ABSTRACT
The Tolosa–Hunt syndrome is a rare clinical condition characterized by painful opthalmoparesis associated with idiopathic granulomatous inflammation of the orbital apex and cavernous sinus. Historically, this condition was thought to result from arteritic changes in the internal carotid artery and cavernous sinus. Modern digital angiographic techniques were unavailable when THS was initially described, and few reports exist on its high-resolution angiographic findings. Painful ophthalmoparesis, especially of the oculomotor nerve, warrants vascular imaging because of the concern for an underlying aneurysm. Here, we describe angiographic findings of THS which may be useful for clinicians when encountering patients presenting with painful ophthalmoplegia.
KEYWORDS: Angiography, cavernous sinus, headache disorders, neuroimaging, oculomotor nerve, Tolosa–Hunt syndrome
Introduction
Tolosa–Hunt syndrome (THS) is a rare condition characterized by painful ophthalmoparesis and idiopathic granulomatous inflammation of the cavernous sinus, classically extremely responsive to corticosteroid administration.1–3 While the magnetic resonance imaging (MRI) findings in THS have been well characterized, the condition’s angiographic features are less known, partly because many existing reports predate the advent of high-resolution digital subtraction angiography (DSA).4–6 Here, we describe angiographic findings of a case of THS and report angiographic improvement in these findings following corticosteroid treatment.
Case presentation
History
A 26-year-old otherwise healthy woman presented with right sided headaches and associated right retrobulbar discomfort, with onset 2 weeks prior to presentation. She had a longstanding history of intermittent, throbbing left-sided headaches with accompanying mild nausea or vomiting, but this was a new headache. She then developed diplopia which progressed to complete oculomotor nerve palsy in the right eye over the course of 2 days. She denied any ocular trauma, recent illness, viral syndrome, fevers, chills, and other constitutional complaints. Past medical history was notable for left parietal sinus pericranii treated in childhood with staged bleomycin sclerotherapy and a history of a left cerebellar developmental venous anomaly. There was no history of ocular surgery. Family history was notable for an indirect carotid-cavernous fistula in her father treated with transvenous coil embolization.
Examination
The eyes were quiet, without chemosis or proptosis. There was complete right ptosis. Uncorrected near visual acuity was 20/20 in the left eye and 20/200 in the right eye. Colour vision was 8/8 in both eyes using Ishihara pseudo-isochromatic plates. The right pupil was fixed at 6.5 mm without an afferent pupillary defect by the reverse APD technique. Confrontation fields were full. There was exotropia and hypotropia of the right eye. Elevation, depression, and adduction were markedly impaired in the right eye, as was intortion. Abduction was full. Intra-ocular pressures were normal at 12 mmHg in the right eye and 16 mmHg on the left. Funduscopic examination was normal without disc pallor or papilledema. The overall examination was consistent with complete right oculomotor and trochlear nerve palsies. The remainder of the neurological exam, including facial sensation, was unremarkable.
Laboratory and radiologic evaluation
Laboratory evaluation including serum chemistry and complete blood counts was unremarkable. Cerebrospinal fluid (CSF) examination was normal with a nucleated cell count of 1 cell/µl; however, a lymphocytic predominance was noted. CSF ACE levels were normal. Serum ANA, ANCA, and serum protein electrophoresis were within normal limits. Serum autoantibodies including SS-A, SS-B, and Anti-Sm were also negative.
An MRI of the brain with and without contrast demonstrated inflammatory stranding and enhancement of the right orbital apex and perineural fat with extension into the lateral wall of the right cavernous sinus (Figure 1). A diagnostic cerebral angiogram was performed with injection into the right internal and external carotid arteries. The right external carotid injection demonstrated enlargement of the terminal branches of the right internal maxillary artery, the arteries of foramina rotundum and ovale, with no narrowing of the cavernous carotid. The distal terminus of these vessels gave rise to a hypervascular network within the lateral wall of the right cavernous sinus indicative of a vascular inflammatory process (Figure 2). This cavernous sinus lesion further received contribution from a branch of the accessory meningeal artery, through the foramen ovale. Notably, there was no evidence of early venous drainage to suggest a carotid-cavernous or other fistula. Additionally, a 3-mm aneurysm of the right meningohyophyseal trunk within the cavernous sinus was noted upon injection into the right internal carotid artery (Figure 3).
Figure 1.
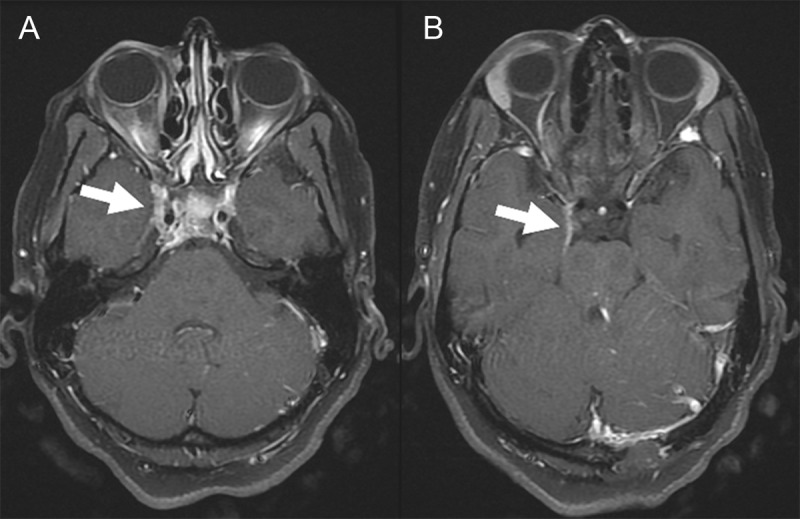
Panels (A, B) Post-contrast T1-weighted MRI of the brain demonstrating pathological enhancement of the right cavernous sinus. The visible left occipital defect is a result of the patient’s prior sinus pericranii.
Figure 2.
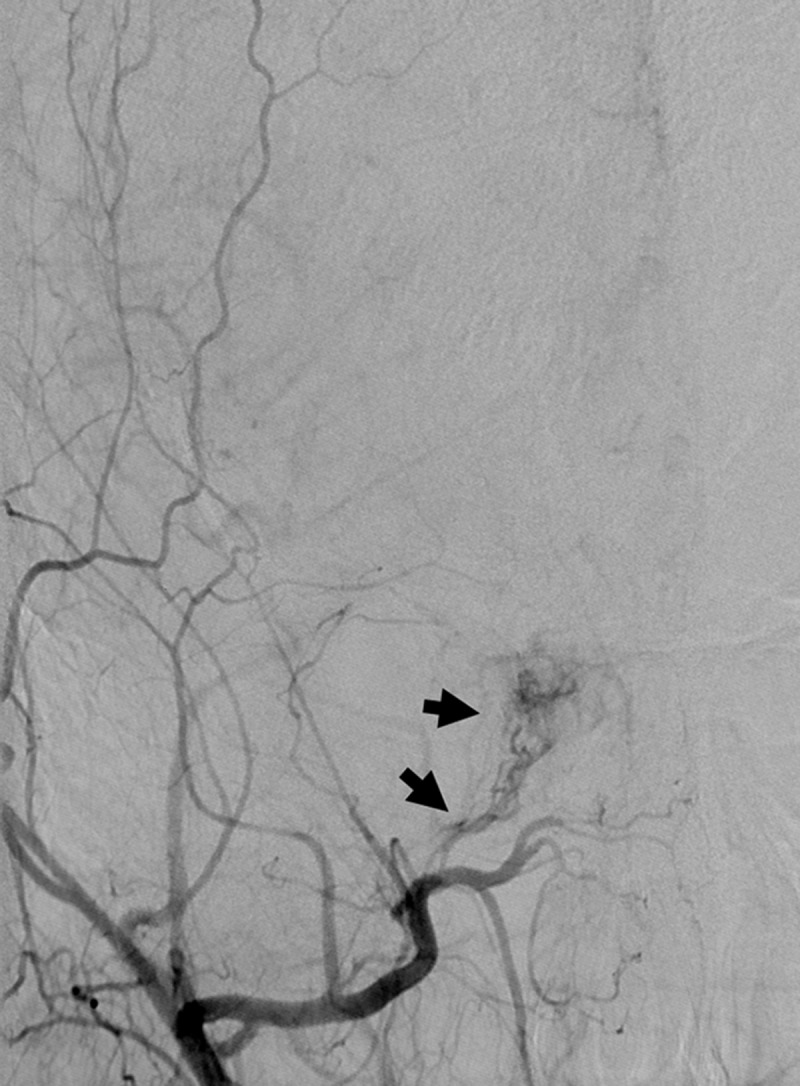
AP projection after contrast injection into the right external carotid artery demonstrates hypervascularity of the right cavernous sinus principally derived from terminal branches of the internal maxillary artery and arteries of foramina rotundum and ovale.
Figure 3.
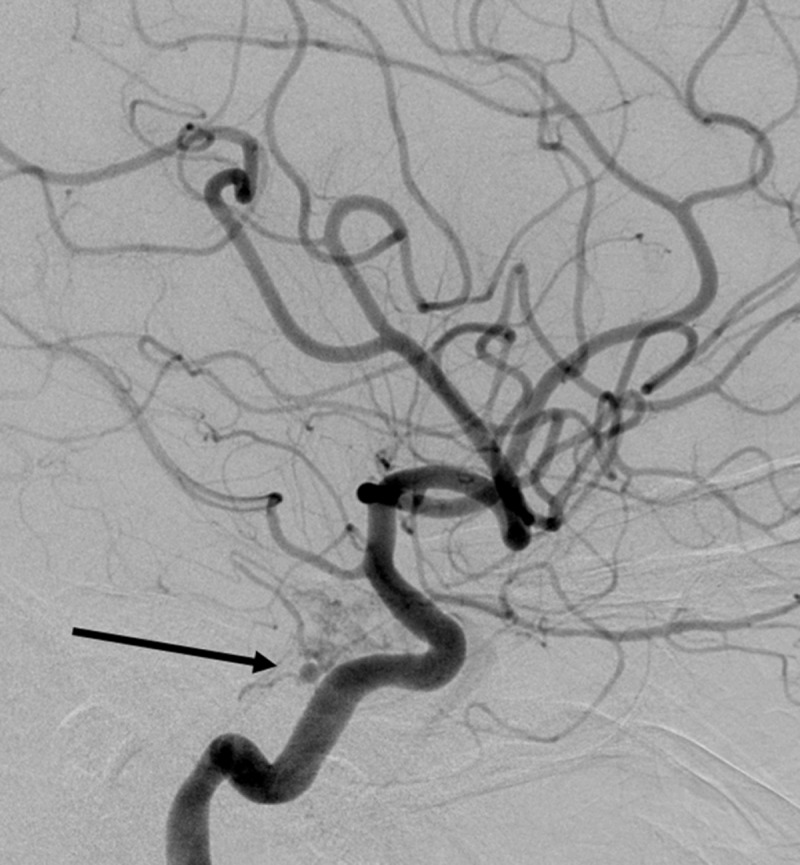
Lateral projection after contrast injection into the right internal carotid artery demonstrating a 3-mm aneurysm arising from the origin of the meningohypophyseal trunk at the cavernous segment of the internal carotid artery.
Clinical course
The patient underwent evaluation as an inpatient by neurology, neurosurgery, ophthalmology, and rheumatology. After rheumatology service ruled out any underlying systemic condition, she was diagnosed with THS and was started on a regimen of oral prednisone 100 mg qd for 3 days, followed by prolonged taper. She reported improvement in her headaches and in eye opening after 2 weeks of treatment. Near visual acuity in the right eye improved to 20/25 once the oculomotor nerve palsy improved and the right eye could accommodate. Four weeks after initial presentation, a follow-up diagnostic cerebral angiogram was performed. This study showed near-complete resolution of the hypervascularity previously seen in the right cavernous sinus. Additionally, the associated right meningohypophyseal trunk aneurysm had involuted (Figure 4). An MRI of the brain performed 7 weeks after presentation demonstrated marked improvement in the stranding in the orbital apex soft tissues and near-complete resolution of the cavernous sinus enhancement (Figure 5).
Figure 4.
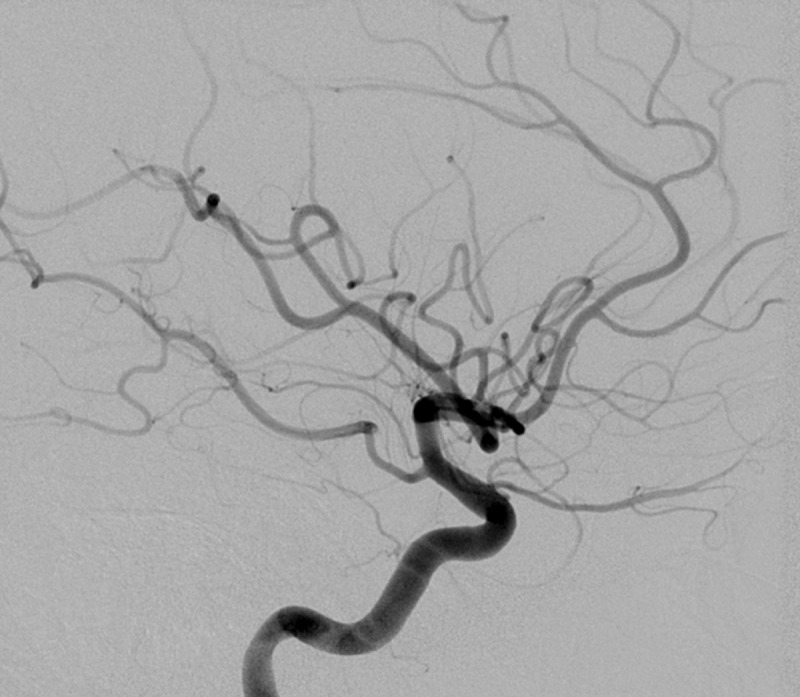
Follow-up internal carotid arteriogram showing interval resolution of the previously demonstrated meningohypophyseal aneurysm after treatment with prednisone. Hypervascularity of the cavernous sinus has also resolved.
Figure 5.
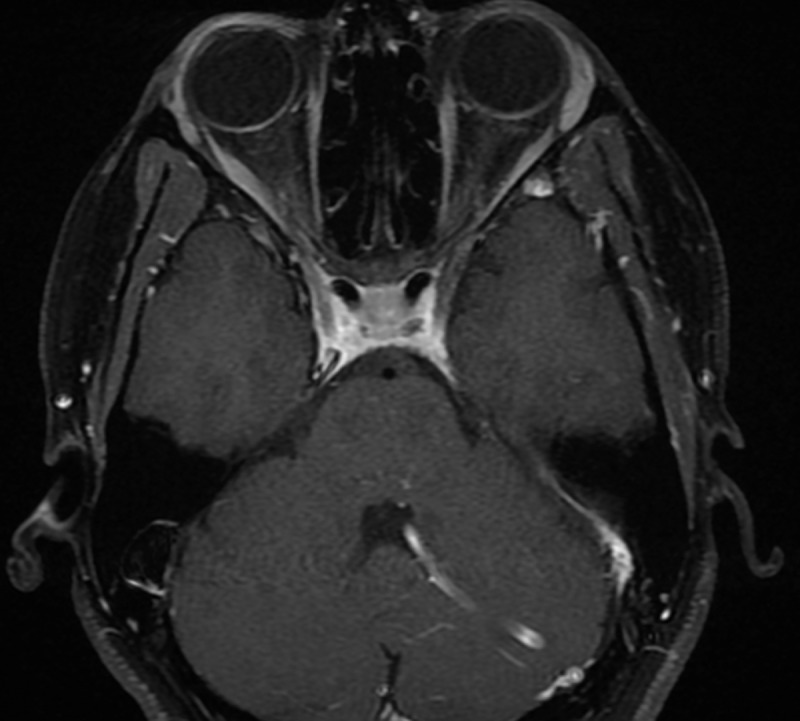
Post-contrast T1-weighted MRI of the brain completed 4 weeks after symptom onset which shows interval resolution of cavernous sinus enhancement.
Discussion
THS was originally described in 1954, in a patient who presented with left orbital pain associated with ipsilateral visual loss and ophthalmoplegia, with stenosis noted in the ICA at angiography. At autopsy, significant granulomatous inflammation of the affected artery was observed.1 In 1961, Hunt and colleagues described a case series of six patients who had presented with painful ophthalmoplegia in conjunction with cavernous sinus inflammation and hence formally characterized the syndrome through a set of diagnostic criteria.2 Numerous case series have validated their original findings, though epidemiological data on incidence remain scant. Technological advances in neuroimaging have provided further anatomical insight allowing more direct visualization of the cavernous sinus; however, the underlying aetiology in THS remains unclear.
Current diagnostic criteria defined by the International Classification of Headache Disorders 3rd edition consist of unilateral orbital pain, paresis of one or more of cranial nerves III, IV, or VI, with demonstrable inflammation of the cavernous sinus.7 THS remains a diagnosis of exclusion. Tumour, vasculitis, neurosarcoidosis, basal meningitis, and diabetic ophthalmoplegia can produce similar findings.8
MRI findings in THS typically show abnormal soft tissue in the orbital apex and marked post-contrast enhancement within the superior orbital fissure and cavernous sinus.9,10 Angiography is undertaken primarily to exclude carotid-cavernous fistulae or cavernous segment aneurysms. While only limited to a handful of case reports, previously described cerebral angiographic findings have included focal narrowing of the cavernous ICA.11 Historically, orbital venography has been used as an adjunct modality in the imaging diagnosis of THS, though now an outdated technique given the widespread use of DSA.10,12
In the absence of accurate recognition and prompt corticosteroid treatment, the natural history of the syndrome is known to be unpredictable. A review of 146 cases shows a recurrence rate of 39%.13 Where systemic steroid therapy is administered, the majority of patients exhibit rapid resolution of symptoms.14 Indeed, it has been previously suggested that resolution of MRI findings following corticosteroid treatment should be considered indicative of THS.4
Here, we describe the high-resolution angiographic findings in a case of THS and report the resolution of these features following high-dose prednisone treatment. Although carotid-cavernous fistula was also in the differential, there was no evidence of early venous drainage to suggest this diagnosis. Biopsy and histological analysis were not performed, given the favourable response to systemic steroid therapy.
To date, only three reports have described the presence of an aneurysm in association with THS.15–17 From a total of four reported aneurysms, a decrease in aneurysm size was observed in two following high-dose steroid administration.15,16 In Kambe and colleagues’ report of a patient with THS and a concurrent bilateral aneurysm, the aneurysm on one side decreased in size following treatment with steroids, while the contralateral side required endovascular intervention; Takasuna and colleagues furthermore reported an increase in aneurysm size following steroid administration15,17 Though the mechanism underpinning aneurysm formation is unknown, we hypothesize that the perivascular inflammatory state led to the vessel wall integrity being compromised, perhaps owing to local matrix metalloproteinase activity.
The presence of an aneurysm in association with inflammatory changes of the cavernous sinus and internal carotid artery, as exhibited in our case, is a very rare finding in this syndrome. In the evaluation of patients with cavernous sinus syndromes, this report may serve as a valuable reference to raise suspicion for THS.
Declaration of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the writing and content of the article.
References
- [1].Tolosa E. Periarteritic lesions of the carotid siphon with the clinical features of a carotid infraclinoidal aneurysm. 1954;17(4):300–302. doi: 10.1136/jnnp.17.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hunt WE, Meagher JN, LeFever HE, Zeman W.. Painful ophthalmoplegia. Its relation to indolent inflammation of the cavernous sinus. 1961;11(1):56. doi: 10.1212/WNL.11.1.56. [DOI] [PubMed] [Google Scholar]
- [3].Zhang X, Zhou Z, Steiner TJ, Zhang W, Liu R, Dong Z, Wang X, Wang R, Yu S. Validation of ICHD-3 beta diagnostic criteria for 13.7 Tolosa-Hunt syndrome: analysis of 77 cases of painful ophthalmoplegia. 2014;34(8):624–632. doi: 10.1177/0333102413520082. [DOI] [PubMed] [Google Scholar]
- [4].Jain R, Sawhney S, Koul RL, Chand P. Tolosa–Hunt syndrome: MRI appearances. 2008;52(5):447–451. [DOI] [PubMed] [Google Scholar]
- [5].Arcaya AA, Cerezal L, Canga A, Polo JM, Berciano J, Pascual J. Neuroimaging diagnosis of Tolosa–Hunt syndrome: MRI contribution. Headache. 1999;39(5):321–325. doi: 10.1046/j.1526-4610.1999.3905321.x. [DOI] [PubMed] [Google Scholar]
- [6].Çakirer S. MRI findings in the patients with the presumptive clinical diagnosis of Tolosa-Hunt syndrome. 2003;13(1):17–28. [DOI] [PubMed] [Google Scholar]
- [7].Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, (beta version). 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- [8].Gladstone JP. An approach to the patient with painful ophthalmoplegia, with a focus on Tolosa–Hunt syndrome. 2007;11(4):317–325. doi: 10.1007/s11916-007-0211-7. [DOI] [PubMed] [Google Scholar]
- [9].Pascual J, Cerezal L, Canga A, De Arcaya AA, Polo JM, Berciano J. Tolosa–Hunt syndrome: focus on MRI diagnosis. 1999;19(25 suppl):36–38. doi: 10.1177/0333102499019S2509. [DOI] [PubMed] [Google Scholar]
- [10].Kline LB, Hoyt WF. The Tolosa–Hunt syndrome. 2001;71(5):577–582. doi: 10.1136/jnnp.71.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sondheimer FK, Knapp J. Angiographic findings in the Tolosa–Hunt syndrome: painful ophthalmoplegia 1. 1973;106(1):105–112. doi: 10.1148/106.1.105. [DOI] [PubMed] [Google Scholar]
- [12].Muhletaler CA, Gerlock AJ Jr. Orbital venography in painful ophthalmoplegia (Tolosa–Hunt syndrome). 1979;133(1):31–34. doi: 10.2214/ajr.133.1.31. [DOI] [PubMed] [Google Scholar]
- [13].Kline LB. The Tolosa–Hunt syndrome. 1982;27(2):79–95. doi: 10.1016/0039-6257(82)90190-4. [DOI] [PubMed] [Google Scholar]
- [14].Smith JL, Taxdal DS Painful ophthalmoplegia*: the Tolosa–Hunt syndrome. 1966;61(6):1466–1472. doi: 10.1016/0002-9394(66)90487-9. [DOI] [PubMed] [Google Scholar]
- [15].Kambe A, Tanaka Y, Numata H, Kawakami S, Kurosaki M, Ohtake M, Kamitani H, Miyata H, Ohama E, Watanabe T. A case of Tolosa–Hunt syndrome affecting both the cavernous sinuses and the hypophysis, and associated with C3 and C4 aneurysms. 2006;65(3):304–307. doi: 10.1016/j.surneu.2005.06.046. [DOI] [PubMed] [Google Scholar]
- [16].Zhou Z, Zhou G, Lu T, Xu G, Liu X. Tolosa–Hunt syndrome with reversible dissection aneurysm. 2010;31(6):777–779. doi: 10.1007/s10072-010-0231-7. [DOI] [PubMed] [Google Scholar]
- [17].Takasuna H, Sasaki R, Shiraishi M, Doi M, Wakui D, Ito H, Oshio K, Tanaka Y. Steroid-resistant Tolosa–Hunt syndrome with a de novo intracavernous aneurysm: A case report. 2016;7(Suppl 30):S779. doi: 10.4103/2152-7806.193925. [DOI] [PMC free article] [PubMed] [Google Scholar]


