Abstract
Possibly through their actions upon glia, peroxisome proliferator-activated receptor agonists (PPAR) have been shown to alter the abuse potential of addictive drugs in several preclinical models. The current study extends this research into the human laboratory as the first clinical study into the effects of the PPAR gamma agonist, pioglitazone, on the abuse potential of nicotine. Heavy smokers were recruited for this 3-week study. Upon admission, participants were randomized to either active (45 mg, n = 14) or placebo (0 mg, n = 13) PIO maintenance conditions for the duration of the study. After 5–7 days of stabilization on a 7 mg nicotine patch, participants began laboratory testing. On the 1st–4th test days, participants could self-administer cigarettes or receive money by making verbal choices for either option. On the 5th day, participants were administered 10 puffs of their usual brand of cigarette in the morning and later chose between smoking and money by making finger presses on a computer mouse in a progressive ratio self-administration task. Later on the 5th day participants also underwent a smoking cue exposure session. The 8th–11th test days were identical to the 1st–4th test days with the exception that during one of the test weeks de-nicotinized cigarettes were available, and during the other nicotinized cigarettes were available. Nicotinized cigarettes were always administered on the 5th and 12th days. On some measures PIO increased indicators of abuse potential, though this effect was typically not statistically significant. However, PIO did significantly reduce measures of craving.
Keywords: Tobacco, Nicotine, Pioglitazone, Glia, Abuse potential
1. Introduction
Cigarette smoking is the leading cause of preventable death in the United States, killing approximately 556,000 Americans each year (United State Department of Health and Human Services, 2014). The five forms of nicotine replacement therapy (transdermal nicotine, gum, lozenge, nasal spray, oral inhaler) aid in smoking cessation by delivering nicotine in order to reduce withdrawal and craving for tobacco (Jain et al., 2013). There are two additional FDA-approved pharmacotherapies designed to aid in smoking cessation by reducing withdrawal and craving, and altering the rewarding effects of nicotine. Sustained-release bupropion inhibits dopamine reuptake in the central nervous system and antagonizes nicotinic acetylcholine receptor function (Henningfield et al., 2005; Warner and Shoiab, 2005). Varenicline is a partial nicotinic acetylcholine receptor agonist that reduces cravings and the pleasurable effects of cigarettes and other tobacco products (Cahill et al., 2013).
All types of pharmacotherapies help smokers quit, yet, the likelihood of successfully quitting remains low. In clinical trials, continuous abstinence rates at 9–12 weeks for the most efficacious pharmacotherapy, varenicline, have been shown to be between 44 and 56% (Kaur et al., 2009). Though the percentage of U.S. adults who smoke cigarettes has declined over the last decade, there remains approximately 40 million active smokers in the U.S., and 1.1 billion worldwide (Jamal et al., 2015; World Health Organization, 2016). There is clearly a need for the continued development of efficacious pharmacological treatments for nicotine dependence.
Many commonly abused drugs have been found to activate glial cells, an effect that has been identified across species (Armstrong et al., 2004; Fantegrossi et al., 2008; Narita et al., 2006; Sekine et al., 2008; Watkins et al., 2005). The activity of addictive drugs upon glial cells may contribute directly to their subjective and physiological effects, and therefore mediate their abuse potential. Research investigating the effects of pharmacological modulation of glial activity has provided results encouraging of their potential as pharmacotherapies for substance-use disorders (see Cooper et al., 2012 and Bachtella et al., 2017 for a reviews).
One glial modulator being investigated for its utility in treating drug abuse is the peroxisome proliferator-activated gamma receptor (PPARγ) agonist, pioglitazone (PIO). The peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that regulate gene expression via ligand-activated transcription factors (Michalik et al., 2006). PPARγ agonists, like PIO, inhibit the expression of cytokines by monocytes/macrophages and microglia (Kielian and Drew, 2003). Preclinical research has also shown that PIO attenuates drug-induced dopamine release in the nucleus accumbens, which is an important system in the development and maintenance of addiction (Di Chiara and Bassareo, 2007; Diana, 2011; de Guglielmo et al., 2015).
The first robust evidence for the role of PPAR agonists in addiction came from rodent studies on alcohol (Le Foll et al., 2013). PPARγ receptor activation by PIO was found to significantly reduce alcohol/ethanol consumption, and relapse to alcohol seeking (Ferguson et al., 2014; Stopponi et al., 2011; Stopponi et al., 2013). Other rodent work has found similarly promising results with opioids, cocaine and methamphetamine (Ciccocioppo et al., 2012; de Guglielmo et al., 2017; Maeda et al., 2007; Miller, 2016). Concerning preclinical findings with nicotine, PPAR alpha agonists have been found to block nicotine reward and induced reinstatement of drug seeking (Mascia et al., 2011; Panlilio et al., 2012). Additionally, genetic deletion of neuronal PPARγ has been shown to reduce anxiety evoked by stress exposure and attenuates the expression of somatic and affective nicotine withdrawal symptoms in rodent models (Domi et al., 2016).
The ability of PIO to alter the effects of nicotine in humans has not been characterized in controlled, clinical laboratory settings. As such, the primary aim of the current study was to examine the subjective and reinforcing effects of nicotine under PIO maintenance [0 (placebo), 45 mg] in daily smokers. Based on the preclinical data, we hypothesized that PIO would decrease indicators of abuse potential. If these hypotheses are supported, PPARγ activation may represent a new pharmacotherapeutic target for the treatment of tobacco use.
2. Methods
2.1. Participant recruitment and selection
Cigarette smokers, not interested in smoking cessation, were recruited using newspaper and online advertisements. After the initial telephone screening, individuals who met preliminary study criteria were scheduled for several in-person screening visits at the New York State Psychiatric Institute (NYSPI). Participants' physical health, mental health, and drug use, were evaluated by a team of psychologists, nurses, and physicians. For participants to be eligible for the study, they needed to meet DSM-IV criteria for nicotine dependence, smoked at least 15 cigarettes per day, with a Fagerstrom score ≥ 5 and carbon monoxide (CO) levels indicating that the participants are, at least, moderate smokers (> 14 parts per million (ppm), Heatherton et al., 1991). Additionally, participants were required to be between the ages of 21 and 55 years, mentally and physically healthy, and not physically dependent on any drugs other than nicotine or caffeine. All study procedures were approved by the Institutional Review Board (IRB) of the New York State Psychiatric Institute (NYSPI; IRB# 6255; ClinicalTrials.gov Identifier: NCT01395797).
2.2. Procedures
2.2.1. Stabilization
Over the course of the 3-week study, participants resided in a secured inpatient unit of the NYSPI. For the first 5–7 days following admission, participants were stabilized on a transdermal nicotine patch and randomized to one of the two PIO conditions for the duration of the trial (0 mg or 45 mg). After stabilization, one 7 milligrams (mg) patch was applied daily at 0800 hours (h) throughout the investigation (Table 1).
Table 1.
Representative study design.
| Week 1 | Week 2 | Week 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5–6 days | M | T | W | Th | F | M | T | W | Th | F |
| Nicotine patch stabilization | Nicotine verbal choice | Nicotine PR choice & cue sessions | Placebo verbal choice | Nicotine PR choice & cue sessions | ||||||
| Nicotine patch 7 mg | ||||||||||
2.2.2. Laboratory testing
At the end of the stabilization week, participants began laboratory testing, which occurred Monday to Friday. Participants completed three types of laboratory sessions over the course of two weeks: verbal choice, progressive ratio (PR) choice, and smoking cue.
2.2.3. Verbal choice sessions
On Monday through Thursday, participants were given an opportunity to choose between $0.50 and 3 puffs on a cigarette every 30 min for 5 h (1000 h, 1030 h, 1100 h, 1130 h, 1200 h, 1230 h, 1300 h, 1330 h, 1400 h, and 1430 h). Participants made a simple “yes” or “no” verbal choice for puffs or money. For half of the choice sessions (Days 1–4 or 8–11), participants smoked de-nicotinized cigarettes and for the other half of the choice sessions, participants smoked cigarettes that contained nicotine. The cigarettes were prepared so they would appear identical in order to maintain a blind to both participants and research staff. The same dose was tested in the procedure for the entire week (i.e. week 1 = active nicotine (nicotinized), week 2 = placebo nicotine (denicotinized)), and the order of nicotinized and de-nicotinized cigarettes was counterbalanced. The de-nicotinized and nicotinized testing phases were included to test for PIO's effects on extinction. No order effects related to this variable were found.
2.2.4. Progressive ratio choice sessions
Friday or Day 5/12 laboratory sessions consisted of two parts (Table 2). In the morning “sample” session (1000h), participants were administered $5 and a cigarette containing nicotine (10 puffs of their usual brand of cigarettes) followed by various assessments of subjective effects, performance tasks, and impulsivity measures (discussed below). These data were collected at baseline and at various time points up to 90 min after smoking the cigarette. Vital signs were continuously monitored during all sessions. During a later “choice” session (approximately 1400 h), participants were given the opportunity to respond for up to $5 or 10 puffs on their usual brand of cigarettes after completing a 10-trial task. During each trial, participants worked for $0.50 or 1 puff on a cigarette. The number of responses (finger presses on a computer mouse) required to receive the first puff was 10, followed by 20, 40, 80, 160, 320, 640, 1280, 2560, and 5120 responses. They had 1 h to complete the task. At the end of the task, participants received whatever fraction of the money or puffs that they earned. They were told that the option to smoke a cigarette would not be available for the remainder of the day.
Table 2.
Day 5/Friday session schedule.
| Sample | Cue/self-admin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| −60 | 0 | 2 | 10 | End | −65 | −25 | 0 | 60 | 70 | End | |
| Physiological monitoring | |||||||||||
| Blood pressure | X | X | X | X | X | X | X | ||||
| Heart rate | X | X | X | X | X | X | X | ||||
| Expired CO | X | X | |||||||||
| Questionnaires | |||||||||||
| DEQ | X | X | |||||||||
| NEB | X | X | X | ||||||||
| QSU | X | X | X | X | X | X | |||||
| VAS | X | X | X | X | X | X | |||||
| Physiologic | X | X | |||||||||
| Drug admin (10 puffs) | X | ||||||||||
| Cue exposure task | Neutral | Smoking | |||||||||
| Self-admin (1 puffs or $0.50) | X | ||||||||||
2.2.5. Cue sessions
At approximately 1300 h of the Friday session, participants completed a drug “cue exposure” session. Two opaque pitchers were placed on the participant's desk at the beginning of the session. Hidden under one pitcher was a glass and a bottle of spring water. An unopened pack of their favorite brand of cigarettes, lighter, matches, and an ashtray were hidden under the second pitcher. During the cue session, participants were first shown the water bottle and asked to look at, hold and sniff it, and take a drink of the water inside. After a 5-minute relaxation period, participants were visually exposed to the smoking cues. A research nurse subsequently instructed participants to open their pack of cigarettes and take one out. They were then instructed to hold the cigarette in their mouth, then hold it in their hand, light it up without placing in their mouth, hold it directly in front of them without smoking, and then finally extinguish it in the ashtray.
2.3. Measures
2.3.1. Subjective effects
Five questionnaires were used to assess subjective drug effects and craving. The drug effects questionnaire (DEQ) was used to measure drug effects. Participants selected among a series of possible answers ranging from –4 (‘Very Bad’) or 0 (‘No Effect’), up to 4 (‘Very Good Effect’). The Nicotine Effects Battery (NEB) assessed similar drug effects using a 0–100 mm Likert Scale. Participants rated each item on a scale from ‘Not at all’ (0 mm) to ‘Extremely’ (100 mm). A visual analog scale (VAS) also used the same anchors to query subjective and physiological drug effects such as “I feel mellow” and “I feel anxious”. A shortened version of the Questionnaire of Smoking Urges (QSU) was used to assess craving for cigarettes (Tiffany and Drobes, 1991). Finally, during the cue session, participants used a 5-point Likert scale to rate the degree to which they experienced events such as “salivating” and “trembling” while manipulating the smoking paraphernalia.
2.3.2. Physiological effects
For safety, heart rate, and blood pressure (systolic and diastolic) were continuously monitored during sessions. During the cue-exposure sessions, galvanic skin response (GSR), skin temperature, and heart rate were assessed immediately before and after active and neutral smoking cue presentation, using an MP100 Telemetry System (Biopac Systems, Santa Barbara, CA). These measures were used as indicators of autonomic arousal (Mendes, 2009).
2.4. Drugs
Pioglitazone hydrochloride (Actos®) tablets (15 mg) were provided by the OMEROS Corporation (Seattle, WA). PIO was over-encapsulated and administered in identical size 00 capsules (manufactured by Capsugel®) in order to maintain a dosing blind. Each daily dose consisted of 3 capsules of active drug and/or lactose-filled placebo, depending on the final target dose (e.g. 45 mg = three 15 mg tablets; 0 mg = three 0 mg tablets). The 3 tablets were administered at 2000 h by a research nurse.
Cigarettes (e.g., Quest 1 and Quest 3 or equivalent brand) used during Verbal Choice sessions were obtained from Vector Tobacco (Mebane, NC). Some of the cigarettes contained nicotine (Quest 1, Vector Tobacco, Mebane, North Carolina) and some were de-nicotinized (Quest 3). Nicotine patches (Nicotine Transdermal System) were obtained from Novartis Pharmaceuticals (Parsippany, New Jersey). All drugs were prepared by the NYSPI Pharmacy.
2.5. Statistical analyses
A priori power analyses were conducted using nQuery Advisor®, Statistical Solutions, to ensure that effects on the outcome measures of interest could be suitably detected. Estimates for our primary measure of reinforcing effects were obtained from a recently completed trial using similar verbal choice procedures. The targeted sample size of 20 per arm was calculated to provide 80% power to detect a 26% between-group difference in average percentage of drug choice, which is an effect size of 0.65. This assumes a standard deviation of 28%. The sample size goal of 40 completers was also estimated to provide 80% power to detect a 500-point between-group difference in progressive ratio breakpoint values, which is an effect size of 0.64. This assumes a standard deviation of 594, and a between-level correlation of 0.60. This difference was used because we anticipated a fairly small difference in the effects of PIO on drug self-administration. Estimates of positive subjective effects were also considered. Twenty completers per arm in each study calculated to provide 83% power to detect a 15-mm difference in ratings of “Liking” as measured by the VAS. This analysis assumes a common standard deviation of differences of 16 mm (e.g., effect size of 0.66).
Categorical and continuous demographics variables were summarized descriptively and compared between the 0 and 45 mg PIO conditions using the chi-square (X2) or t-tests, respectively. The primary statistical analyses were comparisons of acute drug effects and self-administration (from Day 5/Friday session) following placebo (0 mg) and active (15 & 45 mg) PIO maintenance. These comparisons were made using analysis of variance (ANOVA) evaluating peak drug effect (maximal drug effect throughout the session). Independent-samples t-tests were also used to compare PR breakpoint between active and placebo PIO groups. This same method was used to compare mean autonomic arousal following presentation of neutral and active smoking cues. ANOVA was employed to compare nicotine choices between 45 mg and 0 mg PIO groups during the preceding 4 days (Mon-Thurs). Tukey's HSD post-hoc tests were used to identify significant differences between PIO groups. For all analyses, the significance level of α was set at < 0.05. All data analyses were performed using SPSS version 18 (SPSS, 2009) and SuperANOVA (Gagnon et al., 1990).
3. Results
3.1. Participants
A total of 42 smokers were enrolled in the current study. Sixteen participants were randomized into the PIO 45 mg group with 14 completers. One participant was discontinued due to an elevation in liver function tests, and a second after learning she was pregnant. Sixteen participants were randomized into the PIO 0 mg group. One participant was discontinued due to an elevation in liver function tests, one participant withdrew because they could not tolerate the boredom and confinement of the inpatient unit, and one participant was discontinued for unreported reasons. Table 3 displays the major demographic variables for completers of both groups. Ten enrolled participants were included in a 15 mg PIO condition. Enrollment into the 15 mg PIO condition was ceased prior to the end of the grant period in order to increase the sample sizes of the 0 and 45 mg conditions. Of the 10 participants enrolled in the 15 mg condition, 5 completed. The data from these participants are presented in Table 4, but were not included in the comparative statistical analyses due to the potentially biasing effect of such a small sample size.
Table 3.
Sample demographics.
| Participants (%) or median (std. dev.) |
Significance | ||
|---|---|---|---|
|
|
|||
| PIO 0 mg (n = 13) | PIO 45 mg (n = 14) |
||
| Age | 41.6 (10.0) | 44.9 (6.7) | ns |
| Sex | |||
| Male | 12 (92) | 13 (93) | ns |
| Ethnic/racial category | ns | ||
| Asian | – | – | |
| African-American | 6 (46) | 5 (36) | |
| Caucasian | 4 (31) | 6 (43) | |
| Hispanic/Latino | 3 (23) | – | |
| More than one race/unreported | – | 3 (21) | |
| Years of education | 12.1 (1.6) | 12.4 (0.9) | ns |
| Cigarette use | |||
| Cigarette use (cigs/day) | 17.8 (4.6) | 20.5 (4.0) | ns |
| Years of smoking | 24.2 (11.6) | 27.0 (8.1) | ns |
| Longest time without smoking (days) | 165 (345) | 172 (328) | ns |
| Ever quit? (yes) | 6 (46) | 6 (43) | ns |
Table 4.
Drug effects.
| PIO 0 mg | PIO 15 mg | PIO 45 mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De-nicotinized week |
Nicotinized week | De-nicotinized week |
Nicotinized week | De-nicotinized week |
Nicotinized week | |||||||
| Dependent measure | Day 5 | |||||||||||
| Nicotine self-administration | ||||||||||||
| Drug breakpoint | 586 (391) | 652 (266) | 691 (629) | 908 (654) | 960 (373) | 1004 (466) | ||||||
| Subjective effects | ||||||||||||
| BSL* | PK | BSL | PK | BSL | PK | BSL | PK | BSL | PK | BSL | PK | |
| DEQ (−4–4) | ||||||||||||
| Good | 0.1 (0.3) | 2.5 (0.4) | 0.7 (0.3) | 2.3 (0.4) | 0.5 (0.9) | 2.3 (1.5) | 0.6 (1.3) | 2.3 (1.3) | 0.4 (0.3) | 2.3 (0.3) | 0.1 (0.1) | 2.8 (0.3) |
| Like | 0.7 (0.4) | 2.1 (0.5) | 0.4 (0.5) | 2.0 (0.6) | 0.7 (0.6) | 1.7 (1.2) | 0.2 (0.5) | 2.1 (0.9) | 0.3 (0.4) | 1.5 (0.6) | −0.3 (0.2) | 2.1 (0.5) |
| NEB (0–100) | ||||||||||||
| High** | 67.8 (7.7) | 61.5 (10.5) | 70.4 (11.7) | 74.5 (13.5) | 81.7 (7.0) | 81.9 (5.8) | ||||||
| Like | 62.3 (10.8) | 54.6 (8.1) | 61.3 (16.5) | 65.4 (12.4) | 64.1 (10.5) | 71.3 (8.9) | ||||||
| VAS (0–100) | ||||||||||||
| Good | 19.2 (9.8) | 46.8 (11.6) | 19.6 (10.2) | 44.7 (11.2) | 15.2 (12.3) | 36.8 (14.6) | 14.5 (10.6) | 46.7 (13.6) | 11.5 (6.9) | 58.8 (9.6) | 1.8 (1.0) | 61.2 (9.4) |
| High | 5.6 (4.2) | 15.1 (6.5) | 5.6 (3.7) | 27.6 (8.0) | 5.9 (6.0) | 26.9 (14.3) | 6.8 (5.4) | 37.4 (13.4) | 8.6 (5.8) | 26.1 (10.7) | 4.5 (3.8) | 34.8 (9.4) |
| Craving | ||||||||||||
| BSL | TH*** | BSL | TH | BSL | TH | BSL | TH | BSL | TH | BSL | TH | |
| QSU (1–7) | ||||||||||||
| Desire for nicotine | 4.1 (0.7) | 3.3 (0.6) | 4.5 (0.6) | 4.0 (0.7) | 4.1 (1.2) | 3.1 (0.9) | 4.4 (0.9) | 2.2 (1.8) | 4.3 (0.6) | 2.7 (0.5) | 4.3 (0.7) | 2.3 (0.5) |
| Urge | 4.2 (0.6) | 3.4 (0.6) | 4.4 (0.6) | 4.0 (0.6) | 3.8 (1.0) | 2.5 (1.0) | 4.5 (1.0) | 2.0 (0.8) | 4.0 (0.6) | 2.8 (0.6) | 4.5 (0.6) | 2.1 (0.4) |
| Want cigarette | 3.8 (0.6) | 3.2 (0.6) | 3.8 (0.6) | 4.2 (0.7) | 3.6 (0.9) | 2.7 (1.2) | 4.0 (0.9) | 2.0 (0.8) | 3.2 (0.6) | 2.4 (0.6) | 3.3 (0.6) | 1.9 (0.4) |
| Dependent measure | Cue session | |||||||||||
| Neutral | Active | Neutral | Active | Neutral | Active | Neutral | Active | Neutral | Active | Neutral | Active | |
| Physiologic | ||||||||||||
| GSR | 0.7 (0.05) | 0.2 (0.10) | 0.1 (0.03) | 0.1 (0.05) | 0.8 (0.8) | .07 (0.10) | 0.1 (0.05) | 0.1 (0.03) | 0.03 (0.00) | 0.05 (0.01) | 0.03 (0.00) | 0.1 (0.03) |
| HR | 74.5 (2.1) | 74.1 (2.9) | 78.0 (3.9) | 77.0 (3.1) | 74.5 (4.8) | 73.5 (5.5) | 70.2 (8.0) | 75.0 (5.0) | 75.6 (2.4) | 73.7 (2.5) | 68.6 (4.7) | 72.0 (2.7) |
| SKT | 31.6 (1.1) | 32.4 (1.0) | 30.8 (1.1) | 31.4 (1.1) | 30.5 (2.5) | 34.2 (2.0) | 28.5 (2.2) | 30.8 (1.9) | 29.4 (1.2) | 29.8 (1.0) | 28.5 (1.0) | 29.4 (1.0) |
| Craving | ||||||||||||
| QSU: Sum | 36.1 (5.4) | 38.2 (5.7) | 35.5 (4.8) | 32.8 (4.7) | 30.8 (8.8) | 36.3 (4.4) | 32.3 (4.4) | 38.4 (4.9) | 32.9 (4.8) | 36.3 (4.4) | 32.3 (4.4) | 38.4 (4.9) |
| Subjective | ||||||||||||
| Perceived physiologic (0–4) | ||||||||||||
| Faster HR | 1.3 (0.2) | 1.7 (0.3) | 1.7 (0.3) | 1.7 (0.2) | 1.3 (0.4) | 1.8 (0.8) | 2.0 (0.8) | 2.0 (0.6) | 1.4 (0.2) | 2.0 (0.2) | 1.6 (0.3) | 2.0 (0.2) |
| Heavy stomach | 1.1 (0.1) | 1.4 (0.1) | 1.2 (0.1) | 1.5 (0.2) | 1.0 (0.2) | 1.4 (0.6) | 1.0 (0.4) | 1.8 (0.6) | 1.1 (0.1) | 1.3 (0.2) | 1.2 (0.1) | 1.5 (0.2) |
| Salivating | 1.2 (0.1) | 1.5 (0.2) | 1.2 (0.1) | 1.2 (0.2) | 1.4 (0.4) | 1.2 (0.6) | 1.0 (0.4) | 1.2 (0.4) | 1.3 (0.1) | 1.5 (0.3) | 1.4 (0.1) | 1.4 (0.1) |
| Sweating hands | 1.0 (0.0) | 1.0 (0.0) | 1.0 (0.0) | 1.0 (0.0) | 1.0 (0.2) | 1.0 (0.0) | 1.2 (0.4) | 1.1 (0.3) | 1.0 (0.0) | 1.1 (0.1) | 1.0 (0.0) | 1.1 (0.1) |
| Trembling | 1.0 (0.0) | 1.0 (0.0) | 1.0 (0.0) | 1.1 (0.1) | 1.0 (0.2) | 1.2 (0.4) | 1.0 (0.2) | 1.4 (0.6) | 1.0 (0.0) | 1.3 (0.2) | 1.1 (0.1) | 1.3 (0.2) |
Mean baseline (BSL) or mean peak (PK: maximal drug effects throughout the session).
Not measured at baseline.
Mean trough (TH): minimum drug effects throughout the session.
3.2. Reinforcing effects
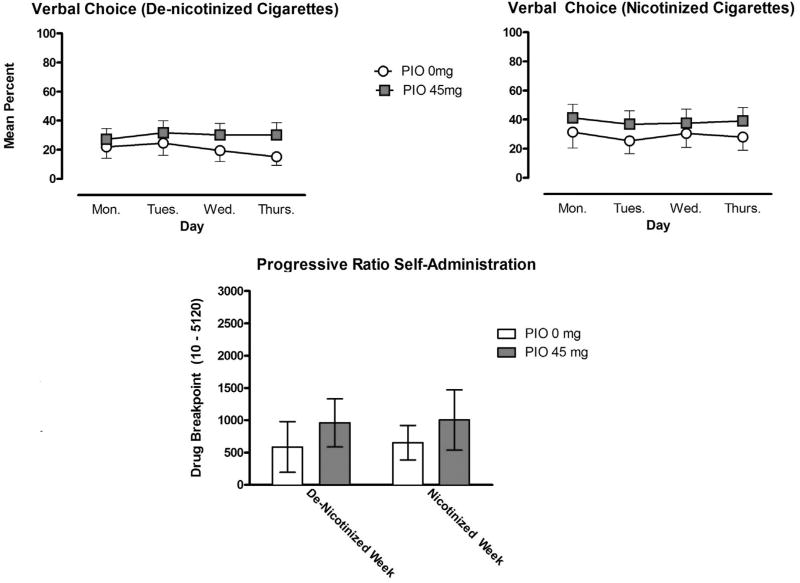
During the first 4 days of testing, nicotinized cigarettes were self-administered at a greater rate in comparison to de-nicotinized cigarettes; this difference did not meet statistical significance. The reinforcing effects of both types of cigarette were maintained throughout the four days of testing (Fig. 1: upper panel). This pattern did not vary as a function of the PIO maintenance condition. On Day 5 (Friday) when participants chose between nicotinized cigarettes and money using a progressive ratio choice procedure, moderate drug breakpoints were observed under all conditions tested. The reinforcing effects of nicotine did not significantly vary as a function of the availability of nicotinized or de-nicotinized cigarettes the preceding week or PIO maintenance (Fig. 1: lower panel).
Fig. 1.
Self-administration (± SEM) of de-nicotinized and nicotinized cigarettes for the pioglitazone (PIO) 0 mg (n = 13) and PIO 45 mg (n = 14) conditions, assessed using the verbal choice procedure during Days 1–4 (upper panel). Self-administration (± SEM) of nicotinized cigarettes on Day 5 using a progressive ratio procedure following 4 days of access to denicotinized and nicotinized cigarettes.
3.3. Positive subjective effects
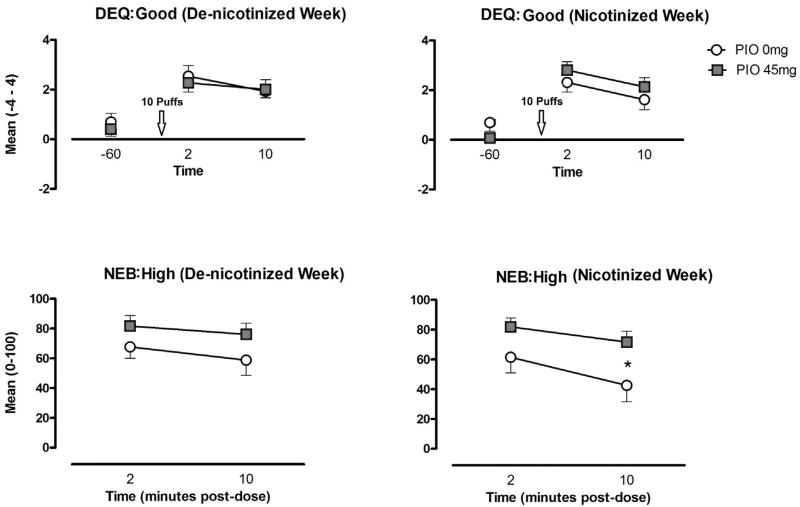
The subjective effects of nicotine (10 puffs of the participants' usual brand of cigarettes) were assessed during the Day 5 sample session that following a week of self-administration of either de-nicotinized or nicotinized cigarettes. In comparison to the pre-dose baseline (−60 min), peak post-dose ratings of “Good” drug effect were significantly increased as measured by the DEQ and VAS scales (p's < 0.05). A similar increase was found on the DEQ ratings of drug “Liking,” (p < 0.05). The NEB, which was only administered at 2 and 10 min post dose, also found robust ratings of “Liked” the dose were also found on the NEB measure (> 50 out of 100). No effect of PIO maintenance condition or the previous week of de-nicotinized vs nicotinized cigarette self-administration was found on these measures.
NEB-measured reports of “High” were also generally robust (> 60) and greater for participants in the 45 mg PIO condition. This difference was only statistically significant following a week of active nicotine self-administration (p < 0.05). In comparison to pre-dose measurement, VAS ratings of “High” increased following smoking. However, the increase only reached statistical significance following a week of nicotinized cigarette self-administration (Fig. 2). PIO typically increased indicators of abuse potential, though this effect only met statistical significance on a single NEB measure (Fig. 2).
Fig. 2.
Mean (± SEM) ratings of “Good” drug effect and “High” for the pioglitazone (PIO) 0 mg and PIO 45 conditions in response to experimenter-administered nicotine on Day 5, following 4 days of access to de-nicotinized and nicotinized cigarettes. * indicates a significance level < 0.05.
3.4. Aversive subjective effects
Aversive drug effects were assessed using the VAS items of “Depressed” and “Anxious,” along with the DEQ measure of “Bad” drug effect. Ratings on all three measures were minimal and did not differ significantly from baseline. These measures were not affected by the previous week's drug (de-nicotinized vs nicotinized) or PIO maintenance.
3.5. Craving
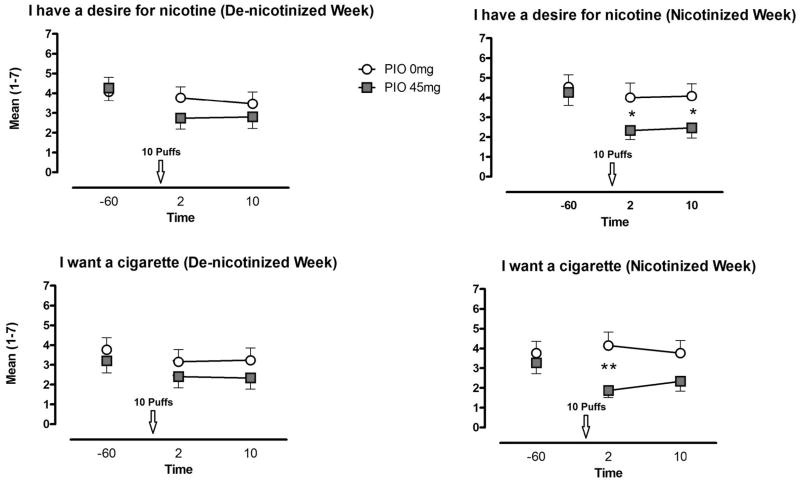
For participants in the PIO 0 mg condition, nicotine administration failed to affect QSU measures of drug craving (“Urge,” “Desire” and “Want”). Conversely, for those in the PIO 45 mg group we observed a decreased in measures of craving following the administration of nicotine. This effect only reached statistical significance following a week of self-administration of nicotinized cigarettes (Fig. 3).
Fig. 3.
Mean (± SEM) ratings of “craving” and “desire” for cigarettes/nicotine for the pioglitazone (PIO) 0 mg and PIO 45 conditions on Day 5, following 4 days of access to de-nicotinized and nicotinized cigarettes. * indicates a significance level < 0.05, ** indicates a significance level < 0.01.
3.6. Subjective response to smoking cues
During the cue sessions, presentation of active smoking cues (vs neutral cues) slightly increased all QSU measures of craving. Similarly, smoking cues slightly increased the participants' assessments of their own “Sweating Hands,” “Salivating,” “Faster Heart Rate,” “Heavy Stomach,” and “Trembling.” Manipulating smoking paraphernalia also slightly increased VAS measure of “Anxious,” while having no effect on measures of “Depressed” and “Mellow.” None of the aforementioned differences met statistical significance, and no effect of PIO maintenance condition or the previous week of de-nicotinized vs nicotinized cigarette self-administration was found.
3.7. Physiological response to smoking cues
Assessment of autonomic arousal during the cue session revealed that smoking cues (vs neutral cues) increased skin conductance. Following a week of de-nicotinized cigarette access, this increase did not meet statistical significance for either PIO condition. Following a week of access to nicotinized cigarettes, the increase only met statistical significance for those in the PIO 45 mg condition. Smoking cues had no observable effect on heart rate or skin temperature.
3.8. Safety measures
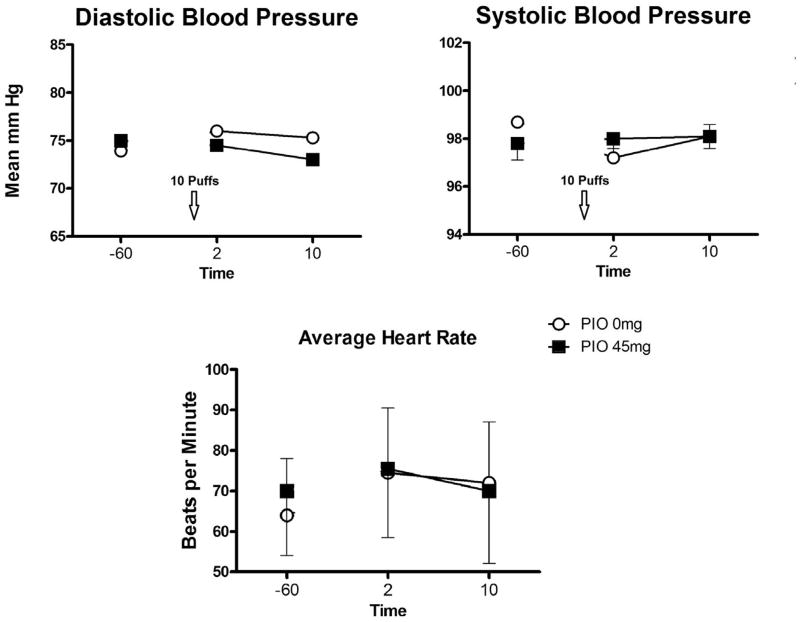
No PIO treatment effect was observed for the heart rate and blood pressure measurements obtained during the week or during the Friday experimental sessions (Fig. 4). The study medication was well tolerated. No serious adverse events were reported and no adverse events were determined to be “probably” or “definitely” related to the study medication or led to discontinuation of the study medication.
Fig. 4.
Mean (± SEM) measures of blood pressure and heart rate on Day 5, following 4 days of access to de-nicotinized and nicotinized cigarettes.
4. Discussion
The current study sought to determine whether pioglitazone, a PPARγ agonist and glial modulator, would alter the subjective and reinforcing effects of smoked nicotine in our sample of heavy smokers. The reinforcing effects of both de-nicotinized and nicotinized cigarettes were maintained throughout the four days of testing in both PIO conditions, thus, PIO did not alter the rate of extinction responding over this relatively short period of observation. Had a more challenging self-administration procedure been employed, in lieu of the simple verbal choice, we may have been able to observe a decrease in drug self-administration over the 4 days.
The progressive ratio self-administration procedure on Day 5 only assessed responses to active nicotine and required participants to make hundreds of responses to receive drug. A moderate breakpoint was observed that was typically greater following a preceding week of smoking nicotinized cigarettes; no effect of PIO maintenance was found. We also did not observe an effect of PIO on direct assessments of the positive or negative subjective effects of experimenter-administered nicotine during this session (10 puffs of their usual brand of cigarette). In comparison to pre-dose baseline, nicotine significantly increased ratings on measures of good drug effect and liking. Participants reported relatively minimal aversive nicotine effects that did not differ from baseline. For participants in the PIO 0 mg condition, nicotine administration failed to affect QSU measures of craving. Meanwhile, those maintained on 45 mg of PIO showed significant decreases in craving, but these effects on craving were only found following a preceding week of smoking nicotinized cigarettes.
The current data suggest that PIO may aid in the satiation of nicotine craving. These clinical observations are consistent with preclinical studies showing that PPARγ agonists blocks reinstatement of heroin-, cocaine- and alcohol-seeking in animal models (Ciccocioppo et al., in preparation; Le Foll et al., 2013; Miller, 2016; Schmitz et al., 2016; Stopponi et al., 2011). The repeated finding of differences in our measures of interest only following a week of smoking nicotinized cigarettes, may suggest a direct physiological effect of nicotine upon their underlying neurobiological mechanisms.
The lack of a significant effect on the study's measures of abuse potential (e.g., drug self-administration, positive subjective effects) is inconsistent with preclinical findings with nicotine. Though this work has primarily focused on PPAR alpha agonist (as opposed to gamma), studies have shown significant attenuation in acquisition and maintenance of self-administration, along with reduced reinstatement to nicotine seeking (Panlilio et al., 2012; Mascia et al., 2011). These differences in PPAR agonist efficacy may be the result of the different PPAP receptor targets, or the unique complexity of drug use behavior among humans.
This investigation also attempted to assess the ability of PIO to reduce reactivity to smoking cues. However, the cue exposure procedure we employed produced only marginal psychological and physiological arousal. One parametric condition that is likely responsible for this finding is the dosing of nicotine in the sample session prior to the cue session. Because of concerns with the overall duration of this inpatient study (3–4 weeks), these two types of sessions were combined in a single day. As a result, participants would have received an administration of nicotine (10 puffs of their usual brand of cigarette) within hours of completing the cue-exposure task. In other studies, the acute administration of nicotine has been found to reduce intentions to smoke and withdrawal-related cravings to a greater extent than placebo among dependent smokers (McGrath et al., 2015). We believe that a greater period of nicotine abstinence preceding the session would greatly increase the salience of the smoking cues. Therefore, the investigators feel that we did not produce a sufficient cue effect upon which PIO could act. Based on the ability of PIO to alter craving during other laboratory sessions, its effects on cue-induced craving should be reassessed, independently of nicotine administration. However, the authors would like to note that the effect size of differences in craving were robust, despite this relatively short abstinence period.
The predictive validity of reductions in craving in the laboratory, to a medication's treatment potential outside of the laboratory, has been less well defined in comparison to reductions in self-administration. As such, the clinical significance of our findings is less clear. However, an effect upon craving may be clinically significant as field studies indicate that such cue-induced cravings are significant contributors to relapse to smoking (Ferguson et al., 2006; Killen and Fortmann, 1997; Shiffman et al., 1997).
There is also data to suggest that a longer PIO maintenance period would lead to a more robust effect. Another pilot clinical study of PIO for cocaine use disorder found that 12 weeks of PIO maintenance (45 mg/day) significantly reduced craving for cocaine (Schmitz et al., 2016). PIO dosing parameters (duration and mg amounts) for the current study were based on its clinical utility for treating diabetes, which may not be related to its effects on glia. Additionally, as research has shown that patients motivated to quit drug use react differently to potential treatment medications; our use of a sample not interested in quitting smoking may have affected our findings (Wells et al., 2010; Tai et al., 2010). For ethical reasons, laboratory drug-choice studies typically do not enroll treatment-seeking participants (Fischman and Johanson, 1998).
Another design feature that may have adversely affected our ability to observe an effect of PIO was the nicotine patch maintenance. Nicotine replacement therapy has been shown to reduce craving and nicotine use; therefore the PIO effect may have been masked by the effect of the nicotine maintenance (Cahill et al., 2013). Because PIO has been found to reduce stress-evoked anxiety and manifestation of nicotine withdrawal symptoms in preclinical studies, future studies should assess the effects of PIO during states of abstinence, rather than in the presence of nicotine. Additionally, due to of its preliminary nature, this study also failed to account for sex differences. There is converging evidence across multiple studies suggesting that sex is a mediating factor in the efficacy of pharmacological interventions for smoking cessation (Smith et al., 2016).
Finally, we did not meet the recruitment goals outlined in our power analysis. Therefore, the study may have been underpowered to detect some differences between the groups. Yet, despite the smaller than anticipated number of completers, the effects sizes related to PIO's effects on craving were medium-to-large (Cohen's d effect sizes: 0.5–0.9). As the first of its kind, this study provides a point of reference for powering future investigations into the therapeutic potential of glial modulators to. All of the limitations mentioned above reflect the difficulty in designing these types of first-in-human trials.
In sum, there is strong evidence that PPARγ receptors and glial-inhibiting compounds in general may play a role in the neurobiological systems important in the various phases of addiction (Cooper et al., 2012; Bachtella et al., 2017). Therefore, medications like PIO may potentially be therapeutically exploitable as novel addiction pharmacotherapies (Cooper et al., 2012; Diana, 2011; Le Foll et al., 2013). Although the effects seen in the current study are limited, as the first clinical investigation for this indication, they are encouraging and provide a direction for further inquiry.
Acknowledgments
The medical assistance of Janet Murray, and Claudia Tindall, along with the technical assistance of Melissa Mahoney, Brian Wade, Johnathan Vogelman, Rachel Luba, Gabriela Madera and Andrew Segoshi is gratefully acknowledged.
Funding
This study was supported by National Institute on Drug Abuse Grant R01DA031022 to Drs. Bisaga and Comer. Pioglitazone was provided by the OMEROS Corporation.
Dr. Bisaga served as an unpaid consultant to Alkermes and received an honorarium from Indivior for an unbranded educational activity. Drs. Comer, Mogali, and Manubay have received compensation (in the form of partial salary support) from studies supported by Alkermes, Braeburn Pharmaceuticals, Cerecor Inc., Endo Pharmaceuticals, Indivior PLC/Reckitt-Benckiser Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, MediciNova, Omeros, and Schering-Plough Corporation. In addition, Sandra Dr. Comer has received compensation from Grunenthal GmbH to conduct a meta-analysis of drug-induced subjective responses and she served as a consultant to the following companies: Analgesic Solutions, AstraZeneca, BioDelivery Sciences International, Cephalon, Clinilabs, Daiichi Sankyo, Egalet, Endo, Inflexxion, Innovative Science Solutions, Janssen, KemPharm, King, Lightlake (now Opiant), Neuromed, Pfizer, and Salix. Dr. Ciccocioppo is the inventor of a number of patent applications, which have been assigned to Omeros, relating to the therapeutic use of PPAR agonists in addiction. He is entitled to receive payments and royalties from Omeros under such licensing arrangement.
Footnotes
Author's contribution
AB, and SDC designed the study and oversaw its completion. JDJ, VM, JM and SM conducted study procedures. SM and MM assisted with data management, cleaning and analysis. RC assisted with interpretation of findings and provided critical revision of the manuscript for important intellectual content. JDJ developed the first draft of the manuscript and all authors reviewed its content and approved the final version for publication.
Declaration of interests
The remaining authors have no conflicts to report.
References
- Armstrong V, Reichel CM, Doti JF, et al. Repeated amphetamine treatment causes a persistent elevation of glial fibrillary acidic protein in the caudate-putamen. Eur. J. Pharmacol. 2004;488:111–115. doi: 10.1016/j.ejphar.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Bachtella RK, Jones JD, Heinzerling CG, Beardsley PM, Comer SD. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend. 2017;180:156–170. doi: 10.1016/j.drugalcdep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD009329.pub2. CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, De Guglielmo G, Melis M, De Luca MA, Li A, Cippitelli A, et al. Activation of PPAR gamma by the anti-diabetic agent pioglitazone reduces opioid reinforcement and opioid-induced activation of the mesolimbic dopamine system. Soc. Neurosci. Abstr. 2012;668:16. [Google Scholar]
- Cooper ZD, Jones JD, Comer SD. Glial inhibitors: a novel pharmacological approach to modulating the behavioral effects of abused substances. Expert Opin. Investig. Drugs. 2012;21:169–178. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr. Opin. Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psych. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domi E, Uhrig S, Soverchia L, Spanagel R, Hansson AC, Barbier E, Heilig M, Ciccocioppo R, Ubaldi M. Genetic deletion of neuronal PPARγ enhances the emotional response to acute stress and exacerbates anxiety: an effect reversed by rescue of amygdala PPARγ function. J. Neurosci. 2016;36(50):12611–12623. doi: 10.1523/JNEUROSCI.4127-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, et al. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J. Consult. Clin. Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Ferguson LB, Most D, Blednov YA, Harris RA. PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology. 2014;86:397–407. doi: 10.1016/j.neuropharm.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Johanson CE. Ethical and practical issues involved in behavioral pharmacology research that administers drugs of abuse to human volunteers. Behav. Pharmacol. 1998 Nov;9(7):479–498. doi: 10.1097/00008877-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, Simpson J. SuperAnova accessible general linear modeling. Yale. J. Biolo. Med. 1990;63:191–192. [Google Scholar]
- de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, Giordano A. PPARγ activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology. 2015;40:927–937. doi: 10.1038/npp.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R. Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology. 2017;234(2):223–234. doi: 10.1007/s00213-016-4452-1. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J. Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. [DOI] [PubMed] [Google Scholar]
- Jain R, Majumder P, Gupta T. Pharmacological intervention of nicotine dependence. Biomed. Res. Int. 2013 doi: 10.1155/2013/278392. http://dx.doi.org/10.1155/2013/278392. [DOI] [PMC free article] [PubMed]
- Jamal A, et al. Current cigarette smoking among adults - United States, 2005–2014. Morb. Mortal. Wkly Rep. 2015;64:1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- Kaur K, Kaushal S, Chopra SC. Varenicline for smoking cessation: a review of the literature. Curr. Ther. Res. 2009;70:35–54. doi: 10.1016/j.curtheres.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Drew PD. Effects of PPARy agonists on central nervous system inflammation. J. Neurosci. Res. 2003;71:315–325. doi: 10.1002/jnr.10501. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp. Clin. Psychopharmacol. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr. Drug Targets. 2013;14(7):768–776. doi: 10.2174/1389450111314070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S. Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology. 2007;32(5):1133–1140. doi: 10.1038/sj.npp.1301213. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol. Psychiatry. 2011;69(7):633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath DS, Peloquin MP, Ferdinand JC, Barrett SP. Acute effects of nicotine on alcohol cue-reactivity in nondependent and dependent smokers. Exp. Clin. Psychopharmacol. 2015;23(1):29–36. doi: 10.1037/a0038606. [DOI] [PubMed] [Google Scholar]
- Mendes WB. Assessing autonomic nervous system activity. In: Harmon-Jones E, Beer JS, editors. Methods in Social Neuroscience. 2009. pp. 118–147. [Google Scholar]
- Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006;58(4):726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- Miller WR. PPARγ agonism attenuates cocaine cue reactivity. Addict. Biol. 2016 doi: 10.1111/adb.12471. http://dx.doi.org/10.1111/adb.12471. [DOI] [PMC free article] [PubMed]
- Narita M, Miyatake M, Narita M, et al. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology. 2012;37(8):1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Land SD, Green CE, Vincent J, Suchtin R, Moeller FG, Cunningham KA, Dineley KT. Pilot clinical trial of PPAR-gamma agonist (pioglitazone) for cocaine use disorder; Abstract Presented at the Annual Meeting of the College on Problems of Drug Dependence; June 2016; Palm Springs, CA. 2016. [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, et al. Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, et al. A day at a time: predicting smoking lapse from daily urge. J. Abnorm. Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA. Sex differences in smoking cessation pharmacotherapy comparative efficacy: a network meta-analysis. Nicotine Tob. Res. 2016;19(3):273–281. doi: 10.1093/ntr/ntw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS I. SPSS 18.0.0 for windows. Chicago, Illinois: [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R. Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol. Psychiatry. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, et al. Activation of PPAR gamma by the anti-diabetic agent pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in the rat. Alcohol. Clin. Exp. Res. 2013;37(8):1351–1360. doi: 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Tai B, Straus MM, Liu D, Sparenborg S, Jackson R, McCarty D. The first decade of the National Drug Abuse Treatment Clinical Trials Network: bridging the gap between research and practice to improve drug abuse treatment. J. Subst. Abus. Treat. 2010;38(Suppl. 1):S4–13. doi: 10.1016/j.jsat.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, CDC; Atlanta, GA: 2014. [Accessed date: 14 October 2016]. www.surgeongeneral.gov/library/reports/50-years-of-progress/ [Google Scholar]
- Warner C, Shoiab M. How does bupropion work as a smoking cessation aid? Addict. Biol. 2005;10:219–231. doi: 10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Wells EA, Saxon AJ, Calsyn DA, Jackson TR, Donovan DM. Study results from the Clinical Trials Network's first 10 years: where do they lead? J. Subst. Abus. Treat. 2010;38(Suppl. 1):S14–30. doi: 10.1016/j.jsat.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. [Accessed date: 15 February 2017];Global Health Observatory (GHO) Prevalence of Tobacco Smoking. 2016 http://www.who.int/gho/tobacco/use/en/