Abstract
Early work in the 1970s by Linus Pauling, a twice-honored Nobel laureate, led to his proposal of using high-dose vitamin C to treat cancer patients. Over the past several decades, a number of studies in animal models as well as several small-scale clinical studies have provided substantial support of Linus Pauling’s early proposal. Production of reactive oxygen species (ROS) via oxidation of vitamin C appears to be a major underlying event, leading to the selective killing of cancer cells. However, it remains unclear how vitamin C selectively kills cancer cells while sparing normal cells and what the molecular targets of high-dose vitamin C are. In a recent article published in Science (2015 December 11; 350(6266):1391–6. doi: 10.1126/science.aaa5004), Yun et al. reported that vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting glyceraldehyde 3-phosphate dehydrogenase (GAPDH) through an ROS-dependent mechanism. This work by Yun et al. along with other findings advances our current understanding of the molecular basis of high-dose vitamin C-mediated cancer cell killing, which will likely give an impetus to the continued research efforts aiming to further decipher the novel biochemistry of vitamin C and its unique role in cancer therapy.
Keywords: Cancer cell killing, Cancer therapy, Dehydroascorbate, Dehydroascorbate reductase, Glucose transporter, Glutathione, Glyceraldehyde 3-phosphate dehydrogenase, Glycolysis, Reactive oxygen species, S-Glutathionylation, Vitamin C
1. OVERVIEW
Vitamin C, also known as ascorbic acid or ascorbate, is a water-soluble molecule synthesized endogenously in animals except humans, monkeys, guinea pigs, and several other animal species [1]. Humans lost this capability as a result of a series of inactivating mutations of the gene encoding gulonolactone oxidase (GULO), a key enzyme in vitamin C biosynthesis. Humans normally acquire vitamin C from dietary sources through a substrate-saturable transporting mechanism (see Section 3). Dietary sources of vitamin C are mainly vegetables and fruits, including brussels sprout, broccoli, cauliflower, bell pepper, chili pepper, lettuce, kale, tomato, citrus fruits (e.g., orange, lemon), strawberry, papaya, kiwifruit, pineapple, and mango, among others. Another source is vitamin C supplement.
Oral vitamin C intake produces plasma concentrations that are tightly controlled; once its oral intake exceeds 200 mg daily, it becomes difficult to further increase the plasma concentrations of vitamin C. The maximal plasma concentration attainable by oral intake of vitamin C has been estimated to be approximately 200 mM though the physiological plasma concentrations of vitamin C in healthy humans range from 40 mM to 100 mM. In contrast, intravenous injection of large doses of vitamin C produces millimolar concentrations of plasma vitamin C [2]. So does intraperitoneal injection of large doses of vitamin C in experimental animals. Under physiological conditions, intracellular levels of vitamin C are in the millimolar range. This is due to selective intracellular accumulation via vitamin C transporting system present in the plasma membrane [3] (see Section 3).
The high intracellular concentrations of vitamin C in mammalian tissues suggest its essential roles in maintaining physiological homeostasis and proper functions of organs and systems. In this article, we first examine the novel biochemical properties and functions of vitamin C, and then discuss recent research evidence supporting its potential role in cancer therapy, which lays a basis for understanding the most recent discovery of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a critical molecular target for vitamin C to kill KRAS and BRAF mutant colorectal cancer cells [4]
2. REDOX FORMS OF VITAMIN C
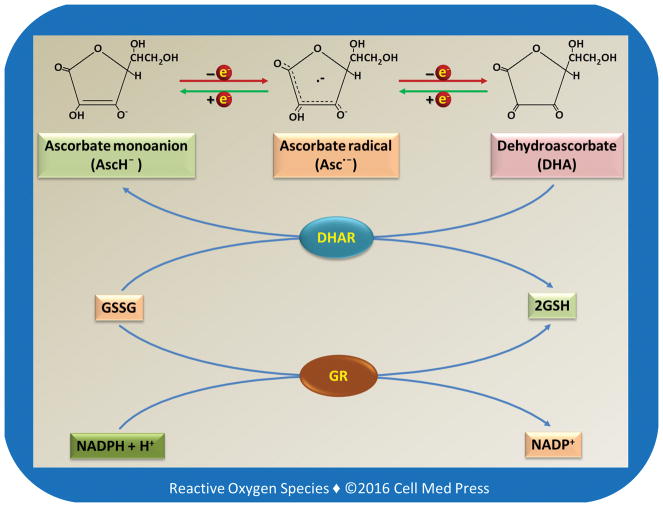
Vitamin C may exist in different redox forms depending on the biological conditions. As illustrated in Figure 1, vitamin C (AscH2) has two ionizable hydroxyl groups. At a physiological pH, vitamin C exists predominantly as a monoanion, i.e., ascorbate monoanion (AscH−). AscH− acts as a reducing agent and is converted to ascorbate radical (Asc•−, also known as semidehydroascorbate) after donation of one electron. After losing another electron, Asc• − is converted to dehydroascorbate (DHA). Asc• − can be reduced back to AscH−. DHA can also be reduced by either one electron to Asc• − or by two electrons to AscH−. The two-electron reduction of DHA to AscH− is catalyzed by dehydroascorbate reductase (DHAR) using the reduced form of glutathione (GSH) as the electron donor. It should be noted that if not specified otherwise, the term vitamin C typically refers to the reduced form of vitamin C, i.e., AscH2 or AscH−.
FIGURE 1. Redox chemistry of vitamin C and its relationship to glutathione.
As illustrated, the 2-electron reduction of DHA to vitamin C is catalyzed by dehydroascorbate reductase (DHAR) with GSH as the electron donor. The oxidized form of glutathione (GSSG) is reduced back to GSH by glutathione reductase (GR) using NADPH as an electron donor.
3. TRANSPORT OF VITAMIN C FROM DIETARY SOURCES INTO CELLS AND SUBCELLULAR ORGANELLES
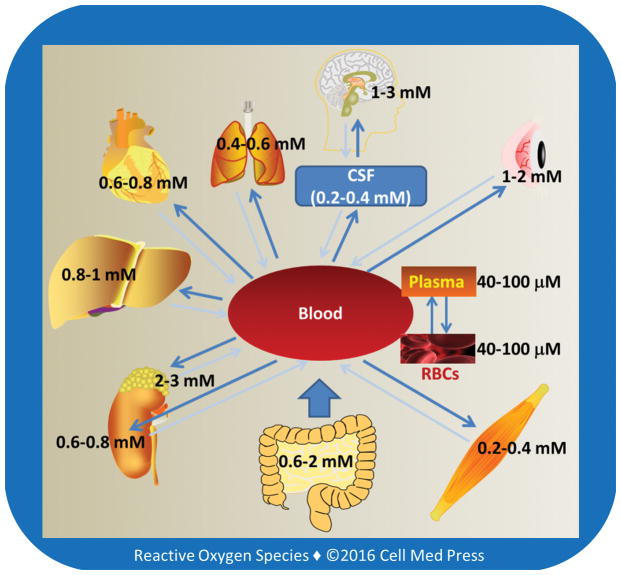
The highest tissue concentrations of vitamin C are found in the brain and in neuroendocrine tissues especially adrenal gland, which may range from 1 mM to 3 mM (Figure 2). These concentrations are 15–50 times higher than those in the plasma [5, 6], pointing to the existence of active transporting mechanisms. Early seminal work by Emmanuel J. Diliberto and coworkers showed that adrenomedullary cells accumulated vitamin C through a saturable and energy-dependent process and that the newly taken-up vitamin C was also secreted from the cells through specific transporter mechanisms [7–10]. It is now well-established that vitamin C enters and accumulates in neurons and other types of cells via two different transporting systems, as described below.
FIGURE 2. Tissue levels of vitamin C.
Human red blood cells (RBCs) express a high number of glucose transporter (GLUT1), but have no sodium-dependent vitamin C transporters (see Section 3.1), and the intracellular concentration of vitamin C in these cells is similar to that in the plasma. The concentration of vitamin C in the cerebrospinal fluid is ~5–10 times higher than that in the plasma. Larger arrows indicate the main direction of vitamin C transportation. As shown, vitamin C accumulates in organs and tissues and the high tissue concentrations are due to high intracellular levels of vitamin C, usually in the millimolar range.
3.1. Sodium-Dependent Vitamin C Transporters
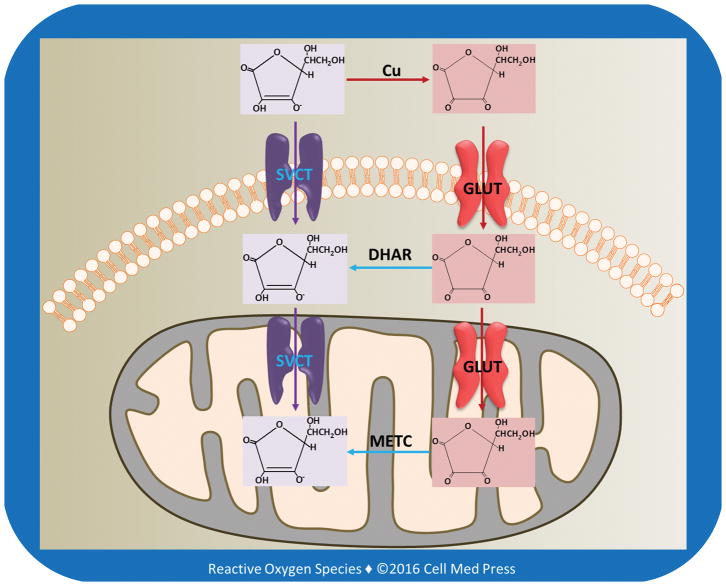
The reduced form of vitamin C is transported into cells via sodium-dependent vitamin C transporters (SVCT1 and SVCT2) (Figure 3). SVCT1 (the product of the SLC23A1 gene in humans) is mainly expressed in intestinal and renal epithelial cells, where it helps to mediate absorption and re-absorption of the vitamin, respectively. SVCT2 (the product of the SLC23A2 gene in humans) is found in cells of most other tissues, including the brain. Both SVCT1 and SVCT2 mediate high affinity, sodium- and energy-dependent transport of vitamin C into cells and are essential to establish steep concentration gradients of vitamin C across the plasma membrane [5]. SVCTs, particularly SVCT2, may also transport vitamin C from the cytosol into the mitochondrial matrix [11].
FIGURE 3. Transport of vitamin C and DHA into cells and mitochondria.
As illustrated, in extracellular milieu vitamin C is oxidized to DHA, likely due to the presence of transition metal ions, especially copper ions. DHA is reduced to vitamin C by DHAR in the cytosol, and DHA in the mitochondrial matrix is reduced to vitamin C by the electrons derived from the mitochondrial electron transport chain (METC).
3.2. Transport of Oxidized Vitamin C by Glucose Transporters
There are two classes of glucose transporting machineries involved in glucose homeostasis in the body: (1) the facilitated transporters or uniporters (commonly known as glucose transporters and abbreviated as GLUTs) and (2) the active transporters or symporters, namely, sodium-glucose transporters (SGLTs, also known as Na+/glucose cotransporters or symporters). The energy for active glucose transport is provided by the sodium gradient across the cell membrane, which is maintained by the Na+/K+ ATPase (also known as Na+/K+ pump) [12]. There are 12 members of the human SGLT gene family, including cotransporters for sugars, anions, vitamins, and short-chain fatty acids. The two most well-known members of SGLT family are SGLT1 and SGLT2, with SGLT1 being expressed in intestinal epithelial and renal proximal tubular cells and SGLT2 expressed predominantly in renal proximal tubular cells. The Na+-electrochemical gradient provided by the Na+/K+ ATPase is utilized to transport glucose into cells against its concentration gradient across the luminal membrane of cells lining the small intestine and the proximal tubules of the kidneys. SGLTs are unable to transport either reduced or oxidized forms of vitamin C. In contrast, several GLUTs (see below) are able to transport oxidized vitamin C.
3.2.1. Transport of Oxidized Vitamin C from Extracellular Milieu into Cells
Fourteen GLUT proteins are expressed in the human, and they include transporters for substrates other than glucose, such as fructose, myoinositol, and urate. The primary physiological substrates for at least half of the 14 GLUT proteins are either uncertain or completely unknown. The four well-established GLUT isoforms, namely, GLUTs 1–4, have been demonstrated to have distinct regulatory and/or kinetic properties that reflect their specific roles in cellular and whole body glucose homeostasis [13, 14]. Several GLUTs, such as GLUTs 1, 3, and 4, have also been shown to transport the oxidized form of vitamin C (i.e., DHA) from extracellular milieu into cells (Figure 3).
Interestingly, among all cells, human erythrocytes express the highest level of GLUT1. However, glucose transport actually decreases during human erythropoiesis despite a more than 3-log increase in GLUT1 transcripts. In contrast, GLUT1-mediated transport of DHA is dramatically enhanced during human erythropoiesis. Stomatin, an integral erythrocyte membrane protein, is responsible for regulating the switch from glucose to DHA transport [15].
Once inside the cells, DHA is reduced to vitamin C via the action of DHAR using GSH as the electron donor (Figure 1). In this reaction, GSH is oxidized to glutathione disulfide (GSSG), which is in turn reduced to GSH by glutathione reductase (GR) using NADPH as the reducing equivalent. NADPH is produced primarily from the pentose phosphate pathway. Although DHA at physiological levels can be reduced to vitamin C by the DHAR/GSH system without causing significant depletion of GSH, DHAR/GSH-catalyzed reduction of large amounts of DHA may cause GSH depletion and thereby accumulation of reactive oxygen species (ROS). In this context, GSH is a major cellular defense in the detoxification of ROS.
3.2.2. Transport of Oxidized Vitamin C from the Cytosol into the Mitochondrial Matrix
Certain GLUT isoforms are also expressed in the mitochondrial inner membrane. GLUT1 is the most extensively studied GLUT isoform in terms of mediating the transport of DHA into the mitochondrial matrix [16, 17]. Once transported into the matrix, DHA is reduced to vitamin C primarily by the mitochondrial electron transport chain (METC) [18] (Figure 3). A recent study suggested that GLUT10 might also be expressed in mitochondria participating in transporting DHA from the cytosol into the mitochondrial matrix [19].
4. VITAMIN C AS A MULTI-TASKING MOLECULE
Since its isolation from adrenal glands by Albert Szent-Györgyi (1893–1986) in 1928 [20], vitamin C has gradually gained a reputation of being a multi-tasking molecule possessing a wide range of distinct biological activities. Of note, Albert Szent-Györgyi won the Nobel Prize in Physiology and Medicine in 1937 for his discoveries of vitamin C and the catalysis of fumaric acid. The multi-tasking activities of vitamin C can be summarized into the following four categories: (1) as a cofactor for various enzymes; (2) as an antioxidant at physiological doses; (3) as a potential pro-oxidant at pharmacological doses; and (4) other emerging novel activities.
4.1. Vitamin C as a Cofactor for Various Enzymes
Vitamin C serves as a cofactor for at least 8 enzymes in mammalian species, including humans. The most notable ones are proline hydroxylase and lysine hydroxylase, which are involved in collagen synthesis. The other enzymes for which vitamin C acts as a cofactor are involved in carnitine synthesis, catecholamine synthesis, peptide amidation, and tyrosine metabolism [18]. Due to its critical role in collagen synthesis, deficiency of vitamin C compromises the integrity of blood vessels, leading to scorbutic gums and pinpoint hemorrhage, characteristic manifestations of vitamin C deficiency.
4.2. Vitamin C as an Antioxidant
The redox chemical properties of vitamin C are responsible for its antioxidant as well as potential pro-oxidant activities. As depicted in Figure 1, the two-electron reduction of DHA to AscH− is catalyzed by DHAR using GSH as the electron donor. This two-electron reduction reaction brings together two highly prevalent intracellular antioxidant molecules: vitamin C and GSH, both of which are present in millimolar concentrations inside the cells. Indeed, vitamin C and GSH cooperate closely to maintain the redox homeostasis of mammalian cells [21]. In addition, the METC, especially the complex III, may also reduce DHA to AscH− [22]. The in vivo significance of this mitochondria-dependent reduction is, however, unclear. As aforementioned, SVCT2 may participate in transporting vitamin C from the cytosol into the mitochondrial matrix. Together, the above findings may help explain the high concentrations of vitamin C in mitochondria and its role in maintaining the redox status of this major ROS-generating organelle. Due to its high instability, DHA, if not rapidly reduced, undergoes hydrolytic ring opening to form 2,3-diketogulonic acid.
In many in vitro systems, vitamin C has been found to scavenge various reactive oxygen and nitrogen species (ROS/RNS), and protect cells from oxidative damage. Vitamin C is able to regenerate a-tocopherol and coenzyme Q from a-tocopherol radical and coenzyme Q radical, respectively, and thereby playing a role in maintaining the antioxidant activities of a-tocopherol and coenzyme Q, two important lipophilic antioxidants in cells. It has recently been demonstrated that vitamin C is also able to reduce 1-Cys peroxiredoxin [23]. Peroxiredoxin is critical for the detoxification of hydrogen peroxide and peroxynitrite. As noted above, vitamin C and GSH cooperate to act as an efficient dual-antioxidant system in mammalian cells [21].
In addition to the above activities involved in detoxifying ROS/RNS, vitamin C is found to inhibit the expression of NADPH oxidase subunit p47phox induced by inflammatory insults, thereby decreasing the formation of ROS from this important cellular source [24]. The inducible expression of inducible nitric oxide synthase (iNOS) in septic mice is also inhibited by vitamin C [25]. It is unlikely that vitamin C directly inhibits the enzyme activity of either NADPH oxidase or iNOS. The reduced expression of the above enzymes or enzyme subunits by vitamin C most likely results from its modulation of cellular redox signaling that is involved in the inducible expression of the above two ROS/RNS-generating enzyme systems.
Interestingly, vitamin C also induces heme oxygenase-1 (HO-1), an enzyme with potent antioxidative and anti-inflammatory activities [26]. While the mechanism involved in the induction of HO-1 remains to be determined, it is likely that vitamin C provokes a pro-oxidant state in cells that subsequently activates HO-1 gene expression. Indeed, HO-1 is known as an oxidative stress-responsive gene, and as described next, vitamin C at pharmacological doses behaves as a pro-oxidant.
4.3. Vitamin C as a Potential Pro-oxidant
Under certain conditions, such as in the presence of redox active metal ions (especially copper ions), vitamin C may behave as a potent pro-oxidant, giving rise to ROS, damaging DNA, and causing protein glycation. Indeed, increased oxidative DNA damage occurs in individuals consuming a large amount of vitamin C [27]. Vitamin C induces decomposition of lipid hydroperoxide to genotoxins even in the absence of redox active metal ions [28]. In a humanized mouse model, vitamin C is found to mediate chemical aging of lens crystallins via the Maillard reaction [29]. In experimental animals, administration of pharmacological doses of vitamin C causes formation of vitamin C radical and ROS in the extracellular fluid and decreases growth of aggressive tumor xenografts [30]. Pharmacological doses of vitamin C have been employed as a potential therapeutic modality for cancer patients, especially those with advanced cancers [31–33] (see Section 5 below). The pro-oxidant activity of vitamin C also makes it an effective agent for killing drug-resistant Mycobacterium tuberculosis [34]. Hence, the pro-oxidant activities of vitamin C may exert either detrimental or beneficial effects dependent on the physiological and pathophysiological conditions.
4.4. Other Emerging Novel Activities of Vitamin C
Vitamin C reduces ferric ion to ferrous ion and thus facilitates iron absorption in the duodenum. Recent studies suggest a role for vitamin C in nucleic acid and histone demethylation, as well as proteoglycan deglycanation. Due to its role in hydroxylation reactions, vitamin C participates in downregulating hypoxia-inducible transcription factor-1a (HIF-1a). In this regard, proline hydroxylation targets HIF-1a for ubiquitin-mediated degradation [35]. Vitamin C may also play a role in stem cell biology. It enhances the reprogramming efficiency of mouse and human fibroblasts transduced with three (Oct4/Klf4/Sox2) or four (Oct4/Klf4/Sox2/cMyc) factors. It also alleviates cell senescence by p53 repression and may accelerate reprogramming by synergizing with epigenetic regulators [36, 37]. A more recent study has identified a novel function of vitamin C in promoting Tet-mediated generation of 5-hydroxyme-thylcytosine (5-hmC), suggesting that the availability of vitamin C may have a profound effect on many cellular functions dictated by DNA demethylation and that vitamin C may act as a critical mediator of the interface between the genome and environment [38, 39]. Moreover, vitamin C at pharmacological doses was shown to inhibit angiogenesis, leading to suppression of tumorigenesis in animal models [40, 41]. These atypical effects of vitamin C along with its unique pro-oxidative activities described above may contribute to the ever-increasing biological functions of this old vitamin in health and disease, and provide more opportunities for employing this multi-tasking molecule for disease intervention, including cancer therapy.
5. VITAMIN C AND CANCER THERAPY
As aforementioned, vitamin C at millimolar concentrations has recently been found to result in marked production of ROS causing selective killing of various types of cancer cells in diverse in vitro and in vivo models [30, 42, 43]. These observations are in line with early clinical studies showing a therapeutic activity of high-dose vitamin C in cancer patients. This section describes the findings related to the utilization of intravenous high-dose vitamin C in the therapeutic intervention of malignancies so as to provide a rationale for continued basic research to understand the molecular basis of vitamin C-based cancer therapy.
5.1. Historical Overview and Current Status
5.1.1. Early Clinical Studies by Ewan Cameron and Linus Pauling versus the Trials at the Mayo Clinic
Early studies conducted by Ewan Cameron and Linus Pauling in the 1970s suggested that large doses (10 g/day via intravenous infusion for about 10 days and orally thereafter) of vitamin C were effective in increasing survival and improving quality of life of terminal cancer patients [31, 32]. Similar findings were also reported by clinical trials in Japan [44]. However, subsequent trials on large doses of vitamin C given orally (10 g/day) at the Mayo Clinic did not reach the same conclusion [45, 46], and the routes of administration (intravenous infusion versus oral intake) may explain the discrepant results. Indeed, as described earlier (Section 1), oral vitamin C intake produces plasma concentrations that are tightly controlled, and the maximal plasma concentration attainable by oral intake of vitamin C has been estimated to be ~200 mM. In contrast, intravenous administration of pharmacological doses of vitamin C results in millimolar plasma concentrations [2], and as stated above (Section 4.3), millimolar concentrations of vitamin C have been shown to selectively kill cancer cells via an ROS-dependent mechanism in experimental models.
5.1.2. Recent Clinical Studies
Recently, Sebastian J. Padayatty and coworkers reported three well-documented cases of advanced cancers in which patients had unexpectedly long survival times after receiving high doses of vitamin C via intravenous administration [47]. Although the findings from this study supported a possible therapeutic efficacy of high intravenous doses of vitamin C in cancer, other possibilities could not be excluded to account for the unexpectedly prolonged survival of the patients. A phase I clinical trial reported in 2008 that high-dose intravenous vitamin C (0.4–1.5 g/kg body weight, three times weekly) was well-tolerated, but failed to demonstrate an anticancer activity when administered to patients with previously treated advanced malignancies [48]. A clinical study reported in 2014, involving 11 patients with acute myeloid leukemia (AML) excluding acute promyelocytic leukemia (APL), i.e., non-APL AML, reported a limited benefit provided by adding pharmacological doses of vitamin C (1 g/day over 30 min after arsenic trioxide, 5 days a week for 5 weeks) to the standard arsenic trioxide regimen [49].
A phase I–II clinical trial in 2015, involving 14 patients with advanced cancer receiving standard chemotherapy, reported that high-dose intravenous vitamin C was safe and generally well tolerated. The pre- and post-chemotherapy pharmacokinetic profiles suggested that tissue uptake of vitamin C increased after chemotherapy, with no increase in urinary oxalic acid excretion. Three patients with different types of cancer experienced unexpected transient stable disease, increased energy, and functional improvement after adding the high-dose vitamin C to standard chemotherapy [50]. Although this phase I–II trial along with the other small-scale clinical studies described earlier neither proved nor disproved the value of high-dose vitamin C in cancer therapy, it did provide practical information and indicate a feasible way to evaluate this plausible but unproven therapy, and if carried out in sufficient numbers, simple studies like this one could identify specific clusters of cancer type, chemotherapy regimen, and high-dose vitamin C in which exceptional responses occur frequently enough to justify appropriately focused large-scale clinical trials [50].
On the other hand, continued efforts aiming to characterize the pharmacokinetic profiles of high-dose vitamin C in cancer patients may lead to the development of optimal effective dosage regimens for cancer treatment. In this regard, a phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous vitamin C in patients with advanced cancer concluded that vitamin C administered intravenously at 1 g/min for 4 consecutive days per week for a total of 4 weeks produced up to 49 mM vitamin C in patients’ blood and was well tolerated. As such, the authors of the study recommended a dose of 70–80 g/m2 for future clinical studies on vitamin C cancer therapy [51].
More recently, a pharmacokinetic study of vitamin C in prostate cancer patients suggested that the elimination of vitamin C from the body followed first-order elimination kinetics throughout the dosing range with supra-physiological concentrations. The target dose of 60 g of intravenous vitamin C produced a peak plasma vitamin C concentration of 20.3 mM. Elimination half-life was 1.87 hr, volume of distribution was 0.19 L/kg, and clearance rate was 6.02 L/hr. Thus the relatively fast first-order elimination with a half-life of about 2 hr made it impossible to maintain vitamin C concentrations in the potentially cancer cell-killing range after infusion stopped in prostate cancer patients with normal kidney function [52]. The study thus proposed a regimen with a bolus loading dose followed by a maintenance infusion based on the calculated clearance to ensure the plasma concentrations of vitamin C was maintained at high (millimolar) concentrations for a prolonged period of time, which might be required for the vitamin to effectively kill cancer cells in the patients [52]. Hence, continued pharmacokinetic studies in cancer patients would enhance our understanding of how high-dose vitamin C behaves in cancer patients. Likewise, mechanistic studies in experimental models will shed more light on the molecular basis of high-dose vitamin C-based cancer therapy as well as guide the clinical research design.
5.1.3. Millimolar Vitamin C Acts as a Pro-Oxidant in Experimental Cancer Models
In the interstitial fluid surrounding tumor cells, millimolar concentrations of vitamin C exert local pro-oxidant effects by mediating hydrogen peroxide formation in experimental models [30, 42, 43]. More recently, data from experimental models showed that millimolar vitamin C, acting as a pro-oxidant, induced DNA damage and depleted cellular adenosine triphosphate (ATP), activated the ataxia telangiectasia mutated (ATM)/adenosine monophosphate-activated protein kinase (AMPK) pathway, and resulted in mammalian target of rapamycin (mTOR) inhibition and death in ovarian cancer cells. The combination of parenteral vitamin C with the conventional chemotherapeutic agents carboplatin and paclitaxel synergistically inhibited ovarian cancer in mouse models and reduced chemotherapy-associated toxicity in patients with ovarian cancer [53]. Hence, experimental studies have provided strong evidence for an important role of oxidative stress in vitamin C-mediated anticancer activity in animal models [54, 55]. In line with this notion, use of ROS-inducing agents other than vitamin C for cancer treatment has also received much attention in recent years [56]. However, a fundamental question remains: what makes vitamin C selectively toxic to cancer cells? The elegant work by Yun et al. [4] suggests that DHA is the pharmaceutically active agent of high-dose vitamin C therapy that induces oxidative stress, and that the selective toxicity of vitamin C to tumor cells stems from high GLUT1 expression combined with KRAS or BRAF oncogene-induced glycolytic addiction. This addiction leads to an energetic crisis and cell death upon inhibition of GAPDH by DHA-induced oxidative stress.
5.2. Discovery of the “DHA–GLUT1–ROS–GAPDH” Machinery in Vitamin C-Induced Cancer Cell Killing
Over 50% of human colorectal cancers carry either KRAS or BRAF mutations and are often refractory to epidermal growth factor receptor-targeting drugs. In their recent article published in Science [4], Yun et al. reported that cultured human colorectal cancer cells harboring KRAS or BRAF mutations were selectively killed when exposed to high concentrations (1–2 mM) of vitamin C. They showed that this selectivity was due to increased uptake of DHA via GLUT1 which is overexpressed in the cancer cells. Increased DHA uptake and the subsequent intracellular reduction by DHAR led to GSH depletion and thereby accumulation of ROS (Figure 1). The resulting oxidative stress led to inhibition of GAPDH in the highly glycolytic KRAS or BRAF mutant cells, causing an energetic crisis and cell death not seen in KRAS and BRAF wild-type cells [4]. Importantly, this novel mechanism of action operated also in in vivo animal models [4].
5.2.1. Uptake of DHA via GLUT1
Given that GLUT1 levels in KRAS and BRAF mutant cells are elevated, Yun et al. hypothesized that the increase in DHA uptake could disrupt redox homeostasis and compromise cellular viability. To test this hypothesis, Yun et al. used a panel of isogenic colorectal cancer cell lines harboring wild-type or mutant alleles of KRAS (HCT116 and DLD1) or BRAF (VACO432 and RKO). They first observed that in culture media, vitamin C was oxidized to DHA with a half-life of ~70 min, and this oxidation could be prevented by adding GSH to the culture media [4]. Oxidation of vitamin C in phosphate-buffered saline (PBS) or culture media has been well-documented in the literature [57]. It has long been demonstrated that trace amounts of transition metal ions, especially copper ions, present in PBS or culture media catalyzes the oxidation of vitamin C to produce DHA and ROS [58]. Prevention of vitamin C oxidation in culture media by adding GSH could be due to the chelation of transition metals ions, especially copper ions by GSH. In this regard, GSH binds copper ions preventing copper ion-mediated redox reactions [59].
By using [14C]-radiolabeled vitamin C, Yun et al. observed that adding GSH to the media to prevent oxidation of vitamin C to DHA abrogated [14C]-vitamin C uptake. Furthermore, [14C]-vitamin C uptake was significantly decreased in both HCT116 and VACO432 cells treated with a GLUT1-specific inhibitor, STF31, and in GLUT1-knockout cells. Glucose also competed with DHA for uptake in the colorectal cancer cells. These results led Yun et al. to conclude that the colorectal cancer cells preferentially import DHA, rather than vitamin C, and that this uptake is mediated by GLUT1 [4]. Indeed, as discussed earlier in Section 3.2, GLUT1 is the best characterized GLUT isoform for mediating the transport of DHA from extracellular milieu into the cytosol as well as that from the cytosol into the mitochondrial matrix.
Given the increased expression of GLUT1 in the mutant cells, Yun et al. next determined whether KRAS or BRAF mutations influenced vitamin C uptake. They observed that the mutant lines took up significantly more [14C]-vitamin C than did their wild-type counterparts. Moreover, they found KRAS and BRAF mutant cells imported DHA faster than they did [14C]-vitamin C, consistent with the observation that vitamin C must first be oxidized to DHA to enter cells through GLUT1. These results led to the conclusion that GLUT1 is the primary means of vitamin C uptake in the colorectal cancer cells and that elevated GLUT1 expression in KRAS or BRAF mutant cells drives increased DHA uptake [4].
5.2.2. Selective Toxicity of Vitamin C to Cells with Mutant KRAS or BRAF Alleles
In view of the augmented uptake of DHA by the mutant lines, Yun et al. then determined if the increased uptake of DHA in KRAS and BRAF mutant cells could affect their survival and growth. They observed that 24 to 48 hr of vitamin C treatment inhibited KRAS and BRAF mutant cell growth and colony formation, but with reduced effects on their wild-type counterparts. They further showed that vitamin C could selectively kill the KRAS and BRAF mutant cells even in the presence of physiological concentrations (5–10 mM) of glucose. In line with the effect of GSH on oxidation of vitamin C to DHA, they found that vitamin C-mediated cancer cell killing was prevented in the presence of exogenously added GSH [4].
5.2.3. Dependence of Vitamin C Toxicity on both High GLUT1 Expression and Glycolytic Addiction
The next question asked by Yun et al. was: could accumulation of high-level of DHA alone be enough for causing cancer cell death? To answer this question, Yun et al. determined if vitamin C was also cytotoxic to colorectal cancer cells with mutations of oncogenes other than KRAS or BRAF, and in wide-type cells overexpressing GLUT1. Unlike KRAS or BRAF, they found that the PIK3CA (another frequently mutated oncogene) genotype did not exhibit augmented sensitivity to vitamin C-mediated cytotoxicity. Importantly, Yun et al. found that although overexpression of GLUT1 in wild-type cells increased vitamin C uptake, it did not sensitize wild-type cells to vitamin C-mediated cytotoxicity. Together, these findings suggested that high GLUT1 expression alone, without oncogene-induced metabolic reprogramming (i.e., glycolytic addiction), is not sufficient to make cells susceptible to vitamin C-induced cytotoxicity [4].
5.2.4. Vitamin C-Induced Cancer Cell Killing: From in vitro Cultured Cells to in vivo Animal Models
To investigate the in vivo relevance of the above findings, Yun et al. next determined if high-dose vitamin C treatment could affect the growth of KRAS and BRAF mutant colorectal cancer cells in mice. To this end, Yun et al. treated the mice bearing established xenografts derived from parental HCT116 and VACO432 cell lines twice a day via intraperitoneal injection of a high-dose vitamin C (4 g/kg body weight) or PBS as the vehicle control for 3–4 weeks. They found that vitamin C treatment significantly reduced tumor growth relative to vehicle control treatment. KRAS and BRAF wild-type isogenic HCT116 and VACO432 cell lines were unable to form xenograft tumors in mice [4]. This finding by Yun et al. is in line with previous studies showing that high-dose vitamin C inhibits tumor growth in diverse animal models (see section 5.1.3).
To directly test the impact of Kras mutation on the sensitivity of tumors to high-dose vitamin C treatment, Yun et al. generated a transgenic model of intestinal cancer, driven by either Apc mutation, or combined Apc and Kras (G12D) mutations. They elegantly demonstrated that whereas Apcflox/flox mice showed no difference in polyp burden after vitamin C treatment, Apcflox/flox/KrasG12D mice had significantly fewer and smaller small intestine polyps, confirming that vitamin C selectively affected Kras mutant tumors. Consistent with the in vitro experiments in the colorectal cancer cell lines, they found that tumors from Apcflox/flox/KrasG12D mice showed higher GLUT1 expression and greater vitamin C uptake than did tumors from Apcflox/flox mice [4]. Collectively, these findings by Yun et al. indicate that the same high-dose vitamin C-dependent cancer cell killing machinery also operates in animal models. Clinical research on high-dose vitamin C therapy in colorectal cancer patients with KRAS mutations is warranted. In this context, a recent study reported a decrease in serum level of Ras protein in cancer patients following high-dose vitamin C treatment [60].
5.2.5. GAPDH as a Molecular Target in Vitamin C-Induced Cancer Cell Killing
Yun et al. then carried out metabolomics studies and found that vitamin C treatment led to inhibition of GAPDH in the KRAS and BRAF mutant cells [4]. They observed that vitamin C caused GSH depletion and ROS accumulation in the mutant lines, which might be responsible for GAPDH inhibition. Considering the dependence of cancer cells with KRAS or BRAF mutations on glycolysis for energy production and survival, Yun et al. next determined if inhibition of GAPDH, an important enzyme in glycolysis, by vitamin C caused energy crisis in the mutant cells [4]. They found that vitamin C treatment caused a rapid decrease in the glycolytic rate in KRAS and BRAF mutant cells, but not in the wild-type cells. Consequently, they found that vitamin C induced a significant drop in ATP levels, with a concomitant increase in AMP levels and activation of AMPK in the KRAS and BRAF mutant cells. The study then demonstrated that the above effects and cell death in the mutant lines were blocked by N-acetylcysteine [4], an antioxidant compound that increases cellular GSH [61], suggesting an oxidative stress mechanism underlying vitamin C-dependent cytotoxicity to the mutant colorectal cancer cells. Notably, Yun et al. showed that supplementing drinking water with N-acetylcysteine over the course of vitamin C treatment abolished the ability of vitamin C to reduce xenograft growth of KRAS mutant cells [4], suggesting that the aforementioned oxidative machinery also operates in vivo.
5.2.6. Vitamin C-Induced S-Glutathionylation of GAPDH
GAPDH is one of the most prominent cellular targets of oxidative modifications when ROS/RNS are formed during metabolism and under stress conditions, and the Cys152 in the active site of the enzyme is subject to redox modifications, leading to enzyme inactivation [62, 63]. Via immunoprecipitating endogenous GAPDH and blotting with an antibody that recognizes S-glutathionylation under nonreducing conditions, Yun et al. observed that vitamin C treatment caused 2–4-fold increases in the GAPDH S-glutathionylation levels in both KRAS and BRAF mutant lines [4]. Protein S-glutathionylation, the reversible formation of mixed disulfides between glutathione and low-pKa cysteinyl residues, not only is a cellular response to mild oxidative/nitrosative stress, but also occurs under basal (physiological) conditions, and likely participates in redox signaling [64]. The increased GAPDH S-glutathionylation levels in vitamin C-treated KRAS and BRAF mutant cells demonstrated by Yun et al. suggested oxidative modifications of GAPDH. Importantly, direct measurement of the GAPDH activity in lysates of vitamin C treated KRAS and BRAF mutant cells by Yun et al. revealed a significant inhibition (by 50%) of GAPDH and co-treatment with N-acetylcysteine blocked the inhibition [4]. It remained unclear if vitamin C-induced inhibition of GAPDH occurred exclusively due to S-glutathionylation. In this regard, multiple studies suggested that S-glutathionylation of GAPDH led to enzyme inhibition [62, 63]. On the other hand, some other studies using purified human GAPDH and [35S]-labelled GSSG showed that S-glutathionylation of GAPDH did not result in inactivation of the enzyme. Rather, the direct oxidation of GAPDH with hydrogen peroxide was responsible for inhibition of the catalytic activity of the enzyme [65].
5.2.7. Vitamin C-Induced NAD+ Depletion
The metabolomics studies by Yun et al. revealed a 19-fold increase in the accumulation of GAPDH substrate glyceraldehyde-3-phosphate (GAP) in vitamin C-treated mutant cells [4]. This led Yun et al. to examine the changes in the levels of NAD+, the substrate for GAPDH to catalyze the oxidation of GAP to form 1,2-bisphosphoglycerate (BPG) (Figure 4). Indeed, they found that vitamin C treatment resulted in NAD+ depletion. They also showed that vitamin C treatment caused activation of poly(ADP-ribose) polymerase (PARP) likely through oxidative DNA damage. This led Yun et al. to conclude that NAD+ depletion in vitamin C-treated cells may be due to the activation of PARP and the subsequent consumption of NAD+ [4]. Yun et al. then showed that vitamin C-induced cell death was partially rescued by pretreatment with a PARP inhibitor, olaparib, or a cell-permeable NAD+ precursor, nicotinamide mononucleotide. These results led Yun et al. to conclude that in KRAS and BRAF mutant cells vitamin C-induced intracellular ROS accumulation compromises the function of GAPDH by both oxidative modifications of the enzyme and the depletion of its cofactor NAD+, eventually resulting in an energetic crisis and cell death [4].
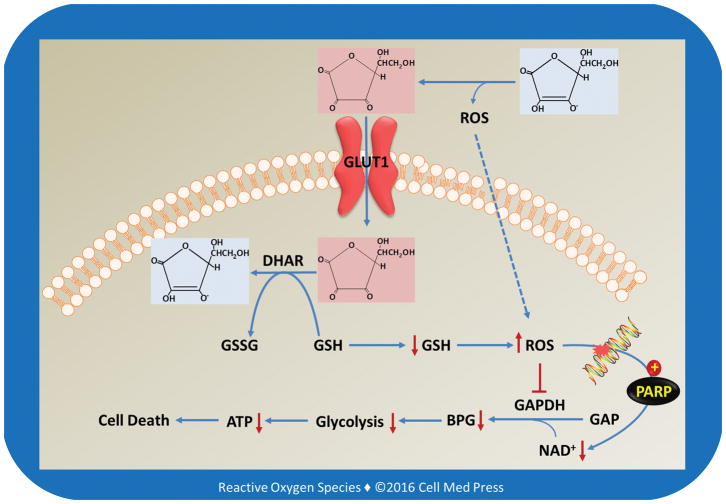
FIGURE 4. Schematic illustration of the molecular and cellular pathways involved in high-dose vitamin C-mediated selective cytotoxicity to cancer cells that both overexpress GLUT1 and have become addicted to glycolysis for energy production.
GAP and BPG denote glyceraldehyde 3-phosphate and 1,3-bisphosphoglycerate, respectively. This scheme is based on Ref. [4].
5.2.8. The Big Picture and Implications of the Study by Yun et al
The elegant study by Yun et al. [4] depicted an important big picture for high-dose vitamin C to selectively kill cancer cells while sparing normal cells (Figure 4). Two critical factors/conditions seem to determine this selectivity of vitamin C-mediated cancer cell killing: (1) the selective accumulation of DHA in cancer cells via a GLUT1-dependent transport mechanism, leading to intracellular GSH depletion and ROS accumulation, and (2) ROS-mediate inhibition of the glycolytic enzyme GAPDH in the background of oncogene-induced metabolic reprogramming (i.e., dependence on glycolysis for survival), resulting in ATP depletion and cell death. The KRAS and BRAF mutant colorectal cancer cells possess the above 2 critical factors and thereby show high susceptibility to high-dose vitamin C-mediated cytotoxicity. It can be further speculated that other types of cancer cells that meet the above 2 conditions would likely be also selectively killed by high-dose vitamin C. Hence, studies are warranted to determine if this unified machinery of “DHA–GLUT1–ROS–GAPDH” found by Yun et al. also operates in other types of cancer cells harboring mutations in oncogenes other than KRAS and BRAF. Since cancer cells typically exhibit altered status of ROS homeostasis and susceptibility to oxidative stress, future studies are also needed to understand how the intrinsic ROS generating and detoxifying systems in cancer cells impact the high-dose vitamin C-dependent tumoricidal activity. Moreover, continued efforts aiming to decipher the molecular basis of other ROS-inducing agents in cancer therapy will likely expand the paradigm of ROS biology and medicine [56, 66].
Acknowledgments
This work was supported by a grant (CA192936) from the U.S. National Cancer Institute/NIH.
ABBREVIATIONS
- AMP
adenosine monophosphate
- AMPK
adenosine monophosphate-activated protein kinase
- ATP
adenosine triphosphate
- BPG
1,2-bisphosphoglycerate
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- GAP
glyceraldehyde 3-phosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLUT
glucose transporter
- GR
glutathione reductase
- GSH
reduced form of glutathione
- GSSG
glutathione disulfide
- HO-1
heme oxygenase-1
- iNOS
inducible nitric oxide synthase
- METC
mitochondrial electron transport chain
- PARP
poly(ADP-ribose) polymerase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SGLT
sodium-glucose transporter
- SVCT
sodium-dependent vitamin C transporter
References
- 1.Bruno EJ, Jr, Ziegenfuss TN, Landis J. Vitamin C: research update. Curr Sports Med Rep. 2006;5(4):177–81. doi: 10.1007/s11932-006-0043-y. [DOI] [PubMed] [Google Scholar]
- 2.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–7. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr. 2005;25:105–25. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 4.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–6. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46(6):719–30. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michels AJ, Hagen TM, Frei B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu Rev Nutr. 2013;33:45–70. doi: 10.1146/annurev-nutr-071812-161246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels AJ, Dean G, Viveros OH, Diliberto EJ., Jr Secretion of newly taken-up ascorbic acid by adrenomedullary chromaffin cells. Science. 1982;216(4547):737–9. doi: 10.1126/science.7079733. [DOI] [PubMed] [Google Scholar]
- 8.Daniels AJ, Dean G, Viveros OH, Diliberto EJ., Jr Secretion of newly taken up ascorbic acid by adrenomedullary chromaffin cells originates from a compartment different from the catecholamine storage vesicle. Mol Pharmacol. 1983;23(2):437–44. [PubMed] [Google Scholar]
- 9.Diliberto EJ, Jr, Heckman GD, Daniels AJ. Characterization of ascorbic acid transport by adrenomedullary chromaffin cells. Evidence for Na+-dependent co-transport. J Biol Chem. 1983;258(21):12886–94. [PubMed] [Google Scholar]
- 10.Knoth J, Viveros OH, Diliberto EJ., Jr Evidence for the release of newly acquired ascorbate and alpha-aminoisobutyric acid from the cytosol of adrenomedullary chromaffin cells through specific transporter mechanisms. J Biol Chem. 1987;262(29):14036–41. [PubMed] [Google Scholar]
- 11.Banhegyi G, Benedetti A, Margittai E, Marcolongo P, Fulceri R, Nemeth CE, et al. Subcellular compartmentation of ascorbate and its variation in disease states. Biochim Biophys Acta. 2014;1843(9):1909–16. doi: 10.1016/j.bbamcr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 13.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298(2):E141–5. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–94. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 15.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, et al. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132(6):1039–48. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Kc S, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005;19(12):1657–67. doi: 10.1096/fj.05-4107com. [DOI] [PubMed] [Google Scholar]
- 17.Szarka A, Balogh T. In silico aided thoughts on mitochondrial vitamin C transport. J Theor Biol. 2015;365:181–9. doi: 10.1016/j.jtbi.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157(7):1097–110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YC, Huang HY, Chang CJ, Cheng CH, Chen YT. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome. Hum Mol Genet. 2010;19(19):3721–33. doi: 10.1093/hmg/ddq286. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter KJ. The discovery of vitamin C. Ann Nutr Metab. 2012;61(3):259–64. doi: 10.1159/000343121. [DOI] [PubMed] [Google Scholar]
- 21.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269(13):9397–400. [PubMed] [Google Scholar]
- 22.Li X, Cobb CE, May JM. Mitochondrial recycling of ascorbic acid from dehydroascorbic acid: dependence on the electron transport chain. Arch Biochem Biophys. 2002;403(1):103–10. doi: 10.1016/S0003-9861(02)00205-9. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro G, Horta BB, Pimenta DC, Augusto O, Netto LE. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc Natl Acad Sci USA. 2007;104(12):4886–91. doi: 10.1073/pnas.0700481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F, Schuster DP, Tyml K, Wilson JX. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic Biol Med. 2007;42(1):124–31. doi: 10.1016/j.freeradbiomed.2006.10.033. S0891-5849(06)00646-0 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R50–6. doi: 10.1152/ajpregu.00564.2002. [DOI] [PubMed] [Google Scholar]
- 26.Huang YN, Wang JY, Lee CT, Lin CH, Lai CC, Wang JY. L-ascorbate attenuates methamphetamine neurotoxicity through enhancing the induction of endogenous heme oxygenase-1. Toxicol Appl Pharmacol. 2012;265(2):241–52. doi: 10.1016/j.taap.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Levine M, Daruwala RC, Park JB, Rumsey SC, Wang Y. Does vitamin C have a pro-oxidant effect? Nature. 1998;395(6699):231. doi: 10.1038/26137. author reply 2. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292(5524):2083–6. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 29.Fan X, Reneker LW, Obrenovich ME, Strauch C, Cheng R, Jarvis SM, et al. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci USA. 2006;103(45):16912–7. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105(32):11105–9. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976;73(10):3685–9. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978;75(9):4538–42. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikirova N, Casciari J, Riordan N, Hunninghake R. Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. J Transl Med. 2013;11(1):191. doi: 10.1186/1479-5876-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilcheze C, Hartman T, Weinrick B, Jacobs WR., Jr Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun. 2013;4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63(8):1764–8. [PubMed] [Google Scholar]
- 36.Shi Y, Zhao Y, Deng H. Powering reprogramming with vitamin C. Cell Stem Cell. 2010;6(1):1–2. doi: 10.1016/j.stem.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–9. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288(19):13669–74. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500(7461):222–6. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikirova NA, Ichim TE, Riordan NH. Anti-angiogenic effect of high doses of ascorbic acid. J Transl Med. 2008;6:50. doi: 10.1186/1479-5876-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeom CH, Lee G, Park JH, Yu J, Park S, Yi SY, et al. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med. 2009;7:70. doi: 10.1186/1479-5876-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102(38):13604–9. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers EJ, Chen S, Chan A. Folate deficiency and plasma homocysteine during increased oxidative stress. N Engl J Med. 2007;357(4):421–2. doi: 10.1056/NEJMc066569. [DOI] [PubMed] [Google Scholar]
- 44.Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl. 1982;23:103–13. [PubMed] [Google Scholar]
- 45.Creagan ET, Moertel CG, O’Fallon JR, Schutt AJ, O’Connell MJ, Rubin J, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: a controlled trial. N Engl J Med. 1979;301(13):687–90. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 46.Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: a randomized double-blind comparison. N Engl J Med. 1985;312(3):137–41. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 47.Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Levine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006;174(7):937–42. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19(11):1969–74. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 49.Aldoss I, Mark L, Vrona J, Ramezani L, Weitz I, Mohrbacher AM, et al. Adding ascorbic acid to arsenic trioxide produces limited benefit in patients with acute myeloid leukemia excluding acute promyelocytic leukemia. Ann Hematol. 2014;93(11):1839–43. doi: 10.1007/s00277-014-2124-y. [DOI] [PubMed] [Google Scholar]
- 50.Hoffer LJ, Robitaille L, Zakarian R, Melnychuk D, Kavan P, Agulnik J, et al. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: a phase I–II clinical trial. PLoS One. 2015;10(4):e0120228. doi: 10.1371/journal.pone.0120228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72(1):139–46. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielsen TK, Hojgaard M, Andersen JT, Poulsen HE, Lykkesfeldt J, Mikines KJ. Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: a pharmacokinetic evaluation. Basic Clin Pharmacol Toxicol. 2015;116(4):343–8. doi: 10.1111/bcpt.12323. [DOI] [PubMed] [Google Scholar]
- 53.Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6(222):222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 54.Frei B, Lawson S. Vitamin C and cancer revisited. Proc Natl Acad Sci U S A. 2008;105(32):11037–8. doi: 10.1073/pnas.0806433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009;47(1):32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Kasiappan R, Safe SH. ROS-Inducing agents for cancer chemotherapy. Reactive Oxygen Species. 2016;1(1):22–37. doi: 10.20455/ros.2016.805. [DOI] [Google Scholar]
- 57.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145(5):532–41. [PubMed] [Google Scholar]
- 58.Mystkowski EM. The oxidation of ascorbic acid in the presence of copper. Biochem J. 1942;36(5–6):494–500. doi: 10.1042/bj0360494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanna PM, Mason RP. Direct evidence for inhibition of free radical formation from Cu(I) and hydrogen peroxide by glutathione and other potential ligands using the EPR spin-trapping technique. Arch Biochem Biophys. 1992;295(1):205–13. doi: 10.1016/0003-9861(92)90507-s. [DOI] [PubMed] [Google Scholar]
- 60.Mikirova N, Riordan N, Casciari J. Modulation of Cytokines in Cancer Patients by Intravenous Ascorbate Therapy. Med Sci Monit. 2016;22:14–25. doi: 10.12659/MSM.895368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830(8):4117–29. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Hildebrandt T, Knuesting J, Berndt C, Morgan B, Scheibe R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol Chem. 2015;396(5):523–37. doi: 10.1515/hsz-2014-0295. [DOI] [PubMed] [Google Scholar]
- 63.Hwang NR, Yim SH, Kim YM, Jeong J, Song EJ, Lee Y, et al. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem J. 2009;423(2):253–64. doi: 10.1042/BJ20090854. [DOI] [PubMed] [Google Scholar]
- 64.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43(6):883–98. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Lind C, Gerdes R, Schuppe-Koistinen I, Cotgreave IA. Studies on the mechanism of oxidative modification of human glyceraldehyde-3-phosphate dehydrogenase by glutathione: catalysis by glutaredoxin. Biochem Biophys Res Commun. 1998;247(2):481–6. doi: 10.1006/bbrc.1998.8695. [DOI] [PubMed] [Google Scholar]
- 66.Li YR, Jia Z, Trush MA. Defining ROS in biology and medicine. Reactive Oxygen Species. 2016;1(1):9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]