Abstract
Background
The leading cause of death for cancer is lung cancer, of which the majority subtype is non-small cell lung cancer (NSCLC). Recent studies have shown long non-coding RNAs are transcribed and contribute to cancer. Previous study has shown that a few single nucleotide polymorphisms (SNPs) in myocardial infarction associated transcript (MIAT) were associated with some diseases or function as competing endogenous RNA (ceRNA) in some cancer.
Patients and methods
We performed bioinformatic methods for analyzing RNA-seq and miRNA-seq data of NSCLC from The Cancer Genome Atlas database. 1352 NSCLC patients and 1320 cancer-free controls for genotyping, and dual luciferase reporter assay, real-time PCR are performed in A549 and H1975 lung cancer cell lines. Results are analyzed by SPSS v16.0.
Results
In the present study, we focus on the role of over-expression MIAT in NSCLC. We confirmed that rs1061451 T>C (allele odds ratio = 0.22; P < 0.01) was associated with NSCLC. Furthermore, we constructed MIAT-centric ceRNA network, and three mRNAs (MYO1B, SGK1 and WNT9A) was identified as targets by MIAT via miR-133a-5p.
Conclusion
C-containing genotypes of MIAT rs1061451 were protective factor of NSCLC, and MIAT, which may act as ceRNA via miR-133a-5p, modulated MYO1B, SGK1 and WNT9A expression level.
Keywords: non-coding RNA, non-small-cell lung cancer, single-nucleotide polymorphism, competing endogenous RNA
Introduction
Thanks to Genomic Projects, it is evident that more than three quarters of the human genome is actively transcribed as non-coding RNAs (ncRNAs),1 which play major biological roles in the regulation of RNA expression by influencing cellular development and other functions.2 Based on the transcript size, ncRNAs can be grouped into microRNA (miRNA, 20–23 nucleotides) and long non-coding RNA (lncRNA, >200 nucleotides).3 Numerous studies have proved that miRNAs can post-transcriptionally repress translation or degrade mRNAs expression via binding to the 3′-untranslated region (3′-UTR) of the target gene.4
In addition, more and more research studies have shifted to lncRNA, which plays vital roles in various physiological and pathological processes.5 Recently, studies identified that lncRNA post-transcriptionally regulates the expression of mRNA by competing for miRNA.38 Interference of dysregulated lncRNA and mRNAs (with complementary sequence of lncRNA) affected the expression level of target mRNAs through miRNAs, together harbored by lncRNAs, and target mRNAs called miRNA response elements (MRE).6,7 This miRNA-regulated lncRNA and mRNA network could be a part of the “competing endogenous RNA (ceRNA) hypothesis” proposed by Salmena et al.8 The ceRNA crosstalk represented that ceRNAs, as miRNA sponges, harbored the same MREs that can be negatively regulated by miRNAs, indicating that ceRNAs communicate with each other by competing for a pool of shared miRNAs.9 Experimental evidence validated that dysregulated expression of key lncRNAs could disturb the balance of ceRNA network, thus leading to the initialization and development of various cancers.10
Lung cancer remains the most common cause of cancer-related mortality worldwide, even with the decline in incidence and mortality rates.11 Non-small-cell lung cancer (NSCLC), of which the two common histologic subtypes are lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC), accounts for ~80% of lung cancer.12 It has been proved that genetic markers play a key role in the occurrence and development of NSCLC, such as single-nucleotide polymorphisms (SNPs), by dysregulating gene expression and molecular pathway.13–16 Therefore, further exploration of lncRNA might complement the physiological and pathological cellular processes.
Myocardial infarction-associated transcript (MIAT) was first identified as lncRNA in 2006. Researchers have previously performed case–control association studies: an SNP in MIAT was significantly associated with myocardial infarction (MI) in Japanese subjects,17 and two SNPs were related to paranoid schizophrenia in the Chinese Han population.18 MIAT might also act as ceRNA in some other diseases.19,20 However, whether MIAT is a part of these two molecular mechanisms in lung cancer development has not been well delineated.
With this in mind, we performed a case–control study of rs1061541 on lncRNA MIAT and dual luciferase reporter assay of interaction between MIAT and miR-548e-3p through rs1061541, and then used bioinformatic methods and experiments to elucidate whether MIAT influences NSCLC by acting as ceRNA. This study focused on these two molecular mechanisms in lung cancer development.
Patients and methods
Analysis of differentially expressed genes (DEGs) in The Cancer Genome Atlas (TCGA) database
The RNA and miRNA expression data (level 3) and the corresponding clinical information of NSCLC patients were retrieved from TCGA database portal (https://tcga-data.nci.nih.gov/tcga/). Both RNA-seq data and miRNA-seq data of tumor tissues and adjacent non-tumor tissues were included, according to the histologic staging data. lncRNA and mRNA transcripts were annotated based on UCSC Genome Browser database (http://www.genome.ucsc.edu) database. After nor-malization of expression data, the DESeq package in R was used to identify the DEGs. Absolute fold change (including upregulation and downregulation) >2 and adjusted P-value < 0.05 were set as cutoffs. Co-expression network and important functional modules were discovered by “Weighted Gene Co-expression Network Analysis” (WGCNA) package.
Construction of ceRNA network
Based on the ceRNA hypothesis, the ceRNA network was constructed by negative regulation between miRNAs and their target genes. DEG expression profiles were processed using Pearson Correlation analysis to measure significantly negative regulation between miRNA and lncRNA or mRNA and positive correlation between lncRNA and mRNA (r > 0.8 in MIAT-mRNAs and r < −0.3 in miRNA-MIAT/mRNA, P < 0.05). In this study, lncRNA–miRNA and mRNA–miRNA interactions were determined according to mirCode (http://www.mircode.org). The intersection of competing lncRNA–miRNA–mRNA was chosen from shared regulatory miRNAs. lncRNA and mRNA were coexpressional and predicted to be targets of miRNAs. The ceRNA network was visualized by Cytoscape v3.4.0.
Patients and samples
The samples in the case–control study included 1352 cases with NSCLC and 1320 controls from Shanghai Jiao Tong University School of Public Health and the Fourth Affiliated Hospital of China Medical University. Cases were histologically confirmed lung cancer without radio- or chemotherapy. Controls without evidence of any cancer were recruited from the same hospital during the same time, based on frequency matched to cases by gender and age (±5 years). This study was approved by the institutional review board of China Medical University, and signed written informed consent forms were provided by each subject.
Genotyping
Genomic DNA samples were extracted according to conventional standard phenol–chloroform extraction. Genotyping of MIAT rs1061541 was detected by the TaqMan SNP genotyping assay using the ABI 7500 FAST real-time polymerase chain reaction (PCR) system (Thermo Fisher Scientific, Waltham, MA, USA) with primer and probe (assay ID C_2467718_1) purchased from Thermo Fisher Scientific. To validate the results, 5% of random samples were retested, with a consistency rate of 100%.
Cell lines and culture
Human lung cancer cell lines, A549 and H1975, purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), were cultured in RPMI-1640 medium with 10% fetal bovine serum (Thermo Fisher Scientific), 100 U/mL penicillin, 100 mg/mL streptomycin in air at 37°C with 5% CO2.
Dual luciferase reporter assay
Transfection was performed in 24-well plates and cultured until attachment, then co-transfection of miR-548e-3p with MIAT-rs1061541-C or MIAT-rs1061541-T mutant plasmids (GeneChem, Shanghai, China) in A549 cells was performed, respectively. Luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega Corporation, Fitchburg, WI, USA) according to the manufacturer’s protocol. Experimental results were normalized by Renilla luciferase activity for each transfected well and repeated three times.
Real-time PCR (RT-PCR)
A549 and H1975 cells were plated in six-well plates and transiently transfected with miR-133a-5p using PolyPlus reagent for 48 h, respectively, according to the manufacturer’s protocol. Total RNA was isolated using RNAiso Plus (KeyGEN BioTECH, Jiangsu, China) and reverse transcribed to cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio, Shiga, Japan) following the manufacturer’s instructions. GAPDH was used as internal reference. The RT-PCR of mRNA was performed using SYBR® Premix Ex Taq™ II (Takara). The RT-PCR of lncRNA was performed using TaqMan Gene Expression Master Mix and MIAT-specific TaqMan primers (assay ID: Hs00402814_m1) according to the manufacturer’s protocol (Thermo Fisher Scientific).
Statistical analysis
In genotype analysis, the χ2 test and Student’s t-test were used to examine difference between cases and controls in demographic variables. Logistic regression model was used to estimate the odds ratios (ORs) and their 95% confidence intervals (CIs). The rest of data were presented as mean ± SD. Student’s t-test (two-tailed) was used to compare two groups, and analysis of variance (ANOVA) with Bonfer-roni analysis was used in three groups. All these analyses were performed using Statistical Products and Services Solutions software (v.16.0, SPSS Inc., Chicago, IL, USA) unless otherwise specified. P-value <0.05 was considered statistically significant.
Results
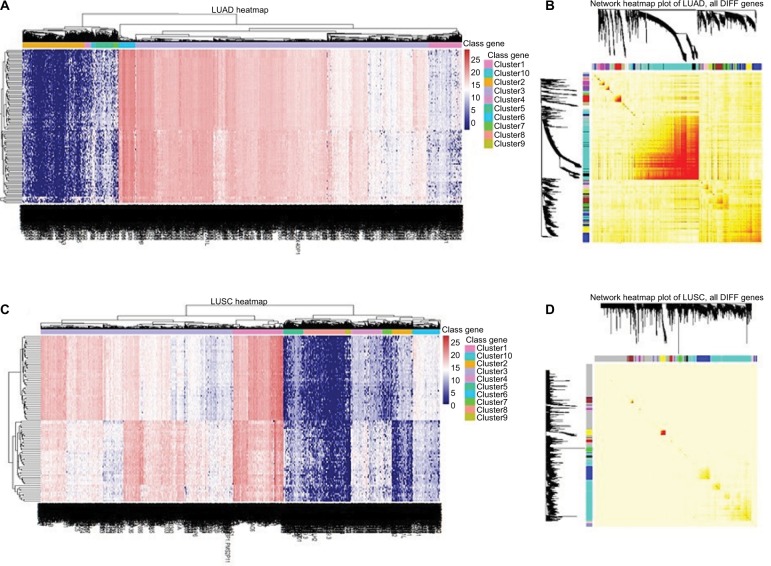
We identified that 161 miRNAs, 107 lncRNAs and 4070 mRNAs were differentially expressed between LUAD tissues and adjacent non-tumor tissues; 308 miRNAs, 86 lncRNAs and 3959 mRNAs were in LUSC tissues and adjacent non-tumor tissues (Figure 1). The expression of MIAT was found to be significantly increased in both histological subtypes.
Figure 1.
Heatmaps of differentially expressed genes in LUAD and LUSC.
Notes: Network visualization plots. (A and C) The heatmap shows the differentially expressed genes (rows: low expression in blue and high in red) in LUAD and LUSC, respectively. (B and D) Visualizing expression pattern network using a heatmap plot by WGCNA (B for LUAD and D, for LUSC). The heatmap depicts the TOM among all differentially expressed genes in the analysis. Light color represents low overlap and progressively darker red color represents higher overlap. Genes that could not be assigned to a module are labeled gray.
Abbreviations: DIFF, differentially expressed; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; TOM, topological overlap matrix; WGCNA, Weighted Gene Co-expression Network Analysis.
MIAT rs1061541 polymorphism in NSCLC
We focused on SNP rs1061541 of lncRNA MIAT according to lncRNASNP (a database of functional SNPs in lncRNAs, http://bioinfo.life.hust.edu.cn/lncRNASNP/). The secondary structure of MIAT transcripts is changed by rs1061541 polymorphisms and illustrated by the RNAfold program in Figure 2. Furthermore, SNP rs1061541 in lncRNA MIAT may create a binding site with miRNA-548e-3p and lead to a novel target site of miRNA-548e-3p and MIAT (Figure 3). To investigate whether MIAT rs1061541 polymorphisms influence the NSCLC, genotyping and Dual luciferase reporter assay were performed.
Figure 2.
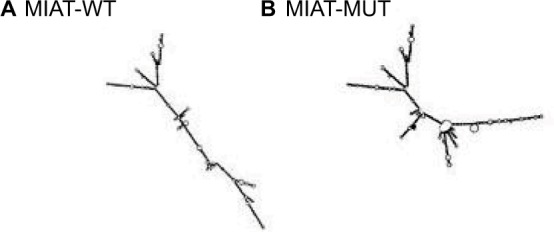
MIAT structure changes based on MIAT-WT (rs1061541-T) and MIAT-MUT (rs1061541-C)
Notes: The secondary structure of MIAT transcripts of (A) MIAT-WT (rs1061541-T) and (B) MIAT-MUT (rs1061541-C).
Abbreviations: MIAT, myocardial infarction-associated transcript; MUT, mutation; WT, wild type.
Figure 3.
Predicted binding sequences of miR-548e-3p to MIAT, rs1061541 shown in bold.
Abbreviation: MIAT, myocardial infarction-associated transcript.
The samples in this study comprised 1352 cases and 1320 controls. Mean values of age of cases and controls (mean ± SD) were 60.1 ± 12.7 and 59.2 ± 15.3 years, respectively. The difference was not statistically significant (t = 1.693, P = 0.091). No significant difference was found in gender or smoking status between cases and controls (P-values were 0.835 and 0.113, respectively). The abovementioned results suggested that case and control groups were comparable in important characteristics.
Table 1 shows the genotype distribution of MIAT SNP rs1061541 in cases and controls, as well as its associations with lung cancer risk. The frequency of the rs1061541 C allele in the controls was 0.191. The allele distributions were consistent with the Hardy–Weinberg equilibrium (χ2 = 0.77 and P = 0.38). For rs1061541 in MIAT gene, carriers of the CC genotype revealed a lower risk of lung cancer compared with the homozygous wild genotype (adjusted OR = 0.46, 95% CI = 0.29–0.74, P = 0.001). Individuals carrying at least one C allele (CT/CC) were less likely to develop lung cancer (adjusted OR = 0.14, 95% CI = 0.11–0.17, P < 0.001). Further analyses were carried out by allele comparison, and the C allele of rs1061541 was found to associate with a decreased risk of lung cancer with an OR of 0.22 (95% CI = 0.18–0.26, P < 0.001).
Table 1.
Genotypes of the single-nucleotide polymorphisms rs1061541 in lung cancer patients and control subjects and association with the risk of lung cancer
| Genotype | Cases, n | Controls, n | OR (95% CI) | P | Adjusted OR (95% CI) | Adjusted P |
|---|---|---|---|---|---|---|
| TT | 1245 | 860 | ||||
| CT | 74 | 417 | 0.12 (0.09–0.16) | <0.001 | 0.10 (0.08–0.14) | <0.001 |
| CC | 33 | 43 | 0.53 (0.33–0.84) | 0.007 | 0.46 (0.29–0.74) | 0.001 |
| CT + CC | 107 | 460 | 0.16 (0.13–0.20) | <0.001 | 0.14 (0.11–0.17) | <0.001 |
| T | 2564 | 2137 | ||||
| C | 140 | 503 | 0.23 (0.19–0.28) | <0.001 | 0.22 (0.18–0.26) | <0.001 |
Note: Adjusted by age, gender and smoking status.
Abbreviations: CI, confidence interval; OR, odds ratio.
Luciferase reporter assay was performed to further investigate whether rs1061541 polymorphisms could influence the expression levels of MIAT by creating binding sites for miR-548e-3p. It was predicted that such functional relevance and only one SNP rs1061541 existed in a potential binding seed in a public database. As shown in Figure 4, co-transfection of miR-548e-3p mimics with MIAT-rs1061541-T mutant plasmids resulted in significantly less luciferase activity; the same result was found in co-transfection of the miR-548e-3p mimics with the MIAT-rs1061541-C. Additionally, no significant difference was observed between MIAT-rs1061541-C and MIAT-rs1061541-T with miR-548e-3p mimics. These results revealed that luciferase activity was significantly reduced by cotransfecting both MIAT-rs1061541-C and MIAT-rs1061541-T constructs in the presence of miR-548e-3p, indicating that MIAT is a target of miR-548e-3p.
Figure 4.
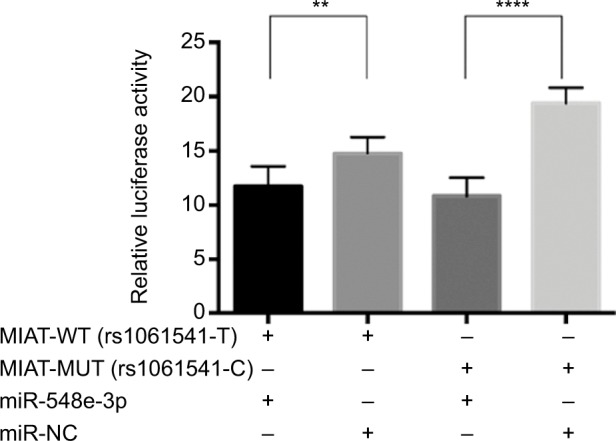
The results of luciferase reporter gene assay between miR-548e-3p and MIAT.
Notes: The effect of rs1061541 on MIAT interaction with miR-548e-3p. Relative reporter gene activity from constructs bearing an MIAT fragment with the rs1061541-T or rs1061541-C allele in the A549 cell lines. “+” means co-transfected with MIAT-WT (rs1061541-T) or MIAT-MUT (rs1061541-C) with miRNA mimics in A549. Both MIAT rs1061541-T and rs1061541-C co-transfected with miR-548e-3p mimics showed less luciferase activity compared with NC controls, respectively. Renilla luciferase/firefly luciferase was calculated and normalized to NC controls as relative luciferase activity. Results are shown as mean ± SD from three times repeated transfection experiments, each with six replicates. **P < 0.01; ****P < 0.0001.
Abbreviations: NC, negative control; MIAT, myocardial infarction-associated transcript; MUT, mutation; WT, wild type.
MIAT-centric ceRNA network
Based on common ceRNA hypothesis, if MIAT absorbs miRNA X away from mRNA Y, the expression levels of MIAT and mRNA Y would be positively correlated; meanwhile, MIAT and mRNA Y would be negatively correlated with miRNA X. To further investigate whether MIAT participated in the development of NSCLC as ceRNA, we performed relevant comparison analysis integrating mRNA, lncRNA and miRNA expression profiles.
A positive correlation was found between MIAT (red node) and myosin IB (MYO1B), serum/glucocorticoid regulated kinase 1 (SGK1) or Wnt family member 9A (WNT9A) (yellow nodes). The miR-133a-5p (green nodes) was negatively related to MYO1B, SGK1, WNT9A or MIAT (Figure 5). Another remarkable signature was that these four RNAs were predicted by mirCode as potential target genes of miR-133a-5p. A dys-regulated lncRNA–miRNA–mRNA ceRNA network could be constructed based on the abovementioned data.
Figure 5.
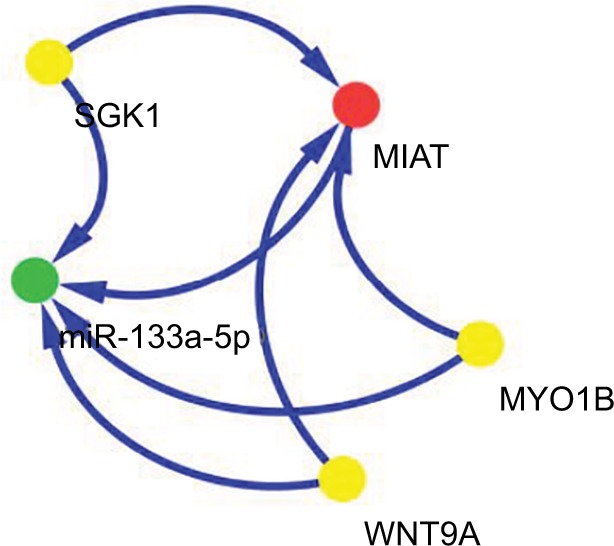
ceRNA network of MIAT in NSCLC.
Note: Red node represents lncRNA; yellow nodes represent mRNAs; and green node represents miRNA.
Abbreviations: ceRNA, competing endogenous RNA; lncRNA, long non-coding RNA; MIAT, myocardial infarction-associated transcript; MYO1B, myosin IB; NSCLC, non-small-cell lung cancer; SGK1, serum/glucocorticoid regulated kinase 1; WNT9A, Wnt family member 9A.
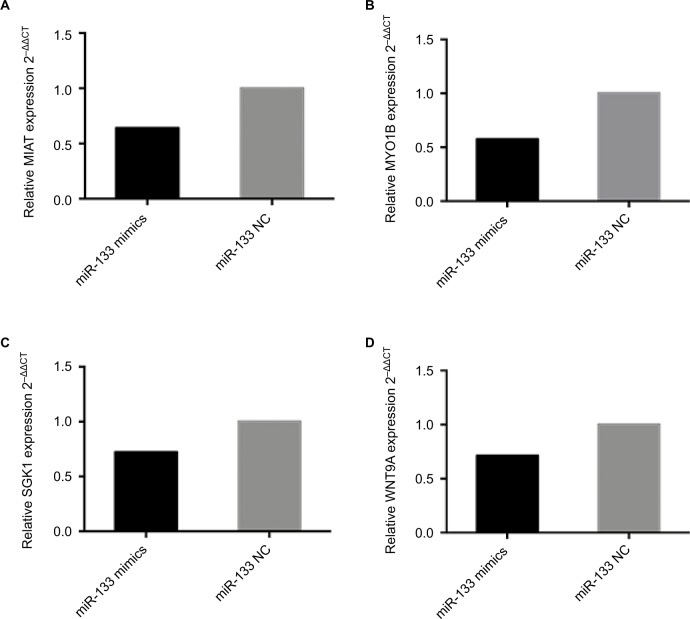
Subsequently, to confirm the reliability and validity of the abovementioned ceRNA network, RT-PCR was performed. We found that overexpression of miR-133a-5p in A549 cells instead of H1975 cells (data not shown) depressed the expression of MYO1B, SGK1, WNT9A and MIAT (Figure 6). Similar results were also shown in RT-PCR and bioinformat-ics analysis, indicating that our ceRNA network is credible.
Figure 6.
The expression levels of ceRNA in A549 following overexpression of miR-133 mimics compared to the negative control.
Notes: MIAT served as a ceRNA by binding miR-133a-5p. The levels of (A) MIAT, (B) MYO1B, (C) SGK1 and (D) WNT9A were significantly decreased, determined by RT-PCR in A549 cells transiently transfected with miR-133a-5p mimics. Results are represented as 2–ΔΔCT related to GAPDH levels from three experiments, each with six replicates. All P values are from two-sided t-tests.
Abbreviations: ceRNA, competing endogenous RNA; MIAT, myocardial infarction-associated transcript; MYO1B, myosin IB; NC, negative control; NSCLC, non-small-cell lung cancer; RT-PCR, real-time polymerase chain reaction; SGK1, serum/glucocorticoid regulated kinase 1; WNT9A, Wnt family member 9A.
Discussion
With the development of microarray and sequencing, non-coding transcripts showed tissue-specific expression profiles. Although some lncRNAs are transcribed, this fact does not necessarily imply that they perform crucial functions. However, a wealth of compelling evidence has proven that lncRNAs regulate gene expression through distinct transcriptional, post-transcriptional or epigenetic mechanisms.21 Recently, emerging evidence indicated that lncRNA transcripts showed biologically important functions, such as LUAD-associated transcript 1 (MALAT1),22,23 Homo sapiens TatD DNase domain containing 1 (TATDN1)24 and smoke and cancer-related long-chain ncRNA1 (SCAL1).25
MIAT, first identified as lncRNA a decade ago, was shown to affect endothelial cell functions17 and diabetic retinopathy.19 In this study, we focused on the lncRNA MIAT pathological processes in the development of NSCLC via two mechanisms.
First, we established a case–control study of the Chinese population to investigate SNPs on lncRNA MIAT in the development of NSCLC. Genotyping results showed that MIAT rs1061541 in the CC genotype (adjusted OR = 0.46, 95% CI = 0.29–0.74, P = 0.001) or the CT + CC (adjusted OR = 0.14, 95% CI = 0.11–0.17, P < 0.001) genotype acted as significantly protective factors compared with the TT genotype. Then, we found that miR-548e-3p could interact with MIAT-rs1061541-T and MIAT-rs1061541-C; hence we demonstrated that there is no clear functional relationship between SNP rs1061541 and miR-548e-3p. Ishii et al reported an association of MIAT rs2301523 with MI patients in a large-scale Japanese population.17 Additionally, MIAT rs1894720 minor allele T carriers showed increased risk for paranoid schizophrenia in the Chinese Han population.18 Together with these findings, the importance of SNP in MIAT miRNAs may not yet be fully discovered.
Second, according to the TCGA project, our results demonstrated that MIAT harbors MRE interacted with miR-133a-5p, and miR-133a-5p targets MYO1B, SGK1 and WNT9A. To do this, we first identified that differentially expressed miRNAs were predicted to target both MIAT and DEGs. For each network, we analyzed the correlation between MIAT-targeted mRNAs, MIAT-miRNAs and mRNAs-miRNAs. The robustness and reliability of the ceRNA network were validated by experiments. The reason why only A549 cells showed the tendency, rather than H1975, may be the different genetic backgrounds between A549 (KRASmut) and H1975 (EGFRmut). The underlying mechanism is probably that Pao et al26 and Massarelli et al’s27 groups established which KRAS mutations are less sensitive to EGFR tyrosine kinase inhibitors (TKIs) than the mutations within EGFR. MIAT-associated ceRNA by binding miR-133a-5p might steadily activate the Ras-Raf-MEK-MAPK pathways through KRAS mutation, resulting in the resistance to treatment with EGFR TKIs. H1975 cells for the threonine at position 790 (T790M) mutation and leucine at position 858 (L858R) are drug-sensitive mutations in EGFR-TKIs, via activating multiple downstream signal transduction pathways to maintain survival and proliferation.28–30 Researchers previously found that MIAT functions as ceRNA competed for miR-150-5p with VEGF in central nervous system disorders31 and sponged the same miRNA to indirectly downregulate the expression of the zinc finger E-box binding homeobox 1 (ZEB1) in NSCLC.32 However, our analysis suggested that potential MIAT-centric ceRNA pathways via miR-133a-5p influence the occurrence and development of NSCLC.
Decreased expression of miR-133a-5p was reported in NSCLC patients.33,34 In this study, we indicated that miR-133a-5p may play a key role in the MIAT-centric ceRNA network. Meanwhile, previous studies suggested that SGK1, the most represented member of the SGK family, showed high expression in NSCLC,35,36 and it was controlled by hormones (including gluco- and mineralocorticoids) and cellular stress (including cell shrinkage).37 Therefore, we represented an MIAT-miR-133a-5p-MYO1B\SGK1\WNT9A transcription ceRNA network, which may be a potential compensatory mechanism in NSCLC by using combined bioinformatics methods and experimental approach. More comprehensive work about MIAT ceRNA network needs to be explored.
Conclusion
This study highlighted that SNPs of lncRNA MIAT have an impact on gene expression and the epigenetic machinery in lung cancer. Based on the first part of our findings, we can conclude that MIAT rs1061451 C-containing genotypes function as a protective factor with the development of NSCLC in Han Chinese populations. Moreover, we used bioinformatic methods and molecular cell biology analyses to characterize functions of the rs1061541, and the results showed that there was no influence of the polymorphisms of rs1061541 in MIAT interaction with miR-548e-3p. The second part of our findings suggested that MIAT, acting as ceRNA, took part in the expression of three mRNAs (MYO1B, SGK1 and WNT9A) by binding miR-133a-5p. These findings suggested that a disturbance between lncRNAs, miRNAs and mRNAs might lead to disease, including cancer.
Acknowledgments
This study was supported by grant no. 81502878 from the National Natural Science Foundation of China and no. 201501017 from the Doctoral Research Project of Liaoning. The authors appreciate the participants in this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 3.Derrien T, Johnson R, Bussotti G, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan SH, Wang LH. Regulation of cancer metastasis by microRNAs. J of Biomedical Science. 2015;22:9. doi: 10.1186/s12929-015-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Ding L, Wang L, et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6(38):41045–41055. doi: 10.18632/oncotarget.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie X, Tang B, Xiao YF, et al. Long non-coding RNAs in colorectal cancer. Oncotarget. 2016;7(5):5226–5239. doi: 10.18632/oncotarget.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C, Wu D, Gao L, et al. Competing endogenous RNA networks in human cancer: hypothesis, validation, and perspectives. Oncotarget. 2016;7(12):13479–13490. doi: 10.18632/oncotarget.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng J, Zhang L, Yuan C, et al. Expression profile analysis of long noncoding RNA in ER-positive subtype breast cancer using microarray technique and bioinformatics. Cancer Manag Res. 2017;9:891–901. doi: 10.2147/CMAR.S151120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 12.Lu C, Chen H, Shan Z, Yang L. Identification of differentially expressed genes between lung adenocarcinoma and lung squamous cell carcinoma by gene expression profiling. Mol Med Rep. 2016;14(2):1483–1490. doi: 10.3892/mmr.2016.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SH, Zhang WJ, Wu XC, et al. Long non-coding RNA Malat1 promotes gallbladder cancer development by acting as a molecular sponge to regulate miR-206. Oncotarget. 2016;7(25):37857–37867. doi: 10.18632/oncotarget.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin Z, Cui Z, Ren Y, Xia L, Li H, Zhou B. MiR-146a polymorphism correlates with lung cancer risk in Chinese nonsmoking females. Oncotarget. 2017;8(2):2275–2283. doi: 10.18632/oncotarget.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lingzi X, Zhihua Y, Xuelian L, et al. Genetic variants in microRNAs predict non-small cell lung cancer prognosis in Chinese female population in a prospective cohort study. Oncotarget. 2016;7(50):83101–83114. doi: 10.18632/oncotarget.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agwa ES, Ma PC. Targeting the MET receptor tyrosine kinase in non-small cell lung cancer: emerging role of tivantinib. Cancer Manag Res. 2014;6:397–404. doi: 10.2147/CMAR.S37345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii N, Ozaki K, Sato H, et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51(12):1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 18.Rao SQ, Hu HL, Ye N, Shen Y, Xu Q. Genetic variants in long non-coding RNA MIAT contribute to risk of paranoid schizophrenia in a Chinese Han population. Schizophr Res. 2015;166(1–3):125–130. doi: 10.1016/j.schres.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Yan B, Yao J, Liu JY, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116(7):1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XH, Yuan YX, Rao SL, Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci. 2016;20(17):3653–3660. [PubMed] [Google Scholar]
- 21.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22(39):8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 24.Zequn N, Xuemei Z, Wei L, et al. The role and potential mechanisms of LncRNA-TATDN1 on metastasis and invasion of non-small cell lung cancer. Oncotarget. 2016;7(14):18219–18228. doi: 10.18632/oncotarget.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thai P, Statt S, Chen CH, Liang E, Campbell C, Wu R. Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines. Am J Respir Cell Mol Biol. 2013;49(2):204–211. doi: 10.1165/rcmb.2013-0159RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13(10):2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 28.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12(21):6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 30.Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66(16):7854–7858. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Q, Shan K, Qun-Wang X, et al. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget. 2016;7(31):49688–49698. doi: 10.18632/oncotarget.10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HY, Zheng FS, Yang W, Lu JB. The long non-coding RNA MIAT regulates zinc finger E-box binding homeobox 1 expression by sponging miR-150 and promoting cell invasion in non-small-cell lung cancer. Gene. 2017;633:61–65. doi: 10.1016/j.gene.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Lan D, Zhang X, He R, et al. MiR-133a is downregulated in non-small cell lung cancer: a study of clinical significance. Eur J Med Res. 2015;20(1):50. doi: 10.1186/s40001-015-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Li J, Chen H, et al. Down-regulation of miR-133a as a poor prognosticator in non-small cell lung cancer. Gene. 2016;591(2):333–337. doi: 10.1016/j.gene.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Abbruzzese C, Mattarocci S, Pizzuti L, et al. Determination of SGK1 mRNA in non-small cell lung cancer samples underlines high expression in squamous cell carcinomas. J Exp Clin Cancer Res. 2012;31:4. doi: 10.1186/1756-9966-31-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiaobo Y, Qiang L, Xiong Q, et al. Serum and glucocorticoid kinase 1 promoted the growth and migration of non-small cell lung cancer cells. Gene. 2016;576(1 pt 2):339–346. doi: 10.1016/j.gene.2015.10.072. [DOI] [PubMed] [Google Scholar]
- 37.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86(4):1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 38.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505(7483):344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]