Abstract
Background
Animal studies suggest that monocyte chemoattractant protein-1 (MCP-1) is a promising biomarker for coronary artery atherosclerosis (CAA), but human studies have been inconclusive.
Objective
To determine potential relationships between plasma MCP-1 and CAA in patients with acute chest pain.
Methods
A secondary analysis of 150 patients enrolled in emergency department (ED) chest pain risk stratification clinical investigations was conducted. Participants with stored blood and known coronary phenotypes (determined by coronary angiography) were selected using stratified randomization such that 50 patients were included into 3 groups: a) no angiographic evidence of CAA b) non-obstructive CAA, and c) obstructive CAA (stenosis ≥70%). Plasma MCP-1 levels were determined by ELISA. The association between MCP-1 and obstructive CAA or any CAA was modeled using logistic regression. Variables in the unreduced model included age, sex, race, prior diagnosis of CAA or acute coronary syndrome (ACS), hyperlipidemia, hypertension, diabetes, smoking, and cardiac troponin I (cTnI) measurement.
Results
Among the 150 participants, 65.3% (98/150) had invasive coronary angiography and 34.7% (52/150) had coronary CT angiography. Myocardial infarction occurred in 27.3% (41/150) and coronary revascularization occurred in 26% (39/150). Each 10 pg/ml increase in MCP-1 measurement was associated with an odds ratio of 1.12 (95%CI: 1.06–1.19) for obstructive CAA. MCP-1 remained a significant predictor of obstructive CAA and any CAA after adjustment for age, sex, race, traditional cardiac risk factors, and cTnI.
Conclusions
MCP-1 is independently associated with CAA among ED patients with chest pain.
Keywords: monocyte chemoattractant protein-1, coronary artery atherosclerosis, chest pain
Introduction
Coronary artery atherosclerosis (CAA) is the leading cause of death in the US and is responsible for $109 billion in healthcare costs annually.(1–3) However, detection of CAA remains challenging and each year approximately 635,000 patients in the US, without previously diagnosed CAA, suffer myocardial infarction (MI) or cardiac death. (4) Emergency Department (ED) patients with chest pain are typically evaluated with electrocardiograms and troponin measures (cTn) to exclude MI and then receive additional screening tests for CAA. However, current CAA screening tests, such as stress testing and coronary angiography, are expensive, expose patients to radiation, or are invasive. A biomarker that could be added to the ED evaluation of patients with chest pain to better predict CAA could greatly improve patient care by aiding the selection of patients who would benefit from stress testing or angiography. Unfortunately, currently available biomarkers, such as C-reactive protein, are weak predictors of CAA and have not been proven useful in an ED setting. (5)
While animal studies suggest that MCP-1 is a promising biomarker for CAA, human studies have yielded mixed results. In the Atherosclerosis Risk in Communities study MCP-1 was associated with incident coronary heart disease independent of traditional cardiovascular risk factors including inflammatory markers, such as C-Reactive Protein. (6–8) In the Dallas Heart Study, MCP-1 levels were associated with computed tomography measurement of coronary artery calcium (CAC) before, but not after adjustment for patient age. (9) Importantly, CAC increases with age and does not always correlate with angiography. Data evaluating the ability of MCP-1 to predict angiographic coronary stenosis is limited. Furthermore, the Atherosclerosis Risk in Communities study and Dallas Heart Study measured MCP-1 among asymptomatic patients and there is only scarce data on MCP-1 performance among patients with acute chest pain. Given these gaps in the literature, this study seeks to determine whether MCP-1 is a predictor of CAA assessed using an angiographic gold standard among patients who presented to the ED for chest pain.
Methods
Study design
A secondary analysis of participants in a registry of ED patients enrolled in chest pain risk stratification clinical trials and investigations was conducted. Participants were enrolled in one of five studies, at institution withheld for review, from 2008 through 2016. Methods from completed studies have been previously described.(10–15) Each study recruited adult patients presenting to the ED with symptoms suggestive of acute coronary syndrome without ST-elevation. Patients with known coronary phenotypes from coronary angiography (computed tomography coronary angiography or invasive coronary angiography) and a banked frozen blood specimen were eligible for inclusion in this analysis. Selection for this analysis was conducted using stratified randomization such that 50 patients were selected for each of the following groups: a) no angiographic evidence of CAA b) non-obstructive CAA, and c) obstructive CAA (stenosis ≥70%), for a total of 150 participants. All participants gave written informed consent at the time of study entry. This analysis was approved by the Internal Review Board of the sponsoring organization.
Study setting
The study institution is a tertiary care academic medical center located in the Piedmont Triad area of North Carolina, serving urban, suburban, and rural populations. The ED is staffed by board certified or board eligible emergency physicians 24 hours per day, 7 days a week who directly provide care and oversee care provided by residents, physician assistants, and nurse practitioners. ED patient volume consists of >90,000 patient encounters per year. Cardiac testing modalities routinely available to study participants included exercise stress echocardiography, dobutamine stress echocardiography, coronary computed tomography angiography, stress nuclear imaging, stress cardiac magnetic resonance imaging, and invasive coronary angiography.
Participants
Patients at least 21 years old presenting with symptoms suggestive of acute coronary syndrome (ACS) were screened during enrollment hours (6 days excluding Saturday, 80 hours/week). Eligibility criteria included the provider ordering a troponin for the evaluation of ACS. Patients were determined ineligible for the following reasons: new ST-segment elevation ≥ 1mm, hemodynamic instability, life expectancy <1 year, a non-cardiac medical, surgical, or psychiatric illness determined by the provider to require admission, prior enrollment, non-English speaking, and incapacity or unwillingness to consent.
Data collection
Data elements were collected prospectively in accordance with standards of Good Clinical Practice, Standardized Reporting Guidelines (16), and Key Data Elements and Definitions (17). Electronic medical records (EMR) were used as the source for data elements reliably contained in the medical record. Study coordinators used electronic data collection templates to prospectively collect and store data from patients and care providers for data elements not reliably present in the EMR.
Follow up was conducted during the index visit using structured record review. At 30 days, a structured record review was followed by a telephone interview using a validated scripted follow-up dialogue to further clarify events since discharge, identify events occurring at other care facilities, and to determine health care utilization since discharge.(18) Outcome events reported at other health care facilities were confirmed using a structured review of those medical records. Incomplete follow up at 30 days was handled using the following algorithm: participants with ongoing visits in the EMR were considered to have complete information and were classified based on the data available in the medical record; participants with no ongoing visits were considered lost to follow up at the point of last contact. For patients enrolled in a clinical trial prior to March 2014, the Social Security Death Master File was used to search for participants unable to be contacted. In the event of discrepancy between a participant’s self-reported event and the medical record, the medical record was considered correct.
Patient Demographics and Risk Factors
For each patient, demographic data and the presence or absence of cardiac risk factors were determined by study coordinators and care providers using a standardized data collection tools. Demographics included age, gender, race (White, African American, or Other), and Ethnicity (Hispanic and Not Hispanic). Cardiac risk factors included hypertension, hyperlipidemia, diabetes, smoking, and Prior AMI or CAD. Patients were considered to have hypertension, hyperlipidemia, and diabetes based on a prior diagnosis and either diet control measures or current antihypertensive, cholesterol/lipid lower, or antihyperglycemic medication use. Current and former smoking was determined based on patient self-report. Prior AMI or CAD was defined by a history of prior AMI, coronary revascularization event (stent, angioplasty, or bypass graft), or angiography demonstrating >70% coronary stenosis.
Cardiac Troponin
Serum troponin measurements were performed using the institutional core-lab contemporary assay. Prior to 7/20/2011 the Beckman Coulter (Brea, California) Access® AccuTnI™ Troponin I Assay using the dxi800s platform was utilized. This assay had assay had a 99th percentile of the upper reference limit (URL) of 0.040 μg/L and 10% coefficient of variation (CV) at 0.060 μg/L. Following 7/20/2011 the ADVIA Centaur platform TnI-Ultra™ (Siemens, Munich, Germany) was used. This assay has a 99th percentile of the upper reference limit (URL) and 10% coefficient of variation (CV) at 0.040 μg/L. These troponin measures were obtained by the ED clinical team and these results were used for both clinical and research purposes.
Monocyte Chemoattractant Protein-1
Each clinical trial participant had a blood specimen collected for research purposes within 3 hours of enrollment. Blood was collected in a 10ml lithium heparin plasma or EDTA tube. Following collection, blood was centrifuged at 3000 ×g at 4 degrees Celsius for 15 minutes. Aliquots (1 ml) of plasma were transferred into cryovials and stored at −70°C until analysis. MCP-1 was measured in freshly first thawed samples using a commercially available enzyme-linked immunosorbent assay (ELISA) (Quantikine® Human CCL2/MCP-1 ELISA; R&D Systems, Minneapolis). Coefficients of variation for MCP-1 were <5.0 %.
Outcome Measures
Obstructive CAA was defined as angiographic demonstration of at least one coronary artery with ≥70% stenosis. Any CAA was defined by angiography demonstrating >0% stenosis in any coronary artery. Myocardial infarction was defined based on the Universal Definition of Myocardial Infarction; a gradual rise of cTnI above the URL with a CV <10% and gradual fall with at least one of the following: a. ischemic symptoms; b. new pathologic Q waves on the Electrocardiogram; c. acute ischemic Electrocardiogram changes (ST segment elevation or depression); d. myocardial imaging demonstrating a new regional wall motion abnormality.(19) Coronary revascularization was defined as coronary artery bypass grafting, stent placement, or other percutaneous coronary intervention. (19) Coronary revascularization was defined as coronary artery bypass grafting, stent placement, or other percutaneous coronary intervention.
Data Analysis
Descriptive statistics were calculated for each variable stratified by CAA status. These included counts and percentages for categorical variables and means and standard deviations for continuous variables. Next, simple logistic regression models were fit examining each risk factor separately in two models, one with obstructive CAA as the outcome and the second with any CAA as the outcome. For each model the odds ratio and corresponding 95% confidence interval for the risk factor of interest were estimated. Next, a backwards selection approach was used for each outcome (obstructive CAD and any CAD) to see which risk factors significantly predicted the outcomes in multiple logistic regression models. ROC curves were generated for the best models and the best models with MCP-1 removed. Predictive characteristics of these models – Area under the curve (AUC), integrated discrimination improvement (IDI) and net reclassification improvement (NRI) were estimated and used to compare the models to determine the incremental impact of MCP-1 in the “best models.”(20) AUC values were compared between models using methods described in DeLong et al.(21) The relationship between MCP-1 and AMI was explored using univariate logistic regression. Spearman Correlation was used to determine whether MCP-1 values were correlated with cTn values or patient age. Statistical analysis was performed using SAS 9.4 (Cary, North Carolina).
Results
Of the 150 patients selected for this cohort, by design, 50/100 (33.3%) had obstructive CAA, 50/150 (33.3%) had non-obstructive CAA (100/150, 66.7% had any CAA), and 50/100 (33.3%) had no angiographic evidence of CAA. During the index visit through the 30 day follow up period MIs occurred in 41/150 (27.3%) of patients with 39/150 (26.0%) of patients undergoing revascularization. Among patients with MI 30/41 (73.2%) occurred in patients with obstructive CAA, 7/41 (17.1%) in patients with non-obstructive (0–70% stenosis) CAA, and 4/41 (9.8%) in patients with no evidence of CAA on angiography (type II MIs). Among patients with revascularization events, 38/39 (97.4%) occurred in patients with obstructive CAA, and 1/39 (2.6%) occurred in a patient with 60% maximal coronary stenosis. Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of the cohort stratified by CAA
| Patient Characteristics | No CAA N=50 |
Non-Obstructive N=50 |
Obstructive CAA N=50 |
Cohort N=150 |
|---|---|---|---|---|
| Age—mean ±SD* | 50±10 | 55±10 | 59±11 | 55±10 |
| Sex | ||||
| Female | 30 (60.0%) | 22 (44.0%) | 17 (34.0%) | 69 (46.0%) |
| Race‡‡ | ||||
| White | 17 (34.0%) | 21 (42.0%) | 35 (70.0%) | 73 (48.7%) |
| African American | 27 (54.0%) | 22 (44.0%) | 12 (24.0%) | 61 (40.7%) |
| Other | 6 (12.0%) | 7 (14.0%) | 3 (6.0%) | 16 (10.7%) |
| Ethnicity | ||||
| Hispanic | 0 (0.0%) | 2 (4.0%) | 1 (2.0%) | 3 (2.0%) |
| Not Hispanic | 50 (100.0%) | 48 (96.0%) | 49 (98.0%) | 147 (98.0%) |
| Risk Factors | ||||
| Smoking | ||||
| Current | 12 (24.0%) | 10 (20.0%) | 14 (28.0%) | 36 (24.0%) |
| Former | 11 (22.0%) | 20 (40.0%) | 14 (28.0%) | 45 (30.0%) |
| Hypertension | 20 (40.0%) | 29 (58.0%) | 39 (78.0%) | 88 (58.7%) |
| Hyperlipidemia | 12 (24.0%) | 30 (60.0%) | 32 (64.0%) | 74 (49.3%) |
| Diabetes | 5 (10.0%) | 13 (26.0%) | 20 (40.0%) | 38 (25.3%) |
| Prior AMI or CAD | 2 (4.0%) | 9 (18.0%) | 19 (38.0%) | 30 (20.0%) |
CAA= Coronary Artery Atherosclerosis, SD = standard deviation, AMI = acute myocardial infarction, CAD = coronary artery disease
Increasing age, male sex, prior diagnosis of CAA or ACS, hypertension, hyperlipidemia, diabetes, were associated with increased odds of obstructive CAA or any CAA. White race and cTnI levels were associated with obstructive CAA, but were not predictive of any CAA. Univariate odds ratios for variables associated with obstructive CAA and any CAA are summarized in Table 2 and 3 respectively.
Table 2.
Univariate analysis for obstructive CAA
| Variables | Odds Ratio (95% CI) | p- value |
|---|---|---|
| MCP-1* | ||
| Per 10 pg/ml | 1.123 (1.062 – 1.188) | <0.0001 |
| Troponin | 1.313 (1.063–1.622) | 0.0114 |
| Age | ||
| Per 1 year | 1.065 (1.030–1.102) | 0.0003 |
| Sex | ||
| Female | 0.476 (0.235–0.962) | 0.0386 |
| Race | ||
| White | 3.99 (1.049, 15.193) | 0.0015 |
| African American | 1.061 (0.26, 4.326) | |
| Ethnicity | ||
| Hispanic | 1.000 (0.088–11.300) | 1.0000 |
| Risk Factors | ||
| Smoking | ||
| Current | 1.36 (0.587–3.149) | 0.718 |
| Former | 0.965 (0.43 – 2.167) | |
| Hypertension | 3.690 (1.699–8.014) | 0.0010 |
| Hyperlipidemia | 2.455 (1.218–4.948) | 0.0120 |
| Diabetes | 3.036 (1.417–6.504) | 0.0043 |
| Prior AMI/CAD | 4.959 (2.125–11.575) | 0.0002 |
MCP-1 = monocyte chemoattractant protein-1, pg/ml = pictogram/milliliter, AMI = acute myocardial infarction, CAD = coronary artery disease
Table 3.
Univariate analysis for any CAA
| Variables | Odds Ratio (95% CI) | p- value |
|---|---|---|
| MCP-1 | ||
| Per 10 pg/ml | 1.123 (1.055 – 1.197) | 0.0003 |
| Troponin | 1.468 (0.957–2.252) | 0.0784 |
| Age | ||
| Per 1 year | 1.074 (1.034–1.115) | 0.0002 |
| Sex | ||
| Female | 0.426 (0.213–0.853) | 0.0160 |
| Race | ||
| White | 1.976 (0.627 – 6.232) | 0.0378 |
| African American | 0.756 (0.244 – 2.342) | |
| Ethnicity | ||
| Hispanic | 1.0000 (0.001–999.99) | 0.986 |
| Risk Factors | ||
| Smoking | ||
| Current | 1.286 (0.552 – 2.993) | 0.272 |
| Former | 1.986 (0.863 – 4.575) | |
| Hypertension | 3.187 (1.575–6.449) | 0.0013 |
| Hyperlipidemia | 5.166 (2.406–11.096) | <0.0001 |
| Diabetes | 4.432 (1.608–12.211) | 0.0040 |
| Prior AMI/CAD | 9.33 (2.124–41.008) | 0.0031 |
MCP-1 = monocyte chemoattractant protein-1, pg/ml = pictogram/milliliter, AMI = acute myocardial infarction, CAD = coronary artery disease
A 10 pg/ml increase in MCP-1 measurement was associated with an odds ratio of 1.123 (95%CI: 1.062–1.188) for obstructive CAA and 1.123 (95%CI: 1.055–1.197) for any CAA. MCP-1 (10 unit change) remained a significant predictor of obstructive CAA (OR:1.149, 95%CI: 1.055 to 1.252, p=0.001) and any CAA (OR: 1.103, 95%CI: 1.020–1.192, p= 0.014) after adjustment for age, sex, race, traditional cardiac risk factors, and cTn. The reduced model derived using a backwards selection procedure for predicting obstructive CAA was significant for race, hyperlipidemia, cTn measurement, and MCP-1 value. The reduced model for any CAA included sex, hyperlipidemia, prior CAA or ACS diagnoses, and MCP-1 measurement. The C-statistics for models of obstructive CAA and any CAA with MCP-1 in the model were 0.863 and 0.829 respectively, compared to 0.817 and 0.756 without MCP-1 in the model. In each case, the chi-square test comparing the likelihood ratio statistics for the model with and without MCP-1 were highly significant (p<0.001) suggesting that the inclusion of MCP-1 significantly improved the goodness of fit of each model. Adding MCP-1 to the Obstructive CAD model produced an AUC improvement of 0.047, which was not statistically significant (p=0.10). However, the IDI and NRI for adding MCP-1 were both highly significant; 0.096 (p=0.001) and 0.52 (p=0.002) respectively. The addition of MCP-1 to the any CAD model resulted in significant improvements in AUC (0.074, p=0.01), IDI (0.114, p<0.0001) and NRI (0.72, p<0.0001). MCP-1 value was not predictive of AMI (OR: 1.042, 95%CI: 0.997–1.090, p=0.07). MCP-1 values did not correlate with cTn values (r=0.06, p=0.50), but were significantly correlated with each year of increasing age (r=0.37, p<0.0001).
Discussion
Results of this secondary analysis of patients in a registry of ED based chest pain clinical trials demonstrate that there was an association between MCP-1 measures and the presence of obstructive or any CAA. This association remained significant after adjustment for traditional CAA risk factors such as age, male sex, hyperlipidemia, prior diagnosis of ACS or CAA, and also cTnI measurement. While, the data from this analysis is promising, given mixed prior human studies and the small sample size of this study, further evaluation is needed to determine whether MCP-1 can play a useful role in the detection of CAA among ED patients with chest pain or be used to detect subclinical disease among the broader population.
MCP-1 is a chemotactic cytokine (chemokine) that is associated with inflammation and monocyte infiltration into tissues.(6) Prior studies in animals have demonstrated a compelling association between MCP-1 and CAA. MCP-1 is highly expressed in arterial atheromatous plaques and is a mediator of monocyte recruitment into the arterial intima where they become macrophages which ingest lipids and may eventually become foam cells.(22) Targeted deletion of MCP-1 in transgenic mouse models is associated with inhibition of atherosclerosis progression.(23,24) There is also evidence that MCP-1 likely plays a role in progression of atherosclerotic disease and may influence plaque instability and rupture.(25–27) For example, Register, et al., demonstrated that increased MCP-1 levels were associated with larger atherosclerotic plaque size and were strongly associated with indices of plaque inflammation and matrix remodeling in the coronary artery intima of female monkeys consuming a Western-like diet.(7)
Despite evidence from animal models of a strong association between MCP-1 and CAA, clinical data is mixed regarding the suitability of MCP-1 as a marker of human atherosclerosis. In the Dallas HEART Study, MCP-1 level was associated with CAC on computed tomography, but after adjustment for age, MCP-1 was no longer a significant predictor. (9) Tang, et al had similar findings in the NHLBI Family Heart Study cohort.(28) However, CAC on imaging does not always correlate with coronary stenosis and it is well known that arteries are more likely to become calcified with increasing age.(9) In another large study from the Atherosclerosis Risk in Communities cohort, MCP-1 was associated with peripheral arterial disease and coronary heart disease events. The association of MCP-1 with coronary events was independent of traditional cardiovascular risk factors including inflammatory markers, such as C-Reactive Protein, but the association with peripheral arterial disease was no longer significant when adjusted for C-Reactive Protein.(8)
While data on MCP-1 in subclinical CAA is mixed, MCP-1 does appear to have prognostic value among patients diagnosed with ACS. Higher MCP-1 values are associated with an increased hazard ratio for death and re-infarction.(29,30) In addition, MCP-1 may also have prognostic value among lower-risk patients without prior ACS. In a study of chest pain unit patients, Mitchell et al, found that a low MCP-1 measurement had a better negative likelihood ratio for ACS events than cTnT, C-Reactive Protein, and other candidate biomarkers.(31)
Our study adds to the existing literature by suggesting that there is a significant relationship between MCP-1 levels and angiographic coronary phenotypes among patients presenting to the ED with acute chest pain. If our finding can be confirmed in a larger study, it is possible that MCP-1 may be a valuable addition to the ED physicians risk stratification paradigm. While cTnI is an excellent marker for acute myocardial injury and infarction, when negative it does not rule out the presence of CAA. Current guidelines recommend objective cardiac testing (stress testing or coronary angiography) among patients presenting to the ED with acute chest pain without ST elevation on electrocardiogram or elevated cTnI measurements. However, stress testing and angiography are expensive, expose patients to radiation, or are invasive. It is possible that a marker of CAA could be used to further stratify which patients are likely or unlikely to benefit from these diagnostic tests or which type of testing is most appropriate.
Limitations
Small sample size and enrollment from a single medical center may limit the generalizability of this analysis. This registry included patients from multiple clinical studies which had similar inclusion criteria, but had differences in design and study procedures. Furthermore, these patients were a highly-selected group (ED patients with chest pain who consented to be included in a clinical study, agreed to having a stored blood sample, and underwent coronary angiography). Therefore, this cohort is not representative of an all comers ED chest pain population. In addition, while blood samples were stored on registry participants for the purpose of evaluating novel biomarkers, none of these trials were designed a-priori for an analysis of MCP-1. For this analysis patients from the registry were selected such that 50 had obstructive CAA, another 50 had non-obstructive CAA, and 50 had no angiographically detectable CAA. While this process was randomized, it is still possible that some selection bias occurred. Our model demonstrating that white race was a significant predictor of obstructive CAA, could be explained in part by confounding since white patients were an average of 8 years older than non-whites. However, white race remained a significant predictor of obstructed CAA even when adjusting for age.
Conclusions
MCP-1 was predictive of obstructive CAA and the presence of any CAA among ED patients with acute chest pain and known coronary phenotypes from angiography. MCP-1 remained predictive of obstructive and any CAA despite adjustment for age, sex, and traditional cardiovascular risk factors. This study requires confirmation in a larger cohort. If our study results are confirmed, it is possible that MCP-1 could serve as a valuable marker alongside cTnI for the early risk stratification of patients with acute chest pain. The potential ability of MCP-1 to predict CAA could enable more informed decisions regarding the need for objective cardiac testing modalities among patients with negative cTnI measures and Electrocardiograms.
Figure 1.
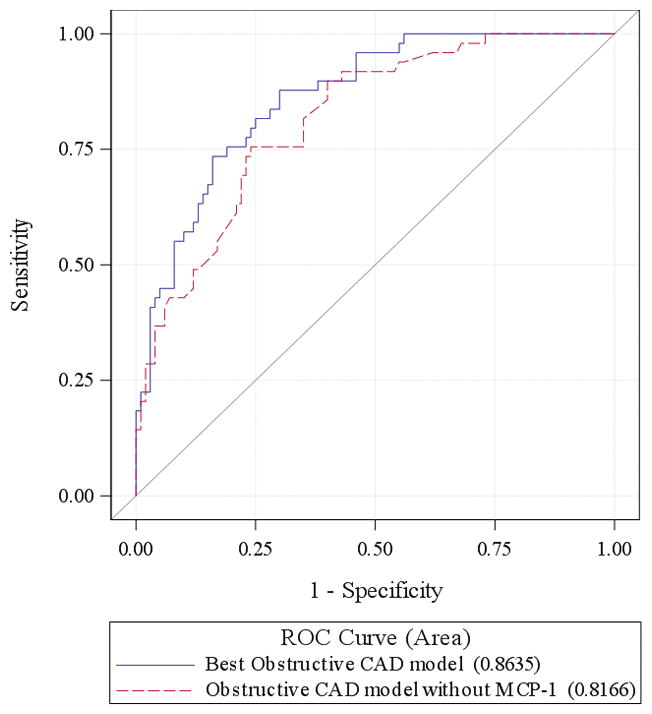
ROC curves for models of obstructive CAD with and without MCP-1.
Figure 2.
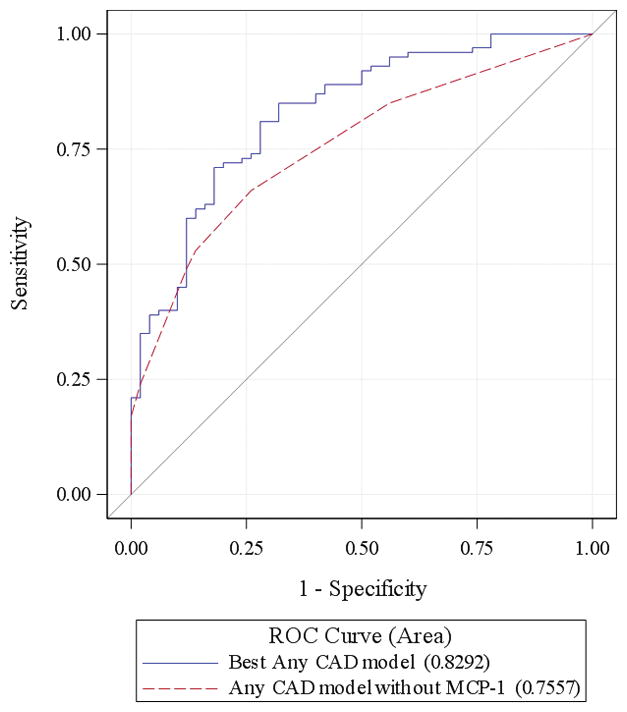
ROC curves for models of any CAD with and without MCP-1.
Acknowledgments
Funding: This study was funded by the Wake Forest Clinical Translational Sciences Institute; Ignition Pilot Award, the AHA Clinical Research Program (0980008N, 12CRP12000001 and 13CRP17090055), NHLBI (R01 HL118263, L30 HL120008).
Dr. Mahler also receives research funding from the AAMC/Donaghue Foundation, Duke Endowment, Abbott Point of Care, and Roche Diagnostics. Use of Research Electronic Data Capture was supported by the Wake Forest Translational Science Institute via a grant from National Center for Catalysis Research (M01 RR007122).
Dr. Mahler is the chief medical officer of Impathiq, inc.
This study was funded by the Wake Forest Clinical Translational Sciences Institute; Ignition Pilot Award, the AHA Clinical Research Program (0980008N, 12CRP12000001 and 13CRP17090055), NHLBI (R01 HL118263, L30 HL120008). Use of Research Electronic Data Capture was supported by the Wake Forest Translational Science Institute via a grant from National Center for Catalysis Research (M01 RR007122).
Footnotes
Reprints not available from the authors
Disclosures
Dr. Mahler also receives research funding from the AAMC/Donaghue Foundation, Duke Endowment, Abbott Point of Care, and Roche Diagnostics. Dr. Mahler has participated in an Advisory Board for Roche Diagnostics and is an author for UpToDate. Dr. Mahler is the chief medical officer Impathiq, Inc.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2008. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;60:1–94. [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 5.Diercks DB, Kirk JD, Naser S, Turnipseed S, Amsterdam EA. Value of high-sensitivity C-reactive protein in low risk chest pain observation unit patients. Int J Emerg Med. 2011;4:37. doi: 10.1186/1865-1380-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Quesada C, Frangogiannis NG. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Current atherosclerosis reports. 2009;11:131–8. doi: 10.1007/s11883-009-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Register TC, Cann JA, Kaplan JR, et al. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. The Journal of clinical endocrinology and metabolism. 2005;90:1734–40. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 8.Hoogeveen RC, Morrison A, Boerwinkle E, et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis. 2005;183:301–7. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–8. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circulation Cardiovascular quality and outcomes. 2015;8:195–203. doi: 10.1161/CIRCOUTCOMES.114.001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller CD, Case LD, Little WC, et al. Stress CMR reduces revascularization, hospital readmission, and recurrent cardiac testing in intermediate-risk patients with acute chest pain. JACC Cardiovasc Imaging. 2013;6:785–94. doi: 10.1016/j.jcmg.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller CD, Hwang W, Case D, et al. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging. 2011;4:862–70. doi: 10.1016/j.jcmg.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller CD, Hwang W, Hoekstra JW, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010;56:209–219. e2. doi: 10.1016/j.annemergmed.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CD, Thomas MJ, Hiestand B, et al. Cholesteryl esters associated with acyl-CoA:cholesterol acyltransferase predict coronary artery disease in patients with symptoms of acute coronary syndrome. Acad Emerg Med. 2012;19:673–82. doi: 10.1111/j.1553-2712.2012.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CD, Hoekstra JW, Lefebvre C, et al. Provider-directed imaging stress testing reduces health care expenditures in lower-risk chest pain patients presenting to the emergency department. Circ Cardiovasc Imaging. 2012;5:111–8. doi: 10.1161/CIRCIMAGING.111.965293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander JE, Blomkalns AL, Brogan GX, et al. Standardized reporting guidelines for studies evaluating risk stratification of emergency department patients with potential acute coronary syndromes. Ann Emerg Med. 2004;44:589–98. doi: 10.1016/S0196064404012806. [DOI] [PubMed] [Google Scholar]
- 17.Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes: A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American College of Emergency Physicians, American Heart Association, Cardiac Society of Australia & New Zealand, National Heart Foundation of Australia, Society for Cardiac Angiography and Interventions, and the Taiwan Society of Cardiology. J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 18.Kline JA, Mitchell AM, Runyon MS, Jones AE, Webb WB. Electronic medical record review as a surrogate to telephone follow-up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med. 2005;12:1127–33. doi: 10.1197/j.aem.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 22.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–7. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Molecular cell. 1998;2:275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 24.Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–8. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 2007;49:2073–80. doi: 10.1016/j.jacc.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 26.Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. European journal of immunology. 2002;32:404–12. doi: 10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Schecter AD, Rollins BJ, Zhang YJ, et al. Tissue factor is induced by monocyte chemoattractant protein-1 in human aortic smooth muscle and THP-1 cells. The Journal of biological chemistry. 1997;272:28568–73. doi: 10.1074/jbc.272.45.28568. [DOI] [PubMed] [Google Scholar]
- 28.Tang W, Pankow JS, Carr JJ, et al. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC cardiovascular disorders. 2007;7:30. doi: 10.1186/1471-2261-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lemos JA, Morrow DA, Blazing MA, et al. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol. 2007;50:2117–24. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 30.de Lemos JA, Morrow DA, Sabatine MS, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–5. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AM, Garvey JL, Kline JA. Multimarker panel to rule out acute coronary syndromes in low-risk patients. Acad Emerg Med. 2006;13:803–6. doi: 10.1197/j.aem.2006.03.553. [DOI] [PubMed] [Google Scholar]


