DOI] [PubMed] [Google Scholar]
- 13.Lou Y-H, Li M, Wang Z-K. Appl. Phys. Lett. 2016;108:053301. [Google Scholar]
- 14.Wang Z-K, Li M, Yuan D-X, Shi X-B, Ma H, Liao L-S. ACS Appl. Mater. Interfaces. 2015;7:9645–9651. doi: 10.1021/acsami.5b01330. [DOI] [PubMed] [Google Scholar]
- 15.Volker SF, Collavini S, Delgado JL. ChemSusChem. 2015;8:3012–3028. doi: 10.1002/cssc.201500742. [DOI] [PubMed] [Google Scholar]
- 16.Meng L, You J, Guo TF, Yang Y. Acc. Chem. Res. 2016;49:155–165. doi: 10.1021/acs.accounts.5b00404. [DOI] [PubMed] [Google Scholar]
- 17.Luo D, Zhao L, Wu J, Hu Q, Zhang Y, Xu Z, Liu Y, Liu T, Chen K, Yang W, Zhang W, Zhu R, Gong Q. Adv. Mater. 2017;29:1604758. doi: 10.1002/adma.201604758. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Trinh MT, Niesner D, Zhu H, Norman Z, Owen JS, Yaffe O, Kudisch BJ, Zhu XY. J Am. Chem. Soc. 2015;137:2089–2096. doi: 10.1021/ja512833n. [DOI] [PubMed] [Google Scholar]
- 19.Shao Y, Fang Y, Li T, Wang Q, Dong Q, Deng Y, Yuan Y, Wei H, Wang M, Gruverman A, Shield J, Huang J. Energy Environ. Sci. 2016;9:1752–1759. [Google Scholar]
- 20.Abate A, Saliba M, Hollman DJ, Stranks SD, Wojciechowski K, Avolio R, Grancini G, Petrozza A, Snaith HJ. Nano Lett. 2014;14:3247–3254. doi: 10.1021/nl500627x. [DOI] [PubMed] [Google Scholar]
- 21.Noel NK, Abate A, Stranks SD, Parrott ES, Burlakov VM, Goriely A, Snaith HJ. ACS Nano. 2014;8:9815–9821. doi: 10.1021/nn5036476. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Buin A, Ip AH, Li W, Voznyy O, Comin R, Yuan M, Jeon S, Ning Z, McDowell JJ, Kanjanaboos P, Sun J-P, Lan X, Quan LN, Kim DH, Hill IG, Maksymovych P, Sargent EH. Nat. Commun. 2015;6:7081. doi: 10.1038/ncomms8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Liu C, Du P, Zheng J, Gong X. Energy Environ. Sci. 2015;8:1245–1255. [Google Scholar]
- 24.Chiang C-H, Wu C-G. Nat. Photonics. 2016;10:196–200. [Google Scholar]
- 25.Zhao Y, Zhou W, Ma W, Meng S, Li H, Wei J, Fu R, Liu K, Yu D, Zhao Q. ACS Energy Lett. 2016;1:266–272. [Google Scholar]
- 26.Shao Y, Xiao Z, Bi C, Yuan Y, Huang J. Nat. Commun. 2014;5:5784. doi: 10.1038/ncomms6784. [DOI] [PubMed] [Google Scholar]
- 27.Tian C, Castro E, Wang T, Betancourt-Solis G, Rodriguez G, Echegoyen L. ACS Appl. Mater. Interfaces. 2016;8:31426–31432. doi: 10.1021/acsami.6b10668. [DOI] [PubMed] [Google Scholar]
- 28.Kitaura S, Kurotobi K, Sato M, Takano Y, Umeyama T, Imahori H. Chem. Commun. 2012;48:8550–8552. doi: 10.1039/c2cc34078j. [DOI] [PubMed] [Google Scholar]
- 29.Meng X, Zhao G, Xu Q, Tan Za, Zhang Z, Jiang L, Shu C, Wang C, Li Y. Adv. Funct. Mater. 2014;24:158–163. [Google Scholar]
- 30.Tao R, Umeyama T, Kurotobi K, Imahori H. ACS Appl. Mater. Interfaces. 2014;6:17313–17322. doi: 10.1021/am5058794. [DOI] [PubMed] [Google Scholar]
- 31.Tao R, Umeyama T, Higashino T, Koganezawa T, Imahori H. ACS Appl. Mater. Interfaces. 2015;7:16676–16685. doi: 10.1021/acsami.5b04351. [DOI] [PubMed] [Google Scholar]
- 32.Tao R, Umeyama T, Higashino T, Koganezawa T, Imahori H. Chem. Commun. 2015;51:8233–8236. doi: 10.1039/c5cc01712b. [DOI] [PubMed] [Google Scholar]
- 33.Zhao F, Meng X, Feng Y, Jin Z, Zhou Q, Li H, Jiang L, Wang J, Li Y, Wang C. J Mater. Chem. A. 2015;3:14991–14995. [Google Scholar]
- 34.Zhang B, Subbiah J, Jones DJ, Wong WWH. Beilstein J. Org. Chem. 2016;12:903–911. doi: 10.3762/bjoc.12.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Z, Geng X, He D, Jia X, Ding L. Energy Environ. Sci. 2016;9:2114–2121. [Google Scholar]
- 36.Cao T, Chen N, Liu G, Wan Y, Perea JD, Xia Y, Wang Z, Song B, Li N, Li X, Zhou Y, Brabec CJ, Li Y. J Mater. Chem. A. 2017;5:10206–10219. [Google Scholar]
- 37.Umeyama T, Miyata T, Jakowetz AC, Shibata S, Kurotobi K, Higashino T, Koganezawa T, Tsujimoto M, Gelinas S, Matsuda W, Seki S, Friend RH, Imahori H. Chem. Sci. 2017;8:181–188. doi: 10.1039/c6sc02950g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y, Chen B, Zhao F, Zheng X, Deng Y, Shao Y, Fang Y, Bai Y, Wang C, Huang J. Adv. Mater. 2017:1700607. doi: 10.1002/adma.201700607. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F, Shi W, Luo J, Pellet N, Yi C, Li X, Zhao X, Dennis TJS, Li X, Wang S, Xiao Y, Zakeeruddin SM, Bi D, Grätzel M. Adv. Mater. 2017:1606806. doi: 10.1002/adma.201606806. [DOI] [PubMed] [Google Scholar]
- 40.Castro E, Martinez ZS, Seong CS, Cabrera-Espinoza A, Ruiz M, Hernandez AG, Valdez F, Llano M, Echegoyen LA. J Med. Chem. 2016;59:10963–10973. doi: 10.1021/acs.jmedchem.6b00994. [DOI] [PubMed] [Google Scholar]
- 41.Cardona CM, Li W, Kaifer AE, Stockdale D, Bazan GC. Adv. Mater. 2011;23:2367–2371. doi: 10.1002/adma.201004554. [DOI] [PubMed] [Google Scholar]
- 42.Mihailetchi VD, Wildeman J, Blom PWM. Phys. Rev. Lett. 2005;94:126602. doi: 10.1103/PhysRevLett.94.126602. [DOI] [PubMed] [Google Scholar]
- 43.Lou Y-H, Wang Z-K, Yuan D-X, Okada H, Liao L-S. Appl. Phys. Lett. 2014;105:113301. [Google Scholar]
- 44.Wang Z, Lou Y, Naka S, Okada H. Appl. Phys. Lett. 2011;98:063302. [Google Scholar]
- 45.Lou Y-H, Xu M-F, Zhang L, Wang Z-K, Naka S, Okada H, Liao L-S. Org. Electron. 2013;14:2698–2704. [Google Scholar]
- 46.You J, Hong Z, Yang Y, Chen Q, Cai M, Song T-B, Chen C-C, Lu S, Liu Y, Zhou H. ACS Nano. 2014;8:1674–1680. doi: 10.1021/nn406020d. [DOI] [PubMed] [Google Scholar]
- 47.Liang P-W, Liao C-Y, Chueh C-C, Zuo F, Williams ST, Xin X-K, Lin J, Jen AKY. Adv. Mater. 2014;26:3748–3754. doi: 10.1002/adma.201400231. [DOI] [PubMed] [Google Scholar]
- 48.Grätzel M. Nat. Mater. 2014;13:838–842. doi: 10.1038/nmat4065. [DOI] [PubMed] [Google Scholar]
- 49.Niu G, Guo X, Wang L. J Mater. Chem. A. 2015;3:8970–8980. [Google Scholar]
- 50.Chen Q, Zhou H, Hong Z, Luo S, Duan H-S, Wang H-H, Liu Y, Li G, Yang Y. J Am. Chem. Soc. 2014;136:622–625. doi: 10.1021/ja411509g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.