Abstract
Technological advances in microfabrication techniques in combination with organotypic cell and tissue models have enabled the realization of microphysiological systems capable of recapitulating aspects of human physiology in vitro with great fidelity. Concurrently, a number of analysis techniques has been developed to probe and characterize these model systems. However, many assays are still performed off-line, which severely compromises the possibility to obtain real-time information from the samples under examination, and which also limits the use of these platforms in high-throughput analysis.
In this review, we focus on sensing and actuation schemes that have already been established or offer great potential to provide in situ detection or manipulation of relevant cell or tissue samples in microphysiological platforms. We will first describe methods that can be integrated in a straightforward way and that offer potential multiplexing and/or parallelization of sensing and actuation functions. These methods include electrical impedance spectroscopy, electrochemical biosensors, and the use of surface acoustic waves for manipulation and analysis of cells, tissue, and multicellular organisms. In the second part, we will describe two sensor approaches based on surface-plasmon resonance and mechanical resonators that have recently provided new characterization features for biological samples, while technological limitations for use in high-throughput applications still exist.
Keywords: Microphysiological Systems: Model systems able to recapitulate aspects of human physiology in vitro; Body-on-Chip: Combination of multiple interconnected organotypic model tissues on a test platform; Microfluidics: Science and technology of fluids in sub-millimeter scale regimes; High-Throughput Screening: Methods commonly applied in drug discovery for the rapid screening of large libraries of compounds, and usually based on the automation of the analysis procedure; Integrated Sensors: Sensors installed and operated within a cell-culture platform; Electrochemical Biosensors: Sensors that produce an electrical signal proportional to the analyte concentration; Electrical Impedance Spectroscopy: Method to measure the dielectric properties of samples; Acoustic-Wave Sensors and Actuators: Devices based on the generation of acoustic waves in piezoelectric materials for sensing and actuation applications
1. Introduction
Over the past ten years, there has been a growing interest in the development of microphysiological systems (MPSs) that are capable of recapitulating aspects of human physiology in vitro.1,2 These systems are key to enhancing our capability to develop disease models and improve the drug-development pipeline, and they also represent a major milestone for testing treatment options for personalized-medicine applications in vitro. In an effort to realize such systems, several microfluidic platforms have been developed with the goal of mimicking physiological conditions in cell cultures.3 Compared to standard static culture conditions, as they are usually applied in well plates or culture dishes, these MPS platforms offer several pivotal features:
physiologically relevant flow conditions4
possibility to mimic flow-induced shear stresses5
realization of cell-to-cell or tissue-to-tissue interactions6
small medium-to-cell/tissue volume ratios, which enable the detection of low-level biomarker secretion7
automated and continuous medium exchange for replenishing nutrients8
extraction and aliquoting of the supernatants for downstream analysis9
comparably simple and straightforward operation.
Furthermore, such MPS platforms can often be combined with novel techniques for controlling culture conditions to enable precise cell manipulations on chip in order to target specific biological questions.10
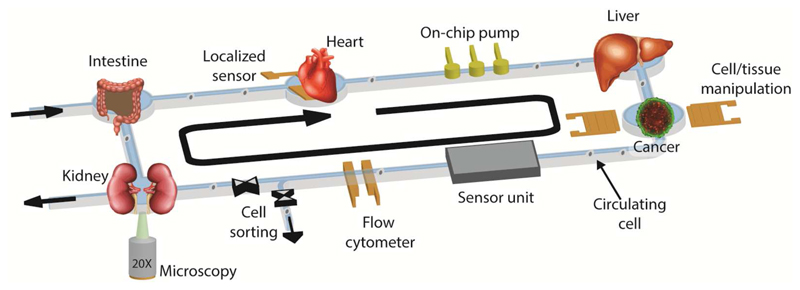
Concurrently, advances in the development of biological model systems, such as the ability to form tissues from primary human cells or induced pluripotent stem cells, have yielded representative models of human tissues and organs for in vitro applications.1,11,12 However, numerous limitations still exist in such systems, in particular for obtaining information or performing manipulation of the tested samples in real time. Several characterization methods, such as viability assays and biomarker quantification to assess either functionality or cytotoxicity are prevailingly performed off-chip and/or may be limited to end-point assays. The addition of on-line features and analysis/manipulation methods and the possibility to parallelize analysis and characterization of the samples would massively add to taking full advantage of these in vitro microphysiological model systems (Fig. 1). The integration of sensors within a culture platform usually entails higher sensitivity and temporal resolution, as analytes are not diluted. Moreover, high spatial resolution can be achieved through integration, so that heterogeneities in the concentrations of metabolites in the overall cell/tissue system can be detected.13
Figure 1.
Schematic representation of an integrated microphysiological system. Multiple interconnected organotypic microtissue models can be co-cultured in the platform to enable tissue-to-tissue interactions. The pumping and related flow mimics physiological shear stress on the tissues. The integrated sensors and actuators enable in situ monitoring, characterization and manipulation of the tissue models and of potentially circulating cells.
This review will present and discuss different classes of sensors and actuators, the use of which in MPSs has already been demonstrated, or which - in our opinion - offer great potential for integration in MPSs, also with respect to high-throughput analysis. As the field is still relatively young, standards for fluidic and electronic connections and for the design of such platforms are yet to be established. Definition of such standards will be imperative to ensure adoption of MPSs in industrial settings.
For this review, we have decided to focus on methods that could be readily addressed and controlled by simple, parallelizable electronic systems and that offer the potential of straightforward integration with cell-culture environments. We will start with a description of electrical impedance spectroscopy and electrochemical biosensors and their applications with a broad range of biological samples. Although highly integrated microelectrode array (MEA) systems have been developed for in vitro and in vivo applications, we will not cover these systems here, as their application is limited to a few cell types, so-called “electrogenic” cells including mostly cardiomyocytes and neuronal cells.14,15 In the second part of this review, we will discuss surface-plasmon-resonance (SPR)-based sensors and mechanical micro- and nanosensors. Although these methods have so far shown limited parallelization potential, they have been successfully operated inside cell-culture environments and provide attractive characterization features for biological samples. Finally, with the exception of SPR, we have decided to not include optical methods, such as fluorescence-based methods or bead-based assays, as the scope of this review would have otherwise become too broad.
2. Electrical Impedance Spectroscopy
Electrical impedance spectroscopy (EIS) is a non-invasive, label-free method to measure the dielectric properties of samples while applying an AC electrical field by means of electrodes. The work on impedance measurements of biological samples was pioneered by Hoeber and Fricke at the beginning of the 20th century.16,17 Following their approach, single-cell impedance measurements on Nitella cells were made in 1937 by Curtis and Cole.18 With the advent of microfluidic systems, integration of electrodes in microfluidic platforms has enabled EIS measurements of a wide variety of biological samples. In this section, we will summarize the different technological approaches for impedance-based characterization of single cells, cell cultures, multi-cellular tissues, and organisms.
2.1. Impedance Cytometers
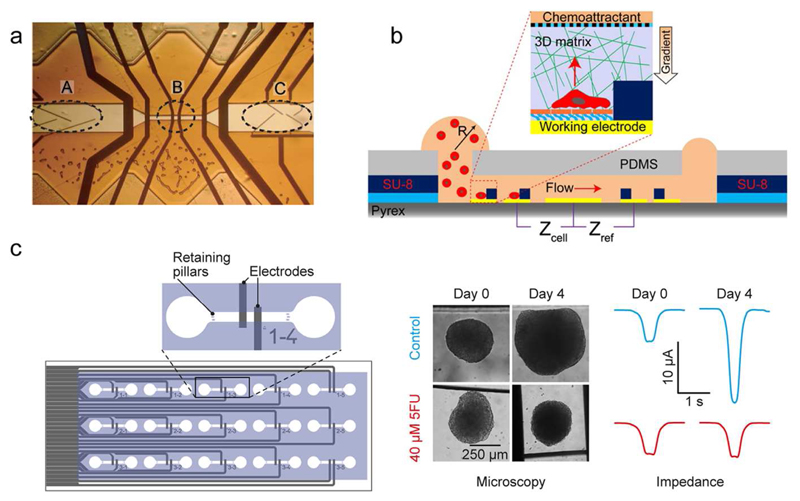
Impedance cytometers are realized by integrating a set of electrodes within a microfluidic channel to perform measurements of single cells in flow-through mode. Impedance cytometers can be used for cell counting and for identification and characterization of different cell types in solution. By probing cells at multiple AC frequencies, different cell characteristics can be extracted: Lower frequency (<1 MHz) impedance measurements provide information on cell size and volume, while higher frequencies (> 1 MHz) are used to investigate the permeability and thickness of cell membranes, cytoplasm conductivity and cell organelles.19 By implementing parallel facing electrodes on the walls of microfluidic channels, Cheung et al. differentiated flowing red blood cells and ghost cells20 (Fig. 2a). Similar multi-frequency measurements were used for distinguishing leukocytes,21 CD4 T-Cells,22 stem cells,23 breast cancer cell lines,24 activated and resting human blood platelets in solution,25 and several other cell types.19,26–29 Finally, microfluidic cell traps equipped with measurement electrodes enable long-term EIS monitoring of non-adherent cells.30–32 Using this approach, Zhu et al. were, for example, able to measure variations in the growth rate of hydrodynamically immobilized single S. pombe cells and identify cell-cycle states.33
Figure 2.
EIS-based sensors. (a) An Iimpedance cytometer by featuring a flow-focusing region (A), measurement electrodes (B), and sorting electrodes (C). Adapted with permission from Cheung et al., Cytometry A 65, 124-132.20 Copyright 2005. (b) Integrated microfluidic device with an ECIS-sensor for studying single-cancer-cell migration in a 3D matrix. Adapted with permission from Nguyen et al., Anal. Chem. 85, 11068-11076.42 Copyright 2013. (c) Microfluidic chip with integrated parallelized EIS monitoring of the size of multiple cancer spheroids (adapted with permission from Bürgel et al., Anal. Chem 88, 10876-10883.51 Copyright 2016) Microtissues rolling over the electrodes generate a peak, whose amplitude is proportional to the size of the spheroids.
2.2. Electrical Cell-Substrate Impedance Sensing
Electrical cell–substrate impedance sensing (ECIS) is a technique used for monitoring the proliferation and viability of cell cultures.34 Cells are seeded and grown on an array of interdigitated electrodes. By measuring variations in the impedance spectra, recorded through the interdigitated electrodes, it is possible to obtain information on cell attachment and cell spreading over the sensor area. Application examples of impedance detection include cytotoxicity assays,35–39 cardiac hypertrophy assays,40 and drinking water toxicity assays.41 Microfluidic devices to study cell proliferation and migration inside 3D matrices, such as hydrogels, with integrated ECIS sensors have also been developed.42 The combination of impedance-based detection and cell/tissue encapsulation within 3D artificial matrices offers great potential for automated real-time monitoring of cytotoxicity drug treatments including drug-diffusion effects43 (Fig. 2b). Finally, ECIS has also found commercial success, as shown by the development of the xCELLigence system by Acea Biosciences, Inc.44 These microtiter plates with interdigitated micro-electrodes integrated at the bottom of the culture wells, called E-Plates®, have found application in the study of drug-induced effects on cardiomyocytes45 and for label-free measurement of the proliferation of different cell types.46
2.3. EIS of Microtissues and Multicellular Organisms
Owing to the rise of 3D multicellular systems as in vitro test models, novel systems for EIS characterization of microtissues have been designed. Molckovsky and Wilson devised a test setup to monitor the effect of photodynamic therapy on liver microtissues by using a pair of needle electrodes between which the microtissues were placed.47 Thielecke et.al. measured the alterations in morphology of cell aggregates due to necrosis and apoptosis by hydrodynamically positioning spheroids in a capillary featuring a four-electrode measurement setup.48 Kloβ et al. developed a higher-throughput platform for measuring drug-induced apoptosis by trapping multiple spheroids in an array of micro-cavities.49 Moreover, the realization of impedance readouts in microfluidic systems, such as open microfluidic hanging-drop networks, demonstrated the feasibility of integrating EIS sensors in MPSs.50 Bürgel et.al developed a multiplexed integrated microfluidic systems that allow for parallelized EIS monitoring of numerous cancer spheroids (Fig. 2c). This platform demonstrates the potential for automated and real-time evaluation of drug toxicity effects through determining spheroid size variations in situ by means of EIS.51
EIS measurements can also be used to characterize and manipulate multicellular organisms, such as trapping, sorting, and counting of C. elegans, combined with subsequent electrophysiology,52–55 or measuring the responses of fish embryos to cryoprotective chemicals, such as methanol and dimethylsulfoxide (DMSO).56,57 Finally, EIS measurements were also used for the detection of parasites in drinking water58 and for testing anthelmintic drugs by monitoring parasite motilities.59,60
In summary, EIS is a label-free, non-invasive analysis method for a wide variety of biological samples, ranging from single cells to multicellular aggregates and organisms. Further integration of EIS methods with MPSs could improve the characterization options, as EIS is a suitable analysis method for highly controlled culturing environments for cells and organisms. Moreover, EIS-detection can easily be parallelized.
3. Electrochemical Biosensors
Electrochemical biosensors consist of a sensor element, which, upon selective recognition and interaction with a biological analyte of interest, produces an electrical signal that is nominally linearly (voltammetry, amperometry, conductometry) or logarithmically (potentiometry) proportional to the analyte concentration.61,62 High selectivity and sensitivity can be provided by the sensor functionalization. A potentially high sensitivity and the possibility of using integrated electronics for the electrical readout render these biosensors very attractive for devising integrated solutions. Furthermore, electrochemical biosensors can be efficiently miniaturized, as has been evidenced by the integration of electrochemical biosensors into the tips of scanning-probe microscopes that then provide highly localized detection of analytes. The corresponding instruments are the so-called scanning electrochemical microscopes (SECMs).63
3.1. Enzyme-Based Electrochemical Sensors
In this class of electrochemical sensors, the sensing electrode is functionalized with an enzyme, which, upon interaction with the target molecule, provides a reaction product that can be detected electrochemically. Several functionalization strategies have been developed for enzyme immobilization on electrodes, which have enabled a wide use of these biosensors in the biomedical and pharmaceutical field.64–66
The flexibility provided by the enzyme functionalization has led to the realization of a large variety of biosensors for the analysis of cell cultures and metabolites in liquid samples.64 Typical examples for cell-culture applications include glucose and lactate electrochemical biosensors, the sensing electrodes of which are functionalized with glucose oxidase or lactate oxidase.67–70 Upon interaction of the oxidase enzyme with the target substrate, hydrogen peroxide is produced, which can then be detected using an amperometric detection scheme. Using an SECM featuring glucose or lactate enzymatic biosensors at the tip, Ciobanu et al. were able to measure the glucose consumption of individual fibroblasts and the lactate secretion of single cancer cells cultured in standard petri dishes.67 Furthermore, several sensor types and units have been developed for the downstream analysis of cell-culture medium. 64,71–74 Frey et al. fabricated a disposable PDMS cartridge for the measurement of glucose and lactate in solution featuring on-line adjustment of the linear response range and sensitivity of the biosensor.68 The same group later developed a novel cartridge sensor for cell-culture applications featuring a limit of detection of less than 5 nM.69 The increased sensitivity was obtained by maximizing the interaction between the solution and the oxidase enzymes by using a microfabricated pillar structure for increasing the functionalized surface area. Furthermore, by varying the flow rate in the cartridge during detection, the sensitivity could be varied to enable a larger detection range.
The development of biocompatible biosensors has also paved the way to a full integration of multi-metabolite sensors within perfused cell culture units.75,76 Ges et al. developed a transparent microfluidic device for trapping and culturing single cells within microfluidic chambers featuring sub-nanoliter volume and an integrated glucose-sensing electrode.75 Using this system, the authors were able to measure the lactate secretion of single mouse fibroblasts during two hours and to monitor metabolic changes upon addition of 2-deoxy-d-glucose), which prevented the generation of pyruvate and the subsequent production of lactate.
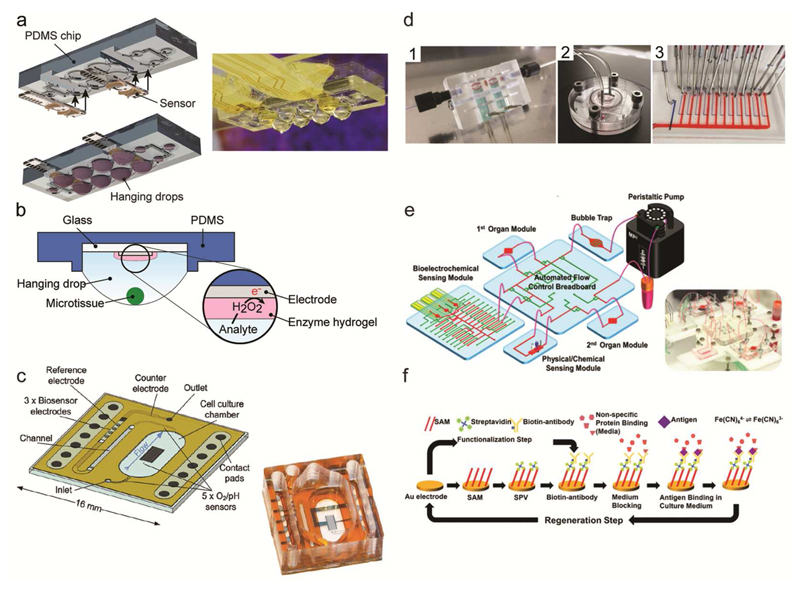
Enzyme-based electrochemical sensors have also been widely applied in MPSs for 3D cell culturing. Misun et al. used a hybrid microfluidic platform, made of PDMS and patterned glass chips that featured glucose and lactate electrochemical sensors, for in situ monitoring of the metabolism of colon cancer (HCT-116) microtissues70 (Fig. 3a-b). Culturing of the tissues in an integrated microfluidic hanging-drop network enabled real-time detection of variations in the microtissue metabolism upon applying different culture conditions. More examples of enzymatic biosensors, used in combination with other types of electrochemical sensors in 3D cell cultures, will be discussed in the “Multiparametric Microphysiometers” subsection.
Figure 3.
Electrochemical biosensors. (a) Schematic view and photograph of sensors integrated in the hanging-drop device for measuring the metabolism of 3D microtissues. The device has been assembled by inserting sensor modules directly into the microfluidic substrate. Adapted with permission from Misun et al., Microsystems and Nanoeng. 2, 16022.70 Copyright 2016. (b) Schematic view showing the location of the biosensor electrode at the ceiling of the hanging drop substrate and the position of the 3D microtissues at the liquid-air interface of the hanging drop. The inset shows the functional enzyme layer on the electrode and the working principle of an enzyme-based biosensor. Adapted with permission from Misun et al.70 (c) Schematic view and photograph of a transparent, integrated microfluidic sensor chip, which features multiple electrochemical sensors. Adapted from Weltin et al. 136 with permission from the Royal Society of Chemistry. (d) Separate biosensor module for glucose and lactate detection (1). Bioreactor for a liver-on-chip culture, which is connected to the sensor unit and to a microfluidic switchboard (2). The microfluidic switchboard, containing flow channels (red) and control channels (blue) (3). Adapted with permission from Bavli et al., PNAS, E2232-E2240.140 (e) Schematic view of the modular MPS by Zhang et al.9 featuring several fluidically interconnected components, including two cell-culturing chambers, a peristaltic pump, a bubble trap, a control breadboard, medium reservoirs and different electrochemical sensors. The photograph shows the complete MPS. (f) Example of electrode functionalization, electrochemical readout and sensor regeneration of an electrochemical immunosensor. Adapted with permission from Zhang et al., PNAS, E2293-E2302.9
Finally, enzymatic bionsensor devices for glucose and lactate detection are also commercially available. C-CIT Sensor AG (Switzerland)77 and Jobst Technologies GmbH (Germany)78 produce sensor units, which can be inserted in cell-culture flasks for real-time monitoring of glucose, lactate and glutamate in solution.79
3.2. Electrochemical Immuno- and Aptasensors
The high specificity provided by antigen–antibody recognition has fueled the development of electrochemical immunosensors.80,81 As for enzyme-based electrochemical biosensors, considerable efforts have been invested into the integration of this class of sensors within microfluidic platforms. Moreover, a large variety of functionalization strategies has been developed to ensure effective and reproducible antibody immobilization on the sensor electrode.82
Several electrochemical methods, such as voltammetry, amperometry, potentiometry, electrochemiluminescence and impedimetric measurements have been used for developing electrochemical immunoassays with the goal of maximizing the electrochemical signal and related sensor sensitivity.83,84 A comprehensive review on the different approaches to realize this class of electrochemical biosensors and on the latest advances in the field has been recently published by Wen et al.85
The sensitivity and specificity as well as the high level of integration and the potential to achieve high-throughput with this class of biosensors has motivated a large range of developments towards in situ detection of small molecules and proteins for in vitro86–88 and in vivo89,90 applications, as well as for diagnostic91–93 and personalized-health94 applications. By combining microbead-based approaches for analyte capture with a fluidically-connected electrochemical sensor, Riahi et al. were able to measure the transferrin and albumin secretion of hepatocytes to monitor the viability and functionality of the cells in a bioreactor for over five days.95 Using aptamer-functionalized electrodes, Shin et al. were able to monitor for several days the drug-induced release of creatine kinase, a cardiac-damage biomarker, from 3D cardiac spheroids derived from human embryonic stem cells.96 It is worth noting that the biomarker detection relied on external sensor units, which were fluidically connected to the culture chamber to measure biomarker concentration at different time points.
3.3. pH Sensors
Maintaining a stable pH is a stringent requirement to ensure viability of biological samples. To this end, a large variety of sensing methods to monitor pH and its variations in vitro and in vivo has been developed.97–100 Electrochemical pH meters can be realized by employing different electronically-conductive oxides, such as platinum oxide, ruthenium oxide, and, most commonly, iridium oxide.101 These sensors mostly rely on potentiometric measurements between a metal-oxide working electrode and a reference electrode. This reference electrode is of critical importance to ensure the stability and reliability of the sensor measurements so that several approaches have been followed to improve its stability upon miniaturization.102–104
Acidification rate measurements of the extracellular environment can be used as a proxy for assessing the metabolism and functionality of the cell/tissue sample under investigation.105 By integrating a microfabricated iridum-oxide pH meter with a microfluidic trapping platform, Ges et al. studied the metabolic activity of single mouse cardiomyocytes for healthy and diseased mice by measuring the acidification rate of the extracellular environment.106 In contrast, Bitziou et al. realized an array of iridium-oxide electrodes on which isolated tissue pieces could be placed.13 The authors used this array of pH sensors to monitor spatial variations in acid secretion of guinea-pig tissue in dependence of tissue morphology and upon drug treatment.
Nano-scale ion-sensitive field-effect transistors (ISFETs) have been demonstrated to be very sensitive pH sensors.107,108 Free ions in solution modulate the charge at the gate oxide surface, which, in turn, induces a modulation in the source-drain current flowing through the device, which is proportional to the ion concentration in solution.109 The ISFET gate electrode is a reference electrode immersed in the analyte solution. The simple sensor readout and the possibility to use established microfabrication techniques render these devices extremely attractive for integration with cell culture settings.110,111 The formation and growth of biofilms,112,113 as well as variations in the bacterial metabolism upon using different sugar sources have been successfully characterized by monitoring the acidification of the extracellular medium through ISFET pH sensors at the bottom of microfabricated cell culture chambers.114–116 ISFET-based pH sensing has been used for label-free monitoring of the antibiotic response and resistance in bacterial populations.117 Finally, Mu et al. also showed that monitoring of acidity can be utilized for the detection of protein concentrations in complex solutions at fM sensitivity by combining indirect sandwich immunoassays with exponential DNA amplification.118
3.4. Electrochemical Oxygen Sensors
Similar to pH measurements, detecting the concentration of dissolved oxygen in cell-culture medium is crucial for monitoring cell-culture conditions. Alterations or strong variations in those culture conditions may lead to unintended cell stress and damage, while they provide, at the same time, an effective strategy for obtaining insights into cellular metabolism and adaptation.119 Oxygen sensing in cell-culture platforms can be performed by using a variety of detection methods,119,120 with optical approaches becoming very popular owing to the possibility of localized monitoring of oxygen concentrations in the platform.121–123 However, the integration of optical sensing methods is limited so that amperometric so-called “Clark” sensors, based on oxygen reduction and hydrogen peroxide production at the surface of a platinum electrode, still find wide application.120
Amperometric measurements of the oxygen consumption of single mouse and bovine embryos at different developmental stages was performed by positioning the embryos over an array of electrochemical sensor electrodes.124,125 The integration of Clark-type oxygen sensors within a microfluidic platform for co-culturing E.coli and neutrophil-like cells enabled the monitoring of the bactericidal activity of the cells and its enhancement upon γ-interferon activation by detecting the variation in the respiratory activity of the bacteria.126 To enable simple integration with standard lab equipment, Kleninger et al. fabricated a Clark-type oxygen sensor, which could be integrated in cell-culture flasks for continuous detection of oxygen levels in the cell environment.127 As a proof-of-concept application of the sensor, the authors monitored oxygen levels in flasks with different cell density, showing that hypoxia can occur, as cell density varies, despite controlled environmental settings. By utilizing a gold electrode protected by a polyethylenimine layer as semipermeable membrane to enhance selectivity, the same group was able to measure the release of superoxide radicals (O2-) from T-47D human breast cancer cells upon stimulation with PMA (phorbol-12-myristate-13-acetate) and subsequent direct oxidation of the reactive oxygen species on the sensing electrode.128
3.5. Multiparametric Microphysiometers
The possibility of integrating - on a single chip - several electrochemical sensors with multiplexing and readout functions has enabled the fabrication of multiparametric electrochemical biosensors.129–134 Eklund et al. fabricated a multi-analyte microphysiometer able to measure oxygen-, lactate- and acidification rates and their variations in cell medium. Applications ranged from hypoxia and cell proliferation studies on cancer cell lines to variations in cell metabolism upon exposure to test substances.76,129,135 The multi-analyte sensor combined different electrochemical sensor methods: lactate and glucose measurements were performed by means of an enzyme-based electrochemical sensor, oxygen consumption rate was determined by measuring the oxygen reduction at the surface of a Nafion-coated electrode, while the acidification-rate sensor was a pH-sensitive light-addressable potentiometric sensor (LAPS). Weltin et al. fabricated a similar multi-analyte sensor featuring an iridium-oxide pH sensor, which was integrated at the bottom of a transparent microfluidic chip for automated drug-screening applications136 (Fig. 3c). McKenzie et al. developed a multi-analyte sensor platform, where the electrode-surface functionalization was carried out by using a screen-printing technique.137 This fabrication approach is of particular interest, as screen-printing could prove an effective method towards the realization of low-cost and mass-produced sensor units, which ensures a high level of reproducibility of the sensor functionalization.
Due to the rising interest and increasing efforts in 3D cell cultures for recapitulating multi-tissue interactions in vitro, multiparametric sensors have also been successfully used for in situ monitoring of the microtissue metabolism, often in conjunction with dedicated microfluidic platforms. Alexander et al. monitored the metabolism of single preformed HepG2 spheroids for over three days by culturing the tissues in a 3D-printed microwell array on top of commercially available BioChips.138 The sensors included electrochemical detection of pH and dissolved oxygen as well as impedance measurements for morphology analysis of the microtissue. A sensor probe equipped with lactate and oxygen electrochemical biosensors was developed by Weltin et al. to enable sensing within standard cell culture plates.139 By bringing the probe into close proximity to the spheroids in the microtiterplate, the authors were able to monitor the metabolism of HepaRG spheroids during three days and to detect variations in lactate secretion caused by drug-induced hepatotoxicity. In contrast, Bavli et al. developed a modular microfluidic platform, where the oxygen concentration was monitored in situ using an optical oxygen probe, while glucose and lactate concentrations were measured on a separate electrochemical sensor unit140 (Fig. 3d). Through the developed platform, early onset of mitochondrial stress of liver microtissues upon exposure to test drugs could be detected in real-time, in contrast to standard detection methods, which rely on endpoint assays. Similarly, Zhang et al. developed a modular multi-organ microfluidic platform, where the different components, the organ-culture chambers, two electrochemical sensor units, and a bubble trap were connected by means of a computer-controlled fluidic-routing breadboard9 (Fig. 3e). The sensor units encompassed one board featuring electrochemical immuno-sensors for biomarker-concentration detection and one board for monitoring the extracellular environment, which included pH and oxygen sensors. Proof-of-concept drug toxicity tests with a three-microtissue model system (liver, cancer, heart) were performed by monitoring biomarker secretion rates. The use of a separate sensor unit for the electrochemical immunosensors enabled the automatic regeneration of the sensor surface for long-term monitoring of biomarker secretion (Fig. 3f). However, the approach by Bavli et al. and Zhang et al. entails a fairly complex overall platform as it requires external or on-chip valves and dedicated pumps for separating and driving the fluids during analyte capture, readout, and sensor regeneration. For high-throughput analysis, e.g., in drug-screening applications compact and integrated solutions are preferable.
4. Acoustic Waves
Surface acoustic waves (SAWs) are acoustic waves (AWs) featuring nanometer amplitudes and a penetration depth of approximately one wavelength in the bulk of the material.141 The acoustic waves are usually generated by mean of interdigitated transducers on the surface of a piezoelectric material.142 Depending on the polarization of the wave, the configuration of the excitation/detection transducers and the substrate properties and orientation, a variety of acoustic-wave devices can be devised with different sensing and actuation capabilities, such as classical Rayleigh-mode SAWs, shear-horizontal SAWs and Lamb-wave-based sensors.141,142 AW-based devices are widely applied in electronics and telecommunications143 and are also used for chemical sensing in gas and liquid samples.141,144 Classical Rayleigh SAW devices are very ineffective when in contact with liquid media due to severe damping of the wave via compressional wave generation, so that prevailingly other modes are used. AW-based biosensor devices have been used to probe and quantify different analytes in solution, including proteins, DNA, and bacteria in complex media (for a review on different AW biosensors and functionalization schemes, see Länge et al.142 and Go et al.141). In cell culture, AW-based devices have found application both as biosensors145–147 and as contactless manipulation tools.148–150 An example includes the use of SAW-induced shear stress as a mean to detach cells from the substrate and to then differentiate between various cell types in a label-free manner by using cell adhesion strength as a type-specific signature.145,146 AW actuators have also been seamlessly integrated with microfluidic platforms,151 which renders this type of sensor particularly suitable to study properties of cells under different culturing conditions that are controlled by microfluidics. Representative examples include the characterization of osteosarcoma SaOs-2 cells, which were exposed to static and dynamic physiological conditions, as well as to different pH and temperature conditions for studying cell adhesion on candidate materials for bone implants.147,152
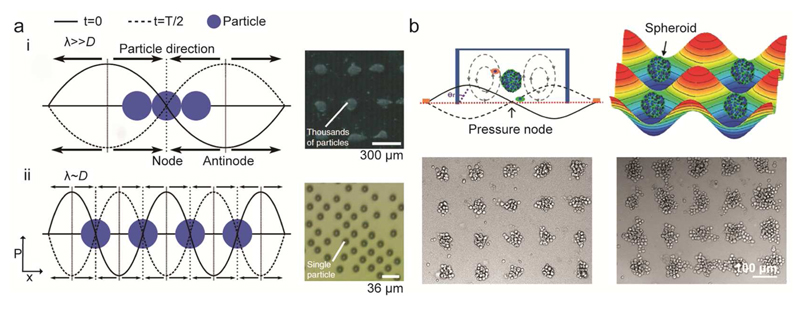
Pressure waves generated by standing surface acoustic waves (SSAW) induce distributions of pressure nodes and antinodes in the solution above the SSAW substrate. This effect can be used for precise contactless handling and patterning of cells using so-called “acoustic tweezers”.153 Collins et al. demonstrated periodic patterning of a large variety of cells, including human lymphocytes, red blood cells, and malaria parasite Plasmodium falciparum and high viability (up to ~90% after 7 hours) of the patterned cells154 (Fig. 4a). Furthermore, precise cell manipulation by SSAW has enabled the realization of custom cell assemblies,155 3D trapping and patterning of live cells for the realization of defined cell culture patterns,156 and the realization of organized co-cultures for the study of heterotypic cell-cell interactions.157
Figure 4.
SAW-based particle or cell manipulation. a) Saw-based single particle/cell patterning. Particles are trapped at the node of the acoustic wave. (i) If the wavelength is much larger than the particle/cell diameter, multiple particles are driven to the force minima. (ii) By tuning the wavelength of the acoustic wave, single particle patterning can be achieved. Adapted with permission from Collins et al., Nat. Communications 6, 8686.154 Copyright 2015. b) Acoustic-tweezer-based formation of spheroids. Suspended cells are aggregated at the trapping positions, defined by the acoustic force, the induced streaming force, gravity and the buoyant force. The photographs at the bottom show the formation of spheroids of different size, which were obtained by using cell suspensions of different cell densities. Adapted from Chen et al.158 with permission from the Royal Society of Chemistry.
Cell patterning is not limited to 2D cell layers, but has also been extended to formation of multicellular 3D spheroids. Chen et al. demonstrated the formation of more than 150 HepG2 spheroids in parallel, which could then be collected for subsequent culturing in standard Petri dishes158 (Fig. 4b). Alhasan et al. developed a SAW centrifugation tool for the rapid generation of spheroids, which can be directly interfaced with standard tissue culture microplates (both 12-well and 96-well plates), so that they would not require any alteration of standard laboratory setups.149 Combination of SAW microcentrifugation and quick gelling hydrogels enabled to form spheroids of human mammary gland carcinoma and rat mesenchymal stem cells within minutes in plastic plates. Furthermore, SAW-based devices were used to follow the proliferation of cells and growth of 3D cellular spheroids: shear-horizontal surface acoustic waves (SH-SAW) traveling through a well containing spheroids experience a size-dependent frequency shift, which enables SAW-based growth monitoring of spheroids over time.159
Besides patterning, SSAWs also enable detection and manipulation of suspended cells in microfluidic channels. Chen et al. realized a SSAW-based flow cytometer, where SSAWs were used for 3D focusing of the flowing cell suspension in the center of the microfluidic channel followed by laser-induced fluorescence detection.160 Ren et al. extended the focusing capability of SSAWs to realize a high-throughput acoustic-based fluorescence-activated cell sorter (FACS) with a sorting rate of up to ~7000 cells per second.161 Moreover, SSAW-based focusing and isolation has been used for sub-micrometer objects: by flowing cell-culture medium in a microfluidic channel between a pair of interdigitated ultrasound transducers, Lee et al. were able to filter and remove nanoscale (<200nm) vesicles in the medium for downstream analysis.162
Finally, by tuning the AW-based excitation parameters, the technique can also be used to realize chemical-free mechanical cell lysing.148 By combining a SAW transducer with a disposable phononic structure, Reboud et al. were able to realize a low-cost device for cell lysis and sample heating for real-time PCR of the rodent malaria parasite Plasmodium berghei in whole blood in an effort to develop a device for point-of-care detection of human pathogens.150
5. Surface Plasmon Resonance
Surface plasmon resonance (SPR) sensors rely on refractive-index variations (at a resolution reaching 10–6 RIUs, Refractive Index Units) at the SPR-sensor surface.163 An electromagnetic wave impinging under total-reflection conditions on a metal-covered surface induces coherent oscillations of the free electrons in the conduction bands of certain metals (Ag, Au) at a metal/dielectric interface.164 These oscillations generate an exponentially decaying electric field (evanescent field) in the medium. Variations of the refractive index in the medium in close proximity (hundreds of nanometers) to the sensor surface induce changes in the characteristics (wavelength, angle, phase, etc.) of the reflected light beam.165 The nature of the detection method and the large versatility in surface functionalization and the possibility to enhance the resolution of SPR by using nanomaterials166–168 have promoted the integration of SPR-based biosensors in microfluidic platforms.163,169
SPR has numerous application in cellular analysis170 and novel SPR techniques, in particular long-range SPR to increase penetration depth, localized SPR to improve spatial resolution (down to nanometer resolution), SPR imaging and phase imaging to increase the multiplexing capability of the sensor, have been developed to address specific applications.171,172
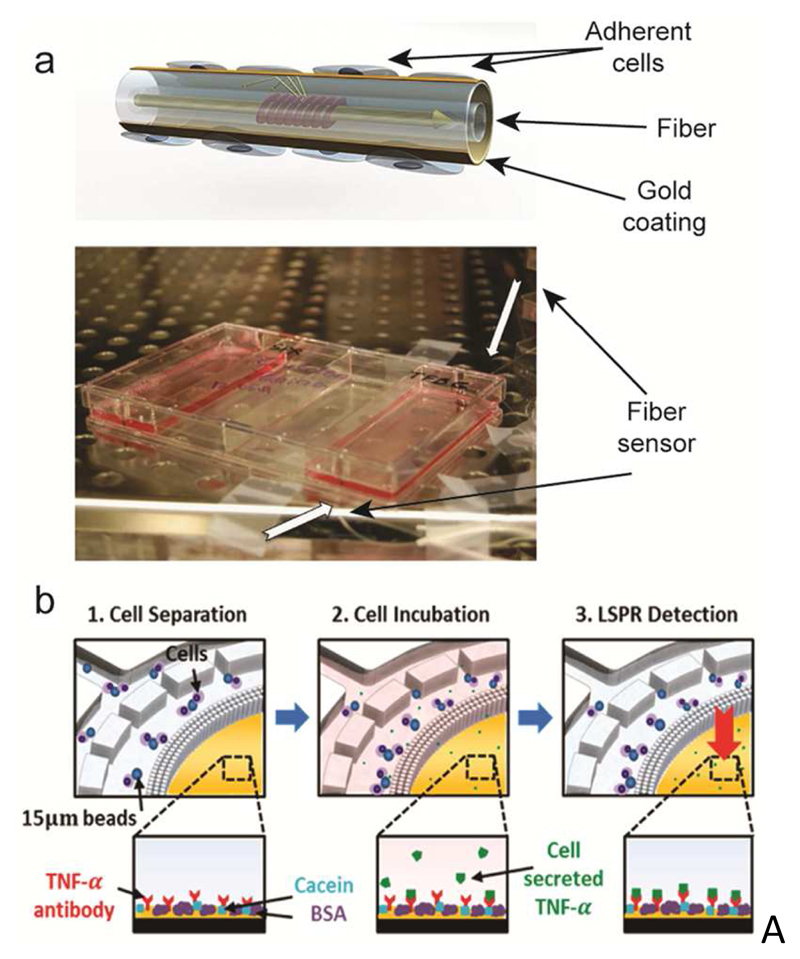
SPR sensors are compatible with cell culturing environments, which renders this technique suitable for real-time monitoring of variations in cell viability and cell characteristics of adherent cells at the sensor surface. Berguiga et al. cultured living C2C12 myoblast cells on the gold surface of a scanning surface plasmon microscope to study the adhesion and motility of the cells for over 50 hours, obtaining diffraction limited (~180 nm) imaging and characterization of the interactions between the cells and the substrate.173 Shevchenko et al. realized a portable optical fiber biosensor to characterize cellular responses, such as cell detachment, serum uptake, and inhibition of cellular metabolism after exposing the cells, cultured on the surface of the plasmonic biosensors, to different stimuli174 (Fig. 5a). The portability and reusability of such biosensors evidences the potential of SPR as a versatile, cost-effective and easy-to-integrate sensing mechanism for cell-culture settings.
Figure 5.
SPR-based sensors. a) A SPR fiber sensor for real-time monitoring of cellular responses, cultured in standard cell-culture flasks. The schematic shows the working principle of the sensor. Cells are cultured on the gold-patterned fiber surface. Light traveling through the waveguide interacts with the adherent cells on the fiber. The photograph shows the integration of the sensor in a cell-culture flask. Adapted with permission from Shevchenko et al., Biosens. Bioelectron. 56, 359-367.174 Copyright 2014. b) LSPR-based detection of cytokine (TNF-α) secretion by immune cells isolated from human blood. Functionalized beads bind to the target cells. (1) The sample then flows through a microfluidic chamber featuring a sieve structure to selectively retain the cells that are bound to beads. (2) Cells are then stimulated to induce cytokine secretion. (3) Cytokine secretion is quantified by the on-chip LSPR sensor functionalized with target-specific antibodies. The insets at the bottom show the LSPR-sensor surface at different analysis stages. Reprinted with permission from Oh et al., ACS Nano 8, 2667-2676.181 Copyright 2014.
Furthermore, the possibility to specifically functionalize the SPR sensor surface enables a characterization of the interactions between specific immobilized ligands on the sensor surface and antigens expressed on the cell membrane. Stojanovic et al. studied the adhesion and spreading of several breast cancer cell lines (HS578T, SKBR3 and MCF7) on the surface of an SPR sensor functionalized with Epithelial Cell Adhesion Molecule (EpCAM) and other ligands of interest.175 Functionalization with different concentrations of ligands enabled to quantify the expression levels of the antigens on the surfaces of the different cells of interest.
To extend the sensitivity of the SPR detector to whole-cell events, the sensor has to be modified by implementing a symmetrical structure of the sensor surface, such as using a dielectric – metal – dielectric structure. This technique, called long-range SPR (LRSPR) increases the penetration depth of the evanescent field to micrometer scales, showing a higher sensitivity to whole-cell responses in comparison to standard SPR.176 As an example of the increased sensitivity of LRSPR to subtle changes within cells, Yang et al. were able to detect and monitor cellular micromotions, i.e., dynamic fluctuations of adherent cells caused by membrane rearrangements of layers of confluent cells, using 3T3 fibroblasts and MDA-MB-231 cancer cells as model systems.177
The label-free nature and the extreme sensitivity of SPR biosensors have sparked great interest in applying this sensing mechanism for cytotoxicity and drug toxicity testing.178 Andersson et al. used a functionalized SPR biosensor to monitor the secretion of cardiac troponin T and other biomarkers from HL-1 and human-embryonic-stem-cell-derived cardiomyocytes upon exposure to cardiotoxic substances.179,180 Culturing of the cells and exposure to test substances was performed separately, followed by manual aliquoting and dosing of the cell medium to the biosensor. Recently, more integrated approaches, featuring cell culture and cell secretion quantification by SPR on the same chip, have been presented. Oh et al. designed a microfluidic platform able to trap immune cells (THP-1 and CD45) from human lysed blood to measure the secretion of TNF-α upon stimulation of the cells181 (Fig. 5b). By selectively trapping cells of interest and performing the analysis on the same platform, the researchers were able to minimize cytokine dilution after secretion and to measure the TNF-α released by as little as 1000 cells in the chip. Li et al. developed a nanoplasmonic biosensor, integrated with a microfluidic module, to monitor the secretion of vascular endothelial growth factor (VEGF) by cancer cells under controlled culture conditions.182 The biosensor showed extreme sensitivity of 145 pg mL-1 (~5 pM) for VEGF detection, and enabled real-time monitoring of cell secretion for over 10 hours.
6. Mechanical Biosensors
Mechanical biosensors are based on the transduction of the variables of interest into a mechanical parameter. Scaling of the sensors to micron and submicron dimensions has enabled mass measurements with very high resolution in terms of measured absolute mass (down to attogram, 1 ag=10-18 g, resolution for particles in liquid183,184 and sub-yoctogram, 1 yg = 10-24 g, for samples in vacuum185), which enabled to monitor processes at molecular scale. Extremely low spring constants can be used to detect low-force (pN) events, and miniaturization enables fast sensor response times (~ms).186 Miniaturization, however, also entails very small detection surfaces or volumes, larger dependence on transducer material and fabrication process fluctuations and larger inter-transducer variations, as well as lower signal-to-noise ratios in many instances. The large absolute mass sensitivity does, therefore, not directly translate into being able to detect very small analyte concentrations. This holds in particular as, at low concentrations, the analyte molecules have to “find” the minute transducer or sensor in order to be detected.
We distinguish between two operation modes:186,187 (i) surface-stress (static) mechanical biosensors, which transduce the reaction of the analyte at or with the sensor surface into stress forces, and (ii) dynamic-mode biosensors, which measure variations in the resonance frequency of the device as a function of interaction between the sensor and the sample.
6.1. Surface-Stress (Static) Mechanical Biosensors
Binding of biomolecules on the functionalized surface of mechanical biosensors, usually cantilever-based microstructures, induces surface stress on the sensor surface, which leads to a bending of the microstructure proportional to the amount of bound analyte. This sensing mechanism has been used to detect a large variety of analytes in solution, such as proteins, DNA, mRNA, and small molecules (see Arlett and Roukes186 and Tamayo et al.187 for more detailed information on the transduction mechanism and a review on the applications of these devices). As an example of this transduction method, Longo et al. were able to detect bacteria population growth and antibiotic resistance in extremely short time frames (~1 hour) by measuring the cantilever-deflection fluctuations induced by the bacteria on the sensor surface.188
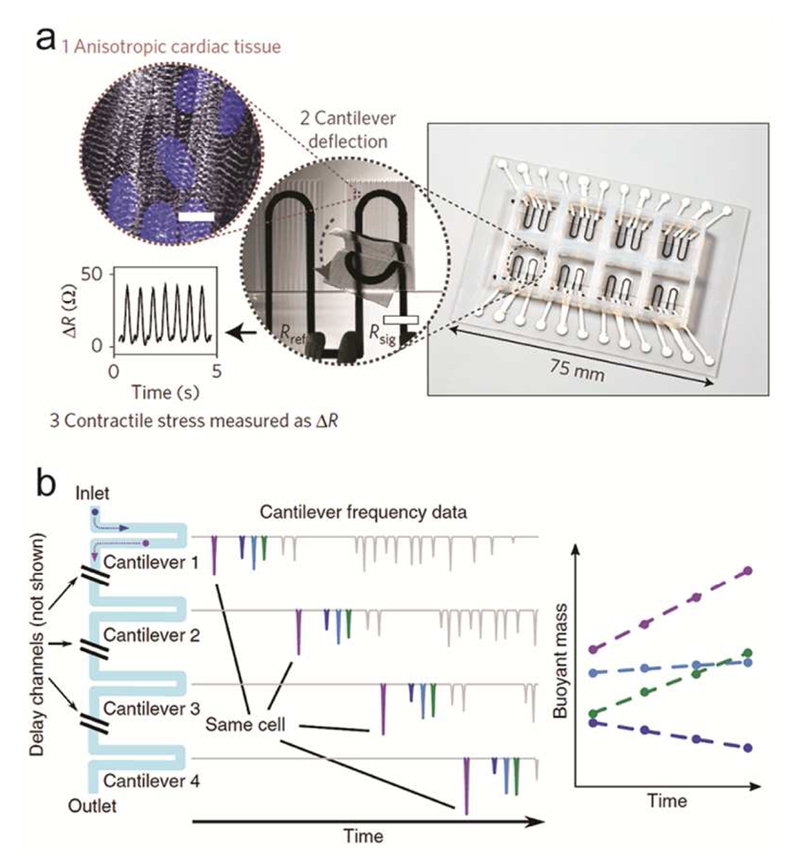
Besides detecting cell and population growth, cantilever sensors have been widely used for investigating cell forces, and in particular for the characterization of muscle cells under different culturing conditions.189–195 The ability to modify the surface of the cantilever and the cell environment during measurement renders these devices particularly suitable for studying drug-induced effects on muscle cells. Wilson et al. demonstrated the possibility of using mechanical biosensors to measure the contractile stress generated by myotubes using skeletal muscles, dissected from thighs of rat fetuses and cultured on the silicon microcantilevers for thirteen days.189 Smith et al. further extended this experimental approach by realizing arrays of functionalized silicon cantilevers for increased throughput, where primary human myoblasts could be cultured, induced into myotubes and exposed to test substances for toxicity testing.191,192 Furthermore, the cantilevers also featured integrated electrodes to study the contraction response of the myotubes to different stimulation patterns, such as varying waveform, intensity and frequency of the stimulation. Lind et al. developed an array of multimaterial (ink-based) cantilevers with integrated strain sensors and a patterned surface to guide the self-assembly of physio-mimetic tissues from human-induced-pluripotent-stem-cell-derived cardiomyocytes (hiPS-CMs) and neonatal rat ventricular myocytes (NRVM)193 (Fig. 6a). Using integrated stress sensors together with engineered surfaces to guide tissue assembly simplified both, the tissue formation and the subsequent data recording. To demonstrate the potential of the device, the group measured the drug-dose response of NRVM to several test substances and characterized the contractile development of hiPS-CMs for twenty-eight days.
Figure 6.
Mechanical sensors. a) Cantilever structure for detecting the contraction of cardiac tissue. The cantilevers feature an engineered surface for formation of a laminar cardiac tissue on the sensor surface and integrated strain sensors for measuring the cantilever deflection. Adapted with permission from Lind et al., Nat. Mater. 16, 303-308.193 Copyright 2016. b) An array of SMR cantilevers is used to detect mass changes of cells as they travel through the array. Cells passing through an SMR cantilever induce a transient frequency shift in the resonance frequency of the cantilever, whose magnitude is proportional to the cell buoyant mass. Measuring the shift amplitudes as the cells travel through the array enables the detection of mass variations at different times. Adapted with permission from Cermak et al., Nat. Biotechnol. 34, 1052-1059.221 Copyright 2016.
6.2. Dynamic-Mode Mechanical Biosensors
One class of dynamic-mode mechanical biosensors translate variations of the mass of the moving sensing element into variations of the phase/resonance frequency of the sensing element. The pioneering work of Sauerbrey in 1959196 on the relationship between frequency change and mass adsorbed on the surface of a quartz crystal resonator inspired the use of quartz crystal microbalances (QCMs) for highly sensitive mass measurements. The measured signal also depends of the coupling of the analyte to the moving quartz surface. For a rigid coupling, there are negligible dissipation losses. In many applications with surface molecule adsorption and soft biofilms, however, the frequency shifts and the energy dissipation (QCM-D) are monitored to elucidate the nature and structural properties of the adsorbed film. Dissipation is determined from the time it takes for the oscillation to stop after disconnecting power.197 QCM-based sensors have found many applications in studying biofilm growth and deposition, antigen-antibody interaction, DNA detection and cell adhesion studies (see Dixon198 and Ferreira et al.199 for reviews on QCMs and Rehman et al.200 for a protocol/example on characterizing cell adhesion by QCM).
Studying cell adhesion and the spreading on surfaces of QCMs was demonstrated on MDCK strains I and II and with Swiss 3T3 fibroblasts.201,202 Subsequent studies on cell-adhesion and changes in cell viscoelasticity upon attachment to QCM surfaces were done to monitor toxicity effects on adherent cells. Marx et al. cultured endothelial cells (ECs) for 24h on a gold-treated surface of a QCM to promote adhesion.203 After exposing the cells to nocodazole, a known microtubule binding drug, the QCM was used to detect changes in cell cytoskeleton, reduction in cell-to-cell contact and in surface coverage. Tan et al. used QCM detection to monitor EC activation by exposure to tumor necrosis factor alpha (TNF-α).204 The binding of ECs with the HL-60 and KG-1 leukemia cells was detected by monitoring the mechanical changes in the ECs, in particular by detecting variations in cell-to-substrate interaction upon cell activation and cell binding. Fatisson et al. used a QCM sensor to provide real-time detection of cytoskeletal changes in live primary ECs in response to different cytomorphic agents, namely Triton-X 100 and bacterial lipopolysaccharide.205 Recently, first attempts to integrate such sensors within microfluidic platforms have been presented.206,207 Thies et al. combined a microfluidic and a sandwich immunoassay to decrease sample consumption to microliter scale, while attaining nanogram analyte resolution.207 The presented immunoassay is of particular interest, as it was designed to present temporary binding of the analyte on the sensor surface to enable multiple use of the sensor surface for kinetic studies without requiring a regeneration step of the active surface.
As frequency variations of mechanical transducers are linearly dependent on the relative mass variations experienced by the transducer element, a drastic reduction in resonant mass is required to reach single-cell resolution. To address this limitation, the group of Rashid Bashir developed an array of micro-electro-mechanical systems (MEMS) sensors with which the mass, growth rate, and other biophysical properties of single adherent cells can be measured and characterized.208–212 Monitoring the growth rate at single-cell level for over 50 hours enabled the detection of differences in growth rate of human colon epithelial cells as a function of cell mass.209 Furthermore, the sensor allowed for quantification of differences in the growth rates of normal and cancerous cells,211 as well as for studying the viscoelastic properties of single human colorectal adenocarcinoma cells (HT-29) that adhered to the sensor surface.210,212
Martínez-Martín et al. developed an optically-excited cantilever resonator able to measure, at millisecond resolution, the total mass of single or multiple adherent mammalian cells, reaching picogram resolution (1 pg = 10-12 g) in liquid environments.213 The resonator was lowered into a culture chamber and individual cells were selected for analysis. The authors used their method to detect the growth arrest of cells upon infection with vaccinia virus. Interestingly, the thickness of the silicon resonator (~1 µm) rendered these devices optically transparent, so that simultaneous optical and mass characterization of the cells was possible on the cantilever.
To measure samples in suspension, high mass resolution can be achieved by embedding the microfluidic structures into the resonant element. These devices, called suspended microchannel resonators (SMRs),214 have been employed to measure mass, growth rates, and size regulation features215 of a large variety of samples, including yeast, bacteria and mammalian cells.214–218 Furthermore, by measuring the variation of the buoyant mass of a cell upon suspension in fluids of different density, a precise measurement the of cell density can be carried out.219 This approach was used to differentiate between healthy and malaria-infected erythrocytes in solution, where cells presented similar masses but differed in density.220 To follow the time-course of cell growth, SMRs have been combined into arrays (10-12 SMRs connected in series), providing characterization of the cell growth over ~3 hours221 (Figure 6b). Single mouse CD8+ T-cells, as well as S. cerevisiae and E. coli cells were measured. Measurements at single-cell resolution allowed for detecting sub-populations of differently growing cells, which is not possible with bulk measurements of cell populations.221
While mass and viscoelasticity measurements have provided great insight into cell behavior and have been successfully used to characterize cellular properties, the integration of these sensors in standard cell-culture platforms for high-throughput analysis is currently limited by the size of the sensor (QCM), or by the complex fabrication and operation of the MEMS devices. Recently, a new method for measuring the mass of single cells in microfluidic platforms has been presented using optically-induced electrokinetics.222,223 Although this method is compatible with integration in cell culture platforms, the measurement requires complex optical elements and electrode setups, which limits applicability in standard cell-culture settings. An extensive description of MEMS- and microfluidic-based methods for measuring the mass of single cells, and a comparison to quantitative phase imaging (not discussed here), has been presented by Popescu et al.224 and Zangle and Teitell.225
7. Conclusion
We have presented several sensing and actuation techniques, which can provide in situ detection and characterization of relevant features of biological samples, ranging from single cells to 3D microtissues and multicellular organisms. A summary of the presented methods including references to specialized reviews on the different sensing mechanism is given in Table 1.
Table 1.
Reference assignment for the different sensors and applications.
| Sensor class | Application | Cited literature |
|---|---|---|
| Electrical Impedance | ||
| Impedance Cytometers | Review on impedance cytometers | [19] |
| EIS analysis of flowing cells | [20–24,26–29] | |
| Long-term analysis of hydrodynamically trapped single cells | [30–32] | |
| Electrical Cell-Substrate Impedance Sensing | Monitoring of cell adhesion and spreading | [34] |
| Cytotoxicity assays | [35–39] | |
| Cell proliferation and migration in 3D matrices | [43] | |
| Commercial ECIS platform and application | [45,46] | |
| Microtissues and Multicellular Organisms | 3D microtissue analysis | [47–51] |
| Manipulation and analysis of multicellular organisms | [52–57,59,60] | |
|
| ||
| Electrochemical Biosensors | Review on electrochemical biosensors | [66] |
| Enzyme-based Sensors | Review on enzymatic biosensors in microfluidics | [64] |
| Downstream analysis of cell-culture media | [64,68,69,71–74] | |
| Sensors integrated in perfused cell-culture units | [75,76] | |
| Commercial sensors | [77,78] | |
| Immuno- and Aptasensors | Review on electrochemical immunosensors | [81,85] |
| Surface functionalization strategies | [82] | |
| Monitoring of biomarker secretion downstream of bioreactors | [95,96] | |
| pH Sensors | Monitoring of cell and tissue metabolism using iridium-oxide | [13,106] |
| ISFET-based pH sensors | [107,108,110,111,114–117] | |
| Electrochemical Oxygen Sensors | Detection of cell metabolism | [124–127] |
| Multiparametric Microphysiometers | Integrated oxygen, lactate, glucose and pH sensors | [76,129,135–137] |
| Probe sensor for detecting lactate and oxygen of 3D microtissues in standard microtiter plates | [139] | |
| Modular multi-organ MPS with multiple electrochemical sensors | [9,140] | |
| Commercial multiparametric sensor | [138] | |
|
| ||
| Surface Acoustic Waves | Review on SAW biosensors | [142] |
| Detection of cell adhesion | [145–147,152] | |
| Acoustic tweezers for manipulation of 2D cell cultures | [154–157] | |
| Formation of 3D spheroids | [149,158] | |
| Focusing of flowing cells | [160,161] | |
| Cell lysing | [148,150] | |
|
| ||
| Surface Plasmon Resonance | Review on SPR for biological and chemical sensing applications | [1165] |
| Scanning SPR microscopy to study cell adhesion and motility | [173] | |
| Portable SPR sensor for application in cell-culture flasks | [174] | |
| Long-range SPR for extended sensitivity to whole-cell events | [176,177] | |
| SPR sensors integrated in microfluidic cell-culture platforms | [181,182] | |
|
| ||
| Mechanical Biosensors | Reviews on mechanical biosensors | [186 187] |
| Surface-Stress (Static) | Detecting cell-population growth | [188] |
| Measuring cell contraction forces | [189–195] | |
| Dynamic-Mode | QCM sensors for studying cell adhesion, spreading and cell-substrate interactions | [201,202 203 204] |
| Measuring mass and growth rate of single adherent cells | [208–213] | |
| Measuring mass, growth rate and density of single flowing cells | [214–218,220,221] | |
The possibility to manipulate and interrogate cells and tissues in a contactless and highly controlled manner and the trend towards large-scale integration will drive the development of future active MPSs. There will be a need of platforms, where sample preparation and analysis can be performed as much as possible in situ and, potentially, in a completely automated fashion using established lab automation techniques, tools and robots.
The possibility to miniaturize and parallelize multiple sensors, and the possibility to use established microfabrication techniques render electrical impedance spectroscopy and electrochemical biosensors the most straightforward approach to realize sensor functions and a high-level of sensor integration in standardized MPSs. SAW-based devices offer similar advantages, however, require specific piezoelectric substrate materials (ZnO, quartz, LiNbO3, LiTaO3) that are not standard in microfabrication.
SPR and mechanical sensors feature comparably low throughput and are not so easy to parallelize and integrate in standard cell-culture settings. However, the very high sensitivity, the label-free nature of the measurements, and the complementary information that these sensors can provide, renders these devices very attractive for targeting specific biological questions.
Finally, the definition of a set of standards for integrated cell culture platforms, including fluidic and electrical connections, employed materials, as well as design and shape of the platforms, will greatly push the development of novel MPSs.
To facilitate acceptance of novel MPSs by industrial players, these platforms should come in standard shapes or formats, such as microtiter-plate or glass-slide formats, and allow for fluid exchange and flow control via pipetting. The platforms would then be compatible with conventional laboratory automation equipment, such as liquid handling robots, which are commonly used by biotech and pharmaceutical companies. Platforms that do not require pumps and a large number of tubing connections can be stacked, which enables economic use of, e.g., incubator space and entails simple handling of multiple plates. Sample or microtissue loading and retrieval by pipetting is preferred, as preformed microtissues can be batch-transferred from formation plates into the MPS platforms without damaging structure and compromising viability. A combination of different tissue types then enables the realization of complex multi-tissue MPSs.
Different plastic materials, such as polystyrene, polypropylene and cyclic-olefin copolymers, have been adopted and validated for use in life science, owing to their versatility in surface functionalization, their biocompatibility and optical transparency. Several methods for low-cost fabrication of such plastic devices already exist, which renders plastics the material of choice for MPSs. Current limitations include facile prototyping of and sensor integration into plastic materials. However, multi-material 3D printing, where different resins and conductive inks can be combined, could provide a possibility to overcome these obstacles and an economically viable approach for academic research laboratories, which cannot afford the high initial tooling costs associated with plastic mass manufacturing.
Similarly to format standards, a set of common rules is also needed for the electrical connections to promote the development of multiple, complementary and compatible readouts and instrumentation. To reduce manufacturing complexity, we envision MPS platforms that feature one connection per integrated sensor/electrode, while the control electronics are external. Although such an approach could result in a large number of connections on a chip, the definition of a set of rules for the contact pins would promote the development of versatile readout instrumentation. Considering a pitch of 2.54 mm (0.1”) for the contacts, the potential number of contact pads would be more than 100 in less than 1 cm length of a well plate. A connection board featuring spring-loaded contact pins could be used to interface to the well plates or platforms, and the same readout instrument could be used with several MPSs featuring different culture chambers but similar or identical sensor types. The use of spring-loaded contacts would also be compatible with automation and the use of robotic arms.
We believe that the introduction of such standards may facilitate the use of MPSs equipped with integrated sensors, for example, in the drug-development pipeline, with the aim to obtain comprehensive compound testing results on representative 3D human-derived tissues.
Acknowledgments
We would like to thank K. Renggli, O. Frey and S. Geissler, all at ETH Zurich, for helpful discussions. Financial support of the European Community (European Research Council Advanced Grant 694829 ‘neuroXscales’) and the Swiss National Science Foundation (SNF project 2-77079-16 “Infected body on a chip”) is acknowledged.
References
- (1).Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- (2).Dehne E-M, Hasenberg T, Marx U. The ascendance of microphysiological systems to solve the drug testing dilemma. Future Sci OA. 2017;3:FSO0185. doi: 10.4155/fsoa-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T. Microfluidic 3D cell culture: From tools to tissue models. Curr Opin Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- (4).Giridharan GA, Nguyen M-D, Estrada R, Parichehreh V, Hamid T, Ismahil MA, Prabhu SD, Sethu P. Microfluidic Cardiac Cell Culture Model (μCCCM) Anal Chem. 2010;82:7581–7587. doi: 10.1021/ac1012893. [DOI] [PubMed] [Google Scholar]
- (5).Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- (6).Frey O, Misun PM, Fluri Da, Hengstler JG, Hierlemann A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat Commun. 2014;5:4250. doi: 10.1038/ncomms5250. [DOI] [PubMed] [Google Scholar]
- (7).Kravchenko-Balasha N, Wang J, Remacle F, Levine RD, Heath JR. Glioblastoma cellular architectures are predicted through the characterization of two-cell interactions. Proc Natl Acad Sci U S A. 2014;111:6521–6526. doi: 10.1073/pnas.1404462111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhang MY, Lee PJ, Hung PJ, Johnson T, Lee LP, Mofrad MRK. Microfluidic environment for high density hepatocyte culture. Biomed Microdevices. 2008;10:117–121. doi: 10.1007/s10544-007-9116-9. [DOI] [PubMed] [Google Scholar]
- (9).Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, Riahi R, Chae S, Hu N, Avci H, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A. 2017;114:E2293–E2302. doi: 10.1073/pnas.1612906114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Duncombe TA, Tentori AM, Herr AE. Microfluidics: Reframing biological enquiry. Nat Rev Mol Cell Biol. 2015;16:554–567. doi: 10.1038/nrm4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sterneckert JL, Reinhardt P, Schöler HR. Investigating human disease using stem cell models. Nat Rev Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- (12).Dutta D, Heo I, Clevers H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- (13).Bitziou E, O’Hare D, Patel BA. Spatial changes in acid secretion from isolated stomach tissue using a pH-histamine sensing microarray. Analyst. 2010;135:482–487. doi: 10.1039/b921296e. [DOI] [PubMed] [Google Scholar]
- (14).Obien MEJ, Deligkaris K, Bullmann T, Bakkum DJ, Frey U. Revealing neuronal function through microelectrode array recordings. Front Neurosci. 2015;8:423. doi: 10.3389/fnins.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Obien MEJ, Gong W, Frey U, Bakkum DJ. CMOS-Based High-Density Microelectrode Arrays: Technology and Applications. Springer; Singapore: 2017. pp. 3–39. [Google Scholar]
- (16).Höber R. Eine Methode, die elektrische Leitfähigkeit im Innern von Zellen zu messen. Pflüger’s Arch für die Gesammte Physiol des Menschen und der Tiere. 1910;133:237–253. [Google Scholar]
- (17).Fricke H, Morse S. the Electric Resistance and Capacity of Blood for Frequencies Between 800 and 4(1/2) Million Cycles. J Gen Physiol. 1925;9:153–167. doi: 10.1085/jgp.9.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Am HJC, Cole KS. Transverse electric impedance of Nitella. J Gen Physiol. 1937;21:189–201. doi: 10.1085/jgp.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sun T, Morgan H. Single-cell microfluidic Impedance cytometry: A review. Microfluid Nanofluidics. 2010;8:423–443. [Google Scholar]
- (20).Cheung K, Gawad S, Renaud P. Impedance spectroscopy flow cytometry: on-chip label-free cell differentiation. Cytometry A. 2005;65:124–132. doi: 10.1002/cyto.a.20141. [DOI] [PubMed] [Google Scholar]
- (21).Holmes D, Pettigrew D, Reccius CH, Gwyer JD, van Berkel C, Holloway J, Davies DE, Morgan H. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip. 2009;9:2881–2889. doi: 10.1039/b910053a. [DOI] [PubMed] [Google Scholar]
- (22).Holmes D, Morgan H. Single cell impedance cytometry for identification and counting of CD4 T-cells in human blood using impedance labels. Anal Chem. 2010;82:1455–1461. doi: 10.1021/ac902568p. [DOI] [PubMed] [Google Scholar]
- (23).Song H, Wang Y, Rosano JM, Prabhakarpandian B, Garson C, Pant K, Lai E. A microfluidic impedance flow cytometer for identification of differentiation state of stem cells. Lab Chip. 2013;13:2300–2310. doi: 10.1039/c3lc41321g. [DOI] [PubMed] [Google Scholar]
- (24).Han A, Yang L, Frazier AB. Quantification of the heterogeneity in breast cancer cell lines using whole-cell impedance spectroscopy. Clin Cancer Res. 2007;13:139–143. doi: 10.1158/1078-0432.CCR-06-1346. [DOI] [PubMed] [Google Scholar]
- (25).Evander M, Ricco AJ, Morser J, Kovacs GTA, Leung LLK, Giovangrandi L, Tseng JL, Subramanyam B, Buckman B, Islam I, Yuan S, et al. Microfluidic impedance cytometer for platelet analysis. Lab Chip. 2013;13:722–729. doi: 10.1039/c2lc40896a. [DOI] [PubMed] [Google Scholar]
- (26).Schade-Kampmann G, Huwiler A, Hebeisen M, Hessler T, Di Berardino M. On-chip non-invasive and label-free cell discrimination by impedance spectroscopy. Cell Prolif. 2008;41:830–840. doi: 10.1111/j.1365-2184.2008.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hassan U, Bashir R. Coincidence detection of heterogeneous cell populations from whole blood with coplanar electrodes in a microfluidic impedance cytometer. Lab Chip. 2014;14:4370–4381. doi: 10.1039/c4lc00879k. [DOI] [PubMed] [Google Scholar]
- (28).Chen J, Zheng Y, Tan Q, Shojaei-Baghini E, Zhang YL, Li J, Prasad P, You L, Wu XY, Sun Y. Classification of cell types using a microfluidic device for mechanical and electrical measurement on single cells. Lab Chip. 2011;11:3174–3181. doi: 10.1039/c1lc20473d. [DOI] [PubMed] [Google Scholar]
- (29).Haandbaek N, Bürgel SC, Rudolf F, Heer F, Hierlemann A. Characterization of single yeast cell phenotypes using microfluidic impedance cytometry and optical imaging. ACS Sensors. 2016;1:1020–1027. [Google Scholar]
- (30).Malleo D, Nevill JT, Lee LP, Morgan H. Continuous differential impedance spectroscopy of single cells. Microfluid Nanofluidics. 2010;9:191–198. doi: 10.1007/s10404-009-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Jang LS, Wang MH. Microfluidic device for cell capture and impedance measurement. Biomed Microdevices. 2007;9:737–743. doi: 10.1007/s10544-007-9084-0. [DOI] [PubMed] [Google Scholar]
- (32).Zhu Z, Frey O, Franke F, Haandbæk N, Hierlemann A. Real-time monitoring of immobilized single yeast cells through multifrequency electrical impedance spectroscopy. Anal Bioanal Chem. 2014:7015–7025. doi: 10.1007/s00216-014-7955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zhu Z, Frey O, Haandbaek N, Franke F, Rudolf F, Hierlemann A. Time-lapse electrical impedance spectroscopy for monitoring the cell cycle of single immobilized S. pombe cells. Sci Rep. 2015;5:17180. doi: 10.1038/srep17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res. 2000;259:158–166. doi: 10.1006/excr.2000.4919. [DOI] [PubMed] [Google Scholar]
- (35).Xing JZ, Zhu L, Gabos S, Xie L. Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol Vitr. 2006;20:995–1004. doi: 10.1016/j.tiv.2005.12.008. [DOI] [PubMed] [Google Scholar]
- (36).Xiao C, Luong JHT. Assessment of cytotoxicity by emerging impedance spectroscopy. Toxicology and Applied Pharmacology. 2005:102–112. doi: 10.1016/j.taap.2004.10.025. [DOI] [PubMed] [Google Scholar]
- (37).Xiao C, Luong JHT. On-line monitoring of cell growth and cytotoxicity using electric cell-substrate impedance sensing (ECIS) Biotechnol Prog. 2003;19:1000–1005. doi: 10.1021/bp025733x. [DOI] [PubMed] [Google Scholar]
- (38).Peters MF, Lamore SD, Guo L, Scott CW, Kolaja KL. Human Stem Cell-Derived Cardiomyocytes in Cellular Impedance Assays: Bringing Cardiotoxicity Screening to the Front Line. Cardiovasc Toxicol. 2015;15:127–139. doi: 10.1007/s12012-014-9268-9. [DOI] [PubMed] [Google Scholar]
- (39).Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JHT. Assessment of Cytotoxicity of Quantum Dots and Gold Nanoparticles Using Cell-Based Impedance Spectroscopy. Anal Chem. 2008;80:5487–5493. doi: 10.1021/ac8004555. [DOI] [PubMed] [Google Scholar]
- (40).Yang M, Lim CC, Liao R, Zhang X. A novel microfluidic impedance assay for monitoring endothelin-induced cardiomyocyte hypertrophy. Biosens Bioelectron. 2007;22:1688–1693. doi: 10.1016/j.bios.2006.07.032. [DOI] [PubMed] [Google Scholar]
- (41).Curtis TM, Widder MW, Brennan LM, Schwager SJ, van der Schalie WH, Fey J, Salazar N. A portable cell-based impedance sensor for toxicity testing of drinking water. Lab Chip. 2009;9:2176–2183. doi: 10.1039/b901314h. [DOI] [PubMed] [Google Scholar]
- (42).Nguyen TA, Yin TI, Reyes D, Urban GA. Microfluidic chip with integrated electrical cell-impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal Chem. 2013;85:11068–11076. doi: 10.1021/ac402761s. [DOI] [PubMed] [Google Scholar]
- (43).Tran TB, Cho S, Min J. Hydrogel-based diffusion chip with Electric Cell-substrate Impedance Sensing (ECIS) integration for cell viability assay and drug toxicity screening. Biosens Bioelectron. 2013;50:453–459. doi: 10.1016/j.bios.2013.07.019. [DOI] [PubMed] [Google Scholar]
- (44).Bird C, Kirstein S. Real-time, label-free monitoring of cellular invasion and migration with the xCELLigence system. Nat Methods. 2009:6. [Google Scholar]
- (45).Nguemo F, Šarić T, Pfannkuche K, Watzele M, Reppel M, Hescheler J. In vitro model for assessing arrhythmogenic properties of drugs based on high-resolution impedance measurements. Cell Physiol Biochem. 2012;29:819–832. doi: 10.1159/000188069. [DOI] [PubMed] [Google Scholar]
- (46).Kho D, MacDonald C, Johnson R, Unsworth C, O’Carroll S, Mez E, Angel C, Graham E. Application of xCELLigence RTCA Biosensor Technology for Revealing the Profile and Window of Drug Responsiveness in Real Time. Biosensors. 2015;5:199–222. doi: 10.3390/bios5020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Molckovsky A, Wilson BC. Monitoring of cell and tissue responses to photodynamic therapy by electrical impedance spectroscopy. Phys Med Biol. 2001;46:983–1002. doi: 10.1088/0031-9155/46/4/306. [DOI] [PubMed] [Google Scholar]
- (48).Thielecke H, Mack A, Robitzki A. A multicellular spheroid-based sensor for anti-cancer therapeutics. Biosens Bioelectron. 2001;16:261–269. doi: 10.1016/s0956-5663(01)00140-3. [DOI] [PubMed] [Google Scholar]
- (49).Kloß D, Kurz R, Jahnke HG, Fischer M, Rothermel A, Anderegg U, Simon JC, Robitzki AA. Microcavity array (MCA)-based biosensor chip for functional drug screening of 3D tissue models. Biosens Bioelectron. 2008;23:1473–1480. doi: 10.1016/j.bios.2008.01.003. [DOI] [PubMed] [Google Scholar]
- (50).Schmid YRF, Bürgel SC, Misun PM, Hierlemann A, Frey O. Electrical Impedance Spectroscopy for Microtissue Spheroid Analysis in Hanging-Drop Networks. ACS Sensors. 2016;1:1028–1035. doi: 10.1021/acssensors.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Bürgel SC, Diener L, Frey O, Kim J-Y, Hierlemann A. Automated, Multiplexed Electrical Impedance Spectroscopy Platform for Continuous Monitoring of Microtissue Spheroids. Anal Chem. 2016;88:10876–10883. doi: 10.1021/acs.analchem.6b01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Chuang H-S, Raizen DM, Lamb A, Dabbish N, Bau HH. Dielectrophoresis of Caenorhabditis elegans. Lab Chip. 2011;11:599–604. doi: 10.1039/c0lc00532k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Zhang B, Li Y, He Q, Qin J, Yu Y, Li X, Zhang L, Yao M, Liu J, Chen Z. Microfluidic platform integrated with worm-counting setup for assessing manganese toxicity. Biomicrofluidics. 2014;8:54110. doi: 10.1063/1.4896663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Hu C, Dillon J, Kearn J, Murray C, O’Connor V, Holden-Dye L, Morgan H. NeuroChip: A Microfluidic Electrophysiological Device for Genetic and Chemical Biology Screening of Caenorhabditis elegans Adult and Larvae. Dupuy D, editor. PLoS One. 2013;8:e64297. doi: 10.1371/journal.pone.0064297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Bakhtina NA, Korvink JG. Microfluidic laboratories for C. elegans enhance fundamental studies in biology. RSC Adv. 2014;4:4691–4709. [Google Scholar]
- (56).Wang RY, Zhang T, Bao Q, Rawson DM. Study on fish embryo responses to the treatment of cryoprotective chemicals using impedance spectroscopy. Eur Biophys J. 2006;35:224–230. doi: 10.1007/s00249-005-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Wang RY, Guan M, Rawson DM, Zhang T. Ultrasound enhanced methanol penetration of zebrafish (Danio rerio) embryos measured by permittivity changes using impedance spectroscopy. Eur Biophys J. 2008;37:1039–1044. doi: 10.1007/s00249-007-0229-0. [DOI] [PubMed] [Google Scholar]
- (58).Houssin T, Follet J, Dei Cas E, Senez V. 2009 IEEE Sensors. IEEE; 2009. Electrochemical impedance spectroscopy for detection of parasites in drinking water; pp. 396–399. [Google Scholar]
- (59).Smout MJ, Kotze AC, Mccarthy JS, Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Neglected Trop Dis. 2010;4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Rinaldi G, Loukas A, Brindley PJ, Irelan JT, Smout MJ. Viability of developmental stages of Schistosoma mansoni quantified with xCELLigence worm real-time motility assay (xWORM) Int J Parasitol Drugs Drug Resist. 2015;5:141–148. doi: 10.1016/j.ijpddr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron. 2001;16:121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- (62).Ronkainen NJ, Halsall HB, Heineman WR. Electrochemical biosensors. Chem Soc Rev. 2010;39:1747–1763. doi: 10.1039/b714449k. [DOI] [PubMed] [Google Scholar]
- (63).Amemiya S, Bard AJ, Fan F-RF, Mirkin MV, Unwin PR. Scanning Electrochemical Microscopy. Annu Rev Anal Chem. 2008;1:95–131. doi: 10.1146/annurev.anchem.1.031207.112938. [DOI] [PubMed] [Google Scholar]
- (64).Mross S, Pierrat S, Zimmermann T, Kraft M. Microfluidic enzymatic biosensing systems: A review. Biosens Bioelectron. 2015;70:376–391. doi: 10.1016/j.bios.2015.03.049. [DOI] [PubMed] [Google Scholar]
- (65).Wang J. Amperometric biosensors for clinical and therapeutic drug monitoring: a review. J Pharm Biomed Anal. 1999;19:47–53. doi: 10.1016/s0731-7085(98)00056-9. [DOI] [PubMed] [Google Scholar]
- (66).Weltin A, Kieninger J, Urban GA. Microfabricated, amperometric, enzyme-based biosensors for in vivo applications. Anal Bioanal Chem. 2016;408:4503–4521. doi: 10.1007/s00216-016-9420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Ciobanu M, Taylor DE, Wilburn JP, Cliffel DE. Glucose and lactate biosensors for scanning electrochemical microscopy imaging of single live cells. Anal Chem. 2008;80:2717–2727. doi: 10.1021/ac7021184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Frey O, Talaei S, van der Wal PD, Koudelka-Hep M, de Rooij NF. Continuous-flow multi-analyte biosensor cartridge with controllable linear response range. Lab Chip. 2010;10:2226–2234. doi: 10.1039/c004851h. [DOI] [PubMed] [Google Scholar]
- (69).Talaei S, van der Wal PD, Ahmed S, Liley M, de Rooij NF. Enzyme SU-8 microreactors: simple tools for cell-culture monitoring. Microfluid Nanofluidics. 2015;19:351–361. [Google Scholar]
- (70).Misun PM, Rothe J, Schmid YRF, Hierlemann A, Frey O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst Nanoeng. 2016;2:16022. doi: 10.1038/micronano.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Moser I, Jobst G, Urban GA. Biosensor arrays for simultaneous measurement of glucose, lactate, glutamate, and glutamine. Biosens Bioelectron. 2002;17:297–302. doi: 10.1016/s0956-5663(01)00298-6. [DOI] [PubMed] [Google Scholar]
- (72).Sassa F, Morimoto K, Satoh W, Suzuki H. Electrochemical techniques for microfluidic applications. Electrophoresis. 2008;29:1787–1800. doi: 10.1002/elps.200700581. [DOI] [PubMed] [Google Scholar]
- (73).Dempsey E, Diamond D, Smyth MR, Urban G, Jobst G, Moser I, Verpoorte EMJ, Manz A, Michael Widmer H, Rabenstein K, Freaney R, et al. Design and development of a miniaturised total chemical analysis system for on-line lactate and glucose monitoring in biological samples. Anal Chim Acta. 1997;346:341–349. [Google Scholar]
- (74).Boero C, Carrara S, Del Vecchio G, Calzà L, De Micheli G. Highly sensitive carbon nanotube-based sensing for lactate and glucose monitoring in cell culture. IEEE Trans Nanobioscience. 2011;10:59–67. doi: 10.1109/TNB.2011.2138157. [DOI] [PubMed] [Google Scholar]
- (75).Ges Ia, Baudenbacher F. Enzyme electrodes to monitor glucose consumption of single cardiac myocytes in sub-nanoliter volumes. Biosens Bioelectron. 2010;25:1019–1024. doi: 10.1016/j.bios.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Eklund SE, Snider RM, Wikswo J, Baudenbacher F, Prokop A, Cliffel DE. Multianalyte microphysiometry as a tool in metabolomics and systems biology. J Electroanal Chem. 2006;587:333–339. [Google Scholar]
- (77).C-CIT Sensors AG – Sensing the chemistry of life. [Accessed on October 29, 2017]; http://c-cit.ch/.
- (78).Biosensors for Glucose and Lactate monitoring at extremly low flow rates. [Accessed on October 29, 2017]; http://www.jobst-technologies.com/.
- (79).Spichiger S, Spichiger-Keller UE. Single-Use Technology in Biopharmaceutical Manufacture. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2011. New Single-Use Sensors for Online Measurement of Glucose and Lactate: The Answer to the PAT Initiative; p. 295.p. 299. [Google Scholar]
- (80).Ugo P, Moretto LM. Electrochemical Immunosensors and Aptasensors. Chemosensors. 2017;5:13. [Google Scholar]
- (81).Piro B, Reisberg S. Recent Advances in Electrochemical Immunosensors. Sensors (Basel, Switz.) 2017;17:794. doi: 10.3390/s17040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Bange A, Halsall HB, Heineman WR. Microfluidic immunosensor systems. Biosens Bioelectron. 2005;20:2488–2503. doi: 10.1016/j.bios.2004.10.016. [DOI] [PubMed] [Google Scholar]
- (83).Kokkinos C, Economou A, Prodromidis MI. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC, Trends Anal Chem. 2016;79:88–105. [Google Scholar]
- (84).Lim SA, Ahmed MU. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: a review. RSC Adv. 2016;6:24995–25014. [Google Scholar]
- (85).Wen W, Yan X, Zhu C, Du D, Lin Y. Recent Advances in Electrochemical Immunosensors. Anal Chem. 2017;89:138–156. doi: 10.1021/acs.analchem.6b04281. [DOI] [PubMed] [Google Scholar]
- (86).Luo X, Xu M, Freeman C, James T, Davis JJ. Ultrasensitive Label Free Electrical Detection of Insulin in Neat Blood Serum. Anal Chem. 2013;85:4129–4134. doi: 10.1021/ac4002657. [DOI] [PubMed] [Google Scholar]
- (87).Lehr J, Fernandes FCB, Bueno PR, Davis JJ. Label-free Capacitive Diagnostics: Exploiting Local Redox Probe State Occupancy. Anal Chem. 2014;86:2559–2564. doi: 10.1021/ac403727h. [DOI] [PubMed] [Google Scholar]
- (88).Liu Y, Tuleouva N, Ramanculov E, Revzin A. Aptamer-based electrochemical biosensor for interferon gamma detection. Anal Chem. 2010;82:8131–8136. doi: 10.1021/ac101409t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Ferguson BS, Hoggarth DA, Maliniak D, Ploense K, White RJ, Woodward N, Hsieh K, Bonham AJ, Eisenstein M, Plaxco KW, Soh HT. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci Transl Med. 2014;5:213ra165. doi: 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc Natl Acad Sci U S A. 2017;114:645–650. doi: 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano. 2012;6:6546–6561. doi: 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Zhou J, Du L, Zou Y, Hu N, Wang P. An ultrasensitive electrochemical immunosensor for carcinoembryonic antigen detection based on staphylococcal protein A—Au nanoparticle modified gold electrode. Sens Actuators B. 2014;197:220–227. [Google Scholar]
- (93).Emami M, Shamsipur M, Saber R, Irajirad R, Turner APF, Mascini M, Marrazza G, Robinson MK, Adams GP. An electrochemical immunosensor for detection of a breast cancer biomarker based on antiHER2–iron oxide nanoparticle bioconjugates. Analyst. 2014;139:2858–2866. doi: 10.1039/c4an00183d. [DOI] [PubMed] [Google Scholar]
- (94).Chen P, Huang N-T, Chung M-T, Cornell TT, Kurabayashi K. Label-free cytokine micro- and nano-biosensing towards personalized medicine of systemic inflammatory disorders. Adv Drug Deliv Rev. 2015;95:90–103. doi: 10.1016/j.addr.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Riahi R, Shaegh SAM, Ghaderi M, Zhang YS, Shin SR, Aleman J, Massa S, Kim D, Dokmeci MR, Khademhosseini A. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci Rep. 2016;6 doi: 10.1038/srep24598. 24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Shin SR, Zhang YS, Kim DJ, Manbohi A, Avci H, Silvestri A, Aleman J, Hu N, Kilic T, Keung W, Righi M, et al. Aptamer-Based Microfluidic Electrochemical Biosensor for Monitoring Cell-Secreted Trace Cardiac Biomarkers. Anal Chem. 2016;88:10019–10027. doi: 10.1021/acs.analchem.6b02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Zhou J, Zhang L, Tian Y. Micro Electrochemical pH Sensor Applicable for Real-Time Ratiometric Monitoring of pH Values in Rat Brains. Anal Chem. 2016;88:2113–2118. doi: 10.1021/acs.analchem.5b03634. [DOI] [PubMed] [Google Scholar]
- (98).Mousavi Shaegh SA, De Ferrari F, Zhang YS, Nabavinia M, Binth Mohammad N, Ryan J, Pourmand A, Laukaitis E, Banan Sadeghian R, Nadhman A, Shin SR, et al. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics. 2016;10:44111. doi: 10.1063/1.4955155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Anderson M, Moshnikova A, Engelman DM, Reshetnyak YK, Andreev OA. Probe for the measurement of cell surface pH in vivo and ex vivo. Proc Natl Acad Sci U S A. 2016;113:8177–8181. doi: 10.1073/pnas.1608247113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Kurzweil P. Metal oxides and ion-exchanging surfaces as pH sensors in liquids: State-of-the-art and outlook. Sensors (Basel, Switz.) 2009;9:4955–4985. doi: 10.3390/s90604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Fog A, Buck RP. Electronic semiconducting oxides as pH sensors. Sens Actuators. 1984;5:137–146. [Google Scholar]
- (102).Kim TY, Hong SA, Yang S. A solid-state thin-film Ag/AgCl reference electrode coated with graphene oxide and its use in a pH sensor. Sensors (Basel, Switz.) 2015;15:6469–6482. doi: 10.3390/s150306469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Kieninger J, Marx A, Spies F, Weltin A, Urban GA, Jobst G. pH micro sensor with microfluidic liquid-junction reference electrode on-chip for cell culture applications. Proc IEEE Sensors. 2009:2009–2012. [Google Scholar]
- (104).Noh J, Park S, Boo H, Kim HC, Chung TD. Nanoporous platinum solid-state reference electrode with layer-by-layer polyelectrolyte junction for pH sensing chip. Lab Chip. 2011;11:664–671. doi: 10.1039/c0lc00293c. [DOI] [PubMed] [Google Scholar]
- (105).Thedinga E, Kob A, Holst H, Keuer A, Drechsler S, Niendorf R, Baumann W, Freund I, Lehmann M, Ehret R. Online monitoring of cell metabolism for studying pharmacodynamic effects. Toxicol Appl Pharmacol. 2007;220:33–44. doi: 10.1016/j.taap.2006.12.027. [DOI] [PubMed] [Google Scholar]
- (106).Ges Ia, Dzhura Ia, Baudenbacher FJ. On-chip acidification rate measurements from single cardiac cells confined in sub-nanoliter volumes. Biomed Microdevices. 2008;10:347–354. doi: 10.1007/s10544-007-9142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293:1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- (108).Zörgiebel FM, Pregl S, Römhildt L, Opitz J, Weber W, Mikolajick T, Baraban L, Cuniberti G. Schottky barrier-based silicon nanowire pH sensor with live sensitivity control. Nano Res. 2014;7:263–271. [Google Scholar]
- (109).Bergveld P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans Biomed Eng BME. 1970;17:70–71. doi: 10.1109/tbme.1970.4502688. [DOI] [PubMed] [Google Scholar]
- (110).Lee C-S, Kim SK, Kim M. Ion-sensitive field-effect transistor for biological sensing. Sensors (Basel, Switz.) 2009;9:7111–7131. doi: 10.3390/s90907111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Pachauri V, Ingebrandt S. Biologically sensitive field-effect transistors: from ISFETs to NanoFETs. Essays Biochem. 2016;60:81–90. doi: 10.1042/EBC20150009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Matsuura K, Asano Y, Yamada A, Naruse K. Detection of Micrococcus Luteus Biofilm Formation in Microfluidic Environments by pH Measurement Using an Ion-Sensitive Field-Effect Transistor. Sensors. 2013;13:2484–2493. doi: 10.3390/s130202484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Ponsonnet L, Boureanu M, Jaffrezic N, Othmane A, Dorel C, Lejeune P. Local pH variation as an initial step in bacterial surface-sensing and biofilm formation. Mater Sci Eng C. 2008;28:896–900. [Google Scholar]
- (114).Pourciel-Gouzy M-L, Sant W, Humenyuk I, Malaquin L, Dollat X, Temple-Boyer P. Development of pH-ISFET sensors for the detection of bacterial activity. Sens Actuators, B. 2004;103:247–251. [Google Scholar]
- (115).Castellarnau M, Zine N, Bausells J, Madrid C, Juárez A, Samitier J, Errachid A. Integrated cell positioning and cell-based ISFET biosensors. Sens Actuators, B. 2007;120:615–620. [Google Scholar]
- (116).Castellarnau M, Zine N, Bausells J, Madrid C, Juárez A, Samitier J, Errachid A. ISFET-based biosensor to monitor sugar metabolism in bacteria. Mater Sci Eng C. 2008;28:680–685. [Google Scholar]
- (117).Ibarlucea B, Rim T, Baek C-K, de Visser JAGM, Baraban L, Cuniberti G. Nanowire sensors monitor bacterial growth kinetics and response to antibiotics. Lab Chip. 2017;17:4283–4293. doi: 10.1039/c7lc00807d. [DOI] [PubMed] [Google Scholar]
- (118).Mu L, Droujinine IA, Lee J, Wipf M, Davis P, Adams C, Hannant J, Reed MA. Nanoelectronic Platform for Ultrasensitive Detection of Protein Biomarkers in Serum using DNA Amplification. Anal Chem. 2017;89:11325–11331. doi: 10.1021/acs.analchem.7b02036. [DOI] [PubMed] [Google Scholar]
- (119).Brennan MD, Rexius-Hall ML, Elgass LJ, Eddington DT. Oxygen control with microfluidics. Lab Chip. 2014;14:4305–4318. doi: 10.1039/c4lc00853g. [DOI] [PubMed] [Google Scholar]
- (120).Wolfbeis OS. Luminescent sensing and imaging of oxygen: fierce competition to the Clark electrode. Bioessays. 2015;37:921–928. doi: 10.1002/bies.201500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Grist SM, Chrostowski L, Cheung KC. Optical oxygen sensors for applications in microfluidic cell culture. Sensors (Basel, Switz.) 2010;10:9286–9316. doi: 10.3390/s101009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Ungerböck B, Charwat V, Ertl P, Mayr T. Microfluidic oxygen imaging using integrated optical sensor layers and a color camera. Lab Chip. 2013;13:1593–1601. doi: 10.1039/c3lc41315b. [DOI] [PubMed] [Google Scholar]
- (123).Lasave LC, Borisov SM, Ehgartner J, Mayr T, Woodley JM, Revsbech NP, Glud RN, Canfield DE, Klimant I. Quick and simple integration of optical oxygen sensors into glass-based microfluidic devices. RSC Adv. 2015;5:70808–70816. [Google Scholar]
- (124).Date Y, Takano S, Shiku H, Ino K, Ito-Sasaki T, Yokoo M, Abe H, Matsue T. Monitoring oxygen consumption of single mouse embryos using an integrated electrochemical microdevice. Biosens Bioelectron. 2011;30:100–106. doi: 10.1016/j.bios.2011.08.037. [DOI] [PubMed] [Google Scholar]
- (125).Wu CC, Saito T, Yasukawa T, Shiku H, Abe H, Hoshi H, Matsue T. Microfluidic chip integrated with amperometric detector array for in situ estimating oxygen consumption characteristics of single bovine embryos. Sens Actuators, B. 2007;125:680–687. [Google Scholar]
- (126).Yamagishi A, Tanabe K, Yokokawa M, Morimoto Y, Kinoshita M, Suzuki H. Microfluidic device coupled with a microfabricated oxygen electrode for the measurement of bactericidal activity of neutrophil-like cells. Anal Chim Acta. 2017;985:1–6. doi: 10.1016/j.aca.2017.07.049. [DOI] [PubMed] [Google Scholar]
- (127).Kieninger J, Aravindalochanan K, Sandvik Ja, Pettersen EO, Urban Ga. Pericellular oxygen monitoring with integrated sensor chips for reproducible cell culture experiments. Cell Prolif. 2014;47:180–188. doi: 10.1111/j.1365-2184.2013.12089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Flamm H, Kieninger J, Weltin A, Urban GA. Superoxide microsensor integrated into a Sensing Cell Culture Flask microsystem using direct oxidation for cell culture application. Biosens Bioelectron. 2015;65:354–359. doi: 10.1016/j.bios.2014.10.062. [DOI] [PubMed] [Google Scholar]
- (129).Eklund SE, Taylor D, Kozlov E, Prokop A, Cliffel DE. A microphysiometer for simultaneous measurement of changes in extracellular glucose, lactate, oxygen, and acidification rate. Anal Chem. 2004;76:519–527. doi: 10.1021/ac034641z. [DOI] [PubMed] [Google Scholar]
- (130).McKenzie JR, Palubinsky AM, Brown JE, McLaughlin B, Cliffel DE. Metabolic multianalyte microphysiometry reveals extracellular acidosis is an essential mediator of neuronal preconditioning. ACS Chem Neurosci. 2012;3:510–518. doi: 10.1021/cn300003r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Brischwein M, Motrescu ER, Cabala E, Otto aM, Grothe H, Wolf B. Functional cellular assays with multiparametric silicon sensor chips. Lab Chip. 2003;3:234–240. doi: 10.1039/b308888j. [DOI] [PubMed] [Google Scholar]
- (132).Weltin A, Kieninger J, Urban G, Moser I, Jobst G, Wego M, Ehret R. 2010 IEEE Sensors. IEEE; 2010. A novel multiparametric microphysiometry system for dynamic cell culture monitoring; pp. 2113–2116. [Google Scholar]
- (133).Weltin A, Kieninger J, Enderle B, Gellner AK, Fritsch B, Urban GA. Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens Bioelectron. 2014;61:192–199. doi: 10.1016/j.bios.2014.05.014. [DOI] [PubMed] [Google Scholar]
- (134).Satoh W, Hosono H, Yokomaku H, Morimoto K, Upadhyay S, Suzuki H. Integrated Electrochemical Analysis System with Microfluidic and Sensing Functions. Sensors. 2008;8:1111–1127. doi: 10.3390/s8021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Eklund SE, Thompson RG, Snider RM, Carney CK, Wright DW, Wikswo J, Cliffel DE. Metabolic discrimination of select list agents by monitoring cellular responses in a multianalyte microphysiometer. Sensors. 2009;9:2117–2133. doi: 10.3390/s90302117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Weltin A, Slotwinski K, Kieninger J, Moser I, Jobst G, Wego M, Ehret R, Urban Ga. Cell culture monitoring for drug screening and cancer research: a transparent, microfluidic, multi-sensor microsystem. Lab Chip. 2014;14:138–146. doi: 10.1039/c3lc50759a. [DOI] [PubMed] [Google Scholar]
- (137).McKenzie JR, Cognata AC, Davis AN, Wikswo JP, Cliffel DE. Real-Time Monitoring of Cellular Bioenergetics with a Multianalyte Screen-Printed Electrode. Anal Chem. 2015;87:7857–7864. doi: 10.1021/acs.analchem.5b01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Alexander F, Eggert S, Wiest J. A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology. 2017:1–12. doi: 10.1007/s10616-017-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Weltin A, Hammer S, Noor F, Kaminski Y, Kieninger J, Urban GA. Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens Bioelectron. 2017;87:941–948. doi: 10.1016/j.bios.2016.07.094. [DOI] [PubMed] [Google Scholar]
- (140).Bavli D, Prill S, Ezra E, Levy G, Cohen M, Vinken M, Vanfleteren J, Jaeger M, Nahmias Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2016:E2232–E2240. doi: 10.1073/pnas.1522556113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Go DB, Atashbar MZ, Ramshani Z, Chang H-C. Surface acoustic wave devices for chemical sensing and microfluidics: A review and perspective. Anal Methods. 2017;9:4112–4134. doi: 10.1039/C7AY00690J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Länge K, Rapp BE, Rapp M. Surface acoustic wave biosensors: A review. Anal Bioanal Chem. 2008;391:1509–1519. doi: 10.1007/s00216-008-1911-5. [DOI] [PubMed] [Google Scholar]
- (143).Hashimoto K. Surface Acoustic Wave Devices in Telecommunications. Springer Berlin Heidelberg; Berlin, Heidelberg: 2000. [Google Scholar]
- (144).Jakubik WP. Surface acoustic wave-based gas sensors. Thin Solid Films. 2011;520:986–993. [Google Scholar]
- (145).Sivanantha N, Ma C, Collins DJ, Sesen M, Brenker J, Coppel RL, Neild A, Alan T. Characterization of adhesive properties of red blood cells using surface acoustic wave induced flows for rapid diagnostics. Appl Phys Lett. 2014;105:103704. [Google Scholar]
- (146).Bussonnière A, Miron Y, Baudoin M, Bou Matar O, Grandbois M, Charette P, Renaudin A. Cell detachment and label-free cell sorting using modulated surface acoustic waves (SAWs) in droplet-based microfluidics. Lab Chip. 2014;14:3556–3563. doi: 10.1039/c4lc00625a. [DOI] [PubMed] [Google Scholar]
- (147).Stamp M, Jötten A, Kudella P, Breyer D, Strobl F, Geislinger T, Wixforth A, Westerhausen C. Exploring the Limits of Cell Adhesion under Shear Stress within Physiological Conditions and beyond on a Chip. Diagnostics. 2016;6:38. doi: 10.3390/diagnostics6040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Salehi-Reyhani A, Gesellchen F, Mampallil D, Wilson R, Reboud J, Ces O, Willison KR, Cooper JM, Klug DR. Chemical-free lysis and fractionation of cells by use of surface acoustic waves for sensitive protein assays. Anal Chem. 2015;87:2161–2169. doi: 10.1021/ac5033758. [DOI] [PubMed] [Google Scholar]
- (149).Alhasan L, Qi A, Al-Abboodi A, Rezk A, Chan PPY, Iliescu C, Yeo LY. Rapid Enhancement of Cellular Spheroid Assembly by Acoustically Driven Microcentrifugation. ACS Biomater Sci Eng. 2016;2:1013–1022. doi: 10.1021/acsbiomaterials.6b00144. [DOI] [PubMed] [Google Scholar]
- (150).Reboud J, Bourquin Y, Wilson R, Pall GS, Jiwaji M, Pitt AR, Graham A, Waters AP, Cooper JM. Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. Proc Natl Acad Sci U S A. 2012;109:15162–15167. doi: 10.1073/pnas.1206055109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Yeo LY, Friend JR. Surface Acoustic Wave Microfluidics. Annu Rev Fluid Mech. 2014;46:379–406. [Google Scholar]
- (152).Hartmann A, Stamp M, Kmeth R, Buchegger S, Stritzker B, Saldamli B, Burgkart R, Schneider MF, Wixforth A. A novel tool for dynamic cell adhesion studies – the De-Adhesion Number Investigator DANI. Lab Chip. 2014;14:542–546. doi: 10.1039/c3lc50916h. [DOI] [PubMed] [Google Scholar]
- (153).Shi J, Ahmed D, Mao X, Lin S-CS, Lawit A, Huang TJ. Acoustic tweezers: patterning cells and microparticles using standing surface acoustic waves (SSAW) Lab Chip. 2009;9:2890–2895. doi: 10.1039/b910595f. [DOI] [PubMed] [Google Scholar]
- (154).Collins DJ, Morahan B, Garcia-Bustos J, Doerig C, Plebanski M, Neild A. Two-dimensional single-cell patterning with one cell per well driven by surface acoustic waves. Nat Commun. 2015;6 doi: 10.1038/ncomms9686. 8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Guo F, Li P, French JB, Mao Z, Zhao H, Li S, Nama N, Fick JR, Benkovic SJ, Huang TJ. Controlling cell-cell interactions using surface acoustic waves. Proc Natl Acad Sci U S A. 2015;112:43–48. doi: 10.1073/pnas.1422068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Guo F, Mao Z, Chen Y, Xie Z, Lata JP, Li P, Ren L, Liu J, Yang J, Dao M, Suresh S, et al. Three-dimensional manipulation of single cells using surface acoustic waves. Proc Natl Acad Sci U S A. 2016;113:1522–1527. doi: 10.1073/pnas.1524813113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Li S, Guo F, Chen Y, Ding X, Li P, Wang L, Cameron CE, Huang TJ. Standing Surface Acoustic Wave Based Cell Coculture. Anal Chem. 2014;86:9853–9859. doi: 10.1021/ac502453z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Chen K, Wu M, Guo F, Li P, Chan CY, Mao Z, Li S, Ren L, Zhang R, Huang TJ. Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers. Lab Chip. 2016;16:2636–2643. doi: 10.1039/c6lc00444j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Wang T, Green R, Nair RR, Howell M, Mohapatra S, Guldiken R, Mohapatra SS. Surface acoustic waves (SAW)-based biosensing for quantification of cell growth in 2D and 3D Cultures. Sensors (Basel, Switz.) 2015;15:32045–32055. doi: 10.3390/s151229909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Chen Y, Nawaz AA, Zhao Y, Huang P-H, McCoy JP, Levine SJ, Wang L, Huang TJ. Standing surface acoustic wave (SSAW)-based microfluidic cytometer. Lab Chip. 2014;14:916–923. doi: 10.1039/c3lc51139a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Ren L, Chen Y, Li P, Mao Z, Huang P-H, Rufo J, Guo F, Wang L, McCoy JP, Levine SJ, Huang TJ, et al. A high-throughput acoustic cell sorter. Lab Chip. 2015;15:3870–3879. doi: 10.1039/c5lc00706b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Lee K, Shao H, Weissleder R, Lee H. Acoustic Purification of Extracellular Microvesicles. ACS Nano. 2015;9:2321–2327. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Hoa XDD, Kirk AGG, Tabrizian M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens Bioelectron. 2007;23:151–160. doi: 10.1016/j.bios.2007.07.001. [DOI] [PubMed] [Google Scholar]
- (164).Homola J, Yee SS, Gauglitz G. Surface plasmon resonance sensors: review. Sens Actuators, B. 1999;54:3–15. [Google Scholar]
- (165).Zeng S, Baillargeat D, Ho H-P, Yong K-T, Pelletier JN, Masson JF, Ito I, Yoshida M, Sakaguchi K, Sugawara F, Banerjee SK, et al. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem Soc Rev. 2014;43:3426–3452. doi: 10.1039/c3cs60479a. [DOI] [PubMed] [Google Scholar]
- (166).Estevez MC, Otte MA, Sepulveda B, Lechuga LM. Trends and challenges of refractometric nanoplasmonic biosensors: A review. Anal Chim Acta. 2014;806:55–73. doi: 10.1016/j.aca.2013.10.048. [DOI] [PubMed] [Google Scholar]
- (167).Guo L, Jackman JA, Yang HH, Chen P, Cho NJ, Kim DH. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today. 2015;10:213–239. [Google Scholar]
- (168).Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Biosensing with plasmic nanostructure. Nat Mater. 2008;7:442–453. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- (169).Wang DS, Fan SK. Microfluidic surface plasmon resonance sensors: From principles to point-of-care applications. Sensors (Basel, Switz.) 2016;16:1175. doi: 10.3390/s16081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Abadian PN, Kelley CP, Goluch ED. Cellular Analysis and Detection using Surface Plasmon Resonance (SPR) Techniques. Anal Chem. 2014;86:2799–2812. doi: 10.1021/ac500135s. [DOI] [PubMed] [Google Scholar]
- (171).Méjard R, Griesser HJ, Thierry B. Optical biosensing for label-free cellular studies. TrAC, Trends Anal Chem. 2014;53:178–186. [Google Scholar]
- (172).Méjard R, Thierry B. Systematic study of the surface plasmon resonance signals generated by cells for sensors with different characteristic lengths. PLoS One. 2014;9:e107978. doi: 10.1371/journal.pone.0107978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Berguiga L, Streppa L, Boyer-Provera E, Martinez-Torres C, Schaeffer L, Elezgaray J, Arneodo A, Argoul F. Time-lapse scanning surface plasmon microscopy of living adherent cells with a radially polarized beam. Appl Opt. 2016;55:1216–1227. doi: 10.1364/AO.55.001216. [DOI] [PubMed] [Google Scholar]
- (174).Shevchenko Y, Camci-Unal G, Cuttica DF, Dokmeci MR, Albert J, Khademhosseini A. Surface plasmon resonance fiber sensor for real-time and label-free monitoring of cellular behavior. Biosens Bioelectron. 2014;56:359–367. doi: 10.1016/j.bios.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Stojanović I, Schasfoort RBM, Terstappen LWMM. Analysis of cell surface antigens by Surface Plasmon Resonance imaging. Biosens. Bioelectron. 2014;52:1–8. doi: 10.1016/j.bios.2013.08.027. [DOI] [PubMed] [Google Scholar]
- (176).Chabot V, Miron Y, Grandbois M, Charette PG. Long range surface plasmon resonance for increased sensitivity in living cell biosensing through greater probing depth. Sens Actuators B. 2012;174:94–101. [Google Scholar]
- (177).Yang C-T, Méjard R, Griesser HJ, Bagnaninchi PO, Thierry B. Cellular micromotion monitored by long-range surface plasmon resonance with optical fluctuation analysis. Anal Chem. 2015;87:1456–1461. doi: 10.1021/ac5031978. [DOI] [PubMed] [Google Scholar]
- (178).Székács I, Horvath R, Székács A. Label-Free Optical Biosensors for Monitoring Cellular Processes and Cytotoxic Agents at Interfaces Using Guided Modes and Advanced Phase-Contrast Imaging Techniques. In: Nikolelis DP, Nikoleli G-P, editors. Biosensors for Security and Bioterrorism Applications. Springer International Publishing; Cham: 2016. pp. 443–468. [Google Scholar]
- (179).Andersson H, Steel D, Asp J, Dahlenborg K, Jonsson M, Jeppsson A, Lindahl A, Kågedal B, Sartipy P, Mandenius CF. Assaying cardiac biomarkers for toxicity testing using biosensing and cardiomyocytes derived from human embryonic stem cells. J Biotechnol. 2010;150:175–181. doi: 10.1016/j.jbiotec.2010.06.023. [DOI] [PubMed] [Google Scholar]
- (180).Andersson H, Kågedal B, Mandenius CF. Monitoring of troponin release from cardiomyocytes during exposure to toxic substances using surface plasmon resonance biosensing. Anal Bioanal Chem. 2010;398:1395–1402. doi: 10.1007/s00216-010-4041-9. [DOI] [PubMed] [Google Scholar]
- (181).Oh BR, Huang NT, Chen W, Seo JH, Chen P, Cornell TT, Shanley TP, Fu J, Kurabayashi K. Integrated nanoplasmonic sensing for cellular functional immunoanalysis using human blood. ACS Nano. 2014;8:2667–2676. doi: 10.1021/nn406370u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Li X, Soler M, Ozdemir CI, Belushkin A, Yesilkoy F, Altug H. Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip. 2017;17:2208–2217. doi: 10.1039/c7lc00277g. [DOI] [PubMed] [Google Scholar]
- (183).Olcum S, Cermak N, Wasserman SC, Christine KS, Atsumi H, Payer KR, Shen W, Lee J, Belcher AM, Bhatia SN, Manalis SR. Weighing nanoparticles in solution at the attogram scale. Proc Natl Acad Sci U S A. 2014;111:1310–1315. doi: 10.1073/pnas.1318602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Modena MM, Wang Y, Riedel D, Burg TP. Resolution enhancement of suspended microchannel resonators for weighing of biomolecular complexes in solution. Lab Chip. 2014;14:342–350. doi: 10.1039/c3lc51058a. [DOI] [PubMed] [Google Scholar]
- (185).Chaste J, Eichler A, Moser J, Ceballos G, Rurali R, Bachtold A. A nanomechanical mass sensor with yoctogram resolution. Nat Nanotechnol. 2012;7:301–304. doi: 10.1038/nnano.2012.42. [DOI] [PubMed] [Google Scholar]
- (186).Arlett JL, Myers EB, Roukes ML. Comparative advantages of mechanical biosensors. Nat Nanotechnol. 2011;6:203–215. doi: 10.1038/nnano.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Tamayo J, Kosaka PM, Ruz JJ, San Paulo Á, Calleja M. Biosensors based on nanomechanical systems. Chem Soc Rev. 2013;42:1287–1311. doi: 10.1039/c2cs35293a. [DOI] [PubMed] [Google Scholar]
- (188).Longo G, Alonso-Sarduy L, Rio LM, Bizzini A, Trampuz A, Notz J, Dietler G, Kasas S. Rapid detection of bacterial resistance to antibiotics using AFM cantilevers as nanomechanical sensors. Nat Nanotechnol. 2013;8:522–526. doi: 10.1038/nnano.2013.120. [DOI] [PubMed] [Google Scholar]
- (189).Wilson K, Das M, Wahl KJ, Colton RJ, Hickman J. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS One. 2010;5:e11042. doi: 10.1371/journal.pone.0011042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Pirozzi KL, Long CJ, McAleer CW, Smith AST, Hickman JJ. Correlation of embryonic skeletal muscle myotube physical characteristics with contractile force generation on an atomic force microscope-based bio-microelectromechanical systems device. Appl Phys Lett. 2013;103:83108. doi: 10.1063/1.4817939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Smith AST, Long CJ, Pirozzi K, Najjar S, McAleer C, Vandenburgh HH, Hickman JJ. A multiplexed chip-based assay system for investigating the functional development of human skeletal myotubes in vitro. J Biotechnol. 2014;185:15–18. doi: 10.1016/j.jbiotec.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Smith AS, Long CJ, McAleer C, Bobbitt N, Srinivasan B, Hickman JJ. Utilization of Microscale Silicon Cantilevers to Assess Cellular Contractile Function In Vitro. J Vis Exp. 2014;92:e51866. doi: 10.3791/51866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park S, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater. 2016;16:303–308. doi: 10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Oyunbaatar NE, Kim DS, Kim ES, Lee BK, Lee DW. Cardiac toxicity screening using polymeric cantilever integrated with cell stimulators. Micro Electro Mech Syst (MEMS), 2017 IEEE 30th Int Conf; 2017. pp. 418–421. [Google Scholar]
- (195).Kim DS, Jeong YJ, Lee BK, Shanmugasundaram A, Lee DW. Piezoresistive sensor-integrated PDMS cantilever: A new class of device for measuring the drug-induced changes in the mechanical activity of cardiomyocytes. Sens Actuators, B. 2017;240:566–572. [Google Scholar]
- (196).Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift für Phys. 1959;155:206–222. [Google Scholar]
- (197).QCM-D | Measurements. [Accessed on November 30, 2017]; http://www.biolinscientific.com/measurements/qcm-d.
- (198).Dixon MC. Quartz crystal microbalance with dissipation monitoring: enabling real-time characterization of biological materials and their interactions. J Biomol Tech. 2008;19:151–158. [PMC free article] [PubMed] [Google Scholar]
- (199).Ferreira GNM, Da-Silva A-C, Tome’ B. Acoustic wave biosensors: physical models and biological applications of quartz crystal microbalance. Trends Biotechnol. 2009;27:689–697. doi: 10.1016/j.tibtech.2009.09.003. [DOI] [PubMed] [Google Scholar]
- (200).Rehman A, Zeng X. Monitoring the Cellular Binding Events with Quartz Crystal Microbalance (QCM) Biosensors. In: Prickril B, Rasooly A, editors. Biosensors and Biodetection: Methods and Protocols, Volume 2: Electrochemical, Bioelectronic, Piezoelectric, Cellular and Molecular Biosensors. Springer New York; New York, NY: 2017. pp. 313–326. [DOI] [PubMed] [Google Scholar]
- (201).Wegener J, Janshoff A, Galla H-J. Cell adhesion monitoring using a quartz crystal microbalance: comparative analysis of different mammalian cell lines. Eur Biophys J. 1998;28:26–37. doi: 10.1007/s002490050180. [DOI] [PubMed] [Google Scholar]
- (202).Wegener J, Janshoff A, Steinem C. The Quartz Crystal Microbalance as a Novel Means to Study Cell-Substrate Interactions In Situ. Cell Biochem Biophys. 2001;34:121–151. doi: 10.1385/CBB:34:1:121. [DOI] [PubMed] [Google Scholar]
- (203).Marx KA, Zhou T, Montrone A, Schulze H, Braunhut SJ. A quartz crystal microbalance cell biosensor: detection of microtubule alterations in living cells at nM nocodazole concentrations. Biosens Bioelectron. 2001;16:773–782. doi: 10.1016/s0956-5663(01)00219-6. [DOI] [PubMed] [Google Scholar]
- (204).Tan L, Lin P, Pezeshkian B, Rehman A, Madlambayan G, Zeng X. Real-time monitoring of cell mechanical changes induced by endothelial cell activation and their subsequent binding with leukemic cell lines. Biosens Bioelectron. 2014;56:151–158. doi: 10.1016/j.bios.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Fatisson J, Azari F, Tufenkji N. Real-time QCM-D monitoring of cellular responses to different cytomorphic agents. Biosens Bioelectron. 2011;26:3207–3212. doi: 10.1016/j.bios.2010.12.027. [DOI] [PubMed] [Google Scholar]
- (206).Conklin G, Ngourn SC, Gerdon AE. Quartz crystal microbalance with microfluidic multi-stream solution control for mineralization kinetic analysis. Sens Actuators, B. 2015;214:174–180. [Google Scholar]
- (207).Thies J-W, Kuhn P, Thürmann B, Dübel S, Dietzel A. Microfluidic quartz-crystal-microbalance (QCM) sensors with specialized immunoassays for extended measurement range and improved reusability. Microelectron Eng. 2017;179:25–30. [Google Scholar]
- (208).Park K, Jang J, Irimia D, Sturgis J, Lee J, Robinson JP, Toner M, Bashir R, Spencer ND, Marobbio CM, Slotboom DJ, et al. “Living cantilever arrays” for characterization of mass of single live cells in fluids. Lab Chip. 2008;8:1034–1041. doi: 10.1039/b803601b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Park K, Millet LJ, Kim N, Li H, Jin X, Popescu G, Aluru NR, Hsia KJ, Bashir R. Measurement of adherent cell mass and growth. Proc Natl Acad Sci U S A. 2010;107:20691–20696. doi: 10.1073/pnas.1011365107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Corbin EA, Adeniba OO, Ewoldt RH, Bashir R. Dynamic mechanical measurement of the viscoelasticity of single adherent cells. Appl Phys Lett. 2016;108:93701. [Google Scholar]
- (211).Corbin EA, Adeniba OO, Cangellaris OV, King WP, Bashir R. Evidence of differential mass change rates between human breast cancer cell lines in culture. Biomed Microdevices. 2017;19:1–7. doi: 10.1007/s10544-017-0151-x. [DOI] [PubMed] [Google Scholar]
- (212).Park K, Mehrnezhad A, Corbin EA, Bashir R. Optomechanical measurement of the stiffness of single adherent cells. Lab Chip. 2015;15:3460–3464. doi: 10.1039/c5lc00444f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Martínez-Martín D, Fläschner G, Gaub B, Martin S, Newton R, Beerli C, Mercer J, Gerber C, Müller DJ. Inertial picobalance reveals fast mass fluctuations in mammalian cells. Nature. 2017;550:500–505. doi: 10.1038/nature24288. [DOI] [PubMed] [Google Scholar]
- (214).Burg TP, Godin M, Knudsen SM, Shen W, Carlson G, Foster JS, Babcock K, Manalis SR. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature. 2007;446:1066–1069. doi: 10.1038/nature05741. [DOI] [PubMed] [Google Scholar]
- (215).Godin M, Delgado FF, Son S, Grover WH, Bryan AK, Tzur A, Jorgensen P, Payer K, Grossman AD, Kirschner MW, Manalis SR. Using buoyant mass to measure the growth of single cells. Nat Methods. 2010;7:387–390. doi: 10.1038/nmeth.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Bryan AK, Goranov A, Amon A, Manalis SR. Measurement of mass, density, and volume during the cell cycle of yeast. Proc Natl Acad Sci U S A. 2010;107:999–1004. doi: 10.1073/pnas.0901851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Feijó Delgado F, Cermak N, Hecht VC, Son S, Li Y, Knudsen SM, Olcum S, Higgins JM, Chen J, Grover WH, Manalis SR. Intracellular Water Exchange for Measuring the Dry Mass, Water Mass and Changes in Chemical Composition of Living Cells. PLoS One. 2013;8:e67590. doi: 10.1371/journal.pone.0067590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Son S, Tzur A, Weng Y, Jorgensen P, Kim J, Kirschner MW, Manalis SR. Direct observation of mammalian cell growth and size regulation. Nat Methods. 2012;9:910–912. doi: 10.1038/nmeth.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Bryan AK, Hecht VC, Shen W, Payer K, Grover WH, Manalis SR. Measuring single cell mass, volume, and density with dual suspended microchannel resonators. Lab Chip. 2014;14:569–576. doi: 10.1039/c3lc51022k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Grover WH, Bryan AK, Diez-Silva M, Suresh S, Higgins JM, Manalis SR. Measuring single-cell density. Proc Natl Acad Sci U S A. 2011;108:10992–10996. doi: 10.1073/pnas.1104651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Cermak N, Olcum S, Delgado FF, Wasserman SC, Payer KR, A Murakami M, Knudsen SM, Kimmerling RJ, Stevens MM, Kikuchi Y, Sandikci A, et al. High-throughput measurement of single-cell growth rates using serial microfluidic mass sensor arrays. Nat Biotechnol. 2016;34:1052–1059. doi: 10.1038/nbt.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Rahman MH, Ahmad IL, Sayahkarajy M, Ahmad MR, Nakajima M, Fukuda T. Lab-on-chip microfluidic system for single cell mass measurement. IECBES 2014, Conf. Proc. - 2014 IEEE Conf. Biomed. Eng. Sci. “Miri, Where Eng. Med. Biol. Humanit. Meet”; 8–10; 2015. [Google Scholar]
- (223).Zhao Y, Lai HSS, Zhang G, Lee G-B, Li WJ, Pries AR, Li WJ, Warburton RJ, Greenaway AH, Ornatsky OI, Balderas RS, et al. Rapid determination of cell mass and density using digitally controlled electric field in a microfluidic chip. Lab Chip. 2014;14:4426–4434. doi: 10.1039/c4lc00795f. [DOI] [PubMed] [Google Scholar]
- (224).Popescu G, Park K, Mir M, Bashir R. New technologies for measuring single cell mass. Lab Chip. 2014;14:646–652. doi: 10.1039/c3lc51033f. [DOI] [PubMed] [Google Scholar]
- (225).Zangle TA, Teitell MA. Live-cell mass profiling: an emerging approach in quantitative biophysics. Nat Methods. 2014;11:1221–1228. doi: 10.1038/nmeth.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]